Abstract
This study examined the contributions of a polymorphism of the corticotropin-releasing hormone receptor type I (CRHR1)gene (rs110402) and a history of child maltreatment—alone and in interaction—to patterns of cortisol reactivity in adolescents. Adolescents between the age of 13 and 17 years with (n = 61) and without (n = 97) a history of child maltreatment were exposed to the Trier Social Stress Test (TSST). Salivary cortisol was assessed at baseline, and 15 and 30 minutes after the start of the speech portion of the TSST. Saliva samples for genotyping rs110402 also were collected. Adolescents with one or more G alleles of rs110402, relative to A allele homozygotes, and those exposed to maltreatment, relative to non-exposed adolescents, exhibited blunted cortisol reactivity to the TSST (although these associations approached, but did not reach, the level of statistical significance when accounting for underlying population structure in our racially and ethnically diverse sample). There was also a trend for a stronger child maltreatment association with cortisol hypo-reactivity among G allele carriers, but this association was not statistically significant. Findings suggest that CRHR1 variation may moderate the downstream effects of child maltreatment on HPA axis function, and implications for understanding mechanisms of risk associated with early adversity are discussed.
Keywords: Cortisol, Child maltreatment, CRHR1, Genetics, Stress reactivity, Trier Social Stress Test, Adolescence
1. Introduction
Child maltreatment is associated with risk for numerous negative outcomes, including mental disorders and adverse physical health outcomes (e.g., Ehlert, 2013). However, considerable variability exists in whether individuals develop health problems following child maltreatment exposure (Ehlert, 2013). Identifying risk and protective factors for negative health sequelae among those exposed to child maltreatment could improve identification of those at greatest risk for experiencing harmful consequences and facilitate better targeting of preventive interventions.
Child maltreatment is associated with numerous neurobiological changes, including hypothalamic-pituitary-adrenal (HPA) axis dysregulation (e.g., Yehuda et al., 2010). The HPA axis regulates neurobiological responses to stress, particularly social and evaluative threats (Dickerson and Kemeny, 2004). Release of corticotropin-releasing hormone (CRH) from the hypothalamus initiates the HPA axis response to stress, which in turn triggers a coordinated neuroendocrine response culminating in cortisol production (Sapolsky et al., 2000). Child maltreatment has been associated with blunted cortisol reactivity to psychosocial stressors (e.g., Carpenter et al., 2007; Fisher et al., 2011; MacMillan et al., 2009). Although the mechanisms underlying this hypo-responsivity are unknown, they might reflect down-regulation of CRH receptors in the pituitary due to hypothalamic CRH hyper-secretion following severe or chronic trauma (Fries et al., 2005; Heim et al., 2001), or elevated cortisol production following trauma resulting in heightened negative feedback sensitivity to glucocorticoids, which inhibits hypothalamic CRH production and terminates the HPA axis response after stress (Sapolsky et al., 2000; Stein et al., 1997). There is evidence for heritability of these neuroendocrine disturbances (Yehuda et al., 2010), and regulators of CRH functioning—including genetic influences—may shape the sensitivity of the HPA axis to child maltreatment.
The CRH type 1 receptor (CRHR1) gene codes for one of two G-protein coupled CRH receptors (Bittencourt and Sawchenko, 2000). The protein encoded by CRHR1 is involved in CRH signal transduction, and variants of the gene bind with differential affinity to CRH (Sakai et al., 1998). Variation in CRHR1 may be associated with risk for psychopathology and other adverse outcomes following child maltreatment. Bradley and colleagues (2008) identified two CRHR1 single nucleotide polymorphisms (SNPs; rs110402 and rs7209436) that interacted with child maltreatment to predict depressive symptoms in adulthood. For each SNP, maltreatment was associated with higher depressive symptoms among those with the common allele (G for rs110402 and C for rs7209436), whereas the rare allele (A for rs110402 and T for rs7209436) was protective in that maltreated homozygotes did not exhibit elevated depressive symptoms compared to non-maltreated homozygotes. Similar results emerged based on the common TAT haplotype (formed by rs7209436, rs110402, and rs242924). The interaction of CRHR1 genotype with child maltreatment in predicting depression has been replicated in several studies (Heim et al., 2009; Polanczyk et al., 2009). CRHR1 variants also have been associated with posttraumatic stress symptoms following pediatric injury trauma (Amstadter et al., 2011).
Given the critical role of CRH in HPA axis regulation, CRHR1 genotype might moderate the effects of child maltreatment on HPA axis reactivity. In young rhesus macaques, CRHR1 polymorphisms were related to increased metabolic activity in the amygdala and hippocampus in response to stress (Rogers et al., 2013). These associations were observed in “healthy” macaques reared in typical environments, suggesting that particular CRHR1 genotypes may be associated with maladaptive stress responses even in the absence of environmental adversity or psychopathology. In humans, differences in brain activity during an emotional word processing task as a function of rs110402 genotype have been observed (Hsu et al., 2012).
Several studies have examined associations between CRHR1 variants and cortisol regulation, although sample characteristics, specific SNPs, and cortisol metrics vary across investigations. For example, healthy adults homozygous for the minor alleles of rs7209436, rs110402, and rs242924 had lower peak cortisol responses to a psychosocial stress task compared to major allele carriers (Mahon et al., 2013). However, this study did not consider child maltreatment history. In investigations of adults reporting child maltreatment, cortisol response to the dexamethasone/CRH test was higher among homozygotes for the major allele of rs110402 compared to minor allele carriers (Heim et al., 2009; Tyrka et al., 2009), although this effect was only observed in men in one study (Heim et al., 2009). In a community sample of preschool-aged children, carriers of the minor (A) allele of rs17763104 exhibited greater cortisol reactivity to a stress task compared to major (G) allele homozygotes (Sheikh et al., 2013). CRHR1 has also been associated with diurnal cortisol rhythms. Youths with two copies of the TAT haplotype and a history of maltreatment exhibited a flatter diurnal cortisol slope than those without maltreatment exposure; no differences as a function of maltreatment history were observed for those with zero or one copies of the haplotype (Cicchetti et al., 2011).
Taken together, existing evidence suggests that CRHR1 variants influence cortisol responses to stress. However, the extent to which CRHR1 polymorphisms moderate the effect of child maltreatment on cortisol reactivity to psychosocial stress is not clear. Furthermore, no studies have examined whether CRHR1 genotype and child maltreatment contribute jointly to cortisol reactivity in adolescents. The HPA axis undergoes significant changes from childhood to adolescence, such that adolescents show increased physiological stress responses compared to children (Stroud et al., 2009). Adolescence is also associated with increased incidence of numerous psychiatric disorders (Kessler et al., 2005), and changes in stress reactivity during adolescence may contribute to vulnerability to psychopathology in at-risk youth (e.g., Spear, 2009). Examining genetic influences on stress reactivity during adolescence may aid in identifying those most susceptible to dysregulation during this developmental stage.
The current study examined associations of a SNP in CRHR1 (rs110402) with cortisol reactivity to a psychosocial stressor in adolescents alone and in interaction with child maltreatment. Based on previous research (e.g., MacMillan et al., 2009; Sheikh et al., 2013), we anticipated significant main effects of genotype and maltreatment history on cortisol reactivity. Furthermore, given that child maltreatment has been associated with maladaptive emotional functioning (e.g., greater depressive symptoms) among carriers of the G allele of rs110402 (e.g., Bradley et al., 2008), we hypothesized that child maltreatment would be associated with blunted cortisol reactivity to psychosocial stress as a function of rs110402 genotype (i.e., primarily among G allele carriers).
2. Method
2.1 Participants
A community-based sample of 168 adolescents aged 13–17 was recruited for participation at schools, after-school programs, medical clinics, and the general community in Boston and Cambridge, MA. Recruitment efforts were targeted to obtain a sample with high variability in exposure to child maltreatment. To do so, we recruited heavily from neighborhoods with high levels of violence and from clinics that served a predominantly low-SES catchment area. Adolescents taking medications known to influence cardiovascular functioning were excluded (n=4). The sample was 56.0% female (n=94) and had a mean age of 14.9 years (SD=1.4). All females were post-menarchal. Approximately one-third of the sample (38.1%, n=64) was from single-parent households. Racial/ethnic composition of the analytic sample was as follows: 41.1% White (n=65), 17.7% Black (n=28), 17.7% Hispanic (n=28), 8.2% Asian (n=13), and 15.2% Biracial or Other (n=24). A total of 7 participants did not complete the experimental procedures and were not included in the analytic sample: 2 participants declined to complete the procedures, and 5 participants began the study procedures but did not complete all tasks. An additional 3 participants were excluded from analysis due to presence of a heart murmur (n=1), severe cognitive impairment (n=1), and presence of a pervasive developmental disorder (n=1). Thus, the final analytic sample included 158 participants.
2.2 Procedure
All participants were run between the hours of 2–7 p.m. because cortisol reaches its diurnal nadir during this time period. Participants were asked to refrain from activities that could influence cortisol levels, including brushing their teeth or drinking caffeinated beverages within 4 hours of their scheduled time and exercising at any point during that day. Participants first completed a 5-minute baseline period in which they were asked to sit quietly. Adolescents then completed questionnaire and interview measures. Informed consent was obtained from the parent/guardian who attended the session, and assent was provided by adolescents.
Participants completed the Trier Social Stress Test (TSST; Kirschbaum et al., 1993), a widely-used stress induction procedure that has been used with children and adolescents (Buske-Kirschbaum et al., 1997; Stroud et al., 2009). The TSST involves three periods. After being told that they would be delivering a speech in front of trained evaluators who would judge their performance, participants were given 5 minutes to prepare their speech. In the current study, participants were asked to talk about the qualities of a good friend and which of those characteristics they did and did not possess. Next, participants delivered a 5-minute speech in front of two evaluators. Evaluators were trained to provide neutral and mildly negative feedback (e.g., appearing bored) during the speech. Finally, participants completed a mental subtraction task out loud in front of the evaluators for 5 minutes. Specifically, participants were asked to count backwards in steps of seven from a three-digit number and were stopped and asked to start again each time they made a mistake. Following the TSST, participants engaged in a 5-minute recovery period during which they were asked to sit quietly. Saliva samples were taken after the initial baseline period, 15 minutes following the beginning of the speech portion of the TSST (reactivity period), and 15 minutes following completion of the recovery period.
2.3 Child Maltreatment
Child maltreatment was assessed using a self-report questionnaire and an interview. First, we administered the Childhood Trauma Questionnaire (CTQ; Bernstein et al., 1997). The CTQ is a 28-item scale that assesses the frequency of maltreatment exposure during childhood and adolescence. Three types of abuse are assessed: physical, sexual, and emotional. The CTQ has excellent psychometric properties including internal consistency, test-retest reliability, and convergent and discriminant validity with interviews and clinician reports of maltreatment (Bernstein et al., 1997; Bernstein et al., 1994). We created a maltreatment severity composite by summing items from each of the abuse sub-scales. This composite demonstrated good reliability in our sample (α = 0.88).
Second, we administered the Childhood Experiences of Care and Abuse (CECA) interview (Bifulco et al., 1994; Bifulco et al., 1997). The CECA assesses multiple aspects of caregiving experiences, including physical and sexual abuse. Inter-rater reliability for maltreatment reports is excellent, and multiple validation studies suggest high agreement between siblings on reports of caregiver behaviors and maltreatment (Bifulco et al., 1994; Bifulco et al., 1997).
We used the CECA and the CTQ to create a dichotomous indicator of exposure to child maltreatment. Participants who reported physical or sexual abuse during the interview or who had a score on any of the three CTQ abuse sub-scales above a previously-identified threshold (Walker et al., 1999) were classified as having experienced child maltreatment. No participant was currently experiencing maltreatment, and the proper authorities were contacted in cases where we had safety concerns.
2.4 Neuroendocrine Measures
Neuroendocrine samples were obtained with cryovial tubes (Immuno-Biological Laboratories [IBL]) using the drool method. Participants expectorated approximately 1.5 ml of saliva into a cryovial with a plastic straw. Saliva samples were stored immediately at −20°C until they were shipped on dry ice to a laboratory in Boston, Massachusetts. Samples were assayed for salivary free cortisol using a commercially available luminescence immunoassay (CLIA; IBL, Hamburg, Germany). Intra-assay and inter-assay coefficients of variance were acceptable (4.24% and 3.34%, respectively).
We assessed cortisol reactivity to the TSST in two ways: 1) by examining cortisol concentrations (nmol/L) across the three assessments, and 2) by examining area under the curve (AUC) with respect to increase. AUC with respect to increase represents the time-dependent change in cortisol relative to the baseline resting value (i.e., it ignores the distance from zero for all measurements; Fekedulegn et al. 2007). It is a measure of total cortisol output, and it is thought to reflect the sensitivity of the cortisol response.
2.5 DNA Extraction and Genotyping
Participants provided saliva samples for DNA collection using Oragene® kits (DNA Genotek, Ontario, Canada) at the beginning of the study session. Consistent with prior research (e.g., Heim et al., 2009), we examined the CRHR1 SNP rs110402 given that this SNP had the strongest signal in previous work on CRHR1 variants and child maltreatment (e.g., Bradley et al., 2008). Furthermore, this SNP has been found to be in high linkage disequilibrium with other associated SNPs in CRHR1; almost complete overlap has been found between rs110402 A allele carriers and carriers of the TAT haplotype (Heim et al., 2009). DNA extraction and SNP genotyping were performed at the Massachusetts General Hospital Psychiatric and Neurodevelopmental Genetics Unit Core Lab using the Sequenom iPLEX Gold® application and MassARRAY® system. The major steps in this process included: 1) primer and multiplex assay design using Sequenom’s MassARRAY® Designer software, 2) DNA amplification by polymerase chain reaction (PCR), 3) post-PCR nucleotide deactivation using shrimp alkaline phosphatase (SAP) to remove phosphate groups from unincorporated dNTPs, 4) single-base extension reaction for allele differentiation, 5) salt removal using ion-exchange resin, and 6) mass correlated genotype calling using SpectroCHIP® array and MALDI-TOF mass spectrometry. Quality control to determine sample and genotyping quality and to potentially remove poor SNPs and/or samples was performed in PLINK, a whole genome association analysis toolset. The call rate for rs110402 was 98.8%. Participants were grouped based on CRHR1 rs110402 G allele carrier status (AA vs. AG+GG). A history of child maltreatment has been associated with maladaptive emotional functioning (e.g., greater depressive symptoms) among carriers of the G allele of rs110402 (Bradley et al., 2008; Polanczyk et al., 2009), and we merged carriers of the G allele into one group to increase power. This approach is common in genetic analyses with smaller sample sizes (cf. Heim et al., 2009).
3. Results
3.1 Participant Characteristics
Descriptive statistics as a function of rs110402 G allele carrier status and child maltreatment history are presented in Table 1. In the full sample, 27.2% of participants were classified as A allele homozygotes and 72.8% were classified as G allele carriers. Genotype frequencies did not deviate significantly from Hardy-Weinberg equilibrium, χ2 (df=1) = 1.62, p = .20. Just over one third of participants (38.6%, n = 61) were classified as having a history of child maltreatment, reflecting our efforts to over-represent maltreated youths in our sample. As shown in Table 1, A allele homozygotes and G allele carriers did not differ significantly in terms of age, gender, White/Non-White racial/ethnic status, or maltreatment history, all ps > .16. The mean age of participants reporting a history of child maltreatment (M = 15.3 years, SD = 1.3) was significantly older than that of participants with no history of maltreatment (M = 14.7 years, SD = 1.4), t(156) = 2.73, p = .01. In addition, the percentage of White participants was significantly lower in the group with a history of maltreatment (39.3%) compared to the group with no maltreatment history (61.9%), χ2(df=1) = 7.62, p = .01.
Table 1.
Participant characteristics as a function of CRHR1 rs110402 genotype and child maltreatment history.
| rs110402 Genotype
|
MAF=0.50a | ||
|---|---|---|---|
| AA (n = 43) | AG+GG (n = 115) | ||
| Age | 15.0 (1.4) | 15.0 (1.4) | p = .93 |
| Percent female | 62.8% | 50.4% | p = .17 |
| Percent White | 48.8% | 54.8% | p = .51 |
| Percent maltreatment history | 30.2% | 41.7% | p = .19 |
| Child Maltreatment History
|
|||
|---|---|---|---|
| Maltreatment (n = 61) | No Maltreatment (n = 97) | ||
| Age | 15.3 (1.3) | 14.7 (1.4) | p = .01 |
| Percent female | 60.7% | 49.5% | p = .17 |
| Percent White | 39.3% | 61.9% | p = .01 |
Note. Age reported in years and standard deviations presented in parentheses. MAF = minor allele frequency.
Genotype frequencies did not deviate significantly from Hardy-Weinberg equilibrium; χ2 (df=1) = 1.62, p = .20.
3.2 Within-Group Cortisol Change Over Time
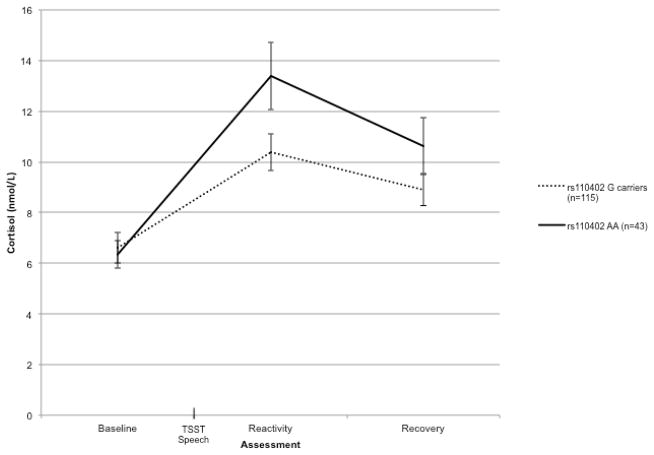
We first examined cortisol reactivity to the TSST by analyzing cortisol concentrations (in nmol/L) at each time point using a 3 (time) x 2 (rs110402 G allele carrier status) x 2 (child maltreatment) repeated measures Analysis of Covariance (ANCOVA), statistically controlling for sex, age, and White/Non-White racial/ethnic status.1 There were no significant main effects or interactions with time for sex, age, or White/Non-White racial/ethnic status. Greenhouse-Geisser corrected degrees of freedom are presented to control for the assumption of sphericity. There was a significant 2-way interaction of time by rs110402 genotype, F(1.36, 205.90) = 4.40, p = .03, η2 = .03. As depicted in Figure 1, compared to A allele homozygotes, G allele carriers had significantly lower cortisol levels at the second cortisol assessment collected 15 minutes after the start of the TSST speech (i.e., the reactivity period), t(156) = 2.09, p = .04. However, there were no significant differences between the cortisol levels of A allele homozygotes and G allele carriers at either baseline or the third (i.e., the recovery period) cortisol assessment, ps > 13. Thus, adolescents with one or more G alleles of rs110402 exhibited lower reactivity to the TSST compared to A allele homozygotes. In other words, possession of the G allele was associated with lower peak cortisol levels in response to the psychosocial stress task.
Figure 1.
Cortisol response to the Trier Social Stress Test (TSST) over time as a function of rs110402 genotype. Error bars represent standard error of the mean.
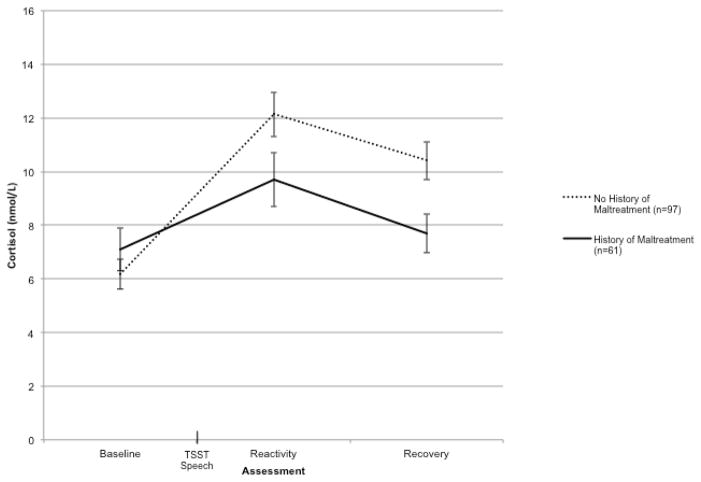
In addition, there was a significant Time x Child Maltreatment interaction, F(1.36, 205.90) = 5.05, p = .02, η2 = .03. As shown in Figure 2, adolescents with a history of child maltreatment exhibited lower cortisol levels at the second [t(156) = 1.83, p = .07] and third [t(146.23) = 2.69, p = .01] cortisol assessments compared to those without a history of maltreatment, although the difference between cortisol levels for the two groups at the second assessment approached, but did not reach, the conventional level of statistical significance. There was no significant difference between those with and without a history of child maltreatment in baseline cortisol levels, p > .33. Thus, in this community sample of adolescents, a history of child maltreatment was associated with blunted cortisol reactivity to a psychosocial stressor.
Figure 2.
Cortisol response to the Trier Social Stress Test (TSST) over time as a function of child maltreatment history. Error bars represent standard error of the mean.
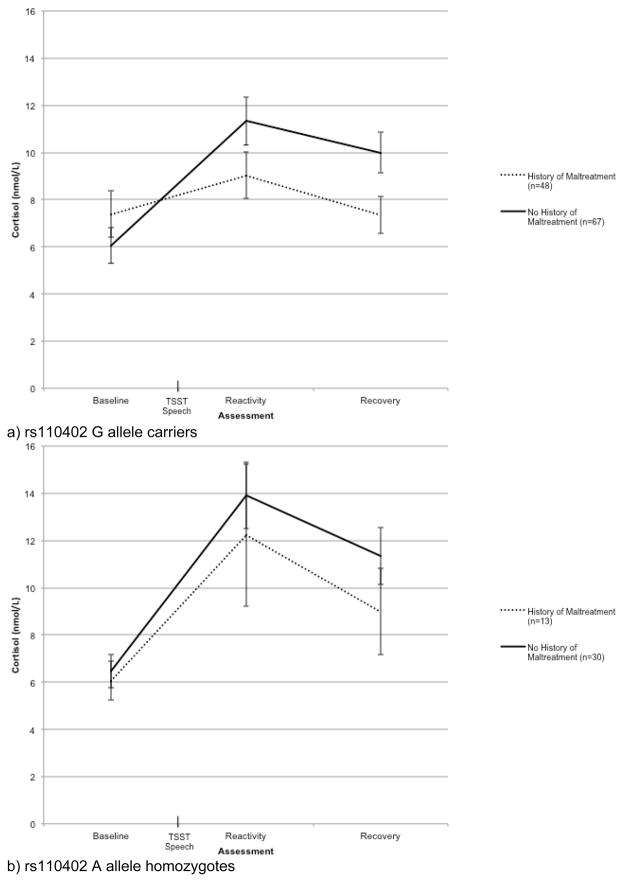
Contrary to our hypothesis, the interaction of time by rs110402 genotype by child maltreatment was not statistically significant, F(1.36, 205.90) = 0.68, p = .45 η2 = .004. However, we were underpowered to detect a significant 3-way interaction in this relatively small sample (power was estimated to be .14). Given our a priori hypothesis that rs110402 genotype would moderate the association between maltreatment history and cortisol reactivity to the TSST, we examined the relationship between child maltreatment and cortisol concentration at the three assessments separately for A allele homozygotes and G allele carriers. Consistent with our prediction, the interaction of time and maltreatment history was statistically significant for those with one or more G alleles but not for A allele homozygotes, F(1.35, 152.88) = 9.16, p = .001, η2 = .08 for G allele carriers, and F(1.35, 55.24) = 0.48, p = .55, η2 = .01 for A allele homozygotes. As shown in Figure 3, a history of child maltreatment was associated with significantly lower cortisol levels at the third (i.e., the recovery period) assessment among G allele carriers, t(112.65) = 2.28, p = .03.
Figure 3.
Effect of child maltreatment history on cortisol response to the Trier Social Stress Test (TSST) over time for rs110402 G allele carriers (a) and A allele homozygotes (b). Error bars represent standard error of the mean.
3.3 Cortisol Area Under the Curve with Respect to Increase
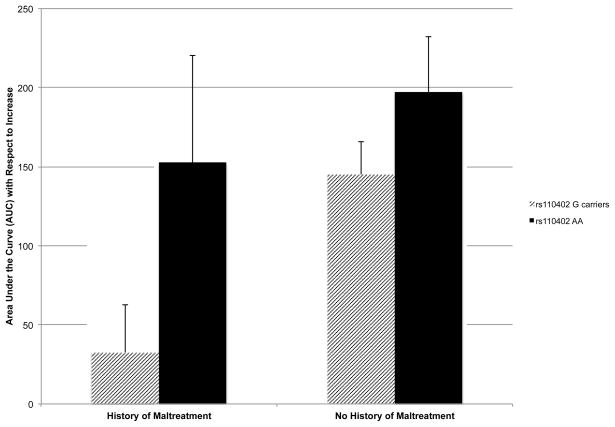
We also examined cortisol reactivity to the TSST by analyzing cortisol AUC with respect to increase using a 2 (rs110402 G allele carrier status) x 2 (child maltreatment) ANCOVA, again statistically controlling for age, sex, and White/Non-White racial/ethnic status.2 As above, none of the covariates was statistically significant. There was a significant main effect of rs110402 genotype, F(1, 151) = 4.95, p = .03, η2 = .03. Adolescents with one or two G alleles had a lower AUC with respect to increase than adolescents with two A alleles (see Figure 4). Furthermore, as shown in Figure 4, a history of child maltreatment was associated with a lower AUC with respect to increase as well, F(1, 151) = 5.29, p = .02, η2 = .03. In other words, possessing one or more G alleles and reporting a history of child maltreatment were associated with blunted cortisol reactivity to the TSST.
Figure 4.
Cortisol area under the curve (AUC) with respect to increase as a function of rs110402 genotype and child maltreatment history. Error bars represent standard error of the mean. Sample sizes for the subgroups are as follows: G carriers with maltreatment history (n = 48), A homozygotes with maltreatment history (n = 13), G carriers without maltreatment history (n = 67), A homozygotes without maltreatment history (n = 30).
We were underpowered to detect a significant 2-way interaction of rs110402 genotype by child maltreatment history (power was estimated to be 0.15), and this interaction term was not statistically significant, F(1, 151) = 0.81, p = .37, η2 = .005. However, we tested our a priori hypothesis that rs110402 genotype would moderate the association between a history of child maltreatment and cortisol reactivity to the TSST by comparing the AUC with respect to increase for those with and without child maltreatment separately for G allele carriers and A allele homozygotes. In line with our prediction, among adolescents with one or two G alleles, those with a history of child maltreatment had a significantly lower AUC with respect to increase than those without a history of maltreatment, t(113) = 3.20, p = .002. In contrast, the difference between AUC with respect to increase for the two maltreatment groups among A allele homozygotes was not statistically significant, t(41) = 0.65, p = .52. In sum, there was evidence that a history of child maltreatment was associated with diminished cortisol reactivity among adolescents with one or more copies of the rs110402 G allele.
3.4 Addressing Population Stratification
Given the racial/ethnic diversity of our sample, we inferred the underlying population structure from 40 ancestry-informative markers in order to address concerns regarding population stratification (i.e., the presence of systematic differences in allele frequencies as a function of subpopulations in the sample; e.g., Pritchard and Rosenberg, 1999). We re-ran analyses covarying the first two principal components from a principal components analysis of the ancestry-informative markers. Overall, the pattern of findings remained similar. The Time x rs110402 Genotype and Time x Child Maltreatment interactions were generally similar in effect size but fell just short of the conventional level of statistical significance, F(1.37, 208.44) = 3.30, p = .06, η2 = .02 and F(1.37, 208.44) = 3.11, p = .07, η2 = .02, respectively. In addition, the Time x Maltreatment History interaction was still significant in G allele carriers, F(1.35, 150.07) = 7.64, p = .003, η2 = .06. In the regression model of AUC with respect to increase, the main effects of rs110402 genotype and history of child maltreatment also approached statistical significance but remained relatively similar in effect size, F(1, 152) = 3.64, p = .06, η2 = .02 and F(1, 152) = 3.30, p = .07, η2 = .02, respectively. Additionally, the effect of history of child maltreatment on AUC with respect to increase was still significant in G allele carriers, F(1, 111) = 8.60, p = .004, η2 = .07. Together, these findings suggested that the associations between rs110402 genotype, history of child maltreatment, and cortisol reactivity to the TSST were not solely due to confounding effects of population structure.
4. Discussion
To the best of our knowledge, this is the first study to document the joint associations between a variant of CRHR1, a history of child maltreatment, and cortisol reactivity to a psychosocial stress task in adolescents. In our sample, possessing one or more G alleles of rs110402 and reporting a history of child maltreatment were both associated with blunted cortisol reactivity to the Trier Social Stress Test (TSST). Furthermore, there was preliminary evidence that CRHR1 genotype moderates the effects of child maltreatment on cortisol reactivity. Among carriers of the G allele of rs110402, a history of maltreatment was associated with cortisol hypo-reactivity to stress. However, no significant association between maltreatment history and cortisol reactivity emerged in A allele homozygotes.
These results add to the growing literature suggesting that variation in an HPA axis-related gene and early adversity are associated with differential patterns of physiological responses to stress. In line with prior research in community samples (Mahon et al., 2013; Sheikh et al., 2013), we found a main effect of rs110402 genotype on cortisol reactivity to a psychosocial stress task. However, whereas prior investigations have detected associations between CRHR1 variants and cortisol reactivity during childhood (Sheikh et al., 2013) and adulthood (Mahon et al., 2013), we extended this pattern of findings to adolescence—a period characterized by changes in HPA axis reactivity to stress (Stroud et al., 2009). Together, these results suggest that variation in CRHR1 may be linked to differences in the sensitivity of the HPA axis even prior to experiencing adversity or psychopathology. This is consistent with the conclusions drawn by Rogers and colleagues (2013) after observing associations between CRHR1 variants and increased amygdala and hippocampal activation in response to a stressor in healthy young macaques. Our finding that adolescents with a history of maltreatment exhibited blunted cortisol reactivity is in accordance with previous research (e.g., Carpenter et al., 2007; Fisher et al., 2011; MacMillan et al., 2009).
The only prior study to examine the interaction between CRHR1 variants and child maltreatment in relation to cortisol in youth found that youth with two copies of the TAT haplotype who had a history of maltreatment exhibited a flatter diurnal cortisol slope than those without a history of maltreatment (Cicchetti et al., 2011). Building on this initial study, we found evidence that a history of child maltreatment was associated with blunted cortisol reactivity primarily for G allele carriers of rs110402 (and not for A allele homozygotes). Given methodological differences between our investigation and that of Cicchetti et al. (2011), direct comparisons of the findings are difficult. For example, Cicchetti et al. (2011) examined the TAT haplotype in CRHR1, whereas we examined variation in a single SNP (rs110402). However, almost complete overlap has been found between rs110402 A allele carriers and carriers of the TAT haplotype (Heim et al., 2009). Additionally, Cicchetti et al. (2011) assessed diurnal cortisol rhythms, whereas we assessed cortisol reactivity to the TSST. Divergent patterns of findings have been observed when examining diurnal cortisol vs. cortisol reactivity to stress; indeed, some groups have been found to exhibit both higher basal cortisol levels and cortisol hyporeactivity to stress (e.g., Fairchild et al., 2008; Gustafsson et al., 2008). Further research that measures diurnal cortisol rhythms and cortisol reactivity within a single investigation is needed to better understand the relationships between CRHR1 variation, child maltreatment, and cortisol.
Additional research is also needed to better elucidate the neurobiological consequences of variation in CRHR1. The rs110402 SNP is located in intron 2 of the CRHR1 gene. Even though functional intronic regulatory variants have been observed (e.g., Hubler and Scammell, 2004), rs110402 is in high linkage disequilibrium with other variants in the gene. Fine mapping of this region might identify variants with functional consequences and provide insights into the mechanisms underlying the association between CRHR1 variation and cortisol reactivity. Furthermore, relatively little is known about the functional consequences of diminished cortisol reactivity. Some research suggests that, compared to more robust cortisol response, low cortisol response to stress may be associated with worse cognitive performance (Carpenter et al., 2007). Additionally, there is some evidence that lower cortisol levels may be associated with increased risk for PTSD (e.g., Yehuda et al., 2010). Nevertheless, more work is required to understand the effects of blunted cortisol reactivity to psychosocial stress on emotional and physical health.
Moreover, our findings need to be interpreted in light of several limitations. First, due to the small sample size, we were underpowered for some analyses, particularly tests of interactions. Thus, even though we found support for planned a priori comparisons between child maltreatment and cortisol reactivity as a function of rs110402 genotype, these results are preliminary and need to be replicated in a larger sample. Furthermore, the small sample size precluded an investigation of whether particular types of child maltreatment (e.g., sexual abuse, physical abuse) were associated with different patterns of cortisol reactivity. We also lacked genotypes for the other SNPs that make up the TAT haplotype in CRHR1, and thus were unable to examine this haplotype. However, as noted above, almost complete overlap has been found between rs110402 A allele carriers and carriers of the TAT haplotype (Heim et al., 2009). In addition, as with all studies of genetic variants in racially and ethnically heterogeneous samples, population stratification is a concern (e.g., Pritchard and Rosenberg, 1999). However, this concern is partially mitigated by the fact that when we modeled the underlying population structure using principal components based on ancestry-informative markers, the overall pattern of results was similar. Nevertheless, the associations between rs110402 genotype and child maltreatment with cortisol reactivity were marginally significant in these analyses, and our findings need to be interpreted in light of this limitation. More research using larger and more homogeneous samples will better clarify the nature of these associations.
Despite these limitations, we believe that our findings may begin to illuminate why some individuals who are maltreated during childhood—and not others—go on to experience poor emotional and physical health. CRHR1 variation may be one factor that moderates the downstream effects of child maltreatment, including associations with cortisol reactivity to psychosocial stressors.
Acknowledgments
Funding Body Agreements and Policies
This research was funded by the National Institute of Mental Health (K01-MH092526 to Dr. McLaughlin and K01-MH092555 to Dr. Sheridan).
Footnotes
The overall pattern of results remained the same when covarying the number of hours since waking and measures of caffeine intake, exercise, and drug use.
Again, the overall pattern of results remained the same when covarying the number of hours since waking and measures of caffeine intake, exercise, and drug use.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Contributors
J.A. Sumner managed the literature searches, conducted the statistical analyses, and wrote the first draft of the manuscript. K.A. McLaughlin and M.A. Sheridan designed the larger investigation, collected the data, and assisted with manuscript preparation. K. Walsh assisted with study design and manuscript preparation. K.C. Koenen assisted with study design and manuscript preparation. All authors contributed to and have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amstadter AB, Nugent NR, Yang B-Z, Miller A, Siburian R, Moorjani P, Haddad S, Basu A, Fagerness J, Saxe G, Smoller JW, Koenen KC. Corticotropin-releasing hormone type 1 receptor gene (CRHR1) variants predict posttraumatic stress disorder onset and course in pediatric injury patients. Dis Markers. 30:89–99. doi: 10.3233/DMA-2011-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein DP, Ahluvalia T, Pogge D, Handelsman L. Validity of the Childhood Trauma Questionnaire in an adolescent psychiatric population. J Am Acad Child Psy. 1997;36:340–348. doi: 10.1097/00004583-199703000-00012. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Fink L, Hondelsman L, Foote J, Lovejoy M. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiat. 1994;151:1132–1136. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- Bifulco A, Brown GW, Harris TO. Childhood Experiences of Care and Abuse (CECA): a retrospective interview measure. J Child Psychol Psyc. 1994;35:1419–1435. doi: 10.1111/j.1469-7610.1994.tb01284.x. [DOI] [PubMed] [Google Scholar]
- Bifulco A, Brown GW, Lillie A, Jarvis J. Memories of childhood neglect and abuse: Corroboration in a series of sisters. J Child Psychol Psyc. 1997;38:365–374. doi: 10.1111/j.1469-7610.1997.tb01520.x. [DOI] [PubMed] [Google Scholar]
- Bittencourt JC, Sawchenko PE. Do centrally administered neuropeptides access cognate receptors?: An analysis in the central corticotropin-releasing factor system. J Neurosci. 2000;20:1142–1156. doi: 10.1523/JNEUROSCI.20-03-01142.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley RG, Binder EB, Epstein MP, Tang Y, Fair HP, Liu W, Gillespie CF, Berg T, Evces M, Newport DJ, Stowe ZN, Heim CM, Nemeroff CB, Schwartz A, Cubells JF, Ressler KJ. Influence of child abuse on adult depression: Moderation by the corticotropin-releasing hormone receptor gene. Arch Gen Psychiat. 2008;65:190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buske-Kirschbaum A, Jobst S, Psych D, Wustmans A, Kirschbaum C, Rauh W. Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosom Med. 1997;59:419–426. doi: 10.1097/00006842-199707000-00012. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Carvalho JP, Tyrka AR, Wier LM, Mello AF, Mello MF, Anderson GM, Wilkinson CW, Price LH. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biol Psychiatry. 2007;62:1080–1087. doi: 10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Oshri A. Interactive effects of corticotropin releasing hormone receptor 1, serotonin transporter linked polymorphic region, and maltreatment on diurnal cortisol regulation and internalizing symptomatology. Dev Psychopathol. 2011;23:1125–1138. doi: 10.1017/S0954579411000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Ehlert U. Enduring psychobiological effects of childhood adversity. Psychoneuroendocrinology. 2013;38:1850–1857. doi: 10.1016/j.psyneuen.2013.06.007. [DOI] [PubMed] [Google Scholar]
- Fairchild G, van Goozen SHM, Stollery SJ, Brown J, Gardiner J, Herbert J, Goodyer I. Cortisol diurnal rhythm and stress reactivity in male adolescents with early-onset or adolescence-onset conduct disorder. Biol Psychiatry. 2008;64:599–606. doi: 10.1016/j.biopsych.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekedulegn DB, Andrew ME, Burchfiel CM, Violanti JM, Hartley TA, Charles LE, Miller DB. Area under the curve and other summary indicators of repeated waking cortisol measurements. Psychosom Med. 2007;69:651–659. doi: 10.1097/PSY.0b013e31814c405c. [DOI] [PubMed] [Google Scholar]
- Fisher PA, Kim HK, Bruce J, Pears KC. Cumulative effects of prenatal substance exposure and early adversity on foster children’s HPA-axis reactivity during a psychosocial stressor. Int J Behav Dev. 2011;36:29–35. doi: 10.1177/0165025411406863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries E, Hesse J, Hellhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30:1010–1016. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Gustafsson PE, Gustafsson PA, Ivarsson T, Nelson N. Diurnal cortisol levels and cortisol response in youths with obsessive-compulsive disorder. Neuropsychobiology. 2008;57:14–21. doi: 10.1159/000123117. [DOI] [PubMed] [Google Scholar]
- Heim C, Bradley B, Mletzko TC, Deveau TC, Musselman DL, Nemeroff CB, Ressler KJ, Binder EB. Effect of childhood trauma on adult depression and neuroendocrine function: Sex-specific moderation by CRH receptor 1 gene. Front Behav Neurosci. 2009;3:1–10. doi: 10.3389/neuro.08.041.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Am J Psychiat. 2001;158:575–581. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- Hsu DT, Mickey BJ, Langenecker SA, Heitzeg MM, Love TM, Wang H, Kennedy SE, Peciña M, Shafir T, Hodgkinson CA, Enoch MA, Goldman D, Zubieta JK. Variation in the corticotropin-releasing hormone receptor 1 (CRHR1) gene influences fMRI signal responses during emotional stimulus processing. J Neurosci. 2012;29:3253–3260. doi: 10.1523/JNEUROSCI.5533-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubler TR, Scammell JG. Intronic hormone response elements mediate regulation of FKBP5 by progestins and glucocorticoids. Cell Stress Chaperon. 2004;9:243–252. doi: 10.1379/CSC-32R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiat. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer D. The ‘Trier Social Stress Test’ - a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Mahon PB, Zandi PP, Potash JB, Nestadt G, Wand GS. Genetic association of FKBP5 and CRHR1 with cortisol response to acute psychosocial stress in healthy adults. Psychopharmacology. 2013;227:231–241. doi: 10.1007/s00213-012-2956-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMillan HL, Georgiades K, Duku EK, Shea A, Steiner M, Niec A, Tanaka M, Gensey S, Spree S, Vella E, Walsh CA, De Bellis MD, Van der Meulen J, Boyle MH, Schmidt LA. Cortisol response to stress in female youths exposed to childhood maltreatment: Results of the Youth Mood Project. Biol Psychiatry. 2009;66:62–68. doi: 10.1016/j.biopsych.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanczyk G, Caspi A, Williams B, Price TS, Danese A, Sugden K, Uher R, Poulton R, Moffitt TE. Protective effect of CRHR1 gene variants on the development of adult depression following childhood maltreatment. Arch Gen Psychiat. 2009;66:978–985. doi: 10.1001/archgenpsychiatry.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard JK, Rosenberg NA. Use of unlinked genetic markers to detect population stratification in association studies. Am J Hum Genet. 1999;65:220–228. doi: 10.1086/302449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J, Raveendran M, Fawcett GL, Fox AS, Shelton SE, Oler JA, Cheverud J, Muzny DM, Gibbs RA, Davidson RJ, Kalin NH. CRHR1 genotypes, neural circuits and the diathesis for anxiety and depression. Mol Psychiatr. 2013;18:700–707. doi: 10.1038/mp.2012.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Yamada M, Horiba N, Wakui M, Demura H, Suda T. The genomic organization of the human corticotropin-releasing factor type-1 receptor. Gene. 1998;219:125–130. doi: 10.1016/s0378-1119(98)00322-9. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Sheikh HI, Kryski KR, Smith HJ, Hayden EP, Singh SM. Corticotropin-releasing hormone system polymorphisms are associated with children’s cortisol reactivity. Neuroscience. 2013;229:1–11. doi: 10.1016/j.neuroscience.2012.10.056. [DOI] [PubMed] [Google Scholar]
- Spear LP. Heightened stress responsivity and emotional reactivity during pubertal maturation: Implications for psychopathology. Dev Psychopathol. 2009;21:87–97. doi: 10.1017/S0954579409000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Yehuda R, Koverola C, Hanna C. Enhanced dexamethasone suppression of plasma cortisol in adult women traumatized by childhood sexual abuse. Biol Psychiatry. 1997;42:680–686. doi: 10.1016/s0006-3223(96)00489-1. [DOI] [PubMed] [Google Scholar]
- Stroud LR, Foster E, Papandonatos GD, Handwerger K, Granger DA, Kivlighan KT, Niaura R. Stress response and the adolescent transition: Performance versus peer rejection stressors. Dev Psychopathol. 2009;21:47–68. doi: 10.1017/S0954579409000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, Price LH, Gelernter J, Schepker C, Anderson GM, Carpenter LL. Interaction of childhood maltreatment with the corticotropin-releasing hormone receptor gene: Effects of HPA axis reactivity. Biol Psychiatry. 2009;66:681–685. doi: 10.1016/j.biopsych.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EA, Unutzer J, Rutter C, Gelfand A, Saunders K, VonKorff M, Koss MP, Katon W. Costs of health care use by women HMO members with a history of childhood abuse and neglect. Arch Gen Psychiat. 1999;56:609–613. doi: 10.1001/archpsyc.56.7.609. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Flory JD, Pratchett LC, Buxbaum J, Ising M, Holsboer F. Putative biological mechanisms for the association between early life adversity and the subsequent development of PTSD. Psychopharmology. 2010;212:405–417. doi: 10.1007/s00213-010-1969-6. [DOI] [PubMed] [Google Scholar]