Abstract
Osteoporosis is a major public health care concern. Although often described as a disease affecting postmenopausal women, researchers and clinicians have emphasized its prevalence in men in recent years. The National Osteoporosis Foundation has stated that up to 25% of men over the age of 50 years will experience a fracture due to osteoporosis. Men who suffer from a major fracture have higher mortality rates than women. Pharmacologic therapy options for treating osteoporosis are limited for men as compared with women, so each medication approved for use in this population represents an important clinical option. In September 2012, the US Food and Drug Administration approved a new indication for denosumab to increase bone mass in men with osteoporosis at high risk for fracture. Denosumab is a fully human monoclonal antibody and novel antiresorptive agent that works by binding receptor activator of nuclear factor kappa-β ligand (RANKL) and inhibiting the signaling cascade that causes osteoclast maturation, activity, and survival. Ultimately, denosumab suppresses bone turnover and increases bone mineral density in both trabecular and cortical bone. Approval for treating osteoporosis in men was based on data from the ADAMO trial which displayed efficacy in increasing bone mineral density at the lumbar spine, total hip, femoral neck, hip trochanter, and one-third radius. Studies indicate that denosumab is effective and safe, and has superior adherence rates and patient satisfaction. Although long-term data and further research on fracture reduction rates in men should be explored, at this time denosumab is one of several appropriate first-line treatment options for men with osteoporosis.
Keywords: denosumab, osteoporosis, men, treatment
Introduction
Forty-four million Americans meet the criteria for osteopenia or osteoporosis, making bone disease a major US public health care concern.1 Although osteoporosis education, prevention, and treatment has historically been aimed at women, in recent years, researchers and health care professionals have begun to focus on its prevalence and effects in men. According to the National Osteoporosis Foundation, up to 25% of men over the age of 50 years will experience a fracture due to osteoporosis, with approximately 80,000 suffering from a broken hip.1 In 2050, the incidence of hip fracture in men is expected to increase by 310% worldwide.2 In men and women over 60 years of age, fracture patients have a significantly higher mortality than the general population, and men suffering any major fracture have a higher mortality rate than women.3–5 The direct medical cost of osteoporosis in 2025 in the US is expected to be as high as $25.3 billion, with men accounting for over 25% of fractures and health care costs.6
Normal male bone development and pathogenesis of osteoporosis in men
Bone is a dynamic tissue which undergoes constant remodeling. It is composed of a mineralized matrix of calcium hydroxyapatite and collagen. Bone homeostasis is primarily maintained by three types of cells, ie, osteocytes, osteoblasts, and osteoclasts. Osteocytes are derived from osteoblasts and are the most common cell type found in bone. Osteocytes play a role in sensing mechanical stresses and damage in the tissue and signaling for action to be taken by osteoblasts or osteoclasts.7 Osteoclasts are responsible for tissue breakdown, while osteoblasts are responsible for anabolic activity. Each of these cell types is regulated by hormonal and chemical factors that alter bone turnover. A central regulatory cytokine is receptor activator of nuclear factor kappa-β ligand (RANKL), which is secreted by osteoblasts and binds to the RANK receptor located on osteoclast precursors and mature osteoclasts. Once bound, the RANK receptor begins a signaling cascade for osteoclast maturation, activity, and survival via several downstream signaling molecules.8 This process increases the amount of bone resorption by osteoclasts. Another signaling molecule, osteoprotegerin, is also secreted by osteoblasts. Osteoprotegerin acts as a decoy receptor for RANKL, thereby inhibiting its catabolic cascade. The balance of osteoprotegerin and RANKL secreted by osteoblasts determines the level of activity of osteoclasts and can be affected by hormones and cytokines, including vitamin D, estrogen, testosterone, glucocorticoids, parathyroid hormone, parathyroid hormone-related protein, interleukins 1, 7, 13, and 17, tumor necrosis factor alpha, interferon-gamma, prostaglandin E2, transforming growth factor beta, and bone morphogenetic protein 2.8
In both genders, prior to puberty, bone mineral density (BMD) and bone mass increase because bone length and diameter increase, particularly in the appendicular skeleton compared with the axial skeleton.7,9 With puberty beginning later in males, the male appendicular skeleton grows larger and as a result younger males have larger BMD.9,10 Once puberty begins, however, BMD increases for males and females equally.9 In males, bone fusion occurs later and the pubertal growth rate is faster and longer than that of a female, so males undergo a longer period of growth yielding longer legs, larger vertebral body size, and higher bone mineral densities.9,11 Puberty is also coupled to faster periosteal apposition and less cortical expansion in males, resulting in cortical thickening and increased bone and medullary diameter.12 In adulthood, loss of trabecular and cortical bone contributes to reductions in bone mass. Briggs et al13 studied volumetric BMD of trabecular and cortical bone for 3 years at the distal radius and distal tibia at baseline and at 3 years for trabecular volumetric BMD at the lumbar spine. Their results demonstrated that most cortical bone loss does not begin until after the age of 75 years in men, but begins much earlier in women. Trabecular bone loss, however, begins in young adults of both genders and continues throughout life, with an acceleration seen in women during perimenopause. Men experience 42% of their total lifetime trabecular bone loss before age 50 years. In addition, the authors conclude that late-onset cortical bone loss is associated with sex steroid deficiency.13 Older men are more likely to become osteoporotic with total testosterone or estradiol deficiency.14 While women tend to lose trabecular bone with age, resulting in trabecular perforation, in men, lower BMD is typically due to reduced bone formation resulting in trabecular thinning.15,16
Risk factors and contributors to osteoporosis in men
Several risk factors have been identified as contributing to the development of osteoporosis in men. In general, the condition is affected by genetics, age, hormones, lifestyle choices, comorbid conditions, and medical therapy. Primary osteoporosis consists of age-related and idiopathic disease.17 The most common secondary causes of osteoporosis in men include corticosteroid therapy, Cushing syndrome, excessive alcohol intake, primary or secondary hypogonadism, low calcium intake, vitamin D deficiency or insufficiency, smoking, and family history of minimal trauma fracture.18 Other secondary correlations include low body mass index, lack of exercise or excessive exercise, some antiepileptic medications, thyrotoxicosis or thyroxine overreplacement, primary hyperparathyroidism, chronic kidney or liver disease, intestinal malabsorption, hypercalciuria, rheumatoid arthritis, ankylosing spondylitis, diabetes mellitus (both type 1 and type 2), multiple myeloma or other monoclonal gammopathies, human immunodeficiency virus and/or its treatment with protease inhibitors, mastocytosis, organ transplantation or immunosuppressive medications, chronic obstructive pulmonary disease, and osteogenesis imperfecta.17,18 Three common groups of men particularly at risk for fracture are those on oral glucocorticoids for 3 months or longer, those receiving androgen deprivation therapy for prostate cancer, and those who have suffered a fragility (osteoporotic fracture) in the past.19
Screening, diagnosis and guidelines for osteoporosis in men
The World Health Organization defines osteoporosis in men using dual-energy X-ray absorptiometry (DXA). The criteria for diagnosis of osteoporosis in men of all ethnic groups is a T-score indicating a BMD >2.5 standard deviations below that of a uniform Caucasian (not race-adjusted) female reference standard.20 Clinical guidelines exist aimed at screening and diagnosing of osteoporosis in men. According to the Endocrine Society clinical practice guideline published in June 2012, men aged 70 years or older or those aged 50–69 years with risk factors, such as low body weight, prior fracture as an adult, or smoking, should be screened for osteoporosis using central DXA.21 The American College of Physicians recommends periodic individual assessment of risk factors for osteoporosis in older men before the age of 65 years, and DXA scans for men who are at increased risk for osteoporosis and are candidates for drug therapy.22 Lastly, the 2013 International Society for Clinical Densitometry recommends testing in all men older than 70 years and in men younger than 70 years if they have a risk factor for low bone mass such as low body weight, prior fracture, high-risk medication use, or a disease or condition associated with bone loss.20 The International Society for Clinical Densitometry further recommends testing in men and women with a fragility fracture, a disease or condition associated with low bone mass/bone loss, those taking medications associated with low bone mass/bone loss, anyone being considered for pharmacologic therapy, those being treated for osteoporosis to monitor treatment, and in those not receiving therapy in whom evidence of bone loss would lead to treatment.20
Overview of current management options for men
Treatment decisions for osteoporosis in men should be based on clinical evaluation, fracture risk assessment, diagnostic workup, and BMD measurements.23 Guidelines currently suggest initiating treatment in men over 50 with a history of spine or hip fractures, those with a T-score of −2.5 or below and men at high risk for fracture based on low BMD and/or clinical risk factors (such as those undergoing androgen deprivation therapy for prostate cancer management).21 Several pharmacologic and nonpharmacologic management options exists for osteoporosis prevention and treatment.
Calcium consumption from dietary sources (with supplementation if necessary) along with appropriate vitamin D levels are recommended for men at risk for or with osteoporosis.21 Dietary supplementation with calcium and vitamin D has been shown to reduce bone loss moderately in the femoral neck, spine, and total body, with a reduced incidence of nonvertebral fractures in men and women 65 years and older.24 The Food and Nutrition Board of the Institute of Medicine recommends 1,000 mg/day of calcium for men aged 51–70 years and 1,200 mg/day for all adults over 70 years. For men and women aged 51–70 years, the recommended vitamin D intake is 600 IU/day. For all adults over 70 years, the recommended vitamin D intake is 800 IU/day.25 Men with vitamin D levels below 30 ng/mL (75 nmol/L) should receive vitamin D supplementation to achieve blood 25(OH)D levels of 30 ng/mL (75 nmol/L) or greater.21,26 Decreased alcohol consumption, smoking cessation, regular weight-bearing exercise, and fall prevention are also important modifiable lifestyle factors for men with osteoporosis or those at high risk.21,23
Several prescription options are also available for the treatment of osteoporosis in men. The American College of Physicians recommends that pharmacologic therapy be offered to those with known osteoporosis and to those who have experienced a fragility fracture in the past.27 The Endocrine Society recommends pharmacologic therapy for all men at high risk of fracture.21 At this time, therapies approved by the US Food and Drug Administration (FDA) for men include bisphosphonates (alendronate, risedronate, and zoledronic acid), teriparatide, and denosumab.
Bisphosphonates are antiresorptive medications typically used as first-line therapy. Alendronate and risedronate are administered orally, usually in a daily or weekly regimen. Zoledronic acid is administered intravenously once annually. Alendronate is effective at increasing BMD in the spine and femoral neck and reducing the incidence of vertebral fractures.28,29 Risedronate is associated with an increase in BMD and a reduction in vertebral and hip fractures.30–32 Zoledronic acid has been shown to decrease bone turnover markers and increase bone density in men.33 It has also been associated with a reduction in the rate of new fractures and improved survival when given within 90 days of hip repair.34 The most common adverse effect with oral bisphosphonates is gastrointestinal irritation. Gastrointestinal symptoms may range from mild reflux, nausea, and vomiting to severe esophageal ulceration. Zoledronic acid may cause flu-like symptoms after intravenous administration. Osteonecrosis of the jaw is also a rare but serious adverse effect of bisphosphonates.
Teriparatide is a recombinant form of human parathyroid hormone amino acids 1–34. It is the only approved anabolic agent available to treat osteoporosis and is given as a once-daily subcutaneous injection. Teriparatide has been shown to increase BMD at the femoral neck, lumbar spine, and hip as well as reduce the incidence of new vertebral fractures.35–37 While generally well tolerated, teriparatide has a black box warning for increased incidence of dose-dependent osteosarcoma in rats, and at least two cases of osteosarcoma in humans who were taking teriparatide have been reported.38
Last, denosumab is a novel biologic agent. A 60 mg dose is administered every 6 months subcutaneously for the treatment of osteoporosis in men. It is a fully human monoclonal antibody manufactured by Amgen Inc., (Thousand Oaks, CA, USA) under the trade name Prolia®. Denosumab was first granted FDA approval in June 2010 for the treatment of postmenopausal women with osteoporosis at high risk of fracture. The following year, in September 2011, the FDA granted an additional indication to increase bone mass in men at high risk for fracture receiving androgen deprivation therapy for nonmetastatic prostate cancer and to increase bone mass in women at high risk for fracture receiving adjuvant aromatase inhibitor therapy for breast cancer. Most recently, in September 2012, denosumab was approved to increase bone mass in men with osteoporosis at high risk for fracture, defined as a history of osteoporotic fracture or multiple risk factors for fracture; or patients who have failed or are intolerant to other available osteoporosis therapy.39 Denosumab is also available under the trade name Xgeva®, which is a 120 mg dose administered subcutaneously every 4 weeks and indicated for the prevention of skeletal-related events in patients with metastases from solid tumors (approved by the FDA in November 2010). The remainder of this review focuses on the pharmacology, efficacy, safety, tolerability, and place in therapy of denosumab in treating osteoporosis in men.
Pharmacology of denosumab
Denosumab is a fully human monoclonal IgG2 antibody genetically engineered in Chinese hamster ovary cells.39 It binds RANKL, preventing it from binding to the RANK receptor on the surface of osteoclast precursor cells and osteoclasts. RANKL is part of the tumor necrosis factor family of proteins and is produced by osteoblasts.8 It is a transmembrane or soluble protein responsible for activating a signaling cascade in osteoclasts to promote bone resorption. High affinity binding of denosumab to RANKL inhibits osteoclast maturation, activity, and survival (Figure 1). The result of this interaction is increased cortical and trabecular bone strength and mass.39 Denosumab is administered as a 60 mg subcutaneous injection every 6 months in the upper arm, upper thigh, or abdomen.
Figure 1.
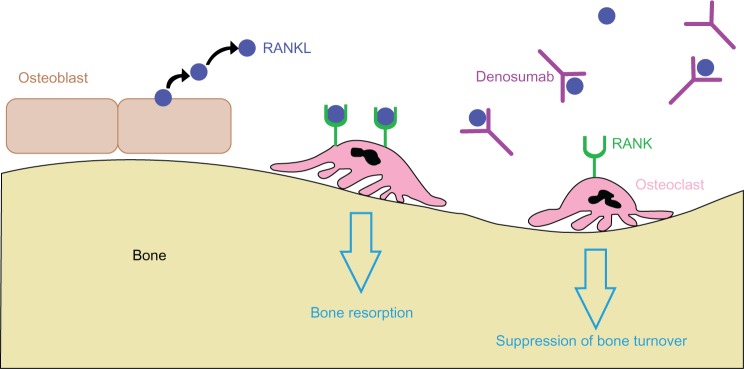
Mechanism of action of denosumab. High affinity binding of denosumab to RANKL inhibits osteoclast maturation, activity, and survival by preventing RANKL from binding the RANK receptor on immature and mature osteoclasts. This decreases bone resorption and suppresses bone turnover.
Abbreviations: RANKL, receptor activator of nuclear factor kappa-β ligand; RANK, receptor activator of nuclear factor kappa-β.
Pharmacokinetic characterization of denosumab was determined in 73 healthy male and female subjects, aged 18–64 years, after a single 60 mg subcutaneous dose was administered (Table 1). The mean maximum denosumab concentration is 6.75 ± 1.89 μg/mL, with a median time to maximum concentration of 10 (range 3–21) days. The mean half-life of denosumab is 25.4 ± 8.5 days. The mean AUC0–16 weeks of denosumab is 316 ± 101 μg*day/mL.39 A meta-analysis of seven Phase I studies, two Phase II studies, and two Phase III studies (n=1,564) determined the subcutaneous bioavailability of denosumab to be 64% with the first-order absorption rate constant ka =0.00883 per hour.40 The central volume of distribution of denosumab is 2.49 L/66 kg, and linear clearance was calculated to be 3.06 mL/hour/66 kg. Lastly, the RANKL degradation rate was determined to be 0.00148 per hour (Table 1). The authors found that a 60 mg fixed dose of denosumab given every 6 months provided similar RANKL inhibition as using weight-based dosing. Finally, they concluded that the nonlinear pharmacokinetic profile of denosumab is likely caused by RANKL binding, and dosing adjustments based on age, gender, or race are not required.40 Denosumab has not been evaluated in patients with hepatic impairment.39 In a study of 55 subjects with renal function ranging from normal to dialysis-dependent, the findings indicated that denosumab does not require any dose adjustment in patients with renal impairment, as no significant changes were seen in pharmacokinetic or pharmacodynamic parameters in this population.41
Table 1.
Pharmacokinetic characterization of denosumab is based on 73 healthy male and female subjects (age 18–64 years) and meta-analysis of seven Phase I studies, two Phase II studies, and two Phase III studies (n=1,564)
| Pharmacokinetic parameter | |
| Cmax | 6.75 ± 1.89 μg/mL |
| Tmax | 10 (range 3–21) days |
| Mean half-life (SD) | 25.4 ± 8.5 days |
| Mean AUC0–16 weeks (SD) | 316 ± 101 μg*day/mL |
| Subcutaneous bioavailability | 64% |
| ka | 0.00883 per hour |
| Central volume of distribution | 2.49 L/66 kg |
| Linear clearance | 3.06 mL/hour/66 kg |
| RANKL degradation rate | 0.00148 per hour |
Abbreviations: Cmax, maximum denosumab concentration; Tmax, median time to maximum concentration; RANKL, receptor activator of nuclear factor kappa-β ligand; SD, standard deviation; AUC, mean area under the concentration-time curve; ka, first-order absorption rate constant.
Pharmacodynamic characterization of subcutaneous denosumab 60 mg is described by the reduction of serum type 1 C-telopeptide (CTX) following administration of denosumab. CTX is a bone resorption marker that when reduced indicates less bone resorption is occurring in the body. Clinical studies indicate that within 3 days of administration, CTX is reduced by approximately 85% (maximal reductions are seen by 1 month).39 Furthermore, CTX levels were substantially lower during a 48-month study of denosumab treatment in 319 postmenopausal women but increased to above baseline upon discontinuation of denosumab. After 24 months without treatment, however, CTX levels returned to baseline levels and were not significantly different from placebo.42 Reinitiation of denosumab in subjects caused decreased CTX levels similar to those seen in subjects initiating denosumab for the first time.39 In a trial of 242 randomized men, CTX levels at day 15 in the denosumab treatment arm were decreased by 81% (placebo −7%, P<0.0001), 6-month CTX levels were decreased 65% from baseline (placebo +3%, P<0.0001), and 12-month CTX levels were decreased 60% (placebo +3%, P<0.0001).43
Efficacy, safety, monitoring, and tolerability of denosumab
ADAMO (a multicenter, randomized, double-blind, placebo controlled Study to Compare the Efficacy and Safety of DenosumAb Versus Placebo in Males With Osteoporosis) is a critical Phase III trial upon which the FDA based its approval for the use of denosumab to treat men with osteoporosis.43 A total of 242 men were randomized 1:1 to receive 60 mg denosumab or placebo every 6 months subcutaneously over 12 months. After the 12-month period, all subjects were assigned to 60 mg denosumab subcutaneously every 6 months in an open-label manner for an additional 12 months (an open-label study is presently ongoing). All subjects received ≥1,000 mg elemental calcium and ≥800 IU vitamin D supplementation throughout the study. All men who participated were ambulatory and between the ages of 30 and 85 (mean 65) years. Included participants had BMD T-scores ≤−2.0 to ≥−3.5 at the lumbar spine or femoral neck or had a prior major osteoporotic fracture and a BMD T-score ≤−1.0 to ≥−3.5 at the lumbar spine or femoral neck, and had at least two lumbar vertebrae, one femur, and one forearm evaluable via DXA. The primary endpoint of the study was evaluation of change in BMD at the lumbar spine from baseline at 12 months. Secondary endpoints were BMD change from baseline of the total hip, femoral neck, hip trochanter, and one-third radius at the 12-month mark. Percent change from baseline of serum CTX at day 15 was also measured, and safety endpoints, including adverse events at month 12, were analyzed.43
At 12 months, BMD at the lumbar spine increased 5.7% from baseline in the denosumab treatment arm versus 0.9% in the placebo group, giving a difference of 4.8% in mean lumbar spine BMD change (confidence interval 4.0%–5.6%, P<0.0001). An increase in BMD at the lumbar spine was also significantly higher at 6 months in the denosumab group (P<0.0001). Further, denosumab significantly increased BMD at the total hip (2.4% versus 0.3%, P<0.0001 versus placebo), femoral neck (2.1% versus 0.0%, P<0.0001 versus placebo), hip trochanter (3.1% versus 0.8%, P<0.0001 versus placebo), and one-third radius (0.6% versus −0.3%, P<0.0144 versus placebo, Table 2). When age, race, geographic region, previous osteoporotic fracture, baseline testosterone level, 10-year major osteoporotic fracture risk, and lumbar spine BMD T-score were controlled for, sensitivity analysis showed that separation from placebo and from baseline was still significant (P<0.0001 for each comparison). Increase in BMD at varying concentrations of testosterone is particularly important due to the role of reduced sex hormones in contributing to osteoporosis in men, particularly those receiving androgen deprivation therapy for hormone-sensitive malignancies, such as prostate cancer. These results are similar to those found comparing denosumab with placebo in men receiving androgen deprivation therapy for prostate cancer, with an increase in BMD at the lumbar spine, total hip, femoral neck, and distal one-third of the radius seen in this population of men as well.44 While fracture risk was not measured in ADAMO, a decreased incidence of new vertebral fractures was seen in androgen-deficient men taking denosumab for 36 months.44 Also, in a study of over 7,800 postmenopausal women, denosumab reduced the relative risk of new radiographic vertebral fracture by 68% (risk ratio 0.32, 95% confidence interval 0.26–0.41, P<0.001), reduced the relative risk of hip fracture by 40% (hazard ratio 0.60, 95% confidence interval 0.37–0.97, P<0.04), and reduced the relative risk of nonvertebral fracture by 20% (hazard ratio 0.80, 95% confidence interval 0.67–0.95, P<0.01).45
Table 2.
Bone mineral density changes after 12 months of subcutaneous denosumab 60 mg versus placebo in ADAMO (n=242 men)
| Denosumab | Placebo | P-value | |
|---|---|---|---|
| ADAMO BMD changes from baseline | |||
| BMD lumbar spine | +5.7% | +0.9% | <0.0001 |
| BMD total hip | +2.4% | +0.3% | <0.0001 |
| BMD femoral neck | +2.1% | 0.0% | <0.0001 |
| BMD hip trochanter | +3.1% | +0.8% | <0.0001 |
| BMD one third radius | +0.6% | −0.3% | <0.0144 |
Notes: Age, race, geographic region, previous osteoporotic fracture, baseline testosterone level, 10-year major osteoporotic fracture risk, and lumbar spine bone mineral density T-score did not confound the data and when controlled for separation from placebo and from baseline were still significant (P<0.0001 for each comparison).
Abbreviations: BMD, bone mineral density; ADAMO, Study to Compare the Efficacy and Safety of DenosumAb Versus Placebo in Males With Osteoporosis.
Median serum CTX levels decreased 81% from baseline at day 15 in the denosumab-treated group versus 7% in the placebo group. At 6 months, serum CTX levels were −65% in the denosumab group versus +3% in the placebo group, and at 12 months serum CTX levels were −60% in the denosumab group and +3% in the placebo group (P<0.0001).43 The authors of ADAMO point out that their efficacy results using denosumab are unique compared with other trials of antiresorptive osteoporosis treatments in men, including those with daily alendronate, weekly risedronate, monthly ibandronate, and annual zoledronic acid.43 While these bisphosphonates gave increases in BMD at the lumbar spine and proximal femur, they did not significantly increase BMD at the one-third radius.28,32,33,46 In contrast, a statistically significant increase was seen at this cortical bone site in ADAMO as well as in denosumab trials in postmenopausal female subjects.42,45 No data exist in men at this time regarding the effects of discontinuation of denosumab; however, discontinuation studies in postmenopausal women indicate no excess fracture risk on discontinuation of denosumab for up to 2 years.47
The most common adverse events (≥5% incidence) reported in ADAMO were back pain, arthralgia, nasopharyngitis, and constipation across the denosumab and placebo groups.43 No incidence of atypical femoral fracture, hypocalcemia, adjudicated osteonecrosis of the jaw, or complications of fracture healing were reported. Serious adverse effects reported included prostate cancer in three men in the denosumab group (two of the three were diagnosed within 3 weeks of first injection and considered not treatment-related; 0 cases of prostate cancer in the placebo group) and arterial limb thrombosis in two men in the denosumab group (0 in placebo group).43 Two clinical fractures occurred in the placebo group while one occurred in the denosumab treatment arm.43
Full prescribing warnings and precautions for denosumab include allergies to drug products with the same active ingredient, hypocalcemia and mineral metabolism, serious infections, dermatologic adverse reactions, osteonecrosis of the jaw, atypical subtrochanteric and diaphyseal femoral fractures, and suppression of bone turnover.39 Denosumab is contraindicated in those with hypocalcemia, and patients taking denosumab should receive adequate calcium and vitamin D supplementation. Those at risk for hypocalcemia (eg, patients with renal impairment or parathyroid disturbances) should have calcium and mineral levels monitored. Hypocalcemia must be corrected prior to starting denosumab.39 Serious infection was not listed by any subjects in ADAMO. However, the FREEDOM (Fracture Reduction Evaluation of Denosumab in Osteoporosis every 6 Months) trial reported higher rates of serious infection leading to hospitalization as well as infections of the skin, abdomen, urinary tract, and ear, and endocarditis in the treatment arm, compared with placebo.45 The same study also found dermatologic adverse reactions, such as dermatitis, rashes, and eczema, to be higher in the denosumab group compared with placebo, although this was not reported in the men who participated in ADAMO.45 Lastly, osteonecrosis of the jaw, suppression of bone turnover, and atypical subtrochanteric and diaphyseal femoral fractures have been reported in patients taking denosumab.39 A Risk Evaluation and Mitigation Strategy program has been established for denosumab and includes distribution of the Prolia medication guide and a communication plan to inform health care providers about the risk of serious infection, suppression of bone turnover, osteonecrosis of the jaw, and dermatologic adverse reactions, although these events are rare.48
Patient-focused perspectives
Men with osteoporosis who are nonadherent to treatment are at risk for poor outcomes, including fractures. The DAPS (Denosumab Adherence Preference Satisfactions) study was designed to analyze adherence, compliance, persistence, patient beliefs, preference, and satisfaction in patients taking denosumab.49 This trial utilized a randomized, crossover design and each patient received denosumab 60 mg subcutaneously every 6 months for 12 months and alendronate 70 mg orally every week for 12 months, one after the other.49 A total of 221 postmenopausal women completed the 24 months of therapy. Results for nonadherence, noncompliance, nonpersistence, and treatment preference, as well as further BMD improvement after crossover all favored denosumab. Nonadherence was 11.9% and 7.5% in year 1 and year 2, respectively, for denosumab, while nonadherence was 23.4% and 36.5% for alendronate in year 1 and year 2, respectively. In addition, of the 198 subjects who preferred one agent over the other, 92.4% (183 subjects) preferred denosumab over once-weekly oral alendronate.49 These results are supported by a recent open-label, prospective study of 142 patients (42 with male osteoporosis) which found that significant increases in BMD at 6 months along with positive reinforcement during doctor-patient interactions, rarity of adverse events, and infrequency of dosing (every 6 months) led to a positive impact on adherence in continuing with denosumab injections every 6 months.50 Finally, although the cost-effectiveness of denosumab has not been evaluated specifically in men with osteoporosis, several studies indicate that treatment with denosumab is a cost-effective alternative to oral bisphosphonates when looking at postmenopausal women with osteoporosis.51,52
Conclusion
Osteoporosis is a major public health care concern, with men representing 29% of fractures in the US and 25% of the costs.6 Sadly, men who suffer a major fracture have higher mortality rates than both the general population and females with major fractures.3 Researchers and health care providers are more aware now than previously of the morbidity, mortality, and cost that osteoporosis is responsible for and its impact on men as well as women. Both lifestyle and pharmacologic treatments should be pursued in men at risk for or with osteoporosis. Dietary consumption and supplementation with calcium and vitamin D at levels recommended by the Food and Nutrition Board of the Institute of Medicine should be initiated. In addition, decreased alcohol consumption, smoking cessation, regular weight-bearing exercise, and fall prevention should be addressed in all men with osteoporosis and those at risk. Denosumab is approved by the FDA to increase bone mass in men with osteoporosis at high risk for fracture. It is the only medication in its class, is a novel biologic, and has a unique mechanism of action. Denosumab is effective in increasing BMD at the lumbar spine, total hip, femoral neck, hip trochanter, and one-third radius in men with osteoporosis, and significantly reduces serum CTX levels. Studies indicate that denosumab is effective and safe, and has superior adherence and patient satisfaction rates, in part because of twice-yearly in-office administration. Denosumab is an appropriate clinical option in men with intolerance or contraindications to bisphosphonates (gastrointestinal complications, hypersensitivity, inability to stand or sit upright, infusion reaction to zoledronic acid, renal impairment). Although long-term data and further research on fracture reduction rates in men should be explored, at this time denosumab is an appropriate first-line option for men with osteoporosis.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.National Osteoporosis Foundation . America’s Bone Health: The State of Osteoporosis and Low Bone Mass in Our Nation. Washington, DC: National Osteoporosis Foundation; 2002. [Google Scholar]
- 2.Gullberg B, Johnell O, Kanis JA. World-wide projections for hip fracture. Osteoporos Int. 1997;7(5):407–413. doi: 10.1007/pl00004148. [DOI] [PubMed] [Google Scholar]
- 3.Center JR, Nguyen TV, Schneider D, Sambrook PN, Eisman JA. Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet. 1999;353(9156):878–882. doi: 10.1016/S0140-6736(98)09075-8. [DOI] [PubMed] [Google Scholar]
- 4.Bliuc D, Nguyen ND, Milch VE, Nguyen TV, Eisman JA, Center JR. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;301(5):513–521. doi: 10.1001/jama.2009.50. [DOI] [PubMed] [Google Scholar]
- 5.Kannegaard PN, van der Mark S, Eiken P, Abrahamsen B. Excess mortality in men compared with women following a hip fracture. National analysis of comedications, comorbidity and survival. Age Ageing. 2010;39(2):203–209. doi: 10.1093/ageing/afp221. [DOI] [PubMed] [Google Scholar]
- 6.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22(3):465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 7.Bonewald LF, Johnson ML. Osteocytes, mechanosensing and Wnt signaling. Bone. 2008;42(4):606–615. doi: 10.1016/j.bone.2007.12.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kearns AE, Khosla S, Kostenuik PJ. Receptor activator of nuclear factor kappaB ligand and osteoprotegerin regulation of bone remodeling in health and disease. Endocr Rev. 2008;29(2):155–192. doi: 10.1210/er.2007-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seeman E. Unresolved issues in osteoporosis in men. Rev Endocr Metab Disord. 2001;2(1):45–64. doi: 10.1023/a:1010054924085. [DOI] [PubMed] [Google Scholar]
- 10.Seeman E. Growth in bone mass and size – are racial and gender differences in bone mineral density more apparent than real? J Clin Endocrinol Metab. 1998;83(5):1414–1419. doi: 10.1210/jcem.83.5.4844. [DOI] [PubMed] [Google Scholar]
- 11.Preece MA, Pan H, Ratcliffe SG. Auxological aspects of male and female puberty. Acta Paediatr Suppl. 1992;383:11–13. [PubMed] [Google Scholar]
- 12.Seeman E. Sexual dimorphism in skeletal size, density, and strength. J Clin Endocrinol Metab. 2001;86(10):4576–4584. doi: 10.1210/jcem.86.10.7960. [DOI] [PubMed] [Google Scholar]
- 13.Riggs BL, Melton LJ, Robb RA, et al. A population-based assessment of rates of bone loss at multiple skeletal sites: evidence for substantial trabecular bone loss in young adult women and men. J Bone Miner Res. 2008;23(2):205–214. doi: 10.1359/JBMR.071020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fink HA, Ewing SK, Ensrud KE, et al. Association of testosterone and estradiol deficiency with osteoporosis and rapid bone loss in older men. J Clin Endocrinol Metab. 2006;91(10):3908–3915. doi: 10.1210/jc.2006-0173. [DOI] [PubMed] [Google Scholar]
- 15.Aaron JE, Makins NB, Sagreiya K. The microanatomy of trabecular bone loss in normal aging men and women. Clin Orthop Relat Res. 1987;215:260–271. [PubMed] [Google Scholar]
- 16.Khosla S, Riggs BL, Atkinson EJ, et al. Effects of sex and age on bone microstructure at the ultradistal radius: a population-based noninvasive in vivo assessment. J Bone Miner Res. 2006;21(1):124–131. doi: 10.1359/JBMR.050916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khosla S, Amin S, Orwoll E. Osteoporosis in men. Endocr Rev. 2008;29(4):441–464. doi: 10.1210/er.2008-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ebeling PR. Clinical practice. Osteoporosis in men. N Engl J Med. 2008;358(14):1474–1482. doi: 10.1056/NEJMcp0707217. [DOI] [PubMed] [Google Scholar]
- 19.Adler RA. Osteoporosis in men: recent progress. Endocrine. 2013;44(1):40–46. doi: 10.1007/s12020-013-9880-7. [DOI] [PubMed] [Google Scholar]
- 20.International Society for Clinical Densitometry Indications for bone mineral density (BMD) testing. [Accessed August 26, 2013]. Available from: http://www.iscd.org/documents/2013/07/2013-iscd-official-positions-adult.pdf.
- 21.Watts NB, Adler RA, Bilezikian JP, et al. Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(6):1802–1822. doi: 10.1210/jc.2011-3045. [DOI] [PubMed] [Google Scholar]
- 22.Qaseem A, Snow V, Shekelle P, Hopkins R, Jr, Forciea MA, Owens DK. Screening for osteoporosis in men: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2008;148(9):680–684. doi: 10.7326/0003-4819-148-9-200805060-00008. [DOI] [PubMed] [Google Scholar]
- 23.Rao SS, Budhwar N, Ashfaque A. Osteoporosis in men. Am Fam Physician. 2010;82(5):503–508. [PubMed] [Google Scholar]
- 24.Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med. 1997;337(10):670–676. doi: 10.1056/NEJM199709043371003. [DOI] [PubMed] [Google Scholar]
- 25.Institute of Medicine . Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 26.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84(1):18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 27.Qaseem A, Snow V, Shekelle P, Hopkins R, Jr, Forciea MA, Owens DK. Pharmacologic treatment of low bone density or osteoporosis to prevent fractures: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2008;149(6):404–415. [PubMed] [Google Scholar]
- 28.Orwoll E, Ettinger M, Weiss S, et al. Alendronate for the treatment of osteoporosis in men. N Engl J Med. 2000;343(9):604–610. doi: 10.1056/NEJM200008313430902. [DOI] [PubMed] [Google Scholar]
- 29.Sawka AM, Papaioannou A, Adachi JD, Gafni A, Hanley DA, Thabane L. Does alendronate reduce the risk of fracture in men? A meta-analysis incorporating prior knowledge of anti-fracture efficacy in women. BMC Musculoskelet Disord. 2005;6:39. doi: 10.1186/1471-2474-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ringe JD, Faber H, Farahmand P, Dorst A. Efficacy of risedronate in men with primary and secondary osteoporosis: results of a 1-year study. Rheumatol Int. 2006;26(5):427–431. doi: 10.1007/s00296-005-0004-4. [DOI] [PubMed] [Google Scholar]
- 31.Sato Y, Iwamoto J, Kanoko T, Satoh K. Risedronate sodium therapy for prevention of hip fracture in men 65 years or older after stroke. Arch Intern Med. 2005;165(15):1743–1748. doi: 10.1001/archinte.165.15.1743. [DOI] [PubMed] [Google Scholar]
- 32.Boonen S, Orwoll ES, Wenderoth D, Stoner KJ, Eusebio R, Delmas PD. Once-weekly risedronate in men with osteoporosis: results of a 2-year, placebo-controlled, double-blind, multicenter study. J Bone Miner Res. 2009;24(4):719–725. doi: 10.1359/jbmr.081214. [DOI] [PubMed] [Google Scholar]
- 33.Orwoll ES, Miller PD, Adachi JD, et al. Efficacy and safety of a once-yearly i.v. Infusion of zoledronic acid 5 mg versus a once-weekly 70-mg oral alendronate in the treatment of male osteoporosis: a randomized, multicenter, double-blind, active-controlled study. J Bone Miner Res. 2010;25(10):2239–2250. doi: 10.1002/jbmr.119. [DOI] [PubMed] [Google Scholar]
- 34.Lyles KW, Colón-Emeric CS, Magaziner JS, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357(18):1799–1809. doi: 10.1056/NEJMoa074941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaufman JM, Orwoll E, Goemaere S, et al. Teriparatide effects on vertebral fractures and bone mineral density in men with osteoporosis: treatment and discontinuation of therapy. Osteoporos Int. 2005;16(5):510–516. doi: 10.1007/s00198-004-1713-3. [DOI] [PubMed] [Google Scholar]
- 36.Gallagher JC, Genant HK, Crans GG, Vargas SJ, Krege JH. Teriparatide reduces the fracture risk associated with increasing number and severity of osteoporotic fractures. J Clin Endocrinol Metab. 2005;90(3):1583–1587. doi: 10.1210/jc.2004-0826. [DOI] [PubMed] [Google Scholar]
- 37.Orwoll ES, Scheele WH, Paul S, et al. The effect of teriparatide [human parathyroid hormone (1–34)] therapy on bone density in men with osteoporosis. J Bone Miner Res. 2003;18(1):9–17. doi: 10.1359/jbmr.2003.18.1.9. [DOI] [PubMed] [Google Scholar]
- 38.Subbiah V, Madsen VS, Raymond AK, Benjamin RS, Ludwig JA. Of mice and men: divergent risks of teriparatide-induced osteosarcoma. Osteoporos Int. 2010;21(6):1041–1045. doi: 10.1007/s00198-009-1004-0. [DOI] [PubMed] [Google Scholar]
- 39.Prolia®(denosumab) Full prescribing information medication guide. Thousand Oaks, CA: Amgen Inc; Sep 10, 2013. [Google Scholar]
- 40.Sutjandra L, Rodriguez RD, Doshi S, et al. Population pharmacokinetic meta-analysis of denosumab in healthy subjects and postmenopausal women with osteopenia or osteoporosis. Clin Pharmacokinet. 2011;50(12):793–807. doi: 10.2165/11594240-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 41.Block GA, Bone HG, Fang L, Lee E, Padhi D. A single-dose study of denosumab in patients with various degrees of renal impairment. J Bone Miner Res. 2012;27(7):1471–1479. doi: 10.1002/jbmr.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller PD, Bolognese MA, Lewiecki EM, et al. Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone. 2008;43(2):222–229. doi: 10.1016/j.bone.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 43.Orwoll E, Teglbjærg CS, Langdahl BL, et al. A randomized, placebo-controlled study of the effects of denosumab for the treatment of men with low bone mineral density. J Clin Endocrinol Metab. 2012;97(9):3161–3169. doi: 10.1210/jc.2012-1569. [DOI] [PubMed] [Google Scholar]
- 44.Smith MR, Egerdie B, Toriz NH, et al. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med. 2009;361(8):745–755. doi: 10.1056/NEJMoa0809003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361(8):756–765. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 46.Orwoll ES, Binkley NC, Lewiecki EM, Gruntmanis U, Fries MA, Dasic G. Efficacy and safety of monthly ibandronate in men with low bone density. Bone. 2010;46(4):970–976. doi: 10.1016/j.bone.2009.12.034. [DOI] [PubMed] [Google Scholar]
- 47.Brown JP, Roux C, Törring O, et al. Discontinuation and associated fracture incidence: analysis from the FREEDOM trial. J Bone Miner Res. 2013;28(4):746–752. doi: 10.1002/jbmr.1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prolia®(denosumab) Risk Evaluation and Mitigation Strategy (REMS) Thousand Oaks, CA, USA: Amgen Inc; [Accessed September 10, 2013]. Available from: http://www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/UCM214383.pdf. [Google Scholar]
- 49.Freemantle N, Satram-Hoang A, Tang ET, et al. Final results of the DAPS (Denosumab Adherence Preference Satisfaction) study: a 24-month, randomized, crossover comparison with alendronate in postmenopausal women. Osteoporos Int. 2012;23(1):317–326. doi: 10.1007/s00198-011-1780-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ringe JD, Farahmand P. Improved real-life adherence of 6-monthly denosumab injections due to positive feedback based on rapid 6-month BMD increase and good safety profile. Rheumatol Int. 2013 Jan 19; doi: 10.1007/s00296-012-2663-2. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 51.Hiligsmann M, Boonen A, Dirksen CD, Ben Sedrine W, Reginster JY. Cost-effectiveness of denosumab in the treatment of postmenopausal osteoporotic women. Expert Rev Pharmacoecon Outcomes Res. 2013;13(1):19–28. doi: 10.1586/erp.12.76. [DOI] [PubMed] [Google Scholar]
- 52.Jönsson B, Ström O, Eisman JA, et al. Cost-effectiveness of denosumab for the treatment of postmenopausal osteoporosis. Osteoporos Int. 2011;22(3):967–982. doi: 10.1007/s00198-010-1424-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


