Abstract
The present study tested the competing hypotheses that adolescents at risk for future substance abuse and dependence by virtue of parental substance use disorders show either weaker or stronger responsivity of brain regions implicated in reward relative to youth without parental history of substance use disorders. Adolescents (N = 52) matched on demographics with and without parental substance use disorders, as determined by diagnostic interviews, who denied substance use in the past year were compared on functional magnetic resonance imaging (fMRI) paradigms assessing neural response to receipt and anticipated receipt of monetary and food reward. Parental-history-positive versus -negative adolescents showed greater activation in the left dorsolateral prefrontal cortex and bilateral putamen, and less activation in the fusiform gyrus and inferior temporal gyrus in response to anticipating winning money, as well as greater activation in the left midbrain and right paracentral lobule, and less activation in the right middle frontal gyrus in response to milkshake receipt. Results indicate that adolescents at risk for future onset of substance use disorders show elevated responsivity of brain regions implicated in reward, extending results from two smaller prior studies that found that individuals with versus without parental alcohol use disorders showed greater reward region response to anticipated monetary reward and pictures of alcohol. Collectively results provide support for the reward surfeit model of substance use disorders, rather than the reward deficit model.
Keywords: fMRI, reward, attention, parental substance use disorder, adolescents
Some theorists posit that individuals with less responsive reward circuitry are more likely to use psychoactive substances because they are compensating for a reward deficit, as drugs of abuse increase dopamine (DA) signaling and activation in mesolimbic reward regions (Volkow, Fowler, & Wang, 2002). Others argue that individuals with more responsive reward circuitry are at greater risk for onset and escalation in use because they experience greater hedonic reward from substance use (Davis & Claridge, 1998).
In apparent support of the reward deficit theory of substance use, adults with versus without alcohol or drug dependence show less striatal D2 receptor availability and sensitivity (Volkow et al., 2001; Volkow, Wang, Fowler, & Logan, 1997; Wang et al., 1997) and less ventral striatal response to anticipating monetary reward (Beck et al., 2009; Wrase et al., 2007). Moreover, human cocaine abusers versus non-abusers show blunted DA release from stimulant drug use (Martinez et al., 2007) and tolerance to the euphoric effects of cocaine (O’Brian, Volkow, & Li, 2006). Even adolescents with only a limited history of substance use show less caudate activation in response to monetary reward relative to non-using adolescents (Stice, Yokum, & Burger, 2013). Further, low striatal D2 receptor availability in primates predicted increased future drug self-administration (Morgan et al., 2002). Yet, animal experiments show that substance use reduces striatal D2 receptor availability (Nader et al., 2006; Porrino, Lyons, Smith, Daunais, & Nader, 2004) and sensitivity of reward circuitry (Ahmed, Kenny, Koob, & Markou, 2002; Kenny, Chen, Kitamura, Markou, & Koob, 2006). These latter findings suggest that the reduced reward circuitry responsivity observed in individuals with substance use disorders in the cross-sectional studies with humans may be a result of chronic substance use rather than represent an initial vulnerability factor.
Other findings appear more consistent with the reward surfeit theory of substance use, which posits that greater reward region responsivity increases risk for substance use. Adults with versus without various substance use disorders show greater activation of reward regions (e.g., amygdala, dorsolateral prefrontal cortex [dlPFC], ventral tegmental area) and attention regions (anterior cingulate cortex [ACC]) and report greater craving in response to substance use cues (e.g., pictures of drugs; Due, Huettel, Hall, & Rubin, 2002; Maas et al., 1998; Myrick et al., 2004). Craving in response to cues correlates with the magnitude of dorsal striatum DA release (Volkow et al., 2006) and activation in the amygdala, dlPFC, ACC, nucleus accumbens (NAcc), and orbitofrontal cortex (OFC) (Maas et al., 1998; Myrick et al., 2004). Cocaine dependent versus non-dependent adults show greater OFC response to monetary reward (Goldstein et al., 2007). One prospective study found that elevated caudate and putamen response to monetary reward predicted future substance use onset (Stice et al., 2013). However, chronic substance use contributes to increased responsivity of reward and attention regions to cues associated with drug reward via conditioning (Robinson & Berridge, 2001), with substance use cues eventually causing DA release in the ventral striatum and medial prefrontal cortex (medial PFC) in substance using animals and humans (Dayas, Liu, Simms, & Weiss, 2007; Di Chiara, 2002; Volkow et al., 2008). These latter findings suggest that substance use may contribute to the hyper-responsivity of reward and attention regions observed in the cross-sectional studies involving humans with substance use disorders and may not constitute an initial vulnerability factor.
To determine if the abnormalities reported in the cross-sectional studies are initial vulnerability factors, researchers have tested whether individuals at high-versus low-risk for substance use disorders due to parental history of these disorders show differential response to reward before experiencing substance use disorders. Streeter et al. (1998) found no differences in fMRI-assessed neural responsivity to benzodiazepine intake for adults with versus without parental history of alcohol dependence. Bjork, Knutson, and Hommer (2008) found that adolescent with versus without parental history of alcohol dependence did not differ on ventral striatum response to monetary reward and anticipated monetary reward. In contrast, Tapert et al. (2003) found that among adolescents who did not meet criteria for alcohol abuse or dependence, those with versus without parental history of alcohol use disorders showed greater activation in the left paracentral, medial frontal, prefrontal, cuneus, and anterior cingulate areas in response to alcohol beverage versus nonalcoholic beverage pictures. Andrews et al. (2011) found that adults with versus without family history of alcohol abuse showed greater caudate activation to the prospect of monetary reward, but less activation of the NAcc, insula, and OFC in response to anticipated monetary reward; this study might have had greater sensitivity because it required family history participants to have substance use disorders in at least one parent and one additional first- or second-degree relative. Yau et al. (2012) found that young adults with versus without parental alcohol use disorders showed lower NAcc response to anticipated monetary reward, but no other differences in responsivity of other brain regions.
Thus, extant parental history studies have provided mixed support for the reward deficit and reward surfeit models of substance use, in that only two of five studies identified aberrant responsivity in classic brain reward regions (e.g., caudate, NAcc), but effects were in opposite directions for these two striatal regions. There was evidence from one study that parental-history-positive individuals showed greater responsivity in a classic attention region (ACC). However, because these studies examined small samples, ranging from 20 to 49 participants, they were only powered to detect large effects. In addition, these parental history studies included individuals who were currently using substances (though the extent of use was typically not well characterized), raising the possibility that substance use might have contributed to the differential neural responsivity across groups. Individuals who use substances show greater responsivity of reward and attention regions to images of the substances they habitually use (Due et al., 2002; Maas et al., 1998; Myrick et al., 2004). Consistent with this interpretation, Tapert et al., (2003) found that elevated ACC responsivity to alcohol images correlated with the number of drinks consumed by the adolescents in the past month, suggesting that use contributed to greater recruitment of this attention region in response to alcohol images. In apparent recognition of this potential interpretational problem, Yau et al. (2012) matched parental-history-positive and -negative participants on habitual substance use. Moreover, prior studies focused primarily on parental alcohol use disorders, and did not include parents with a history of abuse or dependence of other psychoactive substances that occurred without concomitant alcohol use disorders, which affect 21% of those with substance use disorders (Grant et al., 2004). However, at least one study allowed parents with alcoholism to have other substance use disorders (Yau et al., 2012) and others did not expressly state that individuals who had relatives with other types of substance use disorders were excluded (e.g., Andrews et al., 2011).
Accordingly, we conducted a larger study that examined responsivity of reward regions among non-substance using adolescents with versus without a parental history of substance use disorders. We examined response to both a conditioned reward (money) and an unconditioned reward (palatable food) to provide a more comprehensive evaluation of reward region responsivity than prior studies. We also examined response to both receipt and anticipated receipt of money and food because of emerging evidence that abnormalities in both phases of reward are related to disorders of appetitive motivation (Robinson & Berridge, 2001; Stice, Spoor, Bohon, Veldhuizen, & Small, 2008).
Given that a prior study found evidence of increased responsivity of a striatal region for individuals with versus without parental history of alcohol use disorders (Andrews et al., 2011) and that we found that elevated striatal response to monetary reward predicted future substance use onset (Stice et al., 2013), we hypothesized that adolescents with versus without parental substance use disorders would show elevated reward region responsivity to receipt and anticipated receipt of monetary and food reward. However, the fact that studies have also suggested that individuals with versus without parental history of alcohol use disorders showed weaker recruitment of other striatal regions (Andrews et al., 2011; Yau et al., 2012) reduces our confidence in this hypothesis. We anticipated somewhat stronger effects in response to anticipated monetary and food reward because the few parental-history differences that have emerged in reward regions have typically occurred in response to anticipated monetary reward (Andrews et al., 2011; Yau et al., 2012), whereas no parental-history differences emerged in the one study to evaluate receipt of monetary reward (Bjork et al., 2008).
Methods
Participants
Participants were 128 adolescents recruited in a small Western US city via advertisements and flyers that invited youth between the ages of 14 and 17 to participate in a study examining how the brain responds to food and potential causes of overeating. The sample was generally representative of the county from which we recruited in terms of demographics. Adolescents and parents provided written informed consent for this IRB-approved project. Exclusion criteria were current regular use of psychoactive substances (more than once weekly, including nicotine and alcohol) or psychotropic medications, pregnancy, head injury with a loss of consciousness, significant cognitive impairment (e.g., Fetal Alcohol Syndrome), or Axis I psychiatric disorder in the past year.
Measures
Substance use was assessed with 8 items measuring the frequency of use of beer/wine/wine coolers, hard liquor, cigarettes, marijuana, stimulants, downers, inhalants, and hallucinogens in the past year. Sample item: Over the last year how many times did you drink beer, wine, or wine coolers? Response options were: 0 = never, 1 = a few times, 2 = less than monthly, 3 = 1–3 times a month, 4 = 1–2 times a week, 5 = 3–4 times a week, 6 = 5–7 times a week. This scale has shown internal consistency (M α = .86), test-retest reliability (M r = .86), and predictive validity for future substance abuse (Chassin, Rogosch, & Barrera, 1991; Stice, Barrera, & Chassin, 1998).
History of parental substance abuse or dependence was assessed by research assistants via the Structured Clinical Interview for DSM-IV Axis I Disorders, nonpatient version (SCID-I/NP) (First, Spitzer, Gibbon, & Williams, 2002) with one biological parent and via the Family History Research Diagnostic Criteria (FH-RDC) interview for the other biological parent (Endicott, Andreasen, & Spitzer, 1978) who was not interviewed. Because the present study focused primary on neural vulnerability factors that predict future unhealthy weight gain, we streamlined the diagnostic interviews by simply inquiring about whether parents had experienced substance abuse and dependency symptoms related to use of any psychoactive substance, rather than repeat all questions for the numerous substances that are misused. This decision was based on evidence that over 75% of individuals with a primary addiction to one drug (opiates) report regular use of other drugs, including cigarettes, alcohol, cannabis, and benzodiazepines (Lubman et al., 2009). Further, participants who met criteria for certain substance use disorders (e.g., alcoholism) in past parental history studies often met criteria for other substance use disorders (e.g., 60% of the parents who met criteria for alcoholism in the Yau et al., [2012] study also met criteria for another substance use disorder). Substance abuse/dependence diagnoses with the SCID have shown inter-rater agreement in other studies from our research group (κ=.81; Rohde et al., 2007), but we did not collect data on inter-rater agreement of the diagnoses in the present study. Although informant interviews are less accurate than direct interviews (Vandeleur et al., 2008), the FH-RDC has shown inter-rater reliability (κ = .95), test-retest reliability (κ = .74) and criterion validity with diagnoses made with direct interviews with family members (OR = 51.8; Hardt & Franke, 2007; Prescott et al., 2005; Zimmerman, Corvell, Pfohl, & Stangl, 1988). Adolescents were classified as parental-history-positive if one or two biological parents had a history of substance abuse/dependence for any illicit drugs (e.g., marijuana, cocaine, and amphetamines), prescription medications, or alcohol.
fMRI paradigms
Participants were asked to consume their regular meals, but to refrain from eating or drinking caffeinated beverages for 5 hours preceding their scan. Participants were familiarized with the fMRI paradigms prior to scanning. Order of the fMRI paradigms was counterbalanced (as were runs within the paradigms). Female participants were tested for pregnancy if they had been sexually active. Over the counter drug tests confirmed that youth were not using psychoactive drugs on the scan day (tests could detect substance use during the last 3 days).
The monetary reward paradigm (Fig 1A) assessed activation in response to receipt and anticipated receipt of monetary reward, loosely based on the Monetary Incentive Delay (MID) paradigm (Knutson, Taylor, Kaufman, Peterson, & Glover, 2005), though no behavioral responses were required in the variant we developed. First a coin on the left side of the screen alternates between blinking heads (H) and tails (T) 2–4 times for 300ms per blink and then “lands” on either H or T. After 2 seconds, a second coin blinks 4–6 times before landing on H or T. After 3 seconds, a third coin blinks 8–10 times before landing on H or T. After the presentation of the coins, a message appeared saying whether or not the subject won (“You win $3” or “You don’t win”). In total, there were 20 win events (HHH or TTT displays), 30 win anticipation events (HH or TT displays), and 30 reward-neutral events (a single H or T). Participants were informed that they would win $3 when all 3 coins were HHH or TTT before starting the paradigm and that they would receive the money they won at the end of the scan session.
Figure 1.
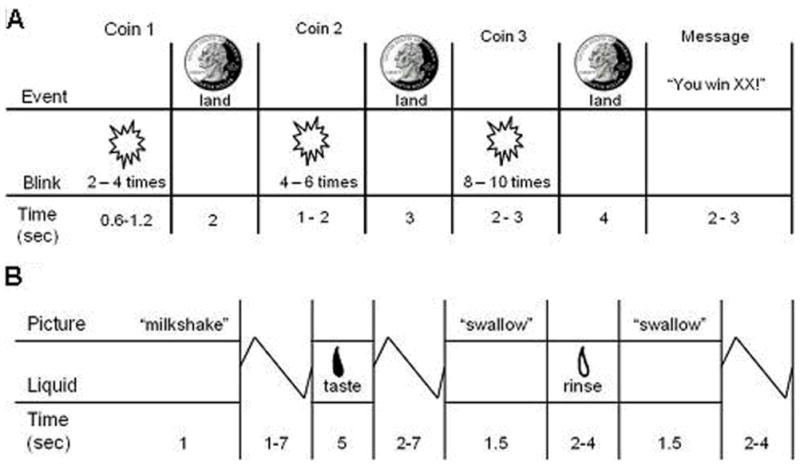
Example of timing and ordering of presentation of A) images and notification of monetary reward during the monetary reward paradigm and of B) pictures and beverages during the food reward paradigm.
The food reward paradigm (Fig 1B; Stice, Yokum, Burger, Epstein, & Small, 2011) assessed response to receipt and anticipated receipt of palatable food. Stimuli were 2 images (glasses of milkshake and water) that signaled impending delivery of either 0.5mL of chocolate milkshake (2 scoops vanilla ice cream, 1 cup 2% milk, and 2 tablespoons chocolate syrup) or tasteless solution (25 mM KCl and 2.5 mM NaHCO3 in distilled water). On 40% of the trials the taste was not delivered following the cue to allow investigation of the neural response to anticipation of a taste that was not confounded with actual receipt of the taste (unpaired trials). There were 30 repeats of both milkshake receipt and tasteless solution receipt, and 20 repeats of both the unpaired milkshake cue and unpaired tasteless solution cue. Cues were presented for 2 seconds, followed by a jitter (1–7 seconds) during which time the screen was blank. Trials ended with another 1–7 sec jitter. Stimuli were presented in 5 separate runs. Tastes were delivered using two programmable syringe pumps controlled by a laptop to ensure consistent delivery rate and amount. Sixty ml syringes filled with milkshake and tasteless solution were connected via Tygon tubing through a wave guide to a custom gustatory manifold that fit into participants’ mouths and delivered the taste to a consistent tongue segment (Fig 2). Participants were instructed to swallow when they saw the ‘swallow’ cue. The next cue appeared 1–7 seconds after the ‘swallow’ cue went off. Participants rated hunger and fullness of the milkshake and tasteless solution before the scan using cross-modal visual analogue scales; these variables were included as covariates for the food reward paradigm. Average activation peaks and maps for main effects analyses revealed that these two fMRI paradigms significantly activated reward regions, including the putamen, pallidum, and OFC (Stice, Yokum, Burger, Epstein, & Smolen, 2012).
Figure 2.
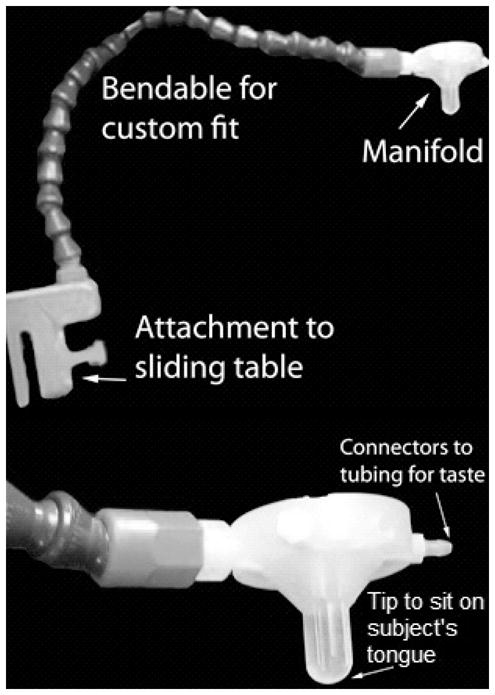
The gustatory manifold was anchored to the table. New tubing and syringes were used for each subject and the mouthpiece was cleaned and sterilized between uses.
Validation measures
Progressive Reinforcement Paradigms
We included the progressive reinforcement paradigm developed by Epstein and colleagues (2003) in which participants work to earn points toward a snack food reward of their choice and monetary reward to provide behavioral validation for the fMRI paradigms and to characterize the parental-history-positive versus -negative groups. Participants first performed a taste test of 1g of each food and rated the pleasantness and intensity of each taste and how much they craved each food on cross-modal visual analogue scales. The pleasantness ratings of these foods were used to provide a behavioral measure of consummatory food reward. We divided the foods in 4 separate food groups based on their saturated (sat) fat and sugar content: a) high-fat/high-sugar foods (M&M’s [6 grams sat fat, 28 grams sugar], Reeses [4.5 grams sat fat, 21 grams sugar], and Kitkat [7 grams sat fat, 21 grams sugar]), b) high-fat/low-sugar foods (Cheetos [2.5 grams sat fat, 1 gram sugar] and Pringles [2 grams sat fat, 0 grams sugar]), c) low-fat/high-sugar foods (Skittles [1.5 grams sat fat, 28 grams sugar], Oreo cookies [2 grams sat fat, 11 grams sugar], Gingersnaps [0.5 grams saturated fat, 11 grams sugar], and Gummy Bears [0 grams sat fat, 18 grams sugar]), and d) low-fat/low-sugar food (popcorn [1 gram sat fat, 0 grams sugar]). Participants then selected the snack food they wanted to earn in the progressive reinforcement task. In the second phase, three boxes varying in shape and color were displayed on a computer screen (similar to a slot machine display). The boxes flipped, rotated and changed in color each time the participant pressed the mouse button. Points were earned each time the shapes matched in color and shape. The task started at a variable ratio 1/4 schedule meaning that, on average, one point was awarded for four button presses. The progressive ratio schedule for the food item doubled (VR8, VR16, VR32, etc.) each time they earned five points. They were told that it would get progressively harder to earn points. The number of points earned for snacks were displayed at the top of the screen. A total of 5 points was worth 1 standard serving of the food. Participants were told to play for as long as they liked. They then repeated this paradigm, but worked for $1 monetary rewards, to provide a behavioral measure of sensitivity to general reward. The break point at which the participant stopped button pressing for food was used as the behavioral measure of receipt and anticipated receipt of food reward (i.e., how many button presses are made in total before the subject stops). A similar monetary reward break point was calculated.
The food reinforcement paradigm has shown 2–7 day test-retest reliability (r = .80 [Epstein et al., 2007]). With regard to the validity of this task, subjects who work more for snack foods eat more food ad lib, subjects who rate the snack foods as more hedonically pleasurable work more for the snack foods, subjects work more for food when they are food deprived, and obese versus lean subjects work for more food (Epstein et al., 2003, 2007; Goldfield & Legg, 2006).
Behavioral Inhibition and Activation Scale (BIS/BAS)
We also included the BAS Reward Responsiveness scale (Carver & White, 1994) to provide validation for the fMRI paradigms and to characterize the parental-history-positive group. The BAS reward responsiveness scale correlated positively with activation of reward valuation regions (OFC, ventral pallidum) in response to images of palatable foods (Beaver et al., 2006).
Imaging acquisition, preprocessing, and analysis
A Siemens Allegra 3T head-only MRI scanner was used to acquire data. Functional scans used a T2* weighted gradient single-shot echo planar imaging (EPI) sequence (TE=30 ms, TR = 2000 ms, flip angle=80°) with an in-plane resolution of 3.0×3.0 mm2 (64×64 matrix; 192×192 mm2 field of view); 32 4mm slices (interleaved acquisition, no skip) were acquired along the AC-PC transverse, oblique plane as determined by the midsagittal section. Structural scans were collected using an inversion recovery T1 weighted sequence (MP-RAGE). High-resolution structural MRI sequences (FOV = 256×256 mm2, 256×256 matrix, thickness = 1.0 mm, slice number ≈ 160) were also acquired.
Data were pre-processed and analyzed using SPM8 (Wellcome Department of Imaging Neuroscience, London) in MATLAB (MATLAB 7.1, The Mathworks Inc.). Functional images were realigned to the mean. Images were normalized to the standard ICBM152 template brain implemented in SPM8. Normalization resulted in a voxel size of 3 mm3 for functional images and 1 mm3 for structural images. Functional images were smoothed with a 6 mm FWHM isotropic Gaussian kernel. Following established procedures (Stice et al., 2011, 2012), activation in response to monetary reward was assessed by contrasting BOLD activation at the time a participant “won” (HHH or TTT displays) versus the reward-neutral coin displays (a single H or T) and anticipation of monetary reward was assessed by contrasting BOLD activation during presentation of the cue signaling a potential win (HH or TT displays) versus during the reward-neutral coin displays. Also following established procedures (Stice et al., 2011, 2012), activation in response to food receipt was assessed by contrasting BOLD activation during receipt of milkshake versus tasteless solution and anticipated food receipt was assessed by contrasting BOLD activation during presentation of the unpaired milkshake cue versus the unpaired tasteless solution cue. Condition-specific effects at each voxel were estimated using general linear models. Vectors of the onsets for each event of interest were entered into a design matrix so that event-related responses could be modeled by the canonical hemodynamic response function (which describes the form of the rapid spike in blood flow that occurs as the body adjusts to deliver oxygen to brain regions in need). Temporal derivatives of the hemodynamic function were included to obtain a better model of the data. A 128 second high-pass filter removed low-frequency noise and slow drifts in the signal.
Individual maps were constructed to compare activations within each participant for the four events described previously. We conducted 2×2 ANOVA models (parental-history-positive versus -negative) by the four events of interest (e.g., win versus reward-neutral coin display). Whole brain analyses were conducted. An overall significance level of p < 0.05, corrected for multiple comparisons across the whole brain, was used. This was accomplished by first estimating the inherent smoothness of gray-matter masked functional data with the 3dFWHMx module in AFNI (Cox, 1996). This smoothness was then used in 10,000 Monte Carlo simulations of random noise at 3mm3 through the gray matter masked data using the 3DClustSim module of AFNI (Cox, 1996, Forman et al., 1995). Results from these simulations indicated that activity surviving a threshold of p < 0.001 and a cluster (k) ≥ 12 was significant corrected for multiple comparisons. Effect sizes (r) were derived from the Z-values (Z/√N).
Results
Characterization of parental-history-positive versus -negative adolescents
Among the 128 adolescents, eighty-seven reported no use of any substances in the past year. Of those, 26 had a parental history of substance use disorders (46.2% males, 86% paternal history, 7% maternal history, 7% paternal and maternal history) and 61 did not (45.9% males). We created a parental-history-negative group (n = 26) that was matched to the parental-history-positive group on the adolescent sex, age, parental education (a proxy for socioeconomic status), and parental BMI ([maternal BMI + paternal BMI]/2. As a result, the sample for the present report consisted of 52 adolescents (M age=14.8 ±1.0; range = 14–17; M BMI=20.8 ±1.8; 25 males; 7.7% Hispanic, 1.9% Native American, 80.8% European Americans, and 9.6% mixed racial heritage). There were no significant differences between parental-history-positive versus -negative adolescents on sex, age, parental education, parental BMI, ethnicity, BAS reward responsiveness, hunger, fullness, pleasantness ratings of the milkshake and tasteless solution, pleasantness ratings of high-sugar snacks and high-fat snacks, or the breakpoints for the progressive reinforcement paradigms (Table 1).
Table 1.
Characterization of parental-history-positive versus -negative adolescents
| Parental-history-positive (n = 26) | Parental-history-negative (n = 26) | |||
|---|---|---|---|---|
|
| ||||
| Percentage | Percentage | F (85) | p | |
| Sex | 46.2% males | 50.0% males | 0.07 | 0.79 |
| Ethnicity: | 0.42 | 0.52 | ||
| - Hispanic | 7.7% | 7.7% | ||
| - American native | 0% | 3.8% | ||
| - Asian | 0% | 0% | ||
| - African American | 0% | 0% | ||
| - Pacific Islander | 0% | 0% | ||
| - Caucasian | 76.9% | 84.6% | ||
| - mixed | 15.4% | 3.8% | ||
| Paternal overweight/obesity | 80.8% | 88.5 | 0.58 | 0.45 |
| Mean ± SD | Mean ± SD | |||||
|---|---|---|---|---|---|---|
| Age | 14.7 | 0.9 | 14.9 | 1.0 | 0.30 | 0.58 |
| Parental education | 3.9 | 0.9 | 4.0 | 0.94 | 0.02 | 0.88 |
| BAS Reward Responsiveness | 17.6 | 1.9 | 17.1 | 2.0 | 0.97 | 0.33 |
| Hunger rating | 7.0 | 4.4 | 7.8 | 3.8 | 0.41 | 0.53 |
| Fullness rating | 5.4 | 3.8 | 6.5 | 3.6 | 1.18 | 0.28 |
| Pleasantness rating milkshake | 13.7 | 2.9 | 14.8 | 2.1 | 2.53 | 0.12 |
| Pleasantness rating tasteless solution | 8.6 | 1.2 | 9.2 | 2.8 | 0.90 | 0.35 |
| Pleasantness rating high sugar snacks | 4.6 | 0.9 | 4.7 | 0.9 | 0.40 | 0.53 |
| Pleasantness rating high fat snacks | 4.5 | 1.2 | 4.8 | 1.1 | 1.12 | 0.30 |
| Breakpoint food reward | 561.5 | 766.2 | 338.5 | 311.1 | 1.89 | 0.18 |
| Breakpoint monetary reward | 1462.0 | 2258.1 | 1343.6 | 2264.5 | 0.04 | 0.85 |
Validation of the fMRI monetary and food reward paradigms
Whole brain analyses were conducted to validate the monetary and food reward paradigms. Average activation maps for the parental-history-positive and -negative groups are shown in Figure 3. These maps confirm that reward regions (i.e. striatum, medial OFC, amygdala) were recruited for each of the key contrasts, with the exception that there were no significant peaks for the milkshake cue > tasteless solution cue contrast. How much participants worked for money during the progressive reinforcement task correlated with activation in regions implicated in reward processing (right posterior cingulate cortex [r = 0.61, p < 0.001], left caudate [r = 0.49, p < 0.001], right putamen [r = 0.42, p < 0.001], left mid insula [r = 0.40, p < 0.001], left mediodorsal thalamus [r = 0.41, p < 0.001]) and attention (bilateral ACC [r left = 0.47, r right = 0.45, p’s < 0.001], left inferior parietal lobe [r = 0.48, p < 0.001]) in response to anticipating winning money. How much participants worked to earn money also correlated with activation in regions implicated in reward processing (right posterior cingulate cortex [r = 0.47, p < 0.001], right caudate [r = 0.45, p < 0.001]) and attention (left inferior parietal lobe [r = 0.46, p < 0.001]) in response to winning money. How much participants worked for their preferred snack food during the progressive reinforcement task correlated with activation in the posterior cerebellar lobe (r = 0.41, p < 0.001) in response to anticipated milkshake receipt and with activation in the caudate (r = 0.44, p < 0.001) in response to milkshake receipt. Participants rated the milkshake (M = 14.3 ±2.5) as significantly more pleasant than the tasteless solution (M = 8.9 ±2.2; t (86) = 14.8, p < 0.001). Further, the BAS reward responsiveness subscale correlated significantly with activation in the dlPFC (r = 0.23, p = 0.03) in response to milkshake receipt and with activation in the ACC in response to winning money (r = 0.22, p = 0.04) and to anticipating winning money (r = 0.24, p = 0.03).
Figure 3.
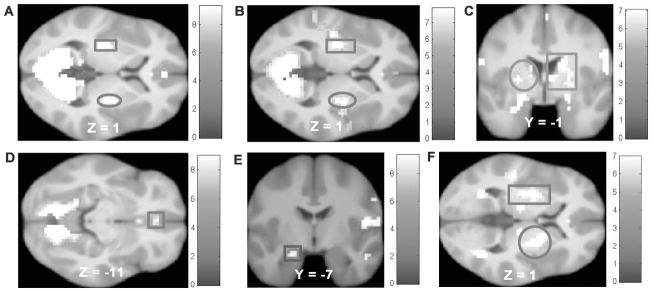
Activation in the parental-history-positive group in A) bilateral putamen (square MNI: −27, −16, 1, Z = 4.94, k = 34, circle MNI: 30, −16, 1, Z = 4.11, k = 77) in response to monetary receipt, B) bilateral putamen (square MNI: −27, −16, 1, Z = 6.43, k = 337, circle MNI: 30, −7, −2, Z = 5.58, k = 293) in response to anticipated monetary receipt, C) bilateral striatum (square MNI peak activation: 27, −10, −2, Z = 5.28, k = 124, circle MNI peak activation: −27, −19, 4, Z = 5.05, k = 62) in response to milkshake receipt. Activation in the parental-history-negative group in D) right medial orbitofrontal cortex (MNI: 0, 38, −11, Z = 3.88, k = 70) in response to monetary receipt, E) left amygdala (MNI: −21, −7, −20, Z = 3.87, k = 12) in response to anticipated monetary receipt, and F) bilateral putamen (square MNI: −24, −4, 4, Z = 4.56, k = 97, circle MNI: 27, −1, 4, Z = 4.58, k = 80) in response to milkshake receipt.
Relation between parental history of substance use disorders and neural responsivity to monetary and food reward
Parental-history-positive versus -negative adolescents exhibited greater activation in the left dlPFC (r = 0.63) and bilateral putamen (r = 0.45–0.57; Fig 4AB) and less activation in the fusiform gyrus (r = 0.58) and inferior temporal gyrus (ITG; r = 0.56) in response to anticipating winning money (Table 2). There were no significant group differences in response to monetary reward receipt or in response to anticipated milkshake receipt. Parental-history-positive versus -negative adolescents showed greater activation in the left midbrain (r = 0.55; Fig 5) and right paracentral lobule (r = 0.53) and less activation in the right MFG (r = 0.50) in response to milkshake receipt (Table 2).
Table 2.
Between-group comparisons for the parental-history-positive (n = 26) versus - negative groups (n = 26) for contrasts from the food reward and monetary reward paradigm
| Contrast and region | H | K | Z value | MNI coordinates | r |
|---|---|---|---|---|---|
| Winning cue > neutral cue | |||||
| Parental-history-positive > parental-history-negative | |||||
| Dorsolateral prefrontal cortex | L | 16 | 4.54 | −39, 14, 31 | 0.63 |
| Putamen | L | 59 | 4.12 | −27, −16, 4 | 0.57 |
| L | 3.97 | −30, −4, −5 | 0.55 | ||
| L | 3.84 | −30, −13, −5 | 0.53 | ||
| R | 20 | 3.83 | 30, −4, −11 | 0.53 | |
| R | 3.27 | 30, −7, 1 | 0.45 | ||
| Parental-history-negative > parental-history-positive | |||||
| Fusiform gyrus | R | 19 | 4.15 | 24, −67, −17 | 0.58 |
| Inferior temporal gyrus | L | 13 | 4.06 | −45, −73, −5 | 0.56 |
| Milkshake receipt > tasteless solution receipt | |||||
| Parental-history-positive > parental-history-negative | |||||
| Midbrain | L | 14 | 3.97 | −15, −22, −14 | 0.55 |
| Paracentral lobule | R | 12 | 3.79 | 0, −31, 58 | 0.53 |
| Parental-history-negative > parental-history-positive | |||||
| Middle frontal gyrus | R | 16 | 3.60 | 36, 47, 19 | 0.50 |
Note: H refers to hemisphere. K = cluster size.
Figure 5.
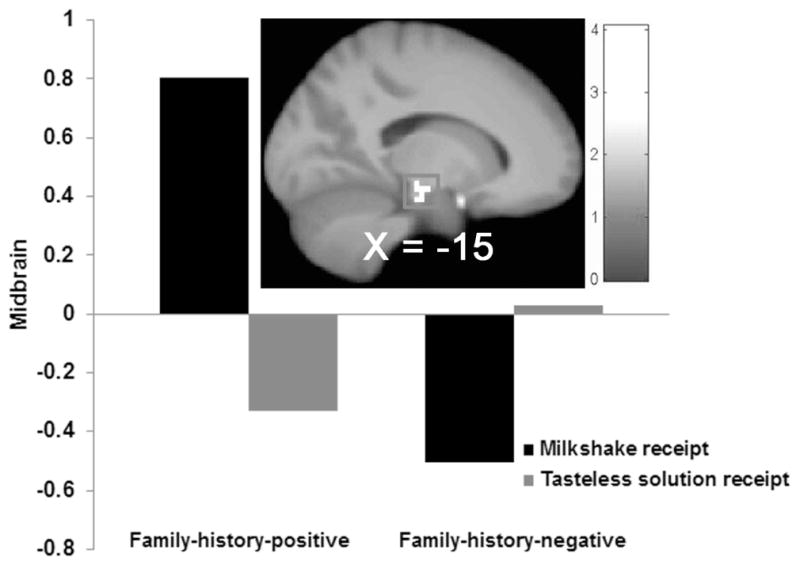
Parental-history-positive versus –negative adolescents exhibited greater activation in the left midbrain (MNI: −15, −22, −14, Z = 3.97, k = 14) in response to milkshake receipt.
Discussion
Adolescents with versus without a parental history of substance use disorders showed greater activation in regions implicated in reward processing (putamen and dlPFC) and less activation in regions implicated in visual attention (fusiform gyrus and ITG) in response to anticipated monetary receipt. The putamen responds to monetary reward (Knutson et al., 2000), appears to be involved in reward learning, and receives inputs from the basolateral amygdala, the hippocampus, and the prefrontal cortex (Everitt & Robbins, 2005). The dlPFC is involved in the representation and integration of goals and reward information (Watanabe & Sakagami, 2007), is directly activated by reward cues (Ballard et al., 2011) and modulates ventral tegmental area and nucleus accumbens activity in response to anticipation of reward (Ballard et al., 2011). The fusiform gyrus has been found to be hypoactive during successful craving resistance (Volkow et al., 2010) and inhibitory control (O’Connor et al., 2012) in response to reward cues. It is thought that hypoactivity in visual attention regions, such as the fusiform gyrus and ITG, is suggestive of attentional disengagement as an aid to withhold response over a rewarding stimulus (O’Conner et al., 2012). Our findings may therefore suggest that adolescents with versus without parental history of substance use disorders show greater reward processing but also greater attentional disengagement in response to anticipated monetary receipt.
Adolescents with versus without a parental history of substance use disorders also showed greater activation in reward (midbrain) and sensorimotor (paracentral lobule) regions and less activation in a region associated with cognitive functioning (MFG) in response to milkshake receipt. The midbrain is part of the cortical-basal ganglia circuit (Haber & Knutson, 2010) and encodes the subjective value of rewards regardless of their type (e.g., food, sex, monetary rewards; Kenny, 2011). The paracentral lobule is associated with sensorimotor functioning and is activated by swallowing (Furlong et al., 2004). Paracentral lobule activation has also been found to be positively correlated with self-reported craving in response to drug cue-reactivity paradigms (Kühn & Gallinat, 2011). The MFG is associated with working memory and cognitive control (Pessoa et al., 2002) and increased MFG activation has been associated with greater inhibitory control in response to food images (McCaffery et al., 2009). These results suggest that adolescents with versus without a parental history of substance use disorders experience greater reward and sensory processing and less inhibitory control in response to receipt of palatable food.
The present results appear to provide support for the reward surfeit model of substance use, which posits that youth at risk for substance use disorders show greater recruitment of reward regions in response to rewarding stimuli, as parental-history positive youth showed greater responsivity in the putamen, midbrain, and dlPFC. These results converge with the findings from Andrews et al., (2011), who found that family-history-positive versus -negative individuals exhibited greater caudate recruitment in response to the prospect of monetary reward. The present findings also converge with evidence that adolescents who exhibited stronger caudate and putamen response to monetary reward showed significantly greater substance use onset over 1-year follow-up (Stice et al., 2013).
In contrast to the present results, two studies found weaker NAcc recruitment in response to anticipated monetary reward (Andrews et al., 2011; Yau et al., 2012) and two other studies that found no difference in reward region responsivity for parental-history-positive versus -negative individuals (Bjork et al., 2008; Streeter et al., 1998). Likewise, although the present results indicated that parental-history-positive youth showed weaker activation of regions implicated in visual attention (fusiform gyrus and ITG) and cognitive control (MFG) in response to anticipated monetary reward, previous studies found evidence of greater activation for parental-history-positive individuals in response to images of alcohol in regions implicated in visual attention (cuneus), attention (ACC), and cognitive control (MFG; Tapert et al., 2003).
There are several factors that might have contributed to the mixed findings. First, there is differential sensitivity due to variation in samples sizes; the studies with the smallest samples did not detect any between-group differences (Bjork et al., 2008 [N=26]; Streeter et al, 1998 [N=20]), whereas the studies with larger samples detected significant between-group differences (Andrews et al., 2011 [N=49]; Yau et al., 2012 [N=40]; the present study [N=52]), though this does not seem to explain the fact that some effects are in opposite directions in striatal regions. Second, some studies examined responsivity to receipt and anticipated receipt of monetary reward (Andrews et al., 2011; Bjork et al., 2008; Yau et al., 2012; the present study), whereas others examined responsivity to images of alcohol (Tapert et al., 2003) and benzodiazepine intake (Streeter et al., 1998). Similarly, previous monetary reward paradigms required a motor response (Andrews et al., 2011; Bjork et al., 2008; Yau et al., 2012), whereas our monetary reward paradigm did not, which may have contributed to the mixed findings because responding to earn money recruits different brain regions and results in greater DA release than simply receiving the monetary reward (Hakyemez, Dagher, Smith, & Zald, 2008; Stice, Yokum, Burger, Epstein, & Smolen, 2012). The fact that our paradigms did not recruit the NAcc (Stice et al., 2012) might therefore explain the inconsistent findings regarding this region. Third, some studies excluded participants based on psychiatric disorders (Bjork et al., 2008; Tapert et al., 2008; Streeter et al., 1998; the present study), whereas others did not mention this exclusion criterion (Andrews et al., 2011; Yau et al., 2012). Fourth, whereas most studies only required an alcohol use disorder in one parent, Andrews et al. (2011) required alcoholism in at least one parent and in at least one first- or second-degree relative. Fifth, variation in age of participants across studies might have contributed to the inconsistent effects, as the participants in the Andrews et al. (2011) and Yau et al. (2012) studies were older than participants in the current study. Indeed, the lower NAcc recruitment in the Yau et al. (2012) study was interpreted as potentially representing a protective effect, as it only appeared in non-drinking children of alcoholics (who were 18–22 years old), who would have been expected to express problem drinking tendencies by this age if were at the greatest risk.
It might appear that a history of substance use contributed to the reduced NAcc responsivity for parental-history-positive versus -negative youth observed previously (Andrews et al., 2011; Yau et al., 2012), given that we only found evidence of greater reward region responsivity in our sample which was restricted to youth who reported no substance use over the past year. However, the fact that Yau et al. (2012) matched family-history-positive and -negative individuals on habitual substance use suggests that this is unlikely.
Also of note, previous studies have reported an increased preference for sweet tastes among individuals with alcoholism and/or a parental history of alcoholism (Kampov-Polevoy, Garbutt, & Janowsky, 1997; Kampov-Polevoy, Garbutt, & Kholitov, 2003; Krahn et al., 2006), although this relation was not observed in other studies (Kranzler, Sandstrom, & Van Kirk, 2001; Scinska et al., 2001). However, adolescents with versus without a parental history of substance use disorders in the present study did not differ on preference ratings for high-sugar or high-fat foods, on how hard they work to earn high-sugar/high-fat foods, or in terms of how palatable milkshake receipt activated the gustatory or oral somatosensory regions. Collectively these data imply that any relation between preferences for sweet tastes and parental substance use disorders is not robust, though the fact that we also included parents with a history of illicit substance use disorders might explain the null findings.
It is important to consider the limitations of the present study. First, although we did not enroll participants with significant developmental delays, we did not expressly screen for fetal alcohol syndrome or maternal drinking during pregnancy. Second, we did not assess whether parents had psychiatric disorders that are often comorbid with substance use disorders. Third, the procedures we used to assess parental substance use disorder history might have resulted in false negative diagnoses either because social desirability biases caused under-reporting or because some parents may yet develop substance use disorders in the future. Fourth, we did not collect data on inter-rater agreement or test-retest reliability for parental substance use disorder diagnoses. Finally, we did not assess lifetime history of substance use among the adolescent participants.
It might be fruitful for future research to test whether substance naïve adolescents with versus without parental history of substance use disorders show aberrant functional connectivity between brain regions, altered volume of reward regions, and aberrant white matter connectivity. One study found that substance naïve adolescents with versus without parental alcoholism showed significantly less functional connectivity of cerebellum regions (implicated in motor control) to the prefrontal cortex, cingulate gyrus, cuneus, putamen, and insula (implicated in executive control, memory, and reward processing; Herting, Fair, & Nagel, 2011), which may partially account for the reduced inhibitory control that characterizes youth with parental history of substance use disorders (Andrews et al., 2011).
In conclusion, the present results suggest that adolescents with versus without parental-history of a substance use disorder showed greater responsivity of reward regions, converging with prior evidence that family-history-positive versus negative individuals showed greater caudate responsivity to the prospect of monetary reward (Andrews et al., 2011) and with evidence that greater caudate and putamen responsivity to monetary reward increased risk for future substance use onset (Stice, Yokum, & Burger, 2013). Collectively, these results provide support for the reward surfeit model of substance use disorder vulnerability, which proposes that elevated responsivity of reward regions increases risk for escalation of substance use that causes negative consequences and dependence.
Figure 4.
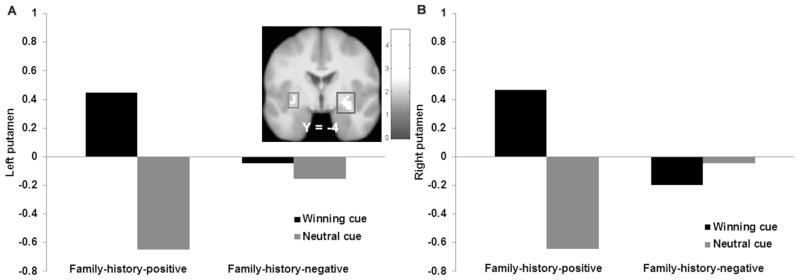
Parental-history-positive versus –negative adolescents exhibited greater activation in A) the left putamen (MNI: −27, −16, 4, Z = 4.12, k = 59) and B) the right putamen (MNI: 30, −4, −11, Z = 3.83, k = 20) in response to anticipated monetary receipt.
Acknowledgments
This project was supported by National Institute of Diabetes and Digestive and Kidney Diseases grant (R01 DK80760, 8/09-7/14). The authors thank the Lewis Center for Neuroimaging at the University of Oregon for their assistance in data collection for these projects.
Footnotes
Research conducted by Eric Stice and Sonja Yokum, Oregon Research Institute, Eugene Oregon.
References
- Ahmed S, Kenny P, Koob G, Markou A. Neurobiological evidence of hedonic allostasis associated with escalating cocaine use. Nature Neuroscience. 2002;5:625–626. doi: 10.1038/nn872. [DOI] [PubMed] [Google Scholar]
- Andrews MM, Meda SA, Thomas AD, Potenza MN, Krystal JH, Worhunsky P, Pearlson GD. Individuals family history positive for alcoholism show functional magnetic resonance imaging differences in reward sensitivity that are related to impulsivity factors. Biological Psychiatry. 2011;69:675–683. doi: 10.1016/j.biopsych.2010.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard IC, Murty VP, Carter RM, MacInnes JJ, Huettel SA, Adcock RA. Dorsolateral prefrontal cortex drives mesolimbic dopaminergic regions to initiate motivated behavior. Journal of Neuroscience. 2011;31:10340–10346. doi: 10.1523/JNEUROSCI.0895-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaver JD, Lawrence AD, van Ditzhuijzen J, Davis MH, Woods A, Calder AJ. Individual differences in reward drive predict neural response to images of food. Journal of Neuroscience. 2006;26:S160–S166. doi: 10.1523/JNEUROSCI.0350-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A, Schlagenhauf F, Wustenberg T, Hein J, Kienast T, Kahnt T, Wrase J. Ventral striatum activation during reward anticipation correlates with impulsivity in alcoholics. Biological Psychiatry. 2009;66:734–742. doi: 10.1016/j.biopsych.2009.04.035. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Hommer DW. Incentive-elicited striatal activation in adolescent children of alcoholics. Addiction. 2008;103:1308–1319. doi: 10.1111/j.1360-0443.2008.02250.x. [DOI] [PubMed] [Google Scholar]
- Carver C, White T. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS scales. Journal of Personality and Social Psychology. 1994;67:319–333. [Google Scholar]
- Chassin L, Rogosch F, Barrera M. Substance use and symptomatology among adolescent children of alcoholics. Journal of Abnormal Psychology. 1991;100:449–463. doi: 10.1037//0021-843x.100.4.449. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Davis C, Claridge G. The eating disorders as addiction: A psychobiological perspective. Addictive Behaviors. 1998;23:463–475. doi: 10.1016/s0306-4603(98)00009-4. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Liu X, Simms JA, Weiss F. Distinct patterns of neural activation associated with ethanol seeking: effects of naltrexone. Biological Psychiatry. 2007;61:979–989. doi: 10.1016/j.biopsych.2006.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G. Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behavioural Brain Research. 2002;137:74–114. doi: 10.1016/s0166-4328(02)00286-3. [DOI] [PubMed] [Google Scholar]
- Due DL, Huettel SA, Hall WG, Rubin DC. Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: Evidence from functional magnetic resonance imaging. American Journal of Psychiatry. 2002;159:954–960. doi: 10.1176/appi.ajp.159.6.954. [DOI] [PubMed] [Google Scholar]
- Endicott J, Andreasen N, Spitzer RL. Family History-Research Diagnostic Criteria (FH-RDC) Washington, DC: National Institute of Mental Health; 1978. [Google Scholar]
- Epstein LH, Truesdale R, Wojckik A, Paluch RA, Raynor HA. Effects of deprivation on hedonics and reinforcing value of food. Physiology & Behavior. 2003;78:221–227. doi: 10.1016/s0031-9384(02)00978-2. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Leddy JJ, Tample JL, Faith MS. Food reinforcement and eating: A multilevel analysis. Psychological Bulletin. 2007;133:884–906. doi: 10.1037/0033-2909.133.5.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nature Neuroscience. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I/NP) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Forman S, Cohen J, Fitzgerald M, Eddy W, Mintun M, Noll D. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Furlong PL, Hobson AR, Aziz Q, Barnes GR, Singh KD, Hillebrand A, Hamdy S. Dissociating the spatio-temporal characteristics of cortical neuronal activity associated with human volitional swallowing in the healthy adult brain. Neuroimage. 2004;22:1447–1455. doi: 10.1016/j.neuroimage.2004.02.041. [DOI] [PubMed] [Google Scholar]
- Goldfield GS, Legg C. Dietary restraint, anxiety, and the relative reinforcing value of snack food in non-obese women. Eating Behaviors. 2006;7:323–332. doi: 10.1016/j.eatbeh.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Tomasi D, Alia-Klein N, Cottone LA, Zhang L, Telang F, Volkow ND. Subjective sensitivity to monetary gradients is associated with frontolimbic activation to reward in cocaine abusers. Drug and Alcohol Dependence. 2007;87:233–240. doi: 10.1016/j.drugalcdep.2006.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant B, Stinson F, Dawson D, Chou P, Dufour M, Kaplan K. Prevalence and co-occurrence of substance use disorders and indepdendent mood and anxiety disorders: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Journal of the American Medical Association. 2004;61:807–816. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakyemez HS, Dagher A, Smith SD, Zald DH. Striatal dopamine transmission in healthy humans during a passive monetary reward task. Neuroimage. 2008;39:2058–2065. doi: 10.1016/j.neuroimage.2007.10.034. [DOI] [PubMed] [Google Scholar]
- Hardt J, Franke P. Validity, reliability and objectivity of the family history method in psychiatry: A meta-analysis. European Psychiatry. 2007;22:49–58. doi: 10.1016/j.eurpsy.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Herting M, Fair D, Nagel B. Altered fronto-cerebellar connectivity in alcohol-naïve youth with a family history of alcoholism. NeuroImage. 2011;54:2582–2589. doi: 10.1016/j.neuroimage.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampov-Polevoy A, Garbutt JC, Janowsky D. Evidence of preference for a high concentration sucrose solution in alcoholic men. American Journal of Psychiatry. 1997;154:269–270. doi: 10.1176/ajp.154.2.269. [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Garbutt JC, Khalitov E. Family history of alcoholism and response to sweets. Alcoholism, Clinical and Experimental Research. 2003;27:1743–1749. doi: 10.1097/01.ALC.0000093739.05809.DD. [DOI] [PubMed] [Google Scholar]
- Kenny P. Reward mechanisms in obesity: New insights and future directions. Neuron. 2011;69:664–679. doi: 10.1016/j.neuron.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny P, Chen S, Kitamura O, Markou A, Koob G. Conditioned withdrawal drives heroin consumption and decreases reward sensitivity. Journal of Neuroscience. 2006;26:5894–5900. doi: 10.1523/JNEUROSCI.0740-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Taylor J, Kaufman M, Peterson R, Glover G. Distributed neural representation of expected value. Journal of Neuroscience. 2005;25:4806–4812. doi: 10.1523/JNEUROSCI.0642-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Westdorp A, Kaiser E, Hommer D. FMRI visualizaitn of brain activity during a monetary incentive delay task. Neuroimage. 2000;12:20–27. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- Krahn D, Grossman J, Henk H, Mussey M, Crosby R, Gosnell B. Sweet intake, sweet-liking, urges to eat, and weight change: Relationship to alcohol dependence and abstinence. Addictive Behaviors. 2006;31:622–631. doi: 10.1016/j.addbeh.2005.05.056. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Sandstrom KA, Van Kirk J. Sweet taste as a risk factor for alcohol dependence. American Journal of Psychiatry. 2001;158:813–815. doi: 10.1176/appi.ajp.158.5.813. [DOI] [PubMed] [Google Scholar]
- Kühn S, Gallinat J. Common biology of craving across legal and illegal drugs – a quantitative meta-analysis of cue-reactivity brain response. European Journal of Neuroscience. 2011;33:1318–1326. doi: 10.1111/j.1460-9568.2010.07590.x. [DOI] [PubMed] [Google Scholar]
- Lubman D, Yücel M, Kettle J, Scaffidi A, MacKenzie T, Simmons J, Allen N. Responsiveness to drug cues and natural rewards in opiate addiction. Archives of General Psychiatry. 2009;66:205–213. doi: 10.1001/archgenpsychiatry.2008.522. [DOI] [PubMed] [Google Scholar]
- Maas LC, Lukas SE, Kaufman MJ, Weiss RD, Daniels SL, Rogers VW, Kukes TJ, Renshaw F. Functional magnetic resonance imaging of human brain activation during cue-induced cocaine craving. American Journal of Psychiatry. 1998;155:124–126. doi: 10.1176/ajp.155.1.124. [DOI] [PubMed] [Google Scholar]
- Martinez D, Narendran R, Foltin R, Slifstein M, Hwang D, Broft A, Laruelle M. Amphetamine-induced dopamine release: Markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. Amercian Journal of Psychiatry. 2007;164:622–629. doi: 10.1176/ajp.2007.164.4.622. [DOI] [PubMed] [Google Scholar]
- McCaffery JM, Haley AP, Sweet LH, Phelan S, Raynor HA, Del Parigi A, Wing RR. Differential functional magnetic resonance imaging response to food pictures in successful weight-loss maintainers relative to normal-weight and obese controls. American Journal of Clinical Nutrition. 2009;90:928–934. doi: 10.3945/ajcn.2009.27924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, Nader MA. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nature Neuroscience. 2002;5:169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Drobes D, Voronin K, George MS. Differential brain activity in alcoholics and social drinkers to alcohol cues: relationship to craving. Neuropsychopharmacology. 2004;29:393–402. doi: 10.1038/sj.npp.1300295. [DOI] [PubMed] [Google Scholar]
- Nader MA, Morgan D, Gage HD, Nader SH, Calhoun TL, Buchheimer N, Mach RH. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nature Neuroscience. 2006;9:1050–1056. doi: 10.1038/nn1737. [DOI] [PubMed] [Google Scholar]
- O’Brian C, Volkow N, Li T. What’s in a word? Addiction vs dependence in DSM-V. American Journal of Psychiatry. 2006;163:764–765. doi: 10.1176/ajp.2006.163.5.764. [DOI] [PubMed] [Google Scholar]
- O’Conner DA, Rossiter S, Yücel M, Lubman DI, Hester R. Successful inhibitory control over an immediate reward is associated with attentional disengagement in visual processing areas. Neuroimage. 2012;62:1841–1847. doi: 10.1016/j.neuroimage.2012.05.040. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Gutierrez E, Bandettini P, Ungerleider L. Neural correlates of visual working memory: fMRI amplitude predicts task performance. Neuron. 2002;35:975–987. doi: 10.1016/s0896-6273(02)00817-6. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D, Smith HR, Daunais JB, Nader MA. Cocaine self administration produces a progressive involvement of limbic, association, and sensorimotor striatal domains. Journal of Neuroscience. 2004;24:3554–3562. doi: 10.1523/JNEUROSCI.5578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott CA, Sullivan PF, Myers JM, Patterson DG, Halberstadt LJ, Walsh D, Kendler KS. The Irish Affected Sib Pair Study of Alcohol Dependence: Study methodology and validation of diagnosis by interview and family history. Alcoholism, Clinical and Experimental Research. 2005;29:417–429. doi: 10.1097/01.alc.0000156085.50418.07. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Rohde P, Lewinsohn PM, Seeley JR, Klein DN, Andrews JA, Small J. Psychosocial functioning of adults who experienced substance use disorders as adolescents. Psychology of Addictive Behaviors. 2007;21:155–164. doi: 10.1037/0893-164X.21.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scinska A, Bogucka-Bonikowska A, Koros E, Polanowska E, Habrat B, Kukwa A, Bienkowski P. Taste responses in sons of male alcoholics. Alcohol and Alcoholism. 2001;36:79–84. doi: 10.1093/alcalc/36.1.79. [DOI] [PubMed] [Google Scholar]
- Stice E, Barrera M, Chassin L. Prospective differential prediction of adolescent alcohol use and problem use: Examining mechanisms of effect. Journal of Abnormal Psychology. 1998;107:616–628. doi: 10.1037//0021-843x.107.4.616. [DOI] [PubMed] [Google Scholar]
- Stice E, Spoor S, Bohon C, Veldhuizen M, Small D. Relation of reward from food intake and anticipated intake to obesity: A functional magnetic resonance imaging study. Journal of Abnormal Psychology. 2008;117:924–935. doi: 10.1037/a0013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Yokum S, Burger KS. Elevated reward region responsivity predicts future substance use onset but not overweight/obesity onset. Biological Psychiatry. 2013;73:869–876. doi: 10.1016/j.biopsych.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Yokum S, Burger KS, Epstein LH, Small DM. Youth at risk for obesity show greater activation of striatal and somatosensory regions to food. Journal of Neuroscience. 2011;31:4360–4366. doi: 10.1523/JNEUROSCI.6604-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Yokum S, Burger K, Epstein L, Smolen A. Multilocus genetic composite reflecting dopamine signaling capacity predicts reward circuitry responsivity. Journal of Neuroscience. 2012;32:10093–10100. doi: 10.1523/JNEUROSCI.1506-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter CC, Ciraulo DA, Harris GJ, Kaufman MJ, Lewis RF, Knapp CM, Renshaw PF. Functional magnetic resonance imaging of alprazolam-induced changes in humans with familial alcoholism. Psychiatry Research. 1998;82:69–82. doi: 10.1016/s0925-4927(98)00009-2. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Cheung EH, Brown GG, Frank LR, Paulus MP, Schweinsburg AD, Brown SA. Neural response to alcohol stimuli in adolescents with alcohol use disorder. Archives of General Psychiatry. 2003;60:727–735. doi: 10.1001/archpsyc.60.7.727. [DOI] [PubMed] [Google Scholar]
- Vandeleur CL, Rothen S, Jeanpretre N, Lustenberger Y, Gamma F, Ayer E, Preisig M. Inter-informant agreement and prevalence estimates for substance use disorders: Direct interview versus family history method. Drug and Alcohol Dependence. 2008;92:9–19. doi: 10.1016/j.drugalcdep.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, Miller EN. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. American Journal of Psychiatry. 2001;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. Role of dopamine in drug reinforcement and addiction in humans: results from imaging studies. Behavioural Pharmacology. 2002;13:355–366. doi: 10.1097/00008877-200209000-00008. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Telang F, Logan J, Jayne M, Swanson JM. Cognitive control of drug craving inhibits brain reward regions in cocaine abusers. Neuroimage. 2010;49:2536–2543. doi: 10.1016/j.neuroimage.2009.10.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang G, Fowler JS, Logan J. Effects of methylphenidate on regional brain glucose metabolism in humans: Relationship to dopamine D2 receptors. American Journal of Psychiatry. 1997;154:50–55. doi: 10.1176/ajp.154.1.50. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. Journal of Neuroscience. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Wong C. Dopamine increases in striatum do not elicit craving in cocaine abusers unless they are coupled with cocaine cues. Neuroimage. 2008;39:1266–1273. doi: 10.1016/j.neuroimage.2007.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Fowler JS, Logan J, Abumrad NN, Hitzemann RJ, Pascani K. Dopamine D2 receptor availability in opiate-dependent subjects before and after naloxone-precipitated withdrawal. Neuropsychopharmacology. 1997;16:174–182. doi: 10.1016/S0893-133X(96)00184-4. [DOI] [PubMed] [Google Scholar]
- Wrase J, Schlagenhauf F, Kienast T, Wustenberg T, Bermpohl F, Kahnt T, Heinz A. Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. Neuroimage. 2007;35:787–794. doi: 10.1016/j.neuroimage.2006.11.043. [DOI] [PubMed] [Google Scholar]
- Yau W, Zubieta J, Weiland B, Samudra P, Zucker R, Heitzeg M. Nucleus accumbens response to incentive stimuli anticipation in children of alcoholics: Relationships with precursive behavioral risk and lifetime alcohol use. The Journal of Neuroscience. 2012;32:2544–2551. doi: 10.1523/JNEUROSCI.1390-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman M, Corvell W, Pfohl B, Stangl D. The reliability of the family history method for psychiatric diagnoses. Archives of General Psychiatry. 1988;45:320–322. doi: 10.1001/archpsyc.1988.01800280030004. [DOI] [PubMed] [Google Scholar]


