Abstract
Patients with head and neck squamous cell carcinoma (HNC) related to human papillomavirus (HPV) represent a growing and distinct patient cohort with unique molecular and epidemiologic characteristics. These patients have markedly improved survival outcomes compared to those with traditional HNC, leading some to advocate for treatment dose reduction. In this article, we review ongoing clinical trials investigating several ways to reduce therapeutic intensity for patients with HPV-positive HNC, discuss the risks and benefits associated with these trials, and summarize the data underlying the advancement of dose reduction trials for patients with HPV-positive HNC.
Keywords: Head and neck cancer, human papillomavirus, radiation, dose reduction
Introduction
Human papillomavirus (HPV) is associated with up to one third of all head and neck squamous cell carcinomas (HNC) and 50–80% of cancers arising in the oropharynx 1, 2. Patients with HPV-positive HNC represent a distinct patient cohort with unique characteristics 3–11. With current trends, it is estimated that HPV-positive HNC will become the dominant etiologic factor for HNC during the coming decades 12. In this article summarize current data suggesting that patients with HPV-positive HNC have markedly improved survival outcomes that may warrant treatment dose reduction, summarize ongoing clinical trials for patients with HPV-positive HNC, describe potential risks and benefits associated with treatment dose reduction, and provide suggestions for future approaches.
Compared to patients with HPV-negative HNC, those with HPV-positive HNC commonly present at a younger age and with a more advanced neck disease 13. Despite this, numerous clinical reports provide striking evidence of improved outcomes in patients with HPV-positive HNC compared to those with HPV-negative HNC 3–10 (reviewed in 11 and 14). The majority of these reports have utilized a combination of immunohistochemistry for p16 and in situ hybridization for high-risk HPV types to identify patients as HPV-positive. Both p16 and HPV status share prognostic significance despite the 15–20% false positive and false negative rate for p16 testing as a surrogate for HPV 15, 16. Several groups have suggested that p16 status retains prognostic significance even in the absence of coincident HPV positivity 4–6, 16. In many of these studies, tumor HPV status is the strongest independent determinant of local and regional control, disease specific survival, and overall survival.
Ongoing clinical trials for patients with HPV-positive HNC
On the basis of the improved survival outcomes seen in patients with HPV-positive HNC, discussions within the major cooperative groups that enroll HNC patients such as the Radiation Therapy Oncology Group (RTOG) and the Eastern Cooperative Oncology Group (ECOG), as well as in head and neck tumor boards across the world, have considered the possibility of dose reduction in patients with HPV-positive HNC. The goals of this approach are to maintain the currently good survival outcomes while minimizing treatment-related morbidity. The trials designed to date vary considerably in their approach: induction chemotherapy with response adapted radiation; alternatives to cisplatin given concurrently with radiation; limiting radiation dose; and integrating minimally invasive surgery into the treatment algorithm.
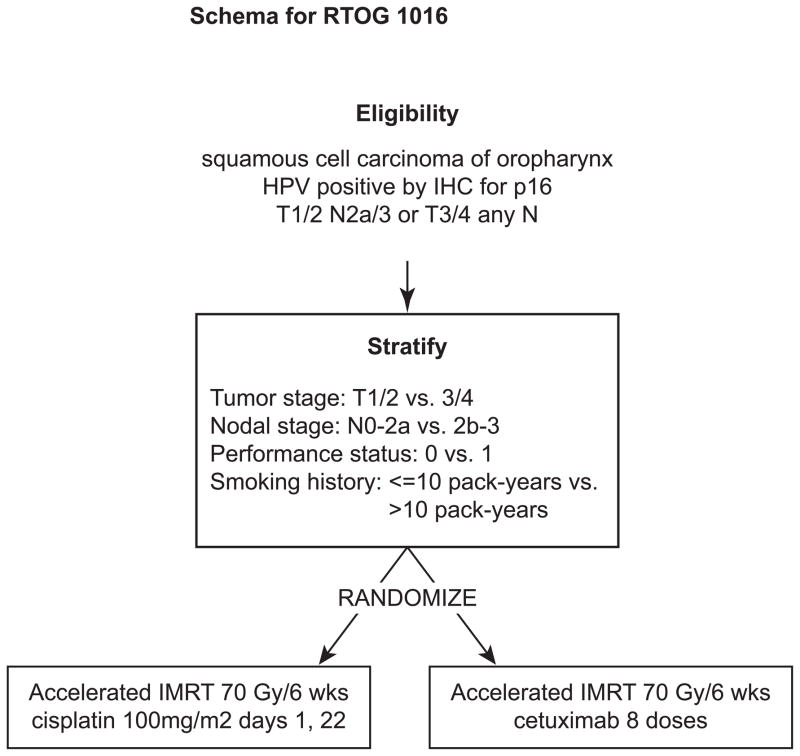
The RTOG has rapidly accrued patients to a phase III trial randomizing patients to cisplatin vs. cetuximab given concurrent with 70 Gy radiation (Figure 1). This study hopes to answer conclusively whether cetuximab can be safely substituted for cisplatin in patients with HPV-positive HNC. In addition, important quality of life metrics are being collected to examine the potential differences in toxicity profiles that may accompany these two treatment approaches. Over 700 patients will to accrue to this important study by Sept 2013, and there is ongoing consideration to expand the enrollment further at the time of this writing. The University of Warwick is leading a similar study in the UK also randomizing HPV-positive HNC patients to cetuximab vs. cisplatin with concurrent radiation.
Figure 1.
Trial schema for RTOG 1016.
Several groups are currently pursuing some form of dose reduction in patients with HPV-positive HNC (Table 1). The ECOG 1308 study was a phase II trial that recently completed accrual and utilized induction chemotherapy to select patients for radiation dose modification (from 66–70 Gy to 54 Gy based on whether they achieved a complete response to induction therapy). While induction chemotherapy is not considered a standard treatment approach in this setting, using it to select patients for dose reduction has been pursued successfully in several other diseases. Several other groups are pursuing variations on the approach of using response-adapted radiation following induction chemotherapy (Table 1). For now this remains an experimental approach, as there is no clear data that HPV-positive HNCs are more sensitive to chemotherapy than HPV-negative HNCs, or that rapid response of tumors predicts for improved radiation sensitivity or improved outcomes in HNC.
Table 1.
Ongoing trials for patients with HPV-positive HNC.
| ClinicalTrials.gov identifier | # of patients | Group/institution | Trial Design |
|---|---|---|---|
| NCT01084083 | 83* | ECOG | Phase 2: neoadjuvant chemo and response adapted radiation (54 or 66–70 Gy) + cetuximab |
| NCT01525927 | 50 | North Shore Long Island Jewish Health System | Phase 2: neoadjuvant TPF and response adapted RT (60 Gy) +/− concurrent chemo |
| NCT01716195 | 50 | Univ. of California Davis | Phase 2: neoadjuvant chemo followed by paclitaxel + response adapted RT (50 or 60 Gy) |
| NCT01663259 | 36 | Univ. of Michigan | Phase 2: weekly cetux + RT (70 Gy) |
| NCT01530997 | 40 | Univ. of North Carolina | Phase 2: radiation with weekly cisplatin followed by supra- selective neck dissection |
| pending | 337 | ECOG | Phase 2: transoral resection - risk adapted postop RT (0 vs. 50 vs. 60 vs. 66Gy with weekly cisplatin) |
| NCT01302834 | 706 | RTOG | Phase 3: randomized to cetuximab vs. cisplatin with concurrent RT (70 Gy in 6 weeks) |
| NCT01874171 | 304 | University of Warwick | Phase 3: randomized to cetuximab vs. cisplatin with concurrent RT (70 Gy in 7 weeks) |
| NCT01706939 | 365 | Mount Sinai School of Medicine | Phase 3: weekly carbo/cetuximab + 56 Gy vs. weekly carbo + 70 Gy |
| NCT01687413 | 496 | Washington Univ. | Phase 3: postop RT (60 Gy) +/− weekly cisplatin |
RTOG: Radiation Therapy Oncology Group; ECOG: Eastern Cooperative Oncology Group; RT: Radiation therapy
trial closed to accrual
Several groups are also pursuing Phase 2 studies investigating some form of therapy dose reduction without induction chemotherapy. The University of Michigan is studying the use of cetuximab + standard dose radiation as an approach to decrease toxicity. This is based, at least in part, on extrapolation of data from the Bonner study suggesting that patients showing greatest benefit from cetuximab + radiation were those with oropharyngeal tumors. While this approach is attractive, the ultimate alteration in toxicity profiles between cetuximab and cisplatin may be modest and there is little data showing that cetuximab alone is efficacious in HPV-positive HNC. At the University of North Carolina (UNC) researchers are studying reduced dose radiation (from 70 Gy to 60 Gy) + weekly cisplatin (30 mg/m2) in patients with HPV-positive HNC. While still very early, the UNC trial has shown promising preliminary results in 18 patients who undergo a mandatory post-treatment biopsy of the primary site and a supra-selective neck dissection to the pre-therapy involved neck (personal communication, Dr. Bhishamjit Chera, Univ of North Carolina).
Three additional randomized trials investigating patients with HPV-positive HNC are currently pending initiation or underway in the US (Table 1). Mount Sinai is leading a group of institutions randomizing 365 patients with HPV-positive HNC to one of two treatment regimens: carboplatin + cetuximab with 56 Gy radiation vs. carboplatin and 70 Gy radiation. This trial, which alters both radiation dose and chemotherapy does not include a standard of care treatment arm, but would be expected to have decreased toxicity compared to standard of care therapy. The ECOG 3311 trial has recently been NCI-approved and is a randomized phase 2 study (projected accrual is 377 patients) investigating whether upfront surgical excision and pathologic staging of all clinically evident disease can permit reduced-dose adjuvant therapy. Various resection techniques are permitted, including transoral robotic surgery (TORS), transoral laser microsurgery (TLM), or traditional headlight excision with neck dissection, with risk stratifying of patients on the basis of T stage, N stage, surgical margins, extent of extracapsular extension (ECE), and number of positive lymph nodes. Patients receive either observation (low risk pathologic stage I–II cohort); 66 Gy radiation and weekly cisplatin (high risk cohort or positive margins, extensive >1mm ECE, or ≥5 metastatic lymph nodes) or are randomized to 50 Gy vs. 60 Gy IMRT (intermediate risk cohort, consisting of minor <1mm ECE, 2–4 metastatic nodes, or close margins). Finally, Washington University School of Medicine is leading a group of institutions studying the use of TLM with removal of all known disease. Patients (n=496) with involved lymph nodes showing ECS are then randomized to postoperative radiation only vs. radiation + weekly cisplatin. Due to the newness of the TORS and TLM approach, late effects are not yet well defined. In addition, as with many highly technical surgical procedures, learning curves are exceptionally important for outcome, and results obtained at academic medical centers may or may not translate effectively into community practice.
Trials undergoing development in the US cooperative group settings continue to separate patients with HPV-positive and HPV-negative HNC. While still in the planning stages, these trials, in general, are attempting to further investigate de-escalation strategies for patients with good prognosis HPV-positive HNC through alteration or elimination of concurrent chemotherapy, modification of radiation dose, or integration of minimally invasive surgery. Until more data becomes available, it is our belief that reducing radiation dose below the current standard of care should only be performed in the setting of controlled clinical trials.
Risks & Benefits of dose reduction
HNC patients receiving lower doses of radiation and/or treated without concurrent chemotherapy will experience less overall toxicity. Because patients with HPV-positive HNCs are often slightly younger and healthier than those with HPV-negative HNC, the value of reducing late toxicities such as swallowing dysfunction, speech impairment, and dental dysfunction is a valuable objective. Potential benefits of dose reduction should be considered with the knowledge that additional radiation treatment modifications have a favorable impact on treatment related morbidity. At the same time, risks of dose reduction must be carefully balanced by the potential gain. It is this balance of risks and benefits (Table 2) that requires careful analysis and consideration as studies proceed with dose reduction approaches.
Table 2.
Potential pros and cons of dose reduction trials in HPV-positive HNC
| Pros | Cons |
|---|---|
| Similar survival outcome? | Worse survival outcome? |
| Decreased toxicity profile | Minimal toxicity difference |
| Decreased cost and resource utilization | Clinical trial resources should be directed to patients with poor outcomes |
| Less interruption of patient work/life | Psychological barrier to delivering “less” treatment |
| Rapidly expanding public health issue - vaccine may never be broadly adopted | Best to focus on vaccine to prevent disease |
The use of intensity modulated radiation therapy (IMRT) as the standard treatment planning technique allows providers to spare structures important for speech, swallowing and salivary function 17, 18. The use of daily image guided radiation (IGRT) allows for the reduction in treatment margins and may also improve the ability to spare normal tissues19. It remains unclear what dosimetric parameters best correlate with toxicity outcomes 20–23, however it is quite clear that delivering less dose results in less toxicity.
In addition to any potential decreases in toxicity, decreasing radiation dose will likely decrease the overall cost of care. The cost savings obtained from safely decreasing the intensity of treatment could in fact be substantial. Fewer health care dollars would need to be spent on chemotherapy or other medications, the need for enteral nutrition, or hospitalization costs associated with management of treatment-related side effects. In addition, shorter treatment courses may reduce time spent away from work and subsequent unemployment. Of the most common reasons associated with a failure to return to work are fatigue, speech dysfunction, and eating difficulties 24, all side effects that could be impacted by decreasing radiation dose. Since nearly 50% of HNC patients fail to return to work 25, 26, minimizing treatment intensity could have significant financial implications.
Alternatively, one can imagine a number of reasons for caution in proceeding with trials focused on this particular HPV-positive subgroup of patients. Most importantly, current treatment approaches, particularly in low level or non-smokers, results in outstanding survival outcomes for patients with HPV-positive HNC. Any attempt to decrease the intensity of therapy incurs a real risk of worsening outcomes. Modern radiation therapy permits improved dose delivery such that toxicity and side effects of therapy are already likely to be improved compared to past techniques. In an era of limited health care resources, should we spend precious clinical trial dollars on a population of patients who are already doing exceptionally well? Perhaps the investment of limited clinical trial resources would be better used to focus on smoking cessation efforts and on those patients with HPV-negative disease in whom incremental improvements in outcome are most needed.
Two separate HPV vaccines are available in the United States. Neither vaccine is likely to be useful in treating patients who have developed HPV-positive HNC as they both target the major capsid protein which is typically lost during transformation. However, both have the potential to decrease the incidence of oral HPV infection and prevent the subsequent development of HNC. Although current recommendations from the Centers for Disease Control and Prevention and the American Academy of Pediatrics are for vaccination of both men and women ages eleven to twenty-six 27, current estimates in the US are that only 35% of the population completes the three injection series as currently recommended 28. Thus, while the vaccine has the potential to reduce the incidence of HPV-positive HNC, it may take decades for the effects of current vaccination to be reflected in cancer rates and the vaccine may never be broadly accepted.
Finally, while the theory of decreasing treatment intensity is attractive, in reality, both patients and physicians may be hesitant to embrace the possibility of worse outcomes for the possibility of improved tolerability. This powerful psychological barrier to dose reduction is well illustrated in a recent structured interview of patients who recently completed chemoradiation for head and neck cancer. Nearly 70% of patients were unwilling to risk a 5% or less drop in survival probability to switch from chemoradiation to radiation alone 29. Dose reduction has been performed in other diseases treated with radiation. Over several decades, and with the support of numerous cooperative group studies, both the total dose and the size of the treatment field have been successfully reduced in the management of Hodgkin Lymphoma 30. A similar approach may be valuable and warranted in head and neck cancer.
Rationale for dose reduction in HPV-positive HNC
The true biologic basis for the improved outcomes seen in HPV-positive HNC patients remains unclear. Enhanced radiation sensitivity has been postulated to underlie improved outcomes 11, 31. However, pre-clinical data supporting this hypothesis are limited and variable with two groups reporting enhanced sensitivity to radiation and two reporting reduced sensitivity to radiation 32–35. We and others have systematically examined HPV-positive HNC cell lines and show a predominant pattern that they are more sensitive to radiation than HPV-negative cells36, 37. Vermeer and colleagues recently suggested an interaction between immunologic recognition of HNC cells and radiation: CD47, a cell surface marker of “self” is decreased in HNC, but not normal cells following radiation leading to an increase in phagocytosis and cell clearance 48.
Several groups have published retrospective analyses to help identify patients in whom dose reduction is a reasonable risk. In general, these risk-profiles identify expected factors such as those with T4 or N3 disease 38. It is also well accepted that tobacco using HPV-positive HNC patients have worse outcomes than non-smoking HPV-positive patients 39, 40. These findings suggest that in tumors with exposure to additional carcinogenic factors, or in which tumor size permits the development of sufficient intratumoral heterogeneity, tumor biology may impact the development of therapeutic resistance.
Several other factors may underlie the difference in outcomes seen between patients with HPV-positive and HPV-negative HNC. For example, if there are systematic differences in tumor oxygenation between HPV-positive and HPV-negative HNC, this difference could explain altered in vivo sensitivity to radiation on the basis of HPV status. Two prospective trials investigating the effect of hypoxic modification on outcomes in patients with head and neck cancer have been analyzed on the basis of HPV status 7, 41. Consistent with there being less hypoxia in HPV-positive tumors, the use of a hypoxic modifier resulted in improved local-regional control in HPV-negative tumors, but had no effect in HPV-positive tumors 7, 41. However, because tumor hypoxia was not directly measured in either of these studies, whether a difference in tumor oxygenation truly underlies these results remains unclear.
Several studies have directly correlated measurements of tumor hypoxia with HPV status, but the results from these studies have been inconsistent. In the Danish Head and Neck Cancer Group (DAHANCA) 5 study, measurement of plasma osteopontin (a marker of hypoxia) was greater in HPV-negative tumors 42. Another group failed to find a correlation between tumor HPV status and carbonic anhydrase IX (upregulated in hypoxic tissues) or tumor pO2 status 43. Molecular imaging has also been used to examine intratumoral hypoxia in patients with head and neck cancer. However, in several recent publications, no correlation between imaging measured hypoxia and HPV status has been seen using dynamic contrast enhanced-MRI (DCE-MRI), proton magnetic resonance spectroscopy ((1)H-MRS), or (18)F-fluoroazomycin arabinoside positron emission tomography/computed tomography (FAZA PET/CT) 44, 45.
Another hypothesis being tested in several current clinical trials (discussed above) is that cetuximab, or other inhibitors of the epidermal growth factor receptor (EGFR), may offer equivalent outcome to the use of cisplatin in patients with HPV-positive HNC. However, there is very little prospective data regarding the value of therapies targeting EGFR in HPV-positive HNC patients. Two large clinical trials have demonstrated somewhat contrasting results. In the Bonner study which demonstrated an overall survival benefit with the addition of cetuximab to radiation in patients with locally advanced HNC, the greatest apparent benefit was in patients whose tumor originated in the oropharynx 46. Unfortunately, insufficient tumor material was available to perform formal HPV testing on these cases, so we are left to consider that many of these patients were likely HPV-positive based simply on the site of origin of their tumor. Alternatively, in the SPECTRUM study which investigated the role of panitumumab, an anti-EGFR antibody, in patients with recurrent or metastatic HNC, there was no apparent benefit to EGFR directed therapy in HPV-positive patients 47. Given the low percentage of patients with HPV-positive HNC who go on to develop recurrent or metastatic disease, it may be that the tumor characteristics of these patients is significantly different from the typical HPV-positive HNC patient meaning that extrapolating from this study to the general population of HPV-positive HNC patients is inadvisable.
Conclusions
In addition to the clinical trials described above, a number of groups are attempting to better understand the biology of HPV-positive HNC using in vitro and in vivo model systems. Unfortunately, at this time, the limited number of available HPV-positive HNC cell lines hampers progress in understanding the sensitivity of these cancers to current and investigational therapeutics. The lack of established HPV-positive HNC cell lines has led us, and others, to develop a patient derived xenograft model system and associated early passage cell strains 49. We are using this system to compare the sensitivity of HPV-positive and HPV-negative HNC to a variety of therapies and to identify biomarkers of response that can be validated in clinical trials.
Future studies of dose reduction for patients with HPV-positive HNC must balance patient preferences with system-wide pressures to limit cost. Using clinical, pathologic, and molecular profiles to determine failure patterns in individual patients will aid the clinician in guiding patients to the most appropriate treatment. Results from ongoing and future clinical trials to evaluate the role of reduced dose radiation or other means of decreasing therapeutic intensity in patients with HPV-positive HNC may not only identify ways in which to maintain good tumor control in good prognosis patients, but also suggest approaches to improve outcomes in those patients at higher risk for failure.
Acknowledgments
Financial support and disclosures: RK is supported by R00 CA160639.
Footnotes
Conflicts of Interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26:612–619. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 2.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, et al. Improved Survival of Patients With Human Papillomavirus-Positive Head and Neck Squamous Cell Carcinoma in a Prospective Clinical Trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 4.Shah NG, Trivedi TI, Tankshali RA, Goswami JV, Jetly DH, Shukla SN, et al. Prognostic significance of molecular markers in oral squamous cell carcinoma: A multivariate analysis. Head & Neck. 2009;31:1544–1556. doi: 10.1002/hed.21126. [DOI] [PubMed] [Google Scholar]
- 5.Reimers N, Kasper HU, Weissenborn SJ, Stützer H, Preuss SF, Hoffmann TK, et al. Combined analysis of HPV-DNA, p16 and EGFR expression to predict prognosis in oropharyngeal cancer. Int J Cancer. 2007;120:1731–1738. doi: 10.1002/ijc.22355. [DOI] [PubMed] [Google Scholar]
- 6.Weinberger PM, Yu Z, Haffty BG, Kowalski D, Harigopal M, Brandsma J, et al. Molecular classification identifies a subset of human papillomavirus--associated oropharyngeal cancers with favorable prognosis. J Clin Oncol. 2006;24:736–747. doi: 10.1200/JCO.2004.00.3335. [DOI] [PubMed] [Google Scholar]
- 7.Rischin D, Young RJ, Fisher R, Fox SB, Le QT, Peters LJ, et al. Prognostic significance of p16INK4A and human papillomavirus in patients with oropharyngeal cancer treated on TROG 02.02 phase III trial. J Clin Oncol. 2010;28:4142–4148. doi: 10.1200/JCO.2010.29.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mellin H, Dahlgren L, Munck-Wikland E, Lindholm J, Rabbani H, Kalantari M, et al. Human papillomavirus type 16 is episomal and a high viral load may be correlated to better prognosis in tonsillar cancer. Int J Cancer. 2002;102:152–158. doi: 10.1002/ijc.10669. [DOI] [PubMed] [Google Scholar]
- 9.Lindel K, Beer KT, Laissue J, Greiner RH, Aebersold DM. Human papillomavirus positive squamous cell carcinoma of the oropharynx: a radiosensitive subgroup of head and neck carcinoma. Cancer. 2001;92:805–813. doi: 10.1002/1097-0142(20010815)92:4<805::aid-cncr1386>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 10.Licitra L, Perrone F, Bossi P, Suardi S, Mariani L, Artusi R, et al. High-risk human papillomavirus affects prognosis in patients with surgically treated oropharyngeal squamous cell carcinoma. J Clin Oncol. 2006;24:5630–5636. doi: 10.1200/JCO.2005.04.6136. [DOI] [PubMed] [Google Scholar]
- 11.Lassen P. The role of Human papillomavirus in head and neck cancer and the impact on radiotherapy outcome. Radiother Oncol. 2010;95:371–380. doi: 10.1016/j.radonc.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 12.Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goon PK, Stanley MA, Ebmeyer J, Steinstrasser L, Upile T, Jerjes W, et al. HPV & head and neck cancer: a descriptive update. Head Neck Oncol. 2009;1:36. doi: 10.1186/1758-3284-1-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ragin CC, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Int J Cancer. 2007;121:1813–1820. doi: 10.1002/ijc.22851. [DOI] [PubMed] [Google Scholar]
- 15.Chau NG, Perez-Ordonez B, Zhang K, Pham NA, Ho J, Zhang T, et al. The association between EGFR variant III, HPV, p16, c-MET, EGFR gene copy number and response to EGFR inhibitors in patients with recurrent or metastatic squamous cell carcinoma of the head and neck. Head Neck Oncol. 2011;3:11. doi: 10.1186/1758-3284-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris SL, Thorne LB, Seaman WT, Hayes DN, Couch ME, Kimple RJ. Association of p16(INK4a) overexpression with improved outcomes in young patients with squamous cell cancers of the oral tongue. Head Neck. 2011;33:1622–1627. doi: 10.1002/hed.21650. [DOI] [PubMed] [Google Scholar]
- 17.Nutting CM, Morden JP, Harrington KJ, Urbano TG, Bhide SA, Clark C, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12:127–136. doi: 10.1016/S1470-2045(10)70290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kam MKM, Leung S-F, Zee B, Chau RMC, Suen JJS, Mo F, et al. Prospective Randomized Study of Intensity-Modulated Radiotherapy on Salivary Gland Function in Early-Stage Nasopharyngeal Carcinoma Patients. J Clin Oncol. 2007;25:4873–4879. doi: 10.1200/JCO.2007.11.5501. [DOI] [PubMed] [Google Scholar]
- 19.Chen AM, Li BQ, Lau DH, Farwell DG, Luu Q, Stuart K, et al. Evaluating the role of prophylactic gastrostomy tube placement prior to definitive chemoradiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 2010;78:1026–1032. doi: 10.1016/j.ijrobp.2009.09.036. [DOI] [PubMed] [Google Scholar]
- 20.Caudell JJ, Schaner PE, Desmond RA, Meredith RF, Spencer SA, Bonner JA. Dosimetric factors associated with long-term dysphagia after definitive radiotherapy for squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2010;76:403–409. doi: 10.1016/j.ijrobp.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 21.Eisbruch A, Kim HM, Feng FY, Lyden TH, Haxer MJ, Feng M, et al. Chemo-IMRT of oropharyngeal cancer aiming to reduce dysphagia: swallowing organs late complication probabilities and dosimetric correlates. Int J Radiat Oncol Biol Phys. 2011;81:e93–99. doi: 10.1016/j.ijrobp.2010.12.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwartz DL, Hutcheson K, Barringer D, Tucker SL, Kies M, Holsinger FC, et al. Candidate dosimetric predictors of long-term swallowing dysfunction after oropharyngeal intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78:1356–1365. doi: 10.1016/j.ijrobp.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levendag PC, Teguh DN, Voet P, van der Est H, Noever I, de Kruijf WJ, et al. Dysphagia disorders in patients with cancer of the oropharynx are significantly affected by the radiation therapy dose to the superior and middle constrictor muscle: a dose-effect relationship. Radiother Oncol. 2007;85:64–73. doi: 10.1016/j.radonc.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 24.Buckwalter AE, Karnell LH, Smith RB, Christensen AJ, Funk GF. Patient-reported factors associated with discontinuing employment following head and neck cancer treatment. Arch Otolaryngol Head Neck Surg. 2007;133:464–470. doi: 10.1001/archotol.133.5.464. [DOI] [PubMed] [Google Scholar]
- 25.de Boer AG, Taskila T, Ojajarvi A, van Dijk FJ, Verbeek JH. Cancer survivors and unemployment: a meta-analysis and meta-regression. JAMA. 2009;301:753–762. doi: 10.1001/jama.2009.187. [DOI] [PubMed] [Google Scholar]
- 26.Ross L, Petersen MA, Johnsen AT, Lundstroem LH, Carlsen K, Groenvold M. Factors associated with Danish cancer patients’ return to work. A report from the population-based study ‘The Cancer Patient’s World’. Cancer Epidemiol. 2012;36:222–229. doi: 10.1016/j.canep.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 27.Committee on Infectious D. HPV vaccine recommendations. Pediatrics. 2012;129:602–605. doi: 10.1542/peds.2011-3865. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Disease C, Prevention. National and state vaccination coverage among adolescents aged 13–17 years--United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61:671–677. [PubMed] [Google Scholar]
- 29.Brotherston DC, Poon I, Le T, Leung M, Kiss A, Ringash J, et al. Patient preferences for oropharyngeal cancer treatment de-escalation. Head Neck. 2013;35:151–159. doi: 10.1002/hed.22930. [DOI] [PubMed] [Google Scholar]
- 30.Meyer RM, Hoppe RT. Point/counterpoint: early-stage Hodgkin lymphoma and the role of radiation therapy. Blood. 2012;120:4488–4495. doi: 10.1182/blood-2012-05-423236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 32.Gupta AK, Lee JH, Wilke WW, Quon H, Smith G, Maity A, et al. Radiation Response in Two HPV-Infected Head-and-Neck Cancer Cell Lines in Comparison to a Non-HPV-Infected Cell Line and Relationship to Signaling Through AKT. International Journal of Radiation Oncology*Biology*Physics. 2009;74:928–933. doi: 10.1016/j.ijrobp.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spanos WC, Nowicki P, Lee DW, Hoover A, Hostager B, Gupta A, et al. Immune response during therapy with cisplatin or radiation for human papillomavirus-related head and neck cancer. Arch Otolaryngol Head Neck Surg. 2009;135:1137–1146. doi: 10.1001/archoto.2009.159. [DOI] [PubMed] [Google Scholar]
- 34.Gubanova E, Brown B, Ivanov SV, Helleday T, Mills GB, Yarbrough WG, et al. Downregulation of SMG-1 in HPV-positive head and neck squamous cell carcinoma due to promoter hypermethylation correlates with improved survival. Clin Cancer Res. 2012;18:1257–1267. doi: 10.1158/1078-0432.CCR-11-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeWeese TL, Walsh JC, Dillehay LE, Kessis TD, Hedrick L, Cho KR, et al. Human papillomavirus E6 and E7 oncoproteins alter cell cycle progression but not radiosensitivity of carcinoma cells treated with low-dose-rate radiation. Int J Radiat Oncol Biol Phys. 1997;37:145–154. doi: 10.1016/s0360-3016(96)00448-8. [DOI] [PubMed] [Google Scholar]
- 36.Kimple RJ, Smith MA, Blitzer GC, Torres AD, Peet C, Saker J, et al. Increased radiation sensitivity in HPV-positive head and neck cancer is due to residual p53 activity. Cancer Research. 2013 [Google Scholar]
- 37.Rieckmann T, Tribius S, Grob TJ, Meyer F, Busch CJ, Petersen C, et al. HNSCC cell lines positive for HPV and p16 possess higher cellular radiosensitivity due to an impaired DSB repair capacity. Radiother Oncol. 2013 doi: 10.1016/j.radonc.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 38.O’Sullivan B, Huang SH, Siu LL, Waldron J, Zhao H, Perez-Ordonez B, et al. Deintensification candidate subgroups in human papillomavirus-related oropharyngeal cancer according to minimal risk of distant metastasis. J Clin Oncol. 2013;31:543–550. doi: 10.1200/JCO.2012.44.0164. [DOI] [PubMed] [Google Scholar]
- 39.Gillison ML, Zhang Q, Jordan R, Xiao W, Westra WH, Trotti A, et al. Tobacco Smoking and Increased Risk of Death and Progression for Patients With p16-Positive and p16-Negative Oropharyngeal Cancer. Journal of Clinical Oncology. 2012 doi: 10.1200/JCO.2011.38.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maxwell JH, Kumar B, Feng FY, Worden FP, Lee JS, Eisbruch A, et al. Tobacco use in human papillomavirus-positive advanced oropharynx cancer patients related to increased risk of distant metastases and tumor recurrence. Clin Cancer Res. 2010;16:1226–1235. doi: 10.1158/1078-0432.CCR-09-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lassen P, Eriksen JG, Hamilton-Dutoit S, Tramm T, Alsner J, Overgaard J, et al. HPV-associated p16-expression and response to hypoxic modification of radiotherapy in head and neck cancer. Radiother Oncol. 2010;94:30–35. doi: 10.1016/j.radonc.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 42.Overgaard J, Eriksen JG, Nordsmark M, Alsner J, Horsman MR, Danish H, et al. Plasma osteopontin, hypoxia, and response to the hypoxia sensitiser nimorazole in radiotherapy of head and neck cancer: results from the DAHANCA 5 randomised double-blind placebo-controlled trial. Lancet Oncol. 2005;6:757–764. doi: 10.1016/S1470-2045(05)70292-8. [DOI] [PubMed] [Google Scholar]
- 43.Kong CS, Narasimhan B, Cao H, Kwok S, Erickson JP, Koong A, et al. The relationship between human papillomavirus status and other molecular prognostic markers in head and neck squamous cell carcinomas. Int J Radiat Oncol Biol Phys. 2009;74:553–561. doi: 10.1016/j.ijrobp.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mortensen LS, Johansen J, Kallehauge J, Primdahl H, Busk M, Lassen P, et al. FAZA PET/CT hypoxia imaging in patients with squamous cell carcinoma of the head and neck treated with radiotherapy: results from the DAHANCA 24 trial. Radiother Oncol. 2012;105:14–20. doi: 10.1016/j.radonc.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 45.Jansen JF, Carlson DL, Lu Y, Stambuk HE, Moreira AL, Singh B, et al. Correlation of a priori DCE-MRI and (1)H-MRS data with molecular markers in neck nodal metastases: Initial analysis. Oral Oncol. 2012;48:717–722. doi: 10.1016/j.oraloncology.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 47.Vermorken J, Stohlmacher J, Oliner K, Villanueva C, Foa P, Winquist E, et al. Safety and Efficacy of Panitumumab (pmab) in HPV Positive (+) and HPV Negative (−) Recurrent/metastatic (R/M) Squamous Cell Carcinoma of the Head and Neck (SCCHN): Analysis of the Phase 3 SPECTRUM Trial. European Journal of Cancer; 2011 Eurpoean Multidisciplinary Cancer Congress; Stockholm, Sweden. 2011. p. 13. [Google Scholar]
- 48.Vermeer DW, Spanos WC, Vermeer PD, Bruns AM, Lee KM, Lee JH. Radiation-induced loss of cell surface CD47 enhances immune-mediated clearance of human papillomavirus-positive cancer. Int J Cancer. 2013;133:120–129. doi: 10.1002/ijc.28015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kimple RJ, Harari P, Torres AD, Yang RZ, Soriano BJ, Yu M, et al. Development and characterization of HPV-positive and HPV-negative head and neck squamous cell carcinoma tumorgrafts. Clinical Cancer Research. 2013;19:855–864. doi: 10.1158/1078-0432.CCR-12-2746. [DOI] [PMC free article] [PubMed] [Google Scholar]