Abstract
Schizophrenia represents a complex, heterogeneous disorder characterized by several symptomatic domains that include positive and negative symptoms, and cognitive deficits. Negative symptoms reflect a cluster of symptoms that remains therapeutically unresponsive to currently available medications. Therefore, the development of animal models that may contribute to the discovery of novel and efficacious treatment strategies is essential. An animal model consists of both an inducing condition or manipulation (i.e., independent variable) and an observable measure(s) (i.e., dependent variables) that are used to assess the construct(s) under investigation. The objective of this review is to describe currently available experimental procedures that can be used to characterize constructs relevant to the negative symptoms of schizophrenia in experimental animals. While negative symptoms can encompass aspects of social withdrawal and emotional blunting, this review focuses on the assessment of reward deficits that result in anhedonia, avolition, and abnormal reward anticipation. The development and utilization of animal procedures that accurately assess reward-based constructs related to negative symptomatology in schizophrenia will provide an improved understanding of the neural substrates involved in these processes.
Keywords: Brain Reward, Animal Models, Effort-Related Choice, Sucrose Preference, ICSS, Contrast Effects
Introduction
In recent years, research has advanced our understanding of the neural substrates that contribute to the neuropathology of schizophrenia. However, there is a distinct lack in our ability to effectively manage the domain of negative symptoms evident in many schizophrenia patients. The negative symptoms domain includes deficit symptoms that reflect a reduction of emotions or behaviors usually present in the healthy population, such as anhedonia, avolition, flat affect or emotional blunting, reductions of verbal fluency or poverty of speech, and defects in social behavior. This cluster of symptoms is largely unresponsive to current antipsychotic medications. The severity of negative symptoms has been suggested to impact and predict the functional and occupational outcome of schizophrenia patients (Ho et al., 1998; Kirkpatrick et al., 2006). Because of the detrimental effects of negative symptoms, coupled with the unmet therapeutic need in addressing this symptomatic domain, much research in recent years has focused on improving the understanding and treatment of negative symptoms associated with schizophrenia (Kirkpatrick et al., 2006).
The purpose of the present review is to describe experimental animal procedures that allow investigators to evaluate constructs thought to be central to the negative symptomatology of schizophrenia. Various experimental manipulations used to induce deficits in reward processes will be discussed briefly, but the focus will be primarily on describing the specific behavioral tasks used to characterize these deficits. Anhedonia, avolition, emotional blunting, alogia, and social withdrawal all fall under the umbrella that encompasses negative symptoms. Some of these features may not be mutually exclusive (e.g., avolition may promote social withdrawal), and some are difficult to assess in animals (e.g., emotional blunting and alogia). Procedures that model social withdrawal in experimental animals are reviewed elsewhere in this Special Issue (Koenig and colleagues, this issue).
Reward Deficits and Anhedonia in Schizophrenia Patients
Anhedonia refers to the inability or diminished capacity to experience pleasure and is thought to reflect deficits in brain reward system function. Anhedonia has long been suggested to be a core symptom of schizophrenia, falling within the cluster of features that encapsulates the negative symptomatology associated with schizophrenia. Anhedonia was originally described by Kraeplin in his first descriptions of dementia praecox (later come to be known as schizophrenia) as a central component of the disorder (Kraepelin, 1921; Bleuer, 1924; Strauss and Gold, 2012), and it is included as a symptom in the Diagnostic and Statistical Manual of Mental Disorders (DSM-V) (American Psychiatric Association, 2013). The definition of anhedonia is sometimes extended incorrectly to encompass additional deficits in reward-related processes, such as the pursuit of pleasure. This extension of the definition of anhedonia should be avoided because the ability to experience pleasure and the desire to engage in pleasurable activities are subserved by distinct neural pathways (Berridge and Robinson, 2003; Der-Avakian and Markou, 2012) and should be treated as separate constructs. Interview-based measures or self-report style questionnaires are often used to assess anhedonia in schizophrenia patients. However, it has been suggested recently that schizophrenia is not associated with diminished capacity to experience pleasure per se (Horan et al., 2006), but rather may reflect deficits in other components of the reward system. Deficits in brain reward function are multifaceted and can include other features in addition to hedonic capacity, such as altered reward prediction, anticipation or valuation. Deficits in any one of these processes may lead to a reduction in the engagement of pleasurable activities, even when the individual still has the capacity to experience pleasure. Given the dissociation between anhedonia and other deficits of brain reward systems, anhedonia may not be as central to schizophrenia as it was once thought to be. Schizophrenia patients have been reported to indeed have the ability to experience affective consummatory pleasure (Gard et al., 2007; Heerey and Gold, 2007), indicating that schizophrenia patients are not necessarily anhedonic. Rather, these patients may have a dissociation of hedonic capacity from motivated behavior (Heerey and Gold, 2007), deficits in reward anticipation (Dowd and Barch, 2012; Gard et al., 2007), and/or a reduced ability to draw upon memories of previous pleasurable activities (Simpson et al., 2012). Deficits in accurately recalling previously pleasurable activities or predicting pleasure from future events likely gives the impression of a global reduction of the capacity to experience pleasure. As a result, these deficits may elicit the false impression of anhedonia, reflected in interview-based questionnaires, while not necessarily being attributable to deficits in the pleasure systems of the brain that mediate hedonic reactions.
Motivational Deficits and Avolition in Schizophrenia Patients
Like anhedonia, a deficit in motivational capabilities, or avolition, is also a symptom included in the original descriptions of schizophrenia (Kraepelin, 1921; Foussias and Remington, 2010). Deficits in a patient’s ability to become motivated and engage in a given task has been suggested to considerably affect their quality of life, leading to a reduced drive to initiate or persist in goal-directed behavior (Barch and Dowd, 2010). Moreover, the severity of these deficits is believed to be closely correlated with the functional outcome of schizophrenia patients (Simpson et al., 2012). In fact, reductions of volition or impairments in the patient’s motivational capabilities have been suggested to be not only one of the most disabling facets of schizophrenia but is also likely to underpin some of the accompanying negative symptoms, such as social withdrawal, and cognitive impairments associated with schizophrenia (Brebion et al., 2009).
To address this fundamental aspect of negative symptomatology and understand the process of avolition, one must explore the factors that drive an organism to become motivated. Reward processing has many facets, including aspects of learning, reward valuation, emotional or affective factors, and purely motivational components (Berridge and Robinson, 2003; Gold et al., 2008). Two of these elements may be particularly important to motivated behavior. First is the affective component. If an organism derives pleasure from something, then it is more likely to expend energy to obtain it (Salamone, 2009). Thus, consummatory pleasure is conducive to motivation because it provides the hedonic component that drives motivated behavior. However, as described above, evidence suggests that the hedonic reactions to evoked stimuli are unimpaired in schizophrenia, and the affective response is still experienced (Gard et al., 2007). Importantly, in addition to consummatory pleasure, incentive motivation contributes to the anticipation of pleasure that will be derived or experienced when future activities are undertaken, and thus guides goal-directed behaviors. This incentive or anticipatory component of motivation ensures that goals are formulated, maintained, and ultimately completed (Barch and Dowd, 2010; Simpson et al., 2012). This construct likely provides the basis for what is traditionally thought of as “motivation,” with deficits in the level of anticipatory motivation reducing the drive to engage in pleasurable activities or social pursuits. Improving the understanding of the neural substrates that underlie motivation and therefore the deficits within this system is essential for the development of effective treatment strategies to attenuate not only avolition but perhaps also additional impairments, such as social withdrawal or cognitive impairments, to which avolition may contribute.
Experimental Animal Procedures
Numerous approaches have been used to induce neurobiological and behavioral abnormalities in experimental animals that are likely to have relevance to schizophrenia. Such manipulations include, but are not limited to, pharmacological models (e.g., targeting dopamine or N-methyl-D-aspartate [NMDA] receptors (Howes and Kapur, 2009; Neill et al., 2010)), neurodevelopmental models (e.g., neonatal phencyclidine [PCP] treatment, neonatal hippocampal lesions, or methylazoxymethanol acetate (Wang et al., 2001; Lipska et al., 2002; Lodge and Grace, 2007)), and various genetic manipulations (for review, see O’Tuathaigh and Waddington, 2010), such as dopamine transporter knockouts (Rodriguiz et al., 2004), dopamine D1 receptor knockouts (Halberstadt and Geyer, 2009), metabotropic glutamate receptor 5 knockouts (Brody et al., 2004), α7-nicotinic acetylcholine receptor knockouts (Young et al., 2011) and dopamine D2 receptor overexpression (Kellendonk et al., 2006).
Once an inducing condition is selected, investigators must also determine the measures that will reflect constructs relevant to the human condition because a model consists of both an inducing condition and the measures that are assessed (see Table 1) (Geyer and Markou, 1995; Markou et al., 2009). Attempting to measure aspects of neuropsychiatric disorders, including schizophrenia, is challenging because these complex disorders have some inherently human aspects, with many facets of the disease that are difficult or impossible to replicate in an animal model. The intrinsically human features of schizophrenia, such as reduced verbal fluency and poverty of speech, is not amenable to modeling in experimental animals. Nevertheless, advances in the assessment of ultrasonic vocalizations in rodents may begin to facilitate investigation into this symptom area. While the remaining symptoms of the disease are readily open to experimental assessment in animals (Ellenbroek and Cools, 2000; Young et al., 2009), researchers are not likely to have the advantage of a “schizophrenia model” that encompasses all dimensions of the disorder (Markou et al., 2009). Instead, researchers may find that focusing on individual constructs (e.g., motivation or attention) and developing procedures that recruit specific constructs are advantageous. However, the specific construct must be accurately assessed in animal procedures, recruiting the same neural substrates as those engaged in humans. If this correspondence in neurosubstrates is achieved, then the translational and predictive validity between species is improved and provides the potential to overcome the translational bottleneck that currently exists between the preclinical and clinical disciplines (Hyman and Fenton, 2003).
Table 1.
Procedures and manipulations used to assess reward-related deficits
| Behavioral Construct | Procedure | Manipulation | Measure | References |
|---|---|---|---|---|
| Anhedonia | Sucrose consumption | Acute PCP (up to 20 mg/kg) | Decreased sucrose intake | Baird et al., 2008 |
| Chronic intermittent PCP (2.58 mg/kg) | No effect | J. Pratt, Personal Communication | ||
| Sub-chronic PCP (2 – 5 mg/kg) tested after washout | No effect | Jenkins et al., 2010 | ||
| Hedonic facial reactions to sucrose | Striatal dopamine D2 over expression | No effect | Ward et al., 2012 | |
| ICSS | Cocaine withdrawal (self-administration procedure) | Elevated ICSS threshold | Markou and Koob, 1991 | |
| Amphetamine withdrawal (up to 15 mg/kg/day experimenter-administered) | Elevated ICSS threshold | Paterson et al., 2000 | ||
| PCP withdrawal (up to 20 mg/mg/day) | Elevated ICSS threshold | Spielewoy and Markou, 2003 | ||
| Avolition | Fixed ratio | Nucleus accumbens dopamine depletion | No effect at low FR, impaired high FR performance | Aberman and Salamone, 1999 |
| Progressive ratio | Striatal dopamine D2 over expression | Lowered breakpoint | Drew et al., 2007; Simpson et al., 2011 | |
| Amphetamine withdrawal (escalating dosing regimen 1 – 10 mg/kg or 10 mg/kg/day for 7 days) | Lowered breakpoint for sucrose (solution and pellet) | Barr and Philips, 1999; Der-Avakian and Markou, 2010 | ||
| Dopamine D1 receptor KO | Impaired learning operant procedure | Young and Geyer, 2010 | ||
| Operant effort-related choice | Nucleus accumbens dopamine depletion | Reduced willingness to work for preferred reward | Salamone et al., 1991 | |
| Systemic haloperidol injection (0.1 mg/kg) | Reduced willingness to work for preferred reward | Salamone et al., 1991 | ||
| Striatal dopamine D2 over expression | Reduced willingness to work for preferred reward | Ward et al., 2012 | ||
| T-maze effort-related choice | Systemic haloperidol injection (0.1 mg/kg) | Biased towards LR arm | Salamone et al., 1994 | |
| Nucleus accumbens dopamine depletion | Biased towards LR arm | Salamone et al., 1994 | ||
| Chronic intermittent PCP administration (2.58 mg/kg) | Biased towards LR arm | J. Pratt, Personal Communication | ||
| Anticipatory Deficits | Anticipatory locomotion | Withdrawal from escalating amphetamine schedule (1 – 12 mg/kg) | Reduced anticipatory side changes, increased post-ejaculatory intervals, no effect on copulatory behavior | Barr et al., 1999 |
| Successive negative contrast | Withdrawal from escalating amphetamine schedule (1 – 10 mg/kg) | Exaggerated negative contrast effect | Barr and Philips, 2002 | |
| Successive positive contrast | Withdrawal from escalating amphetamine schedule (1 – 10 mg/kg) | Failure to develop successive positive contrast | Vacca and Philips, 2005 |
Procedures that Assess Anhedonia
Sucrose preference, consumption, and affective response
When rodents are given a free choice between water and a sucrose solution, a strong preference for the palatable sucrose solution typically develops (Muscat and Willner, 1989). This preference for sucrose may indicate that rodents derive pleasure from drinking a sucrose solution or that sucrose consumption provides an element of reward or reinforcement that encourages the animal to choose sucrose over water. Therefore, the degree of sucrose consumption may be utilized in investigations of hedonic capacity, with reductions of total sucrose consumed perhaps being an indicator of alterations in reward system functioning, potentially reflecting an anhedonic state. One method of assessing sucrose preference is to singly house rodents in a cage with two drinking bottles. One bottle contains normal drinking water while the other contains a sucrose solution. The side that contains the sucrose solution should be counterbalanced and alternated to avoid the development of a side preference (Bergner et al., 2010). Various concentrations of sucrose can be used [(0.7–34% have been reported; (Muscat et al., 1991), but typically 1–2% is used (Overstreet, 2012)], resulting in an inverted-U dose-response curve. Consumption from both bottles is assessed over the course of the experiment (e.g., 1–7 days) to determine the preference for the bottle that contains the sucrose solution. Rodents typically develop a strong preference for the sucrose solution, and more liquid from that bottle is consumed. Rodents that display anhedonic traits via pharmacological, developmental or genetic manipulations show less interest in the sucrose-laced water and a preference score of less than 65% is observed (Strekalova et al., 2004), or simply have reductions of the volume of sucrose solution consumed. This is thought to indicate a deficit in the ability to experience the hedonic properties of sucrose consumption. An alternative method of assessing sucrose consumption or preference is to employ a device that quantifies the number of licks the rodents make during the experimental session. These commercially available lickometers provide a means of easily determining the rate of sucrose consumption (e.g., licks/min).
Studies have indicated that acute exposure to the NMDA receptor antagonist PCP reduced sucrose intake in rats (Turgeon and Hoge, 2003; Baird et al., 2008), suggesting that PCP treatment may result in transient impairment in hedonic capacity. However, reduced sucrose intake occurred after administration of a relatively high dose of PCP (up to 20 mg/kg). Conversely, both chronic intermittent or sub-chronic PCP treatment regimens, utilizing a much lower PCP dose (2.58 or 2 – 5mg/kg), and in the case of the sub-chronic regimen tested after withdrawal, resulted in no alterations in sucrose preference (Jenkins et al., 2010; J. Pratt, Personal Communication). These findings indicate that consummatory hedonic capacity remained intact using PCP treatment regimens that have been validated previously (Jentsch and Roth, 1999; Pratt et al., 2008; Neill et al., 2010) for inducing various cognitive, metabolic and neuropathological changes in rats with relevance to schizophrenia.
Alternatively, assessment of facial reactions after consumption of sucrose has been suggested to reflect the affective reaction to sucrose. Berridge (2000) described that facial responses elicited when sucrose was squirted into a human newborn baby’s mouth consisted of a positive (or hedonic) pattern of lip smacking, tongue protrusions, and relaxation of the facial muscles. The proposed hedonic facial response to sucrose is conserved among species and evident in nonhuman primates, rats and mice (Berridge, 2000; Berridge and Robinson, 2003). While the interpretation of elicited hedonic response is somewhat subjective, experimenters have successfully used this methodology to assess hedonic capacity in rodents. Ward et al. (2012) observed a dose-dependent increase in both consumption rates, and the number of positive facial reactions in both control and dopamine D2 receptor-overexpressing mice as the concentration of sucrose was increased (0.01–1.0 M). These findings suggest that both groups of mice experienced a hedonic response to sucrose. Nonetheless, the validity of inspecting facial expressions in response to a sweet solution has not been evaluated in schizophrenia patients. If this approach is to be utilized clinically, safeguards to limit the risk of experimenter bias due to the subjective nature of the measures should be employed.
Numerous potential limitations are associated with comparing the preference for or consumption of sucrose in rodents with the negative symptomatology observed in schizophrenia, thus limiting the validity of this procedure. First, the sucrose preference test is often used to model anhedonia in reference to clinical depression. As such, a reduction of sucrose preference induced by chronic mild stress is sensitive to attenuation by the chronic administration of antidepressant medications (Willner et al., 1987). However, the benefit of antidepressant treatment in schizophrenia patients remains unclear, with potential advantages only observed when antidepressants are used as adjunctive therapy with antipsychotic medications (Rummel et al., 2006). Second, typical and atypical antipsychotic medications can induce substantial weight gain (Allison et al., 1999). Thus, any potential increase in sucrose preference after antipsychotic treatment would require dissociation of the putative reward-enhancing effects of the medication versus the increased preference for the higher caloric content of the sucrose solution. As an alternative, the preference for a non-caloric saccharin solution may help resolve this issue, because the preference for saccharin occurs independent from alterations of blood glucose levels (Stefurak and Van der Kooy, 1992). Third, the hedonic response to a sucrose solution in humans, assessed by a sweetness rating scale, was similar between schizophrenia patients and healthy volunteers (Berlin et al., 1998). Therefore, models that induce a decrease in sucrose preference or consumption in experimental animals may not necessarily be directly comparable to the deficits, or lack thereof, observed in schizophrenia patients. Using this procedure when modeling negative symptoms should therefore be limited to a negative control, useful in ensuring that the inducing condition utilized does not impair consummatory hedonic reactions and thus impeding translational validity.
Intracranial self-stimulation
The intracranial self-stimulation (ICSS) procedure, originally developed by Olds and Milner (1954), provides an effective methodology to evaluate various pharmacological, environmental, and molecular manipulations on brain reward function in vivo. The procedure involves surgery to implant an electrode into the desired brain region involved in reward function. Commonly, researchers target the lateral hypothalamus or medial forebrain bundle. The animals are then trained to respond on a manipulandum, often a wheel or a lever, located in the operant testing chamber that controls the delivery of the electrical stimulation through the electrode. Then, determination and stabilization of reward thresholds is completed using one of the procedures described below (Markou and Koob, 1993; Stoker and Markou, 2011).
While several methodologies are available for ICSS studies (Liebman, 1983), many have not been validated or may not provide measures of reward function uncontaminated by other processes, such as motor function (Vlachou and Markou, 2010). The greatest limitation of several of these methodologies is that performance assessed by response rates is affected by the subject’s ability to perform the task, and thus does not provide a measure of reward function independent of motor ability (Markou and Koob, 1992). Two validated procedures for assessing reward function in a reward-specific manner include the discrete-trial current-intensity procedure (Esposito and Kornetsky, 1977; Markou and Koob, 1992) and the rate-frequency curve-shift procedure (Gallistel and Freyd, 1987; Carlezon and Chartoff, 2007). Training in the discrete-trial current-intensity procedure begins with a noncontingent reinforcing electrical stimulus being delivered, priming the subject to respond on the manipulandum to receive a second, contingent electrical stimulus that is identical to the noncontingent stimulus. Various current intensities are delivered (typically varied by 5 μA increments) and presented in alternating ascending and descending orders. As the noncontingent current intensity is increased and decreased, the presence or lack of a contingent response, respectively, reflects the animal’s reward threshold, or the minimal current intensity required to elicit a behavioral response (Markou and Koob, 1992; Markou and Koob, 1993; Stoker and Markou, 2011). Current frequency may also be manipulated in this procedure.
The rate-frequency curve-shift procedure provides a different method of assessing reward thresholds. Delivery of the noncontingent stimulus is followed by a response window (e.g., 50 s), during which the number of contingent responses emitted is recorded. After a post-trial timeout period (e.g., 5 s), the frequency of the stimulation is decreased and the next trial begins. Response rates typically vary depending on stimulation frequencies, suggesting a frequency “dose-response” curve (Campbell et al., 1985; Carlezon and Chartoff, 2007). Using this method, the minimal stimulation frequency that sustains responding at a predetermined (arbitrary) rate or an extrapolated “true” frequency threshold that supports responding reflects the animal’s reward threshold (Carlezon and Chartoff, 2007).
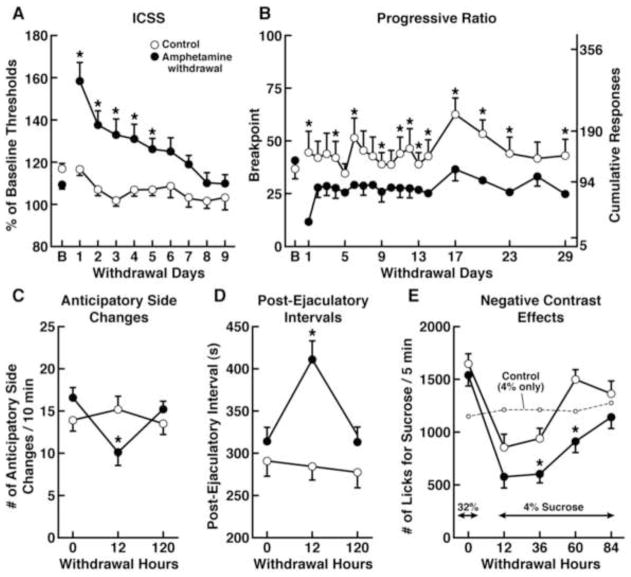
Several parameters are assessed in the ICSS procedure that provide insights into the affective state and motoric ability of the animal (Markou and Koob, 1992). Manipulations that lower the reward threshold (either current or frequency) are suggested to reflect reward enhancement by potentiating brain reward function, such that a lower stimulus intensity is required to elicit the contingent behavioral response for stimulation. For example, cocaine administration is known to be rewarding in humans and lowers reward thresholds in rats (Kornetsky and Esposito, 1979; Markou and Koob, 1992). Conversely, manipulations that elevate reward thresholds reflect deficits in reward function because an increased stimulus intensity is now required to elicit the same behavioral response that was previously elicited at lower current intensities or frequencies under baseline conditions. For example, withdrawal from chronic administration of psychomotor stimulants, including cocaine (Markou and Koob, 1991), amphetamine (Paterson et al., 2000; figure 1A), or PCP (Spielewoy and Markou, 2003), results in ICSS threshold elevations. Importantly, such threshold elevations occurred independently of changes in response latencies, suggesting that ICSS performance alterations were specifically attributable to the depression of brain reward function and not the result of non-reward-specific performance impairment (see below discussion about response latencies). Psychostimulant drug withdrawal is often used to induce an anhedonic depression-like state in experimental animals because psychostimulant withdrawal in humans resembles a major depressive episode, including the expression of anhedonia (American Psychiatric Association, 2000; Barr et al., 2002; Barr and Markou, 2005).
Figure 1.
Impairments relating to various components of reward processing are evident after withdrawal from chronic amphetamine administration. (A) Rats displayed an elevation of ICSS thresholds, reflecting a reward deficit, after withdrawal from chronic amphetamine administration (closed circles), compared to saline-treated controls (open circles). B: Baseline [Modified with permission from Paterson et al., 2000]. (B) Withdrawal from chronic amphetamine treatment significantly reduced the breakpoint for a sucrose pellet in a progressive-ratio schedule task, reflecting a motivational deficit, an effect that persisted for up to 29 days. B: Baseline [Modified with permission from Der-Avakian and Markou, 2010]. (C) Rats trained to anticipate the introduction of a sexually receptive female displayed fewer anticipatory side changes during amphetamine withdrawal, compared to saline-treated controls, reflecting an anticipatory deficit [Taken with permission from Barr and Markou, 2005, and originally from Barr et al., 1999]. (D) Amphetamine withdrawal was also associated with an increased post-ejaculatory interval, reflecting a reduction in an additional component of motivated sexual behavior [Taken with permission from Barr and Markou, 2005, and originally from Barr et al., 1999]. (E) Withdrawal from an escalating amphetamine treatment schedule produced alterations in a successive negative contrast procedure in rats, reflecting an increased negative reaction to reward devaluation. Amphetamine withdrawal significantly reduced consumption of a sucrose solution when the concentration was unexpectedly downshifted (from 32% to 4%) compared to saline-treated rats, and significantly increased the time to return to the consumption levels of control rats [Taken with permission from Barr and Markou, 2005, and originally from Barr and Phillips, 2002].
As previously indicated, ICSS performance may reflect alterations in the motor activity of the experimental subject. The latency between the noncontingent stimulus that initiates each trial and the contingent response provides one indication of the performance capabilities of the animal, with nonspecific performance impairments increasing the response latency (Markou and Koob, 1992). In the rate-frequency procedure, changes in the maximal rate of responding are often indicative of performance deficits. Thus, in both of these procedures, reward thresholds can be calculated that are independent of response rates (Markou and Koob, 1993; Carlezon and Chartoff, 2007).
Procedures that Assess Avolition
Fixed- and progressive-ratio schedules of reinforcement
Procedures to assess the motivational state or willingness to work of an animal often involve responding on an fixed-ratio (FR) or progressive-ratio (PR) schedule of reinforcement (Arnold and Roberts, 1997). When presented with an FR schedule, the animal must press a lever a fixed number of times (e.g., FR1, FR5, etc.) to receive the rewarding stimulus (e.g., food or reinforcing drug). Lower FR schedules can be used to assess hedonic capacity or reward valuation (i.e., reduced desire to obtain the reward may influence responding). Higher FR schedules can be used to assess volition because a greater amount of work is required to obtain the reward (Salamone et al., 2003). Dopamine transmission in the nucleus accumbens is involved with high ratio requirements. Specifically, no difference in the number of lever presses was observed between control and nucleus accumbens dopamine-depleted rats when the FR schedule was low (e.g., FR1 and FR4), suggesting that the desire for food reinforcement was intact. However, when the work load increased (e.g., FR16 and FR64), control rats continued to work for the food reward, whereas nucleus accumbens dopamine-depleted rats did not (Aberman and Salamone, 1999; Salamone et al., 2007).
In a PR schedule, after the delivery of the rewarding stimulus, the response requirement to obtain each subsequent reward exponentially increases. The animals are first trained on a low FR schedule (e.g., FR1, FR2, and then FR5), enabling the animal to associate a lever press with reward retrieval. Once this association is established, the animal is moved to the PR schedule. Various incremental schedules are utilized in different laboratories when conducting PR schedules (e.g., FR is doubled or increased exponentially). Independent of the response requirement progression used, the consistent factor in such schedules is that the response requirement to earn the subsequent reward increases as the PR schedule progresses (Richardson and Roberts, 1996). As such, a greater degree of effort is required to earn the next reward, and alterations in the motivational state of the animal can influence the point at which the animal stops responding, a measure known as the breakpoint (Hodos, 1961). The breakpoint reflects the maximal amount of effort the animal is willing to expend to earn a single rewarding stimulus (Richardson and Roberts, 1996). Some investigators choose to let the PR session continue until the animal stops responding within a specific time period (usually 5–60 min, depending on the type of reinforcer), with the last ratio completed before the animal stopped responding being the breakpoint (Roane, 2008). Alternatively, some investigators utilize sessions with a fixed duration (e.g., 1 h), and the last ratio completed before the session expires is deemed the breakpoint.
Highly reinforcing drugs, such as psychomotor stimulants, support high breakpoints in self-administration PR schedules in a dose-dependent manner (Bedford et al., 1978). However, an inverted-U dose-response curve may be observed because drugs at high doses can become aversive (Roberts and Richardson, 1993; Paterson and Markou, 2005). This pattern of behavior suggests that animals are highly motivated and willing to work to receive psychomotor stimulant drugs. Conversely, a lowering of the breakpoint is often interpreted as either a lowering of the motivational state of the animal or a reduction of the reinforcing properties of the reward stimulus, resulting in a reduced desire to work for the stimulus. Care must be taken when interpreting alterations in breakpoint, however. A lower breakpoint may be attributable to motor impairments that result in an impaired ability to sustain lever-pressing for the extended duration of the session, or difficulties learning the procedure. For example, dopamine D1 receptor KO mice exhibited poor performance in a progressive ratio schedule, due to a failure to learn to respond to a FR1 schedule and not due to altered motivational capabilities (Young and Geyer, 2010). Alternatively, a low breakpoint may be attributable to reduced tolerance for the ever increasing delay between reward presentations. Reductions of the breakpoint in dopamine D2 receptor-overexpressing mice have been demonstrated (Drew et al., 2007; Simpson et al., 2011). To identify whether intolerance for the delay mediated this reduction, Simpson et al. (2011) elegantly increased the delay between reward presentations to mirror the concomitant delay in the PR schedule, rather than increasing the number of lever presses required. No differences in the number of rewards earned in this session were observed. Thus, the lowered breakpoint observed in these mice during the PR schedule was unlikely to reflect intolerance for the increased temporal delay, but may have reflected decreased willingness to work.
Withdrawal from repeated amphetamine administration decreased the breakpoint for a 4% sucrose solution (Barr and Phillips, 1999) or a sucrose pellet (Der-Avakian and Markou, 2010; figure 1B) in rats, suggesting a reduction of the motivation to obtain the sucrose reward. However, a potential caveat with this conclusion is that dissociating a reduction of the breakpoint that is driven by a reduction of the valuation of the sucrose reward (or potentially the hedonic response to sucrose) or a reduction of the desire to expend energy to obtain the sucrose reward (i.e., the drive to work to obtain sucrose) is difficult. Barr and Philips (1999) suggested that the effects observed on the breakpoint after cessation of amphetamine treatment are consistent with the elevations in ICSS thresholds in rats exposed to an identical or similar drug regimen (Leith and Barrett, 1976; Cassens et al., 1981; Paterson et al., 2000). Thus, anhedonia in this case, or at least the hedonic component of motivated behavior, may indeed play a significant role in the lowering of the rats’ breakpoint. The assessment of sucrose preference or the quantification of affective facial responses described above may be used to determine whether the capacity to experience hedonic reactions is still intact after amphetamine withdrawal. As previously discussed, schizophrenia patients may indeed experience evoked hedonia, but have deficits in motivated behavior (Heerey and Gold, 2007). It is suggested that hedonic experience and anticipatory motivation are subserved by distinct neural pathways (Berridge and Robinson, 2003). Therefore, dissociating between these two aspects of reward/motivation is important in experimental animal procedures (Simpson et al., 2012; Ward et al., 2012).
Effort-related choice procedures
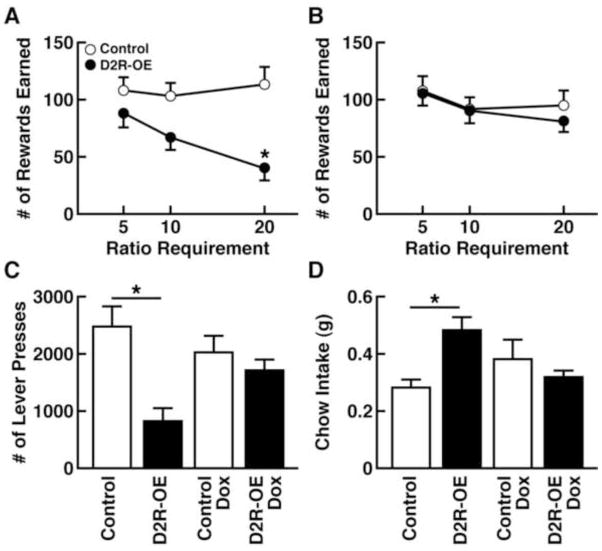
Motivation can be further assessed using an operant-based effort-related choice procedure adapted from standard PR schedules of reinforcement. Such tasks determine the willingness to work for a preferred reward as the response requirements increase, compared with the desire to consume a less-preferred, yet freely available, reward (Salamone et al., 1991). The difference in the intrinsic value of the reward options introduces an element of cost/benefit assessment to determine whether the reward is worth the amount of work required to obtain it. Given the choice, rodents prefer to consume a highly palatable reward (e.g., sucrose pellets or evaporated milk powder) over standard laboratory chow (Salamone et al., 1991; Ward et al., 2012). This preference persists even when the rodent must press a lever on a low FR schedule (e.g., FR1 or FR5) to obtain the more-preferred reward, although the lab chow is still freely available (Salamone et al., 1991). However, alterations in the willingness to work for the preferred reward begin to emerge when the level of work required to obtain the preferred reward increases. When the FR is increased to FR10 or FR20, the rodents consume less of the preferred reward and opt to eat the freely available lab chow (Salamone et al., 1997; Salamone et al., 2007). Salamone and colleagues (1991) demonstrated that depletion of dopamine from the accumbens, or systemic administration of the dopamine D2 receptor antagonist, haloperidol, biased rodents away from the preferred reward, indicating cost/benefit assessment is sensitive to dopaminergic manipulations. Ward and colleagues (2012) demonstrated that control mice and D2 receptor-overexpressing mice both earned comparable numbers of the desirable reward when the work required was minimal. However, instead of using an FR schedule, Ward and colleagues (2012) utilized a random ratio (RR) schedule that varied the number of lever-presses required for each reward. When an average number of five responses was required for reward delivery (RR-5), both groups of mice chose to work for the highly palatable reward rather than settling for the lab chow. However, as the level of work required increased to RR-20, the number of desirable rewards earned by control mice remained constant, whereas the amount of desirable rewards earned by the D2 receptor-overexpressing mice was reduced (see Fig. 2A), an effect that was absent when the transgene was turned off (see Fig. 2B). The low number of lever presses for the desirable reward in D2 receptor-overexpressing mice (see Fig. 2C) was accompanied by increased consumption of the freely available lab chow (see Fig. 2D). These data suggest that striatal D2 receptor-overexpressing mice were less willing to work for the more preferred reward when the level of work required increased, instead preferring to settle for the less desired, freely available reward (Ward et al., 2012).
Figure 2.
Overexpression of the dopamine D2 receptor (D2R-OE) in the striatum of mice resulted in reduced willingness to work for a desirable reward as the workload was increased compared with a less desirable, yet freely accessable, reward. (A) As the workload increased from Ratio Requirement 5 (RR-5) to RR-20, the number of preferred rewards earned by the control mice (open circles) remained relatively constant. However, the D2R-OE mice (closed circles) earned fewer rewards with increasing workload. (B) When the transgene was turned off, no differences were observed between groups of mice in the number of rewards earned, confirming that the reduction in the willingness to work was due to overexpression of striatal dopamine D2 receptors. (C) Performance during the RR-20 session was significantly decreased in D2R-OE mice, exemplified by the decrease in lever presses during this high workload session. (D) D2R-OE mice consumed more chow during the RR-20 session compared to control mice, confirming that the impaired performance of D2R-OE mice was not due to decreased appetite or hedonic capacity for food. Figures modified with permission from Ward et al., 2012.
While many of the same putative confounds that exist in progressive ratio experiments may also exist for effort-related choice procedures, this procedure may more readily address the potential confound of satiety. It is possible that lowered breakpoints may reflect changes in satiety rather than reduced willingness to work. However, the choice procedure may avoid this confounding issue in a way not available in progressive ratio experiments. When the animal refuses to expend energy for the preferred reward, it usually opts for the less-preferred reward. Therefore, the amount of lab chow consumed increases, suggesting that satiety cannot explain the lowered breakpoint (Salamone et al., 1991; Salamone et al., 2003).
An alternative to an operant testing chamber to assess effort-related choice is a T-maze cost/benefit procedure (Salamone et al., 1994; Izquierdo and Belcher, 2012). Different reinforcement densities are located within the two arms of the T-maze. For example, one of the arms may contain one or two pellets and is deemed the low-reward (LR) arm. The other arm is the high-reward (HR) arm and typically contains four food pellets. The rats are trained in the T-maze by first discriminating between the LR and HR arms. Given a free choice, rats typically choose the HR to obtain the high-density reward. Once the rats consistently choose the HR arm, the effort-related choice component is introduced. A small barrier (15 cm) is placed in the HR arm, and the height of the barrier is progressively increased by 5 cm until the target barrier height is reached (Salamone et al., 1994; Bardgett et al., 2009), and so the only way to obtain the HR is to climb the barrier. Untreated rats are likely to opt for the HR arm, navigate the barrier, and consume the high-density reward (Salamone et al., 1994).
Performance within the T-maze cost/benefit procedure is sensitive to dopaminergic manipulations. Rats that receive low-dose haloperidol chose the LR arm when a barrier was present within the HR arm, indicating a reluctance to expend effort for a desirable reward when a freely available, yet less-preferred, reward was available (Salamone et al., 1994). In addition, Salamone and co-workers (1994) observed an increase in the selection of the LR arm in rats after dopamine depletion in the nucleus accumbens when the barrier was present in the HR arm. Interestingly, when the LR arm contained no pellets and the HR arm still contained four pellets, nucleus accumbens dopamine-depleted rats navigated the barrier to obtain the food reward (Cousins et al., 1996). Thus, given the choice between food and no food, nucleus accumbens dopamine-depleted rats still chose to expend energy to retrieve the food reward. However, when these same rats had the option for food but a choice between differing reward densities (e.g., two pellets instead of four), depletion of nucleus accumbens dopamine altered choice behavior, and the rats preferred the easier LR option (Cousins et al., 1996; Salamone et al., 2007).
In addition to dopaminergic manipulations that influence choice behavior in the T-maze cost/benefit procedure, chronic intermittent PCP treatment (Morris et al., 2005; Pratt et al., 2008) biased rats away from the HR arm with the barrier and made them settle for the LR option. Importantly, the preference for sucrose in PCP-treated rats was also assessed and the reduced preference to expend energy for the HR and opt for the LR occurred without a concomitant reduction of sucrose consumption (J. Pratt, Personal Communication). This pattern of results suggests that the hedonic capacity associated with consuming palatable food remained intact, but the motivation to expend energy to obtain a highly desired reward was impaired in a HR/LR choice scenario. Although this effect was transient (evident 24 h but absent 72 h after PCP treatment), these findings suggest that cost/benefit procedures that assess motivation are also amenable to impairment after NMDA receptor antagonist administration, representing a potential method of modeling motivational impairments in experimental animals with relevance to schizophrenia.
Procedures that Assess Anticipation and Expectancy of Rewards
Anticipatory locomotion
After repeated exposure to a sexually receptive female rat in a specially designed, two-level chamber, male rats demonstrate anticipatory searching behavior, characterized by moving from level to level in search of the female rat immediately prior to introduction of the female rat (Mendelson and Pfaus, 1989; Pfaus and Phillips, 1991). This increase in anticipatory locomotor activity has been suggested to be a useful indicator of the sexual anticipatory state of the male rat.
Consistent with previous findings (Mendelson and Pfaus, 1989; Pfaus and Phillips, 1991), male rats exhibited a high level of side changes in a single-level box (as opposed to the two-level chamber described above) after repeated testing, reflecting searching behavior in anticipation of the introduction of the female rat (Barr et al., 1999). Searching behavior can be deemed preparatory behavior, initiated when the rat is anticipating the introduction of the female rat. Conversely, sexual copulation is interpreted as consummatory behavior because the animal is engaging in the activity for which it was previously preparing. Withdrawal from an escalating amphetamine dose regimen reduced the number of anticipatory side changes prior to the introduction of the receptive female rat (Barr et al., 1999; figure 1C). Furthermore, the post-ejaculatory intervals were also longer in amphetamine-withdrawn animals (see Fig. 1D). Interestingly, despite the apparent reduction of preparatory behavior or sexual motivation during amphetamine withdrawal, consummatory behavior was not affected, and both groups of rats engaged in copulation once the female was introduced. Therefore, amphetamine withdrawal in this case shows specificity for inducing impairment in the anticipation of sexual behavior rather than impairing the physical ability to engage in copulation (Barr et al., 1999). Thus, this task may provide a valid methodology for dissociating between the hedonic act of consummation and anticipatory or incentive components of reward-seeking behavior. Increased anticipatory locomotor activity was also observed in rats (Mendoza et al., 2005) and mice (Hsu et al., 2010) during the anticipation of a highly palatable meal, suggesting that any hedonic activity and not simply sexual copulation may induce preparatory behavior prior to consummation in rodents. Furthermore, Barbano and Cador (2005) found that the consummatory and anticipatory components of feeding behavior were differentially modulated by opioid and dopamine neurotransmission, respectively, reflecting distinct neural substrates that underlie the various subgroups of reward-related behaviors.
Successive contrast effects
Flaherty (1996) proposed a multi-stage theory of contrast to describe the phenomenon first identified by Vogel et al. (1968). When subjects are shifted from a highly desirable and anticipated reward (e.g., 32% sucrose solution) to an unexpected and less desirable substance (e.g., 4% sucrose solution), they will consume less of the solution compared with subjects that have only experienced the less-preferred reward. This phenomenon is termed successive negative contrast (Pellegrini and Mustaca, 2000). The opposite also holds true for animals that are trained to receive a reward of consistently low value and unexpectedly receive a reward of higher value. In the latter case, the subjects consume greater quantities of the substance than subjects that were exposed only to the high-value reward (Flaherty, 1996), a phenomenon known as successive positive contrast. These alterations in responses when faced with unexpected variations in reward valuation provide indications of the affective state of the animal. Successive negative contrast can be observed in various mammalian species (Pellegrini and Mustaca, 2000) and emerges after alterations in quality (Elliott, 1928), density (Crespi, 1942), and frequency of reward (Reynolds and Limpo, 1968). Although various alterations may possibly induce successive negative contrast effects, the consistent theme is that effects emerge after the unexpected alteration in reward and the unexpected downshift in the reward value. The suppression of consummatory behavior after the unexpected devaluation of reward has been suggested to reflect a negative affective state, possibly resembling frustration or disappointment, induced by a disruption of reward expectancy (Flaherty, 1982).
Barr and Philips (2002) demonstrated that when rats were unexpectedly downshifted from a sucrose concentration of 32% to 4%, they consumed less sucrose than rats that that were only exposed to a 4% sucrose solution. Interestingly, however, rats that underwent withdrawal from an escalating amphetamine administration regimen exhibited increased negative contrast and consumed even less of the 4% sucrose solution when downshifted from the 32% solution compared with non-amphetamine-treated controls (see Fig. 1E). Furthermore, no difference was found in sucrose consumption between amphetamine-withdrawing and control rats that were not downshifted (e.g., were exposed only to 4% sucrose consumption). Therefore, the exaggerated successive negative contrast observed in the downshifted amphetamine-treated animals reflects a potentiation of the negative affective response when the subject unexpectedly received a devalued reward.
In addition to the exaggerated successive negative contrast during amphetamine withdrawal, rats withdrawn from an escalating dose regimen of amphetamine did not develop successive positive contrast when they were unexpectedly upshifted from a reward with low intrinsic value (e.g., 4% sucrose solution) to one with high intrinsic value (e.g., 32% sucrose solution), an effect that was evident in saline-treated animals (Vacca and Phillips, 2005). When unexpectedly shifted to a 32% sucrose concentration, control animals exhibited an exaggerated response and consumed more sucrose after the unexpected upshift compared with saline-treated animals that were only exposed to the 32% sucrose solution. Although amphetamine withdrawing animals indeed consumed more sucrose after the upshift, amphetamine withdrawal significantly decreased sucrose consumption compared with saline-treated, upshifted animals. Withdrawal from amphetamine had no effect on performance in rats maintained on the 32% sucrose solution, suggesting that the inhibition of the development of successive positive contrast was not attributable to gross abnormalities in hedonic capacity but rather to the specific alteration in reward valuation.
Withdrawal from an escalating dose of amphetamine, therefore, highlights the dual nature of successive contrast effects, in which exaggerated negative contrast or blunted positive contrast is observed during withdrawal (Barr and Phillips, 2002; Vacca and Phillips, 2005). Thus, this pharmacological model provides compelling evidence that alterations in reward valuation and expectation are measurable after a manipulation, psychomotor stimulant withdrawal, that is associated with a depression-like state (American Psychiatric Association, 2000).
Reward, Schizophrenia and Neurobiological Mechanisms
Dopamine plays a critical role within the circuits of the brain reward system. Therefore, manipulations that perturb the dopaminergic system are likely to elicit reward-related abnormalities. Dysregulation of the dopaminergic system has long been thought to play a fundamental role in schizophrenia pathophysiology (Howes and Kapur, 2009). Cortical hypodopaminergic transmission is thought to contribute to cognitive impairment, and subcortical hyperdopaminergic transmission is hypothesized to mediate psychotic episodes. Moreover, the disruption of subcortical dopamine signaling may also contribute to negative symptomatology and reward-related deficits, albeit in a complex manner. Both amphetamine withdrawal (Barr and Markou, 2005; see figure 1) or dopamine depletion within the nucleus accumbens (Salamone et al., 1991; Salamone et al., 1994) induced deficits in several reward-related tasks. In addition though, striatal dopamine D2 receptor overexpression induced reductions of the willingness to work for a reward, as reflected in effort-related choice procedures (Ward et al., 2012; see figure 2). An optimal level of dopamine signaling may therefore be essential for reward function, with alterations in the balance of dopaminergic transmission, either excessive or insufficient transmission, in discrete brain regions leading to reward-related deficits. It is therefore important to note that blockade of dopamine D2 receptors after haloperidol administration also reduced performance in tasks that assess effort-related choice (Salamone et al., 1991; Salamone et al., 1994). Current antipsychotic drugs effectively ameliorate the psychotic symptoms of schizophrenia, but the blockade of dopamine D2 receptors by these medications may in fact exacerbate impairments in motivation. Thus, research should also focus on understanding the true reward deficits that stem from schizophrenia neuropathology and those that arise from the side-effects of currently used antipsychotics with high affinity for dopamine D2 receptors.
In addition to aberrant dopaminergic transmission, glutamatergic hypofunction represents another prominent hypothesis about the neurobiology underlying schizophrenia pathogenesis (Olney et al., 1999). As discussed above, NMDA receptor antagonists are routinely used as inducing conditions when modeling schizophrenia. Therefore, it is notable that glutamatergic and dopaminergic circuits interact to modulate reward-related learning (Sutton and Beninger, 1999; Beninger and Gerdjikov, 2005). Also of great interest is the observation that sub-chronic administration of PCP decreased prefrontal dopaminergic activity (Jentsch et al., 1997a; Jentsch et al., 1997b) and induced sub-cortical dopaminergic hyper-responsivity (Jentsch et al., 1998), potentially mirroring the dopaminergic disturbances in schizophrenia. The interaction between glutamatergic and dopaminergic transmission may therefore play an important role in brain reward deficits observed after NMDA receptor antagonism.
Future Considerations
Evidence suggests that schizophrenia patients discount value of future rewards more than healthy controls (Gold et al., 2008), indicating that alterations in value representation may exist in this patient population. This phenomenon is known as delay discounting and can readily be assessed in experimental animals (Floresco et al., 2008). PCP withdrawal has recently been shown to induce impulsive choice for saccharin in monkeys (Carroll et al., 2013). However, to our knowledge, there is limited information available with respect to inducing conditions of relevance to schizophrenia and their ability to induce delay discounting deficits in rodents. Investigations into this research question could facilitate further elucidation of the neurobiology of alterations in reward representation in relation to schizophrenia pathophysiology.
In addition to delay discounting, schizophrenia patients have shown alterations in rapid learning from response feedback (Gold et al., 2008; Gold et al., 2013). Furthermore, a modest correlation between the severity of negative symptoms and reversal learning errors has been observed in patients (Murray et al., 2008). Procedures investigating probabilistic decision making (e.g., win/stay lose/shift behavior) or probabilistic reversal learning in animals can be used to investigate these shifts in representation of reward value evident in schizophrenia. In addition, Gold and colleagues (2013) recently identified that schizophrenia patients exhibited abnormal effort-cost computations, with impairments greatest in patients with elevated negative symptoms. While various tasks already exist that enable the assessment of effort-cost relationships in animals (see Effort-related choice procedures section), the procedure described by Gold and colleagues (2013) is novel in that it included an element of probabilistic reward. To our knowledge, no equivalent animal procedure encompassing probabilistic reward with effort-related choice exists at present. Future efforts to validate an animal analog of this task, along with investigations of impairments after schizophrenia-relevant inducing conditions, would be of great interest.
It is also interesting to note that current preclinical cost/benefit procedures typically utilize physical effort (e.g., climbing a barrier or extended lever presses) as a means of increasing the difficulty level between reward choices. Because motivated behavior may not always involve physical effort, it has been suggested that these approaches may have limited analogy to real-life situations. Human effort discounting tasks have often shown to increase cognitive, rather than physical, effort (Botvinick et al., 2009; Croxson et al., 2009). Thus, Cocker and colleagues (2012) sought to develop an animal procedure that provided different reward densities after different levels of cognitive effort. It would be of interest to explore the effects of schizophrenia-relevant inducing conditions, and investigate how they impair performance in animals after increased cognitive effort.
Conclusions
The development of animal models of negative symptoms that display translational validity continues to represent a major challenge for preclinical researchers. With the development of increasingly sophisticated and biologically relevant animal models that address specific aspects of negative symptoms, and use relevant inducing conditions, there is improved understanding of the underlying neural substrates of the negative symptomatology of schizophrenia. As discussed above, an animal model of a psychiatric disorder consists of both an inducing condition (independent variable) and measures (dependent variables) (Geyer and Markou, 1995; Markou et al., 2009; see Table 1). Manipulations that induce deficits in select aspects of reward function have etiological validity for schizophrenia in terms of engaging the same neurosubstrates hypothesized to be involved in the generation and expression of the negative symptoms of schizophrenia. The present review only tangentially addressed the issue of the manipulations that may be used to induce reward and other deficits that relate to the negative symptomatology of schizophrenia. Instead, the focus was centered on procedures that enable the assessment anhedonia, avolition, and anticipatory deficits that may underlie some of the negative symptoms of schizophrenia that patients experience.
With regard to modeling reward deficits that are related to schizophrenia, numerous considerations should be made. Emerging evidence suggests that anhedonia in its truest form (i.e., impairments in the ability to experience pleasure) may not necessarily be core to schizophrenia. Rather, deficits in the ability to anticipate the pleasure that will be derived from future activities or the motivation required for goal-directed behaviors that facilitate the engagement and undertaking of pleasurable activities is more likely to represent a cardinal feature of negative symptomatology. Therefore, when assessing the effects of an experimental manipulation, researchers may find it advantageous to utilize a number of tests (e.g., sucrose preference in conjunction with effort-related choice) ensuring that hedonic capacity remains intact when attempting to identify deficits in anticipatory motivation (e.g., Ward et al., 2012). This approach supports an accurate dissociation of behaviors that are impaired in schizophrenia with those that are not, in an effort to improve translational validity. For this purpose, we included experimental animal procedures that assess various aspects of reward processing, including procedures that assess the anticipation of rewards based on expected valuations. These procedures may not be considered measures of hedonic capacity or motivation but indeed reflect the impairments in reward anticipation and valuation that are observed in schizophrenia patients (Gard et al., 2007; Dowd and Barch, 2012).
Ultimately, understanding the underlying neural substrates of the impaired motivational and anticipatory systems associated with schizophrenia may lead to the identification of novel therapeutics. Moreover, these procedures will enable the investigation of the effects of chronic treatment with putative medications to treat negative symptoms. Such approaches will ideally address the negative symptoms of schizophrenia and improve the functional outcome of patients with this debilitating disorder.
Acknowledgments
Funding for the preparation of this manuscript was provided by NIH grant 2R01 MH62527 to AM. The NIH had no further role in the writing of the manuscript and in the decision to submit the paper for publication.
The authors would like thank Mike Arends for proof reading the manuscript and Janet Hightower for assistance with the figures.
AM has received contract research support from Bristol Myers Squibb Co., Pfizer, and AstraZeneca, and consulting fees / honoraria from Pfizer, AstraZeneca and Abbott GmbH and Company during the past 3 years. The remaining authors have no financial disclosures.
SAB wrote the original draft of the manuscript. AD and AM provided input and assisted in the revisions of the manuscript and figure composition. All authors contributed to and have approved the final manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aberman JE, Salamone JD. Nucleus accumbens dopamine depletions make rats more sensitive to high ratio requirements but do not impair primary food reinforcement. Neuroscience. 1999;92:545–552. doi: 10.1016/s0306-4522(99)00004-4. [DOI] [PubMed] [Google Scholar]
- Allison DB, Mentore JL, Heo M, Chandler LP, Cappelleri JC, Infante MC, Weiden PJ. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry. 1999;156:1686–1696. doi: 10.1176/ajp.156.11.1686. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-V. 5. American Psychiatric Association; Washington DC: 2013. [Google Scholar]
- Arnold JM, Roberts DCS. A critique of fixed and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacol Biochem Behav. 1997;57:441–447. doi: 10.1016/s0091-3057(96)00445-5. [DOI] [PubMed] [Google Scholar]
- Baird JP, Turgeon S, Wallman A, Hulick V. Behavioral processes mediating phencyclidine-induced decreases in voluntary sucrose consumption. Pharmacol Biochem Behav. 2008;88:272–279. doi: 10.1016/j.pbb.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbano MF, Cador M. Differential regulation of the consummatory, motivational and anticipatory aspects of feeding behavior by dopaminergic and opioidergic drugs. Neuropsychopharmacology. 2005;31:1371–1381. doi: 10.1038/sj.npp.1300908. [DOI] [PubMed] [Google Scholar]
- Barch DM, Dowd EC. Goal representations and motivational drive in schizophrenia: the role of prefrontal-striatal interactions. Schizophr Bull. 2010;36:919–934. doi: 10.1093/schbul/sbq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardgett ME, Depenbrock M, Downs N, Points M, Green L. Dopamine modulates effort-based decision making in rats. Behav Neurosci. 2009;123:242–251. doi: 10.1037/a0014625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr AM, Fiorino DF, Phillips AG. Effects of withdrawal from an escalating dose schedule of d-amphetamine on sexual behavior in the male rat. Pharmacol Biochem Behav. 1999;64:597–604. doi: 10.1016/s0091-3057(99)00156-2. [DOI] [PubMed] [Google Scholar]
- Barr AM, Markou A. Psychostimulant withdrawal as an inducing condition in animal models of depression. Neurosci Biobehav Rev. 2005;29:675–706. doi: 10.1016/j.neubiorev.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Barr AM, Markou A, Phillips AG. A “crash” course on psychostimulant withdrawal as a model of depression. Trends Pharmacol Sci. 2002;23:475–482. doi: 10.1016/s0165-6147(02)02086-2. [DOI] [PubMed] [Google Scholar]
- Barr AM, Phillips AG. Withdrawal following repeated exposure to d-amphetamine decreases responding for a sucrose solution as measured by a progressive ratio schedule of reinforcement. Psychopharmacology. 1999;141:99–106. doi: 10.1007/s002130050812. [DOI] [PubMed] [Google Scholar]
- Barr AM, Phillips AG. Increased successive negative contrast in rats withdrawn from an escalating-dose schedule of D-amphetamine. Pharmacol Biochem Behav. 2002;71:293–299. doi: 10.1016/s0091-3057(01)00664-5. [DOI] [PubMed] [Google Scholar]
- Bedford JA, Bailey LP, Wilson MC. Cocaine reinforced progressive ratio performance in the rhesus monkey. Pharmacol Biochem Behav. 1978;9:631–638. doi: 10.1016/0091-3057(78)90214-9. [DOI] [PubMed] [Google Scholar]
- Beninger R, Gerdjikov T. Dopamine-glutamate interaction in reward-related incentive learning. In: Schmidt W, Reith MA, editors. Dopamine and Glutamate in Psychiatric Disorders. Humana Press; 2005. [Google Scholar]
- Bergner CL, Smolinsky AN, Hart PC, Dufour BD, Egan RJ, Laporte JL, Kalueff AV. Mouse models for studying depression-like states and antidepressant drugs. Methods Mol Biol. 2010;602:267–282. doi: 10.1007/978-1-60761-058-8_16. [DOI] [PubMed] [Google Scholar]
- Berlin I, Givry-Steiner L, Lecrubier Y, Puech AJ. Measures of anhedonia and hedonic responses to sucrose in depressive and schizophrenic patients in comparison with healthy subjects. Eur Psychiatry. 1998;13:303–309. doi: 10.1016/S0924-9338(98)80048-5. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Measuring hedonic impact in animals and infants: microstructure of affective taste reactivity patterns. Neurosci Biobehav Rev. 2000;24:173–198. doi: 10.1016/s0149-7634(99)00072-x. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Bleuer E. Textbook of Psychiatry. Macmillan; New York: 1924. [Google Scholar]
- Botvinick MM, Huffstetler S, McGuire JT. Effort discounting in human nucleus accumbens. Cognitive, Affective, and Behavioral Neuroscience. 2009;9:16–27. doi: 10.3758/CABN.9.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brebion G, Bressan RA, Pilowsky LS, Davis AS. Depression, avolition, and attention disorders in patients with schizophrenia: associations with verbal memory efficiency. J Neuropsychiatry Clin Neurosci. 2009;21:206–215. doi: 10.1176/jnp.2009.21.2.206. [DOI] [PubMed] [Google Scholar]
- Brody SA, Dulawa SC, Conquet F, Geyer MA. Assessment of a prepulse inhibition deficit in a mutant mouse lacking mGlu5 receptors. Mol Psychiatry. 2004;9:35–41. doi: 10.1038/sj.mp.4001404. [DOI] [PubMed] [Google Scholar]
- Campbell KA, Evans G, Gallistel CR. A microcomputer-based method for physiologically interpretable measurement of the rewarding efficacy of brain stimulation. Physiol Behav. 1985;35:395–403. doi: 10.1016/0031-9384(85)90315-4. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Chartoff EH. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc. 2007;2:2987–2995. doi: 10.1038/nprot.2007.441. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Kohl EA, Johnson KM, Lanasa RM. Increased impulsive choice for saccharin during PCP withdrawal in female monkeys: Influence of menstrual cycle phase. Psychopharmacology (Berl) 2013;227:413–424. doi: 10.1007/s00213-012-2963-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassens G, Actor C, Cling M, Schildkraut JJ. Amphetamine withdrawal: effects on threshold of intracranial reinforcement. Psychopharmacology. 1981;73:318–322. doi: 10.1007/BF00426458. [DOI] [PubMed] [Google Scholar]
- Cocker PJ, Hosking JG, Benoit J, Winstanley CA. Sensitivity to cognitive effort mediates psychostimulant effects on a novel rodent cost/benefit decision-making task. Neuropsychopharmacology. 2012;37:1825–1837. doi: 10.1038/npp.2012.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins MS, Atherton A, Turner L, Salamone JD. Nucleus accumbens dopamine depletions alter relative response allocation in a T-maze cost/benefit task. Behav Brain Res. 1996;74:189–197. doi: 10.1016/0166-4328(95)00151-4. [DOI] [PubMed] [Google Scholar]
- Crespi LP. Quantitative variation of incentive and performance in the white rat. Am J Psychol. 1942;55:467–517. [Google Scholar]
- Croxson PL, Walton ME, O’Reilly JX, Behrens TE, Rushworth MF. Effort-based cost-benefit valuation and the human brain. The Journal of Neuroscience. 2009;29:4531–4541. doi: 10.1523/JNEUROSCI.4515-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der-Avakian A, Markou A. Withdrawal from chronic exposure to amphetamine, but not nicotine, leads to an immediate and enduring deficit in motivated behavior without affecting social interaction in rats. Behav Pharmacol. 2010;21:359–368. doi: 10.1097/FBP.0b013e32833c7cc8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der-Avakian A, Markou A. The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci. 2012;35:68–77. doi: 10.1016/j.tins.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd EC, Barch DM. Pavlovian reward prediction and receipt in schizophrenia: relationship to anhedonia. PloS One. 2012;7:e35622. doi: 10.1371/journal.pone.0035622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew MR, Simpson EH, Kellendonk C, Herzberg WG, Lipatova O, Fairhurst S, Kandel ER, Malapani C, Balsam PD. Transient overexpression of striatal D2 receptors impairs operant motivation and interval timing. J Neurosci. 2007;27:7731–7739. doi: 10.1523/JNEUROSCI.1736-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenbroek BA, Cools AR. Animal models for the negative symptoms of schizophrenia. Behav Pharmacol. 2000;11:223–233. doi: 10.1097/00008877-200006000-00006. [DOI] [PubMed] [Google Scholar]
- Elliott MH. The Effect of Change of Reward on the Maze Performance of Rats. University of California Press; Berkeley: 1928. [Google Scholar]
- Esposito R, Kornetsky C. Morphine lowering of self-stimulation thresholds: lack of tolerance with long-term administration. Science. 1977;195:189–191. doi: 10.1126/science.831268. [DOI] [PubMed] [Google Scholar]
- Flaherty C. Incentive contrast: a review of behavioral changes following shifts in reward. Anim Learn Behav. 1982;10:409–440. [Google Scholar]
- Flaherty CF. Incentive Relativity. Cambridge University Press; Cambridge: 1996. [Google Scholar]
- Floresco SB, Onge JRS, Ghods-Sharifi S, Winstanley CA. Cortico-limbic-striatal circuits subserving different forms of cost-benefit decision making. Cognitive, Affective, and Behavioral Neuroscience. 2008;8:375–389. doi: 10.3758/CABN.8.4.375. [DOI] [PubMed] [Google Scholar]
- Foussias G, Remington G. Negative symptoms in schizophrenia: avolition and Occam’s razor. Schizophr Bull. 2010;36:359–369. doi: 10.1093/schbul/sbn094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallistel CR, Freyd G. Quantitative determination of the effects of catecholaminergic agonists and antagonists on the rewarding efficacy of brain stimulation. Pharmacol Biochem Behav. 1987;26:731–741. doi: 10.1016/0091-3057(87)90605-8. [DOI] [PubMed] [Google Scholar]
- Gard DE, Kring AM, Gard MG, Horan WP, Green MF. Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophr Res. 2007;93:253–260. doi: 10.1016/j.schres.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA, Markou A. Animal models of psychiatric disorders. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The Fourth Generation of Progress. Raven Press; New York: 1995. pp. 787–798. [Google Scholar]
- Gold JM, Waltz JA, Prentice KJ, Morris SE, Heerey EA. Reward processing in schizophrenia: a deficit in the representation of value. Schizophr Bull. 2008;34:835–847. doi: 10.1093/schbul/sbn068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Strauss GP, Waltz JA, Robinson BM, Brown JK, Frank MJ. Negative symptoms of schizophrenia are associated with abnormal effort-cost computations. Biol Psychiatry. 2013;74:130–136. doi: 10.1016/j.biopsych.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Geyer MA. Habituation and sensitization of acoustic startle: opposite influences of dopamine D1 and D2-family receptors. Neurobiol Learn Mem. 2009;92:243–248. doi: 10.1016/j.nlm.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerey EA, Gold JM. Patients with schizophrenia demonstrate dissociation between affective experience and motivated behavior. J Abnorm Psychol. 2007;116:268–278. doi: 10.1037/0021-843X.116.2.268. [DOI] [PubMed] [Google Scholar]
- Ho BC, Nopoulos P, Flaum M, Arndt S, Andreasen NC. Two-year outcome in first-episode schizophrenia: predictive value of symptoms for quality of life. Am J Psychiatry. 1998;155:1196–1201. doi: 10.1176/ajp.155.9.1196. [DOI] [PubMed] [Google Scholar]
- Hodos W. Progressive ratio as a measure of reward strength. Science. 1961;134:943–944. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- Horan WP, Kring AM, Blanchard JJ. Anhedonia in schizophrenia: a review of assessment strategies. Schizophr Bull. 2006;32:259–273. doi: 10.1093/schbul/sbj009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: Version III. The final common pathway. Schizophr Bull. 2009;35:549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CT, Patton DF, Mistlberger RE, Steele AD. Palatable meal anticipation in mice. Plos One. 2010;5:e12903. doi: 10.1371/journal.pone.0012903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Fenton WS. Medicine: What are the right targets for psychopharmacology? Science. 2003;299:350–351. doi: 10.1126/science.1077141. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Belcher AM. Rodent models of adaptive decision making. Methods Mol Biol. 2012;829:85–101. doi: 10.1007/978-1-61779-458-2_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins TA, Harte MK, Reynolds GP. Effect of subchronic phencyclidine administration on sucrose preference and hippocampal parvalbumin immunoreactivity in the rat. Neuroscience Letters. 2010;471:144–147. doi: 10.1016/j.neulet.2010.01.028. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Redmond DE, JR, Elsworth JD, Taylor JR, Youngren KD, Roth RH. Enduring cognitive deficits and cortical dopamine dysfunction in monkeys after long-term administration of phencyclidine. Science. 1997a;277:953–955. doi: 10.1126/science.277.5328.953. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Tran A, Le D, Youngren KD, Roth RH. Subchronic phencyclidine administration reduces mesoprefrontal dopamine utilization and impairs prefrontal cortical-dependent cognition in the rat. Neuropsychopharmacology. 1997b;17:92–99. doi: 10.1016/S0893-133X(97)00034-1. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR, Roth RH. Subchronic phencyclidine administration increases mesolimbic dopaminergic system responsivity and augments stress- and psychostimulant-induced hyperlocomotion. Neuropsychopharmacology. 1998;19:105–113. doi: 10.1016/S0893-133X(98)00004-9. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Roth RH. The neuropsychopharmacology of phencyclidine: From NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1999;20(3):201–225. doi: 10.1016/S0893-133X(98)00060-8. [DOI] [PubMed] [Google Scholar]
- Kellendonk C, Simpson EH, Polan HJ, Malleret G, Vronskaya S, Winiger V, Moore H, Kanel ER. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron. 2006;49:603–615. doi: 10.1016/j.neuron.2006.01.023. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Fenton WS, Carpenter WT, Jr, Marder SR. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull. 2006;32:214–219. doi: 10.1093/schbul/sbj053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornetsky C, Esposito RU. Euphorigenic drugs: effects on the reward pathways of the brain. Fed Proc. 1979;38:2473–2476. [PubMed] [Google Scholar]
- Kraepelin E. Dementia praecox and paraphrenia. J Nerv Ment Dis. 1921;54:384. [Google Scholar]
- Leith N, Barrett R. Amphetamine and the reward system: evidence for tolerance and post-drug depression. Psychopharmacologia. 1976;46:19–25. doi: 10.1007/BF00421544. [DOI] [PubMed] [Google Scholar]
- Liebman JM. Discriminating between reward and performance: a critical review of intracranial self-stimulation methodology. Neurosci Biobehav Rev. 1983;7:45–72. doi: 10.1016/0149-7634(83)90007-6. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Aultman JM, Verma A, Weinberger DR, Moghaddam B. Neonatal damage of the ventral hippocampus impairs working memory in the rat. Neuropsychopharmacology. 2002;27:47–54. doi: 10.1016/S0893-133X(02)00282-8. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J Neurosci. 2007;27:11424–11430. doi: 10.1523/JNEUROSCI.2847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A, Chiamulera C, Geyer MA, Tricklebank M, Steckler T. Removing obstacles in neuroscience drug discovery: the future path for animal models. Neuropsychopharmacology. 2009;34:74–89. doi: 10.1038/npp.2008.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A, Koob GF. Postcocaine anhedonia: an animal model of cocaine withdrawal. Neuropsychopharmacology. 1991;4:17–26. [PubMed] [Google Scholar]
- Markou A, Koob GF. Construct validity of a self-stimulation threshold paradigm: effects of reward and performance manipulations. Physiol Behav. 1992;51:111–119. doi: 10.1016/0031-9384(92)90211-j. [DOI] [PubMed] [Google Scholar]
- Markou A, Koob GF. Intracranial self-stimulation thresholds as a measure of reward. In: Sahgal A, editor. Behavioural Neuroscience: A Practical Approach. Vol. 2. IRL Press; Oxford: 1993. pp. 93–115. [Google Scholar]
- Mendelson SD, Pfaus JG. Level searching: a new assay of sexual motivation in the male rat. Physiol Behav. 1989;45:337–341. doi: 10.1016/0031-9384(89)90136-4. [DOI] [PubMed] [Google Scholar]
- Mendoza J, Angeles-Castellanos M, Escobar C. Entrainment by a palatable meal induces food-anticipatory activity and c-Fos expression in reward-related areas of the brain. Neuroscience. 2005;133:293–303. doi: 10.1016/j.neuroscience.2005.01.064. [DOI] [PubMed] [Google Scholar]
- Morris BJ, Cochran SM, Pratt JA. PCP: from pharmacology to modelling schizophrenia. Curr Opin Pharmacol. 2005;5:101–106. doi: 10.1016/j.coph.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Murray GA, Cheng F, Clark L, Barnett JH, Blackwell AD, Fletcher PC, Robbins TW, Bullmore ET, Jones PB. Reinforcement and reversal learning in first-episode psychosis. Schizophr Bull. 2008;34:848–855. doi: 10.1093/schbul/sbn078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscat R, Kyprianou T, Osman M, Phillips G, Willner P. Sweetness-dependent facilitation of sucrose drinking by raclopride is unrelated to calorie content. Pharmacol Biochem Behav. 1991;40:209–213. doi: 10.1016/0091-3057(91)90541-9. [DOI] [PubMed] [Google Scholar]
- Muscat R, Willner P. Effects of dopamine receptor antagonists on sucrose consumption and preference. Psychopharmacology. 1989;99:98–102. doi: 10.1007/BF00634461. [DOI] [PubMed] [Google Scholar]
- Neill JC, Barnes S, Cook S, Grayson B, Idris NF, McLean SL, Snigdha S, Rajagopal L, Harte MK. Animal models of cognitive dysfunction and negative symptoms of schizophrenia: focus on NMDA receptor antagonism. Pharmacol Ther. 2010;128:419–432. doi: 10.1016/j.pharmthera.2010.07.004. [DOI] [PubMed] [Google Scholar]
- O’Tuathaigh CMP, Waddinton JL. Mutant mouse models: phenotypic relationships to domains of psychopathology and pathobiology in schizophrenia. Schizophr Bull. 2010;36:243–245. doi: 10.1093/schbul/sbq004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol. 1954;47:419–427. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- Olney JW, Newcomer JW, Farber NB. NMDA receptor hypofunction model of schizophrenia. Journal of Psychiatric Research. 1999;33:523–533. doi: 10.1016/s0022-3956(99)00029-1. [DOI] [PubMed] [Google Scholar]
- Overstreet DH. Modeling depression in animal models. In: Kobeissy FH, editor. Psychiatric Disorders: Methods and Protocols. Vol. 829. Humana Press; New York: 2012. pp. 125–144. series title: Methods in Molecular Biology. [DOI] [PubMed] [Google Scholar]
- Paterson N, Markou A. The metabotropic glutamate receptor 5 antagonist MPEP decreased break points for nicotine, cocaine and food in rats. Psychopharmacology. 2005;179:255–261. doi: 10.1007/s00213-004-2070-9. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Myers C, Markou A. Effects of repeated withdrawal from continuous amphetamine administration on brain reward function in rats. Psychopharmacology. 2000;152:440–446. doi: 10.1007/s002130000559. [DOI] [PubMed] [Google Scholar]
- Pellegrini S, Mustaca A. Consummatory successive negative contrast with solid food. Learn Motiv. 2000;31:200–209. [Google Scholar]
- Pfaus JG, Phillips AG. Role of dopamine in anticipatory and consummatory aspects of sexual behavior in the male rat. Behav Neurosci. 1991;105:727–743. doi: 10.1037//0735-7044.105.5.727. [DOI] [PubMed] [Google Scholar]
- Pratt JA, Winchester C, Egerton A, Cochran SM, Morris BJ. Modelling prefrontal cortex deficits in schizophrenia: implications for treatment. Br J Pharmacol. 2008;153(Suppl 1):S465–S470. doi: 10.1038/bjp.2008.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds GS, Limpo AJ. Negative contrast after prolonged discrimination maintenance. Psychonom Sci. 1968;10:323–324. [Google Scholar]
- Richardson NR, Roberts DCS. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Roane HS. On the applied use of progressive-ratio schedules of reinforcement. J Appl Behav Anal. 2008;41:155–161. doi: 10.1901/jaba.2008.41-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DS, Richardson N. Self-administration of psychomotor stimulants using progressive ratio schedules of reinforcement. In: Boulton AA, Baker GB, Wu PH, editors. Animal Models of Drug Addiction. Vol. 24. Humana Press; Totawa NJ: 1993. pp. 233–269. series title: Neuromethods. [Google Scholar]
- Rodriguiz RM, Chu R, Caron MG, Wetsel WC. Aberrant responses in social interaction of dopamine transporter knockout mice. Behav Brain Res. 2004;148:185–198. doi: 10.1016/s0166-4328(03)00187-6. [DOI] [PubMed] [Google Scholar]
- Rummel C, Kissling W, Leucht S. Antidepressants for the negative symptoms of schizophrenia. Cochrane Database Syst Rev. 2006;3:CD005581. doi: 10.1002/14651858.CD005581.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD. Dopamine, effort, and decision making: theoretical comment on Bardgett et al (2009) Behav Neurosci. 2009;123:463–467. doi: 10.1037/a0015381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology. 2007;191:461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote S, Weber SM. Nucleus accumbens dopamine and the regulation of effort in food-seeking behavior: implications for studies of natural motivation, psychiatry, and drug abuse. J Pharmacol Exp Ther. 2003;305:1–8. doi: 10.1124/jpet.102.035063. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Cousins MS, Bucher S. Anhedonia or anergia? Effects of haloperidol and nucleus accumbens dopamine depletion on instrumental response selection in a T-maze cost/benefit procedure. Behav Brain Res. 1994;65:221–229. doi: 10.1016/0166-4328(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Cousins MS, Snyder BJ. Behavioral functions of nucleus accumbens dopamine: empirical and conceptual problems with the anhedonia hypothesis. Neurosci Biobehav Rev. 1997;21:341–359. doi: 10.1016/s0149-7634(96)00017-6. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Steinpreis RE, McCullough LD, Smith P, Grebel D, Mahan K. Haloperidol and nucleus accumbens dopamine depletion suppress lever pressing for food but increase free food consumption in a novel food choice procedure. Psychopharmacology. 1991;104:515–521. doi: 10.1007/BF02245659. [DOI] [PubMed] [Google Scholar]
- Simpson EH, Kellendonk C, Ward RD, Richards V, Lipatova O, Fairhurst S, Kandel ER, Balsam PD. Pharmacologic rescue of motivational deficit in an animal model of the negative symptoms of schizophrenia. Biol Psychiatry. 2011;69:928–935. doi: 10.1016/j.biopsych.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson EH, Waltz JA, Kellendonk C, Balsam PD. Schizophrenia in translation: dissecting motivation in schizophrenia and rodents. Schizophr Bull. 2012;38:1111–1117. doi: 10.1093/schbul/sbs114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielewoy C, Markou A. Withdrawal from chronic phencyclidine treatment induces long-lasting depression in brain reward function. Neuropsychopharmacology. 2003;28:1106–1116. doi: 10.1038/sj.npp.1300124. [DOI] [PubMed] [Google Scholar]
- Stefurak TL, Van Der Kooy D. Saccharin’s rewarding, conditioned reinforcing, and memory-improving properties” mediation by isomorphic or independent processes? Behavioral Neuroscience. 1992;106:125–139. doi: 10.1037//0735-7044.106.1.125. [DOI] [PubMed] [Google Scholar]
- Stoker AK, Markou A. The intracranial self-stimulation procedure provides quantitative measures of brain reward function. In: Gould TJ, editor. Mood and Anxiety Related Phenotypes in Mice: Characterization Using Behavioral Tests, Volume II. Vol. 63. Humana Press; New York: 2011. pp. 307–331. series title: Neuromethods. [Google Scholar]
- Strauss GP, Gold JM. A new perspective on anhedonia in schizophrenia. Am J Psychiatry. 2012;169:364–373. doi: 10.1176/appi.ajp.2011.11030447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strekalova T, Spanagel R, Bartsch D, Henn FA, Gass P. Stress-induced anhedonia in mice is associated with deficits in forced swimming and exploration. Neuropsychopharmacology. 2004;29:2007–2017. doi: 10.1038/sj.npp.1300532. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Beninger RJ. Psychopharmacology of conditioned reward: Evidence for a rewarding signal at D1-like dopamine receptors. Psychopharmacology. 1999;144:95–110. doi: 10.1007/s002130050982. [DOI] [PubMed] [Google Scholar]
- Turgeon SM, Hoge SG. Prior exposure to phencyclidine decreases voluntary sucrose consumption and operant performance for food reward. Pharmacol Biochem Behav. 2003;76:393–400. doi: 10.1016/j.pbb.2003.08.019. [DOI] [PubMed] [Google Scholar]
- Vacca G, Phillips AG. Inhibition of successive positive contrast in rats withdrawn from an escalating dose schedule of D-amphetamine. Int J Comp Psychol. 2005;18:298–306. [Google Scholar]
- Vlachou S, Markou A. Intracranial self-stimulation. In: Olmstead MC, editor. Animal Models of Drug Addiction. Vol. 53. Humana Press; New York: 2010. pp. 3–56. series title: Neuromethods. [Google Scholar]
- Vogel JR, Mikulka PJ, Spear NE. Effects of shifts in sucrose and saccharine concentrations on licking behavior in the rat. J Comp Physiol Psychol. 1968;66:661–666. doi: 10.1037/h0026556. [DOI] [PubMed] [Google Scholar]
- Wang C, McInnis J, Ross-Sanchez M, Shinnick-Gallagher P, Wiley JL, Johnson KM. Long-term behavioral and neurodegenerative effects of perinatal phencyclidine administration: implications for schizophrenia. Neuroscience. 2001;107:535–550. doi: 10.1016/s0306-4522(01)00384-0. [DOI] [PubMed] [Google Scholar]
- Ward RD, Simpson EH, Richards VL, Deo G, Taylor K, Glendinning JI, Kandel ER, Balsam PD. Dissociation of hedonic reaction to reward and incentive motivation in an animal model of the negative symptoms of schizophrenia. Neuropsychopharmacology. 2012;37:1699–1707. doi: 10.1038/npp.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology. 1987;93:358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- Young JW, Powell SB, Risbrough V, Marston HM, Geyer MA. Using the MATRICS to guide development of a preclinical cognitive test battery for research in schizophrenia. Pharmacol Ther. 2009;122:150–202. doi: 10.1016/j.pharmthera.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Geyer MA. Action of Modafinil - Increased motivation via the dopamine transporter inhibition and D1 receptors? Biological Psychiatry. 2010;67:784–787. doi: 10.1016/j.biopsych.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Meves J, Tarantino I, Caldwell S, Geyer MA. Delayed procedural learning in 37-nicotinic acetylcholine receptor knockout mice. Genes, Brain and Behavior. 2011;10:720–733. doi: 10.1111/j.1601-183X.2011.00711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]