Abstract
A clinical need exists for a cell delivery device that supports long term cell viability, cell retention within the device and retrieval of delivered cells if necessary. Previously, cell isolation devices have been based on hollow fiber membranes, porous polymer scaffolds, alginate systems, or micro-machined membranes. We present the development and characterization of a novel dual porosity electrospun membrane based device, which supports cellular infiltration and vascularization of its outer porous layer and maintains cellular isolation within a lumen bounded by an inner low porosity layer. Electrospinning conditions were initially established to support electrospun fiber deposition onto nonconductive silicone surfaces. With these parameters established, devices for in vivo evaluations were produced using nylon as a nonconductive scaffold for deposition of dual porosity electrospun fibers. The outer porous layer supported the development of a penetrating microcirculation and the membrane supported the transfer of insulin from encapsulated sustained release pellets for four weeks. Viable cells implanted within the device could be identified after two weeks of implantation. Through the successful demonstration of survival and cellular isolation of human epithelial cells within the implanted devices and the ability to use the device to deliver insulin, we have established the utility of this device toward localized cell transplantation. The Cell Delivery Device establishes a platform to test the feasibility of approaches to cell dose control and cell localization at the site of implantation in the clinical use of modified autologous or allogeneic cells.
Keywords: cellular isolation, vascularized cell delivery, electrospinning, neovascularization, porosity
Introduction
Cell and tissue based therapies provide a novel therapeutic approach to address pathologies arising from lack of a specific protein or biochemical. Examples of therapeutic proteins amenable to delivery via a cell based system include insulin, erythropoietin, growth hormone and coagulation factors Translation of this technology to clinical practice has been hampered by challenges including the lack of technology to regulate the dose of cells, their long-term fate and efficacy, the effect of migration of cells to unintended sites. Of equal importance the viability of transplanted cells depends on a functional microcirculation to provide nutrients and to provide a route for cell specific product release into the circulation. Several strategies have emerged to address these issues including the direct encapsulation of individual cells and polymer based devices to enclose the cells 1–9.
The viability and integration of implanted tissues, exemplified by islet, parathyroid tissue and lipoaspirated fat is equally dependent on microvascular perfusion of the implanted tissue. Biomaterials have been developed that support peri-implant angiogenesis and porous implant neovascularization. Brauker et al., previously reported a novel porous biomaterial construct that provided both cellular isolation and peri-implant material angiogenesis. Biomaterial modifications have also been reported that support both peri-implant angiogenesis and intra-implant neovascularization.12–14.
Based on these previous studies our goal is to develop a non-degradable, electrospun, cell-delivery vehicle to support rapid re-vascularization of encapsulated cells and/or tissues. Design parameters include a device capable of withstanding manipulation during surgical implantation, the ability to support the formation of a microcirculation around and within the outer wall of the implant, the ability of the newly formed microcirculation to support the egress of therapeutic agents (e.g. proteins) into the general circulation. An additional design parameter is the ability to remove the device, and encapsulated cells if necessary. Here, we describe a novel process of direct electrospinning polymer fibers on two non-conductive surfaces, silicone tubing and nylon mesh. Electrospinining conditions were established that support the formation of an inner electrospin layer of limited porosity to support cell encapsulation and an outer electrospun layer with higher porosity that supports accelerated formation of a functional microcirculation. We report the ability of this cellular isolation device to stimulate both a peri-implant angiogenesis and neovascularization of the porous electrospun biomaterial. The function of the device toward delivery of insulin was also evaluated. The device is intended as an enabling technology targeted at transplantation of islets, allogeneic cells, and genetically re-programmed allogeneic or autologous cells.
Methods
Two polymers, silicone tubing (Si) and nylon mesh (Ny), were evaluated for their ability to provide a stable, semi rigid scaffold for subsequent deposition of electrospun fibers. These scaffold materials differ in handling characteristics, silicone tubing is more rigid and kink resistant than nylon, and have different porosity characteristics. The nylon mesh was used without further modification while the silicone tubing was modified, as described below, to create trans-wall porosity.
Perforated silicone tube Cell Delivery Vehicle (Si-CDV)
To demonstrate the ability to electrospin fibers directly on non-conducting surfaces, dual porosity nylon fibers were electrospun onto a silicone tube interposed over a conducting mandrel. Approximately 2 mm diameter perforations were made 2 mm apart with an electrocautery tip in a 1 inch long piece of implant grade silicone tubing (ID: 0.062″, OD: 0.095″, Allied Biomedical, Ventura, CA), Multiple silicone tubes were then mounted on a 12.5 cm long stainless steel hypodermic tube mandrel (18G, Small Parts Inc.) and loaded on the electrospinning platform.
Heat sealed nylon mesh tube Cell Delivery Vehicle (Ny-CDV)
A rectangular piece of nylon mesh (50μm, Sefar-Nitex, Small Parts Inc. Logansport, IN) approximately 1 inch long, was folded and thermally sealed at the two long edges to form a cylindrical sheath that fit tightly over an 18G steel mandrel. The sealed edges were trimmed to leave very little overhang as seen in a circular cross-section. Each steel mandrel could accommodate up to 5 such devices for the electrospinning process. Nylon fibrils were electrospun over this mesh sheath using the procedure described in the preceding sections. Subsequently, the devices were removed from the mandrel. One end of the devices was sealed first using thermal sealing, and second, a layer of UV-activated adhesive (Loctite 3211, Rocky Hill, CT) was cured over the entire seam to create a complete seal at the tube end. A polyethylene tube (PE-90, Clay-Adams, Parsippany, NJ) 1.5 inches long was inserted 3 mm into the open end of the tube and secured in place using UV-cured glue (Loctite 3211, Rocky Hill, CT). A blunt needle hub (20G) was inserted into the free end of the PE tube to facilitate loading of cells prior to implantation. The devices were sterilized with 70% ethanol and washed with sterile PBS and culture media prior to implantation. A similar larger diameter electrospun tube was made using a 9 mm mandrel. A small cylindrical section was cut, flattened, and used to enclose insulin releasing pellets (LinBit) as described later, by thermally sealing the four edges of this CDV around the pellets.
Electrospun dual porosity membranes
A rotating mandrel based electrospinning technique as described previously 15 was used to manufacture dual porosity electrospun devices. Briefly, nylon 6,6 pellets (Sigma-Aldrich, St. Louis, MO) were dissolved in formic acid with gentle agitation to get 15% and 40% nylon by weight solutions. A trace amount of pyridine was added to the solution to enhance the electrospinning process. The inner low-porosity cellular isolation layer was spun using 15% nylon solution dispensed through an 18G needle at a rate of 1.0 ml/hr. The collector, a needle mandrel, rotated about its long axis at approximately 1000 rpm, and translated from side to side at a low setting (slider set at 20% of maximum) and placed 10 inches away from the dispensing needle. The outer porous layer of thicker fibrils was immediately deposited over this layer using 40% nylon solution dispensed at 0.5 ml/hr, with the mandrel rotating slower (about 20% of the previous speed) while translating rapidly from side to side (slider at 90 % of maximum).
Scanning Electron Microscopy (SEM)
Samples of the electrospun device membranes were processed for SEM, sputter coated and evaluated in a scanning electron microscope (JOEL, Tokyo) 15. Digital micrographs of the inner and outer layers were imported into Metamorph image processing suite (Molecular Devices, Sunnyvale, California, USA) and line and polygon elements were used to determine the fiber diameters and porosity of thirty-two samples per micrograph. The polygonal elements were aligned at the boundaries of clearly identifiable pores and the areas estimated. The areas were then used to calculate the diameters of equivalent circles, assuming the pores to be circular16. Pooled images from two to three each of the Si-CDV and Ny-CDVs were used for this analysis. The mean inner and outer electrospun fiber diameters and the pore sizes were compared between devices using a two-tailed t-test, assuming unequal variance. Axial images were used to make approximate estimates of the membrane thickness.
Characterization of leakage and pressure response of tubular CDVs
Performance of tubular electrospun devices were characterized under flow and pressure following methods recommended for vascular graft evaluation (ANSI/AAMI/ISO 7198:1998/2001). An in-line pressure transducer (DTX Plus TNF-R, Becton Dickinson, Franklin Lakes, NJ, USA) was calibrated before each use in conjunction with a signal conditioning system (Gould 6600 Series, Gould Instrument Systems, Valley View, OH, USA), a data acquisition unit (PowerLab/4SP, ADInstruments, Colorado Springs, CO, USA), and Chart software (version 5.4.2, ADInstruments). The Si-CDVs were mounted on barbed fittings at each end and anchored with sutures to prevent slippage. Ny-CDVs were mounted between two 0.5″ long Teflon needle hubs and glued in place taking care to leave only the tip of the Teflon needle inside the CDV. The inlet of each CDV was connected to the pressure transducer, which itself was connected to a 60 ml syringe driven by a syringe pump (BS-8000, Braintree Scientific, Braintree, MA, USA). The other end of the CDV was connected to a 3-way stop valve leading to an outlet tube. The Si-CDVs were tested both in their dry state and after denucleation by immersion in 70% ethanol. All Ny-CDVs were denucleated with ethanol prior to testing. For leakage testing, the outflow valve was opened, and distilled water was flowed through the lumen of the CDVs at controlled rates (1, 5, and 10ml/min) for 1 minute each, successively. The outflow was transferred to a measuring cylinder and any fluid leaking across the membrane was collected into a pipet basin placed below the CDVs. Outflow volumes and leaks into the basin if any were measured, taking care to dry the basin and collection cylinders between each test. Pressures were simultaneously recorded for each flow rate and the equilibrium pressures in the devices at the respective flow rates were calculated. The outflow was occluded by closing the stopcock valve and water was flowed either manually from a syringe or at a controlled rate of 1ml/min from the syringe pump to measure the burst or maximum pressure.
Biocompatibility and support of cell growth (in vitro)
Si-CDVs were sanitized in 70% ethanol, washed with sterile PBS, and then soaked in culture media (HaCaT complete media: M199, 10% FBS, with Penicillin, streptomycin, and Fungizone). The media was then replaced with HaCaT cell suspension and cells permitted to seed the surface of the device. The CDVs were then incubated for 7 – 10 days to allow for a HaCaT cell monolayer to form.
Evaluation of Cellular Isolation Efficacy (in vivo)
Cell Delivery Vehicles were evaluated using a mouse model. All animal surgeries were performed in accordance with protocols approved by the University of Louisville animal review committee and the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication, 9th Edition, 2011). During all animal surgeries, adequate anesthesia was maintained by monitoring lack of tactile toe pinch reflex and/or respiration. After anesthesia was induced (Isoflurane 1–3%-O2 balance), a subcutaneous pouch was created by skin incision and blunt dissection, on the back of adult mice. Different mouse species were utilized that included: 1. Immune incompetent spontaneously diabetic mice: Rag 1-Akita (Rag1 knockout mice lack mature B and T lymphocytes and were used as a xenograft transplant model), 2. Immune competent diabetic mice: Akita (the Ins2Akita mouse line contains a dominant mutation that results in spontaneous diabetes with a rapid onset) and 3. Control mice; C57B6). CDVs were implanted into a subcutaneous pouch, the skin incision closed and animals recovered. The silicone scaffold based cell delivery vehicles (Si-CDV) were used in the first experimental series to assess both tissue responses and cell isolation capabilities. This assessment involved three distinct steps that included: 1) Implantation of the Si-CDVs, without cells, subcutaneously in mice (C57B6) followed by a 5 week implant phase, 2) Injection of cells (HaCaT) into the lumen of the Si-CDVs followed by an additional 6 week implant period and 3). Explantation of the devices. The final dual porosity design utilized nylon mesh as a scaffold (Ny-CDV) and these devices were evaluated using an in vivo implant model. The Ny-CDVs were pre-loaded with cells (HaCaT), the delivery tube sealed, and the devices implanted subcutaneously in Rag 1-Akita, immunocompromised and diabetic mice and explanted after a period of 2 weeks.
Histology
Implants were removed, fixed in 3 % formalin and prepared for sectioning as previously described. Hematoxylin and eosin stained sections of the explanted CDVs were examined for presence of inflammation, fibrous capsule, vascularization, tissue incorporation, and necrosis. These observations were scored on a scale of 1 to 4, encompassing a spectrum of none, mild, moderate, and severe grade progressively, as previously established for characterization of polymeric implants 17 Scores were compiled from two observers for each sample. Additional sections were treated with antibody to cytokeratin (Cytokeratin 18 –RCK 108, Mouse anti human IgG1), followed by an HRP-conjugated secondary antibody and then developed with DAB and counterstained with hematoxylin to identify HaCaT cells.
Functional vascularity assessment
Ny-CDVs were subjected to assessment of functional vascularity using a laser Doppler blood flow imager (Moor model LDI2, Moor Instruments, Wilmington, DE, USA). Blood flow around the Ny-CDV was registered as a colormap, and is directly proportional to both the velocity and the concentration of red blood cells. The blood flow was calculated prior to device implantation, immediately after device implantation and after the skin was reflected back prior to explantation.
Functional insulin diffusion
To assess the ability of CDVs to act as a delivery system for bioactive agents, controlled release delivery pellets containing insulin (LinBit insulin release pellet, LinShin Inc., Toronto, Canada) were implanted into two test groups. Test group 1 (N=2 mice) received a Ny-CDV device containing 2 LinBit pellets per device, the device sealed and then implanted in the subcutaneous tissue of Akita mice. In Test group 2 the LinBit insulin release pellets were implanted, two pellets per implant site, directly into the tissue (i.e. no delivery device) of Akita, diabetic Control mice, with no LinBit pellet implantation included Akita (diabetic mice) and non-diabetic control mice. All the animals were monitored for their blood sugar levels (BSL) at least twice a week for 4 weeks.
Results
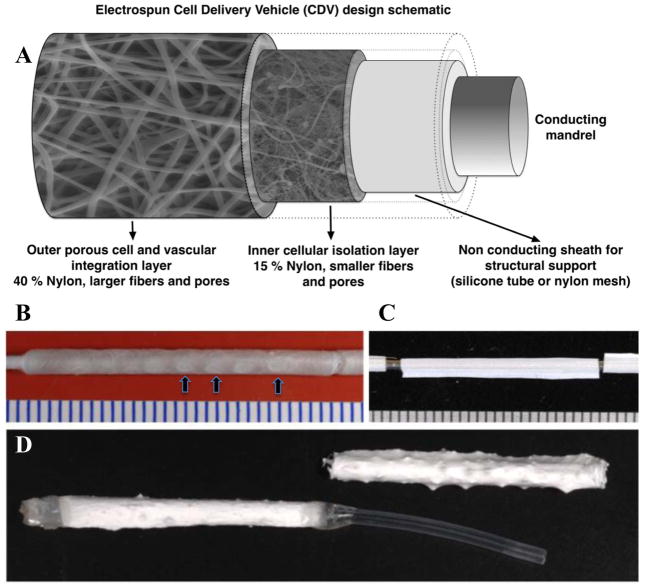
Traditional electrospinning techniques utilize an electrically conductive surface that acts as an attractant surface for the deposition of electrospun fibers 15,18. Here we used a non-conductive interface over the conductive mandrel core to establish a novel variation of this technique. The electrospinning system developed is illustrated in Figure 1A. The electrical potential difference between the exposed parts of the spinning metal mandrel, covered with the perforated non-conductive tubes, and the solution dispensing hub was sufficient to support polymer fibril deposition onto the spinning silicone or nylon membrane tubes. The conditions for electrospinning are diagrammed in Figure 1A and include the deposition of a low porosity layer followed by deposition of a higher porosity layer. Gross images of the CDVs are illustrated in Figure 1B (Silicone- CDV) and Figure 1C (Nylon-CDV). The electrospun fibers were deposited over the perforations in the non-conductive sheath creating openings to the lumen of the devices. The Si-CDV was constructed using a silicone tube into which holes or windows were created using a pattern that created three holes in the circumferential plane and approximately 10 windows in the longitudinal direction resulting in a total of approximately 30 windows per CDV. In Figure 1B three of these windows are identified using arrows. The CDV formed using nylon mesh is illustrated in Figure 1C. The 18 g mandrel used as a conducting surface is observed at each end of this Ny-CDV. A completed Ny-CDV is illustrated in Figure 1D. One end of the device is thermally sealed and PE-90 polyethylene tubing is glued to the other end using UV activated glue.
Figure 1.
A) Schematic of components forming the electrospun Cell Delivery Vehicle. B) A Si-CDV illustrating a thin layer of electrospun nylon. The “windows” in the underlying silicone tube are visible and three have been identified with arrows. C) A nylon sheath of 50 um porosity over an 18G steel mandrel. D) A Ny-CDV immediately post electrospinning step (Right) and in a fully assembled state (Left) with a sealed end and a PE delivery tube in place. All scale bars are in mm.
Morphologic assessment of CDVs
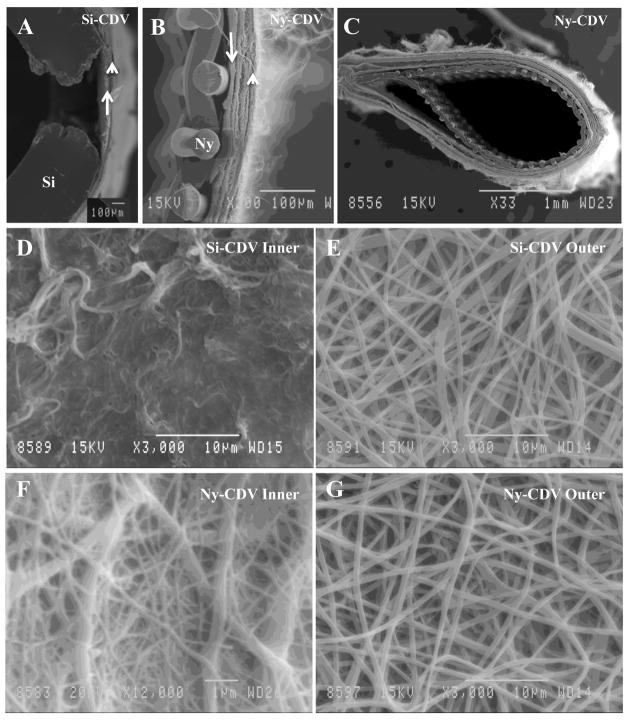
The CDVs constructed using either silicone or nylon as a scaffold were evaluated using scanning electron microscopy. Figure 2A illustrates the Si-CDV structure in a cross section that illustrates the window formed in the silicone and the deposition of low porosity electrospun nylon (arrow) and higher porosity electrospun nylon (arrowhead). Figure 2B is an SEM micrograph of a nylon scaffold based CDV. This is a cross section illustrating the nylon fibers (Ny) and the low porosity electrospun fibers (arrow) and high porosity electrospun fibers (arrowhead). A lower magnification SEM of the Ny-CDV (Figure 2C) illustrates the nylon scaffold and electrospun layers that constitute the device. Scanning electron microscopic evaluation of the electrospun layers of the CDVs revealed differences in the porosity and fiber size of the Si-CDV (Figure 2D inner; Figure 2E outer) compared to the Ny-CDV (Figure 2F inner; Figure 2G outer). Comparison of the inner and outer fiber characteristics of the two CDVs (Table 1) revealed that the Ny-CDVs had a smaller inner fiber diameter (p = 0.028) and pore size (p = 0.084). In comparison, the outer electrospun layer exhibited a fiber diameter that was slightly larger (p = 0.061) in the Ny-CDVs without a significant difference in the pore diameters (p = 0.589). The wall thickness of the dual electrospun membranes calculated from SEM images was comparable between the Si-CDVs (118.2 ±131.8 μm (SE)) and the Ny-CDVs (104.4 ±114.1 μm (SE)).
Figure 2.
Scanning Electron Microscopic Images of the Cell Delivery Vehicles A) The wall of an Si-CDV device illustrating one of the windows created in the silicone (Si) tubing using a thermal probe. The inner lining of the electrospun material is identified with an arrow and the outer layer is identified using an arrowhead. B) High magnification view of the wall of a Ny-CDV showing the inner supporting nylon mesh fabric and the two electrospun layers. The inner layer is identified by an arrow and one of the outer layers identified by an arrowhead. C) Lower magnification SEM illustrating a cross section through an Ny-CDV device D) Higher magnification SEM illustrating the inner surface of an SI-CDV device. E) Outer layer of the Si-CDV device illustrating the electrospun nylon fibers. F) Inner layer of Ny-CDV device illustrating the electrospun fibers. G) Outer layer of Ny-CDV illustrating larger fibrils and pores similar in size and density to the Si-CDV.
Table 1.
Characterization of Electrospun Nylon Layers of the Cell Delivery Vehicles
| Si CDV Pore Size1 (Standard deviation) microns | Ny CDV Pore Size1 (Standard deviation) microns | Si CDV Fiber Size2 (Standard deviation) microns | Ny CDV Fiber Size2 (Standard deviation) microns | |
|---|---|---|---|---|
| Outer Electrospun Layer | 2.04 (0.02) | 1.91 (0.36) | 0.57 (0.03) | 0.66 (0.05) |
| Inner Electrospun Layer | 0.39 (0.03) | 0.25 (0.01) | 0.22 (0.02) | 0.12 (0.01) |
Pore size was quantified from scanning electron micrographs imported into Metamorph image processing software. Measurements were calibrated using the scale bar embedded in the image. Pores (i.e., void areas bordered by electrospun fibers) were outlined, forming polygonal regions. The area of each region (i.e., the pore area) was calculated. The areas were then used to determine diameters of equivalent circles (i.e., assume the pore areas to be circular and calculate diameters). The resulting diameters are the “pore sizes.” Results are based on 3 devices for outer layer and 2 devices for inner layer and 32 measurements per device.
Fiber size was quantified from representative scanning electron micrographs imported into Metamorph image processing software. Measurements were calibrated using the scale bar embedded in the image. Results are based on 3 devices for outer layer and 2 devices for inner layer and 32 measurements per device.
Mechanical integrity and biocompatibility evaluation of the CDVs
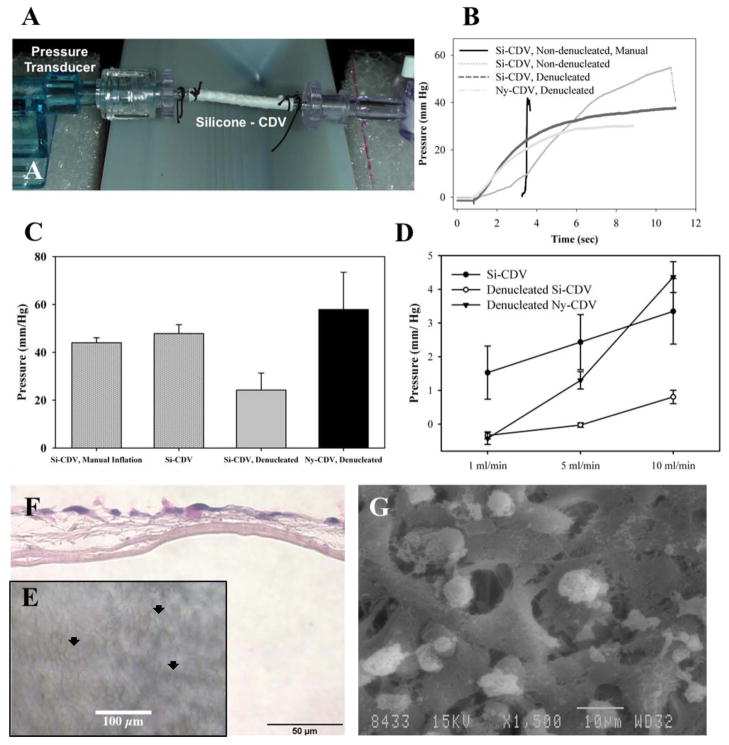
The cellular delivery devices have been designed to provide controlled fluid permeability across the device wall to permit nutrient exchange between encapsulated cells/tissues and the microcirculation that forms on the external surface of the devices. Techniques for loading a CDV with cells involve both flow through and internal pressurization of the device. Therefore, devices were evaluated for integrity by placing test devices in a fluid delivery pathway (Figure 3A). With the outflow path occluded, water was delivered to the devices either manually with a syringe or at 1ml/min by a syringe pump while monitoring internal pressure. Devices were monitored until they burst or reached a maximum pressure. A sharp slope-change in the exponentially increasing pressure curve was considered to be evidence of a break (see Figure 3B, Si-CDV manual flow for example)). Approximately half of all CDVs tested (all non-denucleated) exhibited a catastrophic burst characterized by a rapid change in the pressure curve (Figure 3B). The remaining devices (all denucleated with ethanol) exhibited a leakage through the device rather than failure, and the corresponding plateau pressures were recorded (Figure 3B). The pressure at failure was identified for all devices and provided in Figure 3C. Si-CDVs tested in the non-denucleated state showed an overall average failure pressure of 46.3 mmHg, and the manual inflation and controlled inflation groups were not statistically different (t-test, p = 0.436). Denucleated Si-CDVs exhibited a lower failure pressure (24.23 mm Hg) compared to the non-denucleated devices (t-test, p = 0.059). The average failure pressure of Ny-CDVs was 57.9 mm Hg.
Figure 3.
Characterization of CDVs. A) Flow testing setup with in-line pressure transducer on one end, showing an Si-CDV mounted between two barb connectors and an valve at the other end. The tubing is connected to a continuous flow pump. B) Comparison of steady state intraluminal pressure development during manual inflation and continuous flow inflation with a syringe pump. The CDV devices were evaluated both dry and after denucleation using ethanol C) CDV devices were subjected to constant low flow infusion and steady state intraluminal pressure measured.. D) Peak pressure at failure (burst or major fluid leakage) of CDVs when inflated at constant flow rate against a blocked outflow. E) Biocompatibility of Si-CDV was evaluated in vitro using human HaCaT epithelial cells seeded onto CDVs in vitro. The optical characteristics of the Si-CDV permit visualization of cells on the surface of the devices using phase contrast imaging. Arrowheads identify examples of cellular coverage. F) H&E stained paraffin processed section of the in-vitro cell layer showing growth of cells but lack of penetration through inner low porosity electrospun layer. G) SEM image of cells growing and attaching to larger outer fibers of a Si-CDV in-vitro.
For leakage testing, the outflow path was opened, and water was flowed through the device lumens at controlled rates. The equilibrium pressures as a function of flow rates were very small (< 5 mm of Hg) with an increase in pressure at corresponding increased flow rates (Figure 3D). All pressure response and leakage tests have been reported to provide characterization of the device filling and flow pressure, which can aid in determination of safe device loading strategies. The cellular biocompatibility of the CDV devices was evaluated in vitro using the human epithelial cell line HaCaT. Figures 3E–G illustrate the adherence and viability of the human epithelium on the CDVs in vitro.
Characterization of device biocompatibility in vivo
Prior to the evaluation of cell delivery the Si-CDVs (Figure 4A) and Ny-CDVs were implanted subcutaneously and tissue interaction evaluated after a 5 week implantation. The animals tolerated the implants with no sign of aberrant tissue responses in the area of the implants. Figure 4A illustrates a Si-CDV at the time of explant. The windows in the silicone can be observed visually and are identified with arrows in the image. Figures 4B and C illustrate the Ny-CDV at explant. All the implants exhibited significant revascularization as evidence by patent microvascular structures identified with arrowheads in the images.
Figure 4.
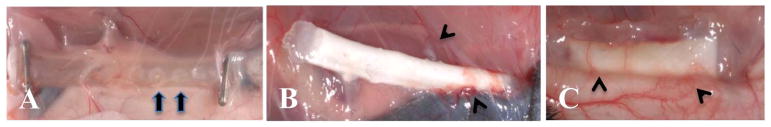
In vivo characterization of CDVs. A) Si-CDV showing ends sealed with hemoclips and loose tissue infiltration and vascularization. The windows in the silicone tubing are evident (arrows) B) Ny-CDV with cells at the time of explant showing focal areas of higher vascularity (arrowheads). C) LinBit insulin pellets within a dual electrospun membrane Ny-CDV implanted subcutaneously. The extensive peri-implant angiogenic response is evident (arrowheads).
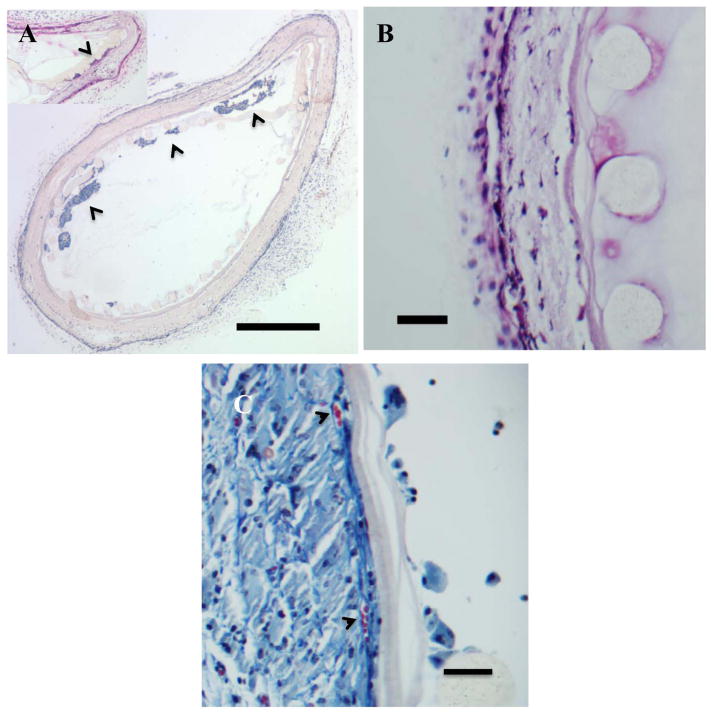
After 5 weeks of implantation Si-CDVs were injected with HaCaT cells suspended in 150 μl of sterile PBS at a concentration of 1 million cells/ml, using a 25G needle. Based on results from the in vitro burst strength studies, the injection process utilized low-pressure injection of cells. These devices were explanted after another 6 weeks (total 11 weeks) and processed for immunohistochemistry (cytokeratin 18) to identify the presence of HaCaT cells. The innermost nylon mesh is highly porous and only offers structural stability to the device. Cells injected into the device were also localized in the space between this innermost structural mesh and inner low porosity electrospun membrane (Figure 5A). Higher magnification light microscopy revealed (Figure 5B) revealed the penetration of cells into the outer porous electrospun surface of the CDVs and a lack of cell penetration into the inner low porosity electrospun layer. No evidence of cell penetration was found in the lumen of the CDVs. Cytokeratin expression confirmed the localization of HaCaT within the Ny-CDV (Figure 6B). The host reactions to implanted CDVs were found comparable based on our morphometric scores [Table 2]. Both cellular incorporation and vascularization trended lower for the Ny-CDV than the Si-CDV with comparable but lower inflammatory and capsular presence. This could be attributable to the lack of inner membrane integrity leading to a heightened cellular invasion combined with the longer implant times for Si-CDVs (data not shown). Both devices showed a few foreign body giant cells as part of the infiltrating cell population. Several cell lined tubular structures were identified on the outer porous layer, especially towards the outer margins (Figure 5B). Our previous study of electrospun materials has revealed similar structures to be areas of vascularization15. Evidence that the electrospun fibers support neovascularization is provided in figure 5C. The arrowheads identify vascular structures and the patency of these vessels can be inferred from the presence of red blood cells in the lumen of the vessels.
Figure 5.
In vivo characterization of CDVs. Hematoxylin and eosin stained sections of Ny-CDV devices implanted in a subcutaneous position in the mouse.. A) Ny-CDVs showing retention of delivered HaCaT (arrowheads) within the Ny-CDV. No breach of the inner cell isolation layer is apparent and no evidence of HaCat cells outside the device was found. Note that the inner nylon sheet is for structural support only and the pores are large enough to allow cells to move across. Inset shows one of the devices with cells at the inner electrospun surface. (Bar = 500 μm) B) Ny-CDV illustrating the components of the CDV: 1- Nylon mesh with the cylindrical fibers, 2- Inner low porosity electrospun layer, 3- Outer high porosity and tissue integration layer. Cells are evident penetrating through the outer porous electrospun layer but do not penetrate the low porosity layer. (Bar = 50 μm). C). Masson’s trichrome stained section of a Ny-CDV illustrating the neovascularization of the porous electrospun layer. Arrowheads identify two vascular profiles at the interface between the high porosity and low porosity electrospun coating. (Bar = 50 μm).
Figure 6.
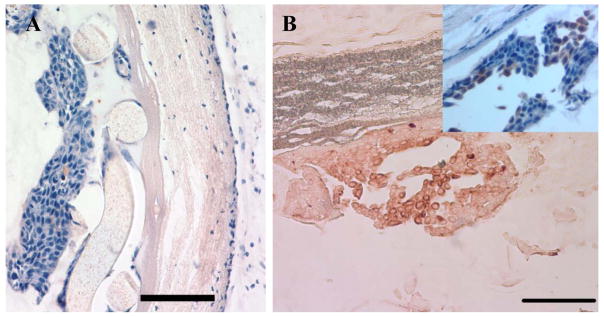
Cellular isolation in the CDV in vivo. A) Light photomicrograph of a hematoxylin and eosin stained section of a Ny-CDV illustrating cellular isolation of human HaCaT epithelial cells separated from the host tissue by the intact inner low porosity membrane. (Left to Right: CDV lumen to host tissue) (Bar = 100 μm) B) Cytokeratin 18 expressing HaCaT cells exhibit a brown reaction product and are localized within CDV lumen (Scale 100μm). Inset: HaCaT cells counterstained with hematoxylin to illustrate the localization of the HaCaT cells within the lumen of the device and the viability of the cells.. (Bar = 100 μm)
Table 2.
Characterization of Tissue Responses to Implanted Cell Delivery Vehicles1
| CDV | Group | Inflammation2 | Capsule3 | Vascularization4 | Incorporation5 | Necrosis6 |
|---|---|---|---|---|---|---|
| Si-CDV | HaCaT Injected | 3 | 2.25 | 3 | 3 | 1 |
| Ny-CDV | HaCaT Injected | 2.375 | 2 | 2.75 | 2.25 | 1 |
| No Cells | 2.5 | 2 | 2.625 | 2.25 | 1 |
Hematoxylin and eosin stained histological sections of implants were evaluated using a morphometric scoring system. The scale used for each assessment is described below.
Inflammation was assessed by evaluating sections for the presence of macrophages and foreign body giant cells. Scale is 1 to 4 where 1 = no inflammatory cells and 4 = intense inflammatory response.
Capsule formation was assessed by evaluating sections for the presence of an avascular fibrous capsule. Scale is 1 to 4 where 1 = no capsule present and 4 = thick, dense capsule present.
Vascularization was assessed by evaluating sections for the presence of vascular profiles. Scale is 1 to 4 where 1 = no vascular elements present and 4 = highly vascularized tissue.
Incorporation was assessed by evaluating sections for the presence cellularized tissue in association with and within the porous outer layer of the CDV. Scale is 1 to 4 where 1 = no tissue associated with the device and 4 = complete incorporation of device with tissue.
Necrosis was assessed by evaluating sections for the presence of viable and non-viable tissue. Scale is 1 to 4 where 1 = viable issue and 4 = non-viable tissue characterized by cell debris and/or cells exhibiting programmed cell death/apoptosis..
Functional Insulin Diffusion
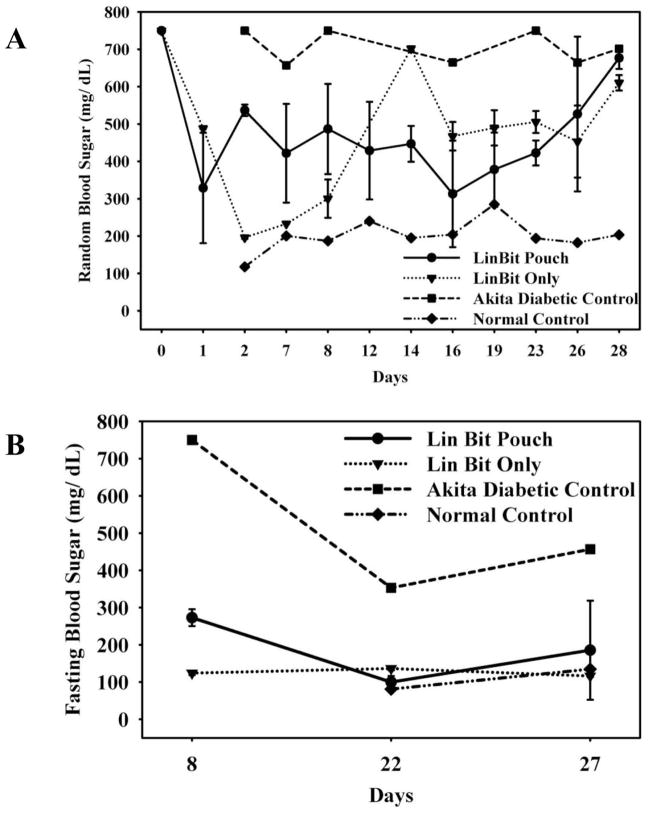
The ability of the CDVs to deliver a therapeutic protein in vivo was evaluated using an indirect method. CDVs were prepared with an insulin controlled release pellet, the CDVs implanted in mice and the functional release of insulin into the circulation evaluated indirectly by measuring blood glucose levels. Figure 7A illustrates the evaluation of blood glucose levels in blood samples taken at varying times throughout the day. Diabetic animals receiving the LinBit insulin release pellets either as pellets alone or pellets within the CDVs all exhibited a reduction in blood glucose as compared to untreated diabetic animals. For comparison the random time point blood glucose levels of a control/non diabetic animal are provided in this figure. These studies were repeated in the same test groups evaluating blood glucose assessments following a glucose fasting period (Figure 7B). Again diabetic animals receiving the LinBit insulin pellets either alone or following encapsulation in the CDVs exhibited reduced blood glucose levels compared to the untreated diabetic animals. Using a 2-tailed T-test, with unequal variance, we determined that the random BSL was significantly lower for the pellet only group (p<0.05, P = 0.022) as compared to the LinBit CDV group only on the 2nd day post implantation. This delay may be explained by the altered degradation kinetics of the pellets now tightly confined within the nylon pouch,. Predictably, at the end of approximately 4 weeks of implantation, the pellets lost their efficacy and the random BSL levels were elevated. Additionally, fasting BSL levels, after 5 hours of fasting after 1 week of implantation were lower for pellet only group reproducing the trend described above (p<0.05, P = 0.083). At three and four weeks after implantation, there were no significant differences in BSL between the LinBit treated groups, however both groups exhibited a significantly reduced BSL compared untreated diabetic control animals. This lowering of blood sugar to levels comparable to freely implanted pellets provides indirect evidence of insulin diffusion out of the CDVs into the circulation.
Figure 7.
In vivo functional characterization of electrospun dual layer CDVs. Comparison of insulin release and activity in directly implanted insulin pellets (LinBit), and insulin pellets enclosed within a CDV made of electrospun dual layer membranes. A) Random blood sugar level (BSL) measurements illustrating comparable BSL levels of both insulin pellet containing groups.(● – LinBit insulin pellets in CDV pouch N=2); ▼- LinBit insulin pellets implanted alone N=2);. ■ – Akita Diabetic Control; ◆Normal Control.
B) Fasting BSL levels of the same groups illustrating the ability of the CDV electrospun membranes to allow passage of insulin across the barrier. (n = 2 for insulin pellet groups, Fasting BSL at 5 hours at 8 days and at 12 hours for 22 and 27 days).
Characterization of functional vascularity
Two Ny-CDVs each with and without encapsulated HaCaT cells were evaluated for local vascularity using a Laser Doppler perfusion system. No significant differences in blood flow between the two groups were observed prior to, or immediately post implantation (Data not shown). After two weeks implantation the skin around the CDVs was reflected and Laser Doppler images obtained. Figure 8 illustrates the peri-implant perfusion present in association with these devices. Figure 8A is the machine-generated image of the CDV at the time of skin reflection, and Figures 8B (HaCaT laden Ny-CDV) C) cell free Ny-CDV, and D) an insulin pellet containing CDV illustrate the perfusion heat maps. The relative blood flow observed in each of the test groups did not differ significantly, but the mean flow around the HaCaT containing CDVs was about 1.75 times higher than the empty CDVs. However, the median flow around the HaCaT containing Ny-CDVs, after reflection of the skin, was higher than the cell free devices at 2 weeks (t-test, p = 0.0136).
Figure 8.
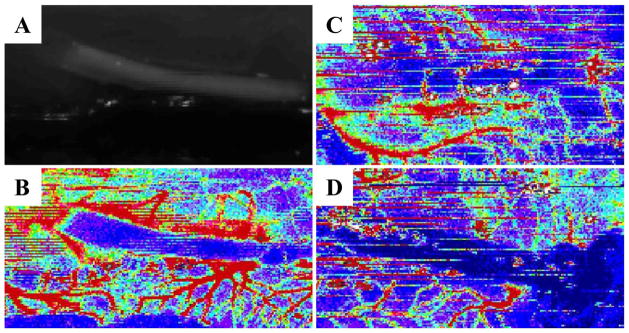
Laser Doppler imaging of implanted CDVs. Vascularity around implanted Ny-CDV represented by heat maps of blood flow measured using a laser Doppler imager. Low flow is blue and high flow is red) A) Ny-CDV gross image at explant at day 14. Heat maps of blood flow around- B) a HaCaT laden Ny-CDV, C) cell free Ny-CDV, and D) an insulin pellet containing CDV (day 30).
Statistical Analysis
Data is expressed as the mean ± standard error mean. Mean differences between test groups was determined using a 2-tailed T-test, with unequal variance.
Discussion
Numerous challenges remain before cell based therapies become accepted as a standard of care. The work described here addresses several technology needs including a cell delivery system that maintains injected cells in a device permitting control of cell dosing, limits cell migration away from the site of injection and permits removal of the delivery device and associated cells if necessary. The cell delivery device tested has a unique dual-electrospun fiber structure that supports neovascularization of the external surface of the device and isolates cells within a lumen entrapped by a lower porosity electrospun fiber. Isolating modified or therapeutic cells within a barrier that supports viability and allows nutrient and secretory molecule exchange, but not direct migration across the barrier, can accelerate clinical translation of such cell based therapeutic alternatives. The use of membrane porosity based sequestration of in vivo delivered cells has been proposed by several investigators, with emphasis on both the immuno-isolation and the vascularization of the device 2,11,19. However, most approaches involve micro-manufacturing techniques with thermal or polymeric adhesive based fusion of membrane layers or polymer-based micro-encapsulation techniques. Electrospun polymeric membranes on the other hand offer an alternative approach for control over choice of functionalizable biomaterials, fiber size, orientation, thickness, and membrane porosity, ideal for reproducibility and scale-up.
Prior to in vivo evaluation, the Cell Delivery Vehicles were evaluated by scanning electron microscopy and pressure systems for porosity and burst strength/leakage, respectively. The electrospinning system designed permitted the deposition of a lower porosity fiber layer onto a silicone or nylon tubular structure. A layer of higher porosity fibers was deposited directly onto the inner, low porosity fibers. This hybrid structure exhibited resistance to delamination from the fibers of the backbone material as well as at the interface between the high and low porosity fibers. Burst strength and water leakage assessments indicate the devices maintained integrity well beyond the pressures necessary to fill the internal cavity.
The conditions for the deposition of electrospun fibers as the outer device surface were designed to create a pore structure that supports cellular penetration and integration. The results indicate that the pore structure created supports cellular penetration. In order to establish a more quantitative assessment of pore size of this outer layer we established a morphometric assessment of pore size using scanning electron microscopic images. The data show that the electrospinning conditions resulted in a porosity of ~2 μm, and yet showed cellular infiltration. As mammalian cells have cell dimensions that are greater than 2 μm, the ability of cells to migrate into and within this electrospun material would seem to be sterically inhibited. The observation that cell infiltration occurs as well as the observations that formation of new blood vessels within the electrospun material occurs indicates the fiber dimensions do not inhibit cellular migration. The morphometric assessment used does not take into consideration the three dimensional structure of these porous surfaces and therefore most likely underestimates the size of the pores. Moreover, the electrospun fibers are not rigid and cells most likely can increase the pore size by physical mechanisms. It is also conceivable that this cell isolation device can be functionalized by covalent localization of pro-angiogenic factors or enveloped completely in autologous adipose derived microvessels to accelerate the formation of a functional vascular bed prior to injection of cells or islets 20–22.
One of the target areas of the CDV technology is delivery of cells that produce a therapeutic agent that has systemic biological activity such as growth hormones, coagulation factors, erythropoietin and insulin. To evaluate the ability of the CDVs to both restrict the migration of cells from and into the therapeutic cell compartment and to maintain the viability of implanted cells, we used an immortalized human epithelial cell line (HaCaT). The retention of viable HaCaT cells within the CDV was assessed by immunocytochemistry using an antibody that recognizes the intermediate filament cytokeratin 18. Our analysis indicated that the CDV provides complete isolation of the injected cells and no migration of cells out of the delivery device was observed. Furthermore, cell viability was maintained confirmed by the lack of cellular necrosis and the presence of cells with normal histologic structure. Any solution to maintain cell viability while providing cellular isolation must also include measures to improve neovascularization and minimize the diffusion distance while providing stable cellular isolation. Allowing pre-vascularization of the electrospun device requires an appropriate post implantation delivery method. Injecting cells or islets directly into the device after a period of implantation offers the most efficient delivery route. The proposed concept device is designed to be modular, and thus used either individually or in series or parallel with multiple similar units, which entails flow through the device with or without pressure. Larger devices designed around semipermeable membranes of appropriate porosity offer the opportunity to accommodate larger implant volumes, but must also contend with limitations of vascularization and diffusion 23. Use of smaller hollow fibers or micro-encapsulation of islets involves logistical challenges as reviewed by Ohgawara 4. More recently, melt electrospinning is growing as a technique to impart controlled three dimensional structure to electrospun membranes and even in the manufacture of tubular scaffolds24. It is likely that comparable tubular devices can be produced by similar techniques, but an inner structural support mesh will likely still be required to support the relatively thin inner cell-isolating layer. We have previously demonstrated that dual porosity electrospun nylon membranes can support cellular incorporation and vascularization in the high porosity exterior layers and prevent infiltration of the low porosity inner layer, without significant separation of the layers 15. Here we also demonstrate the ability of the tubular CDV to maintain implanted cell viability, provide cellular isolation from the host, and provide evidence of functional diffusion of solutes (insulin) delivered from the CDVs to the circulation.
Acknowledgments
Funding from NIH # DK 078175 to SKW and JBH.
References
- 1.Li RH. Materials for immunoisolated cell transplantation. Adv Drug Deliv Rev. 1998;33(1–2):87–109. doi: 10.1016/s0169-409x(98)00022-2. [DOI] [PubMed] [Google Scholar]
- 2.Desai TA, Chu WH, Rasi G, Sinibaldi-Vallebona P, Guarino E, Ferrari M. Microfabricated biocapsules provide short-term immunoisolation of insulinoma xenografts. Biomed Microdevices. 1999;1(2):131–8. doi: 10.1023/A:1009948524686. [DOI] [PubMed] [Google Scholar]
- 3.Desai TA, Hansford DJ, Chu WH, Huen T, Ferrari M. Investigating islet immunoisolation parameters using microfabricated membranes. Materials Research Society Symposium Proceedings. 1998;530:7–12. [Google Scholar]
- 4.Ohgawara H. Strategies for immunoisolation in islet transplantation: challenges for the twenty-first century. J Hepatobiliary Pancreat Surg. 2000;7(4):374–9. doi: 10.1007/s005340070032. [DOI] [PubMed] [Google Scholar]
- 5.Wang T, Lacik I, Brissova M, Anilkumar AV, Prokop A, Hunkeler D, Green R, Shahrokhi K, Powers AC. An encapsulation system for the immunoisolation of pancreatic islets. Nat Biotechnol. 1997;15(4):358–62. doi: 10.1038/nbt0497-358. [DOI] [PubMed] [Google Scholar]
- 6.Iwata H, Morikawa N, Ikada Y. Permeability of filters used for immunoisolation. Tissue Eng. 1996;2(4):289–98. doi: 10.1089/ten.1996.2.289. [DOI] [PubMed] [Google Scholar]
- 7.Dionne KE, Cain BM, Li RH, Bell WJ, Doherty EJ, Rein DH, Lysaght MJ, Gentile FT. Transport characterization of membranes for immunoisolation. Biomaterials. 1996;17(3):257–66. doi: 10.1016/0142-9612(96)85563-3. [DOI] [PubMed] [Google Scholar]
- 8.Grundfest-Broniatowski SF, Tellioglu G, Rosenthal KS, Kang J, Erdodi G, Yalcin B, Cakmak M, Drazba J, Bennett A, Lu L, et al. A new bioartificial pancreas utilizing amphiphilic membranes for the immunoisolation of porcine islets: a pilot study in the canine. ASAIO J. 2009;55(4):400–5. doi: 10.1097/MAT.0b013e3181a8deba. [DOI] [PubMed] [Google Scholar]
- 9.Erdodi G, Kang J, Yalcin B, Cakmak M, Rosenthal KS, Grundfest-Broniatowski S, Kennedy JP. A novel macroencapsulating immunoisolatory device: the preparation and properties of nanomat-reinforced amphiphilic co-networks deposited on perforated metal scaffold. Biomed Microdevices. 2009;11(1):297–312. doi: 10.1007/s10544-008-9236-x. [DOI] [PubMed] [Google Scholar]
- 10.Brauker JH, Carr-Brendel VE, Martinson LA, Crudele J, Johnston WD, Johnson RC. Neovascularization of synthetic membranes directed by membrane microarchitecture. J Biomed Mater Res. 1995;29(12):1517–24. doi: 10.1002/jbm.820291208. [DOI] [PubMed] [Google Scholar]
- 11.Brauker J, Martinson LA, Hill RS, Young SK, Carr-Brendel VE, Johnson RC. Neovascularization of immunoisolation membranes: the effect of membrane architecture and encapsulated tissue. Transplant Proc. 1992;24(6):2924. [PubMed] [Google Scholar]
- 12.Laschke MW, Mussawy H, Schuler S, Kazakov A, Rucker M, Eglin D, Alini M, Menger MD. Short-term cultivation of in situ prevascularized tissue constructs accelerates inosculation of their preformed microvascular networks after implantation into the host tissue. Tissue Eng Part A. 2011;17(5–6):841–53. doi: 10.1089/ten.TEA.2010.0329. [DOI] [PubMed] [Google Scholar]
- 13.Mikos AG, Sarakinos G, Lyman MD, Ingber DE, Vacanti JP, Langer R. Prevascularization of porous biodegradable polymers. Biotechnol Bioeng. 1993;42(6):716–23. doi: 10.1002/bit.260420606. [DOI] [PubMed] [Google Scholar]
- 14.Chen X, Aledia AS, Ghajar CM, Griffith CK, Putnam AJ, Hughes CC, George SC. Prevascularization of a fibrin-based tissue construct accelerates the formation of functional anastomosis with host vasculature. Tissue Eng Part A. 2009;15(6):1363–71. doi: 10.1089/ten.tea.2008.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krishnan L, Clayton LR, Boland ED, Reed RM, Hoying JB, Williams SK. Cellular immunoisolation for islet transplantation by a novel dual porosity electrospun membrane. Transplant Proc. 2011;43(9):3256–61. doi: 10.1016/j.transproceed.2011.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leong MF, Rasheed MZ, Lim TC, Chian KS. In vitro cell infiltration and in vivo cell infiltration and vascularization in a fibrous, highly porous poly(D,L-lactide) scaffold fabricated by cryogenic electrospinning technique. J Biomed Mater Res A. 2009;91(1):231–40. doi: 10.1002/jbm.a.32208. [DOI] [PubMed] [Google Scholar]
- 17.Salzmann DL, Kleinert LB, Berman SS, Williams SK. Inflammation and Neovascularization Associated with Clinically Used Vascular Prosthetic Materials. Cardiovascular Pathology. 1999;7(6):63–71. doi: 10.1016/s1054-8807(98)00003-9. [DOI] [PubMed] [Google Scholar]
- 18.Zucchelli A, Focarete ML, Gualandi C, Compagnoni A, Chelli A, Poletti R. Porcelain Enamelled Steel Sheets as Smart Collectors in the Electrospinning Process for the Production of Polymer Nanofibrous Mats for Biomedical Applications. XXI International Enamellers Congress; Shanghai, China. 2008. pp. 346–48. [Google Scholar]
- 19.Pauley RG, McLarty DL, Khare AR, Sternberg S, Boggs DR, Neuenfeldt S, Jones M, Brauker JH, Martinson LA. 6060640. Multiple layer, formed in place, immunoisolation membrane structures for implantation of cells in host tissue patent. 2000
- 20.Gruionu G, Stone AL, Schwartz MA, Hoying JB, Williams SK. Encapsulation of ePTFE in prevascularized collagen leads to peri-implant vascularization with reduced inflammation. J Biomed Mater Res A. 2010;95(3):811–8. doi: 10.1002/jbm.a.32925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams SK, Kleinert LB, Hagen KM, Clapper DL. Covalent modification of porous implants using extracellular matrix proteins to accelerate neovascularization. J Biomed Mater Res A. 2006;78(1):59–65. doi: 10.1002/jbm.a.30659. [DOI] [PubMed] [Google Scholar]
- 22.Williams SK, Kleinert LB, Patula-Steinbrenner V. Accelerated neovascularization and endothelialization of vascular grafts promoted by covalently bound laminin type 1. J Biomed Mater Res A. 2011;99(1):67–73. doi: 10.1002/jbm.a.33138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griffith CK, Miller C, Sainson RC, Calvert JW, Jeon NL, Hughes CC, George SC. Diffusion limits of an in vitro thick prevascularized tissue. Tissue Eng. 2005;11(1–2):257–66. doi: 10.1089/ten.2005.11.257. [DOI] [PubMed] [Google Scholar]
- 24.Brown TD, Slotosch A, Thibaudeau L, Taubenberger A, Loessner D, Vaquette C, Dalton PD, Hutmacher DW. Design and fabrication of tubular scaffolds via direct writing in a melt electrospinning mode. Biointerphases. 2012;7(1–4):13. doi: 10.1007/s13758-011-0013-7. [DOI] [PMC free article] [PubMed] [Google Scholar]