Abstract
Exogenous ciliary neurotrophic factor (CNTF) promotes motor neuron (MN) survival following trauma and in genetic models of MN disease. Unconditional disruption of the mouse CNTF receptor α (CNTFRα) gene leads to MN loss, demonstrating a developmental role for endogenous CNTF receptor signaling. These data also suggest that CNTF receptors may promote adult MN survival and that appropriately manipulating the receptors could effectively treat adult MN disorders. This effort would greatly benefit from a better understanding of the roles played by CNTF receptors in adult MNs. We have previously found that adult onset disruption of CNTFRα in facial MNs of “floxed CNTFRα” mice by AAV-Cre vector injection leads to significantly more MN loss than in identically treated controls. While indicating that CNTF receptors can promote adult MN survival, the data did not distinguish between potential roles in MN maintenance versus roles in protecting MNs from the injection associated trauma or the toxicity of the chronic Cre recombinase (Cre) produced by the AAV-Cre. Here we used an inducible Cre gene construct to produce adult-onset CNTFRα disruption in facial MNs without the traumatic and toxic effects of the AAV-Cre procedure. The MNs survive without CNTFRα, even when challenged by facial nerve crush or the injection-associated trauma, thereby suggesting, in conjunction with our previous study, that endogenous CNTF receptor signaling can protect MNs against toxic insult, such as that produced by chronic Cre. The data also indicate that in vivo CNTF receptors play very different roles in adult and embryonic MNs.
INDEXING TERMS: CNTF receptor α, facial motor neurons, Cre recombinase, mouse, inducible Cre
Ciliary neurotrophic factor receptor α (CNTFRα) is an essential ligand binding subunit of the CNTF receptor, which is composed of CNTFRα, leukemia inhibitory factor receptor β (LIFRβ), and gp130 (Ip et al., 1992; Davis et al., 1993). While LIFRβ and gp130 are found in other related receptors, CNTFRα is unique to CNTF receptors and is required for all known forms of CNTF receptor signaling, regardless of the ligands or signaling pathways involved (Davis et al., 1993; Elson et al., 2000; Derouet et al., 2004).
CNTF is one of the most potent neuroprotective factors for embryonic motor neurons (MNs) in vitro (Lindsay et al., 1994). In vivo, exogenously administered CNTF protects MNs following early postnatal axotomy (Sendtner et al., 1990). It also protects MNs in genetic models of MN disease (Sendtner et al., 1992; Ikeda et al., 1995; Sagot et al., 1995), and during developmental naturally occurring MN death (Oppenheim et al., 1991). These data suggest that endogenous CNTF receptor signaling may help maintain MNs during development and in adulthood. Moreover, the data suggest that such an endogenous system may also protect MNs from the effects of insults in adulthood including trauma and MN diseases. If so, manipulating this signaling may be a valuable tool in the treatment of these conditions.
While the above results obtained through exogenous CNTF administration are suggestive, manipulation of endogenous CNTF receptors in vivo is required to directly determine the role or roles played by endogenous CNTF receptor signaling. Unconditional disruption of the CNTFRα gene in mice leads to uniform death within 24 hours of birth (DeChiara et al., 1995) accompanied by a 30%–50% reduction in MNs, indicating that endogenous CNTF receptor signaling is essential for embryonic MN survival/development. However, this finding may reflect an embryonic function of CNTF receptors that is not required in the adult. To address this issue we have previously used Cre/loxP technology to produce an adult-onset CNTFRα gene disruption in MNs and thereby examine the role of CNTF receptors in the adult, independent of any contributions the receptors make to MN survival during development (Lee et al., 2008). Adult facial MNs in “floxed CNTFRα” mice were infected with an AAV vector driving Cre recombinase (Cre) expression. We found that almost all MNs that lose CNTFRα die within 4 months. Therefore, CNTF receptor signaling can play an essential role in adult MN survival. However, we also found that the AAV-Cre infection, which chronically produces Cre in infected cells, can, at high concentration, lead to some MN death in control mice, albeit significantly less than that observed in the floxed mice. These results are consistent with previous reports of cellular toxicity associated with long-term exposure to Cre activity (e.g., Loonstra et al., 2001; Kaspar et al., 2002) and they raise the possibility that endogenous CNTF receptor signaling may promote the survival of adult MNs challenged by this type of toxic insult. Similarly, the AAV-Cre injection of the facial nucleus necessarily involved neurotrauma that may have also challenged the MNs such that CNTF receptors were required for their survival. Therefore, while the study demonstrated that adult CNTF receptor signaling can promote MN survival, it did not distinguish between the possibility that the signaling is required to maintain adult MNs even in the absence of insult, as seen during development, and the possibility that the MN survival role for CNTF receptor signaling is restricted to situations in which the MNs are challenged by insult such as the traumatic penetrating injury and the toxic chronic Cre associated with the AAV-Cre. This previous study also did not examine the role of the receptors in the context of other forms of MN insult.
The present studies were designed to address these questions. We utilized an inducible Cre gene construct and the floxed CNTFRα mice to produce an adult-onset disruption of the CNTFRα gene in facial MNs without the neurotrauma and chronic Cre expression seen with the AAV-Cre approach. We also separately challenged these MNs with the AAV-Cre associated penetrating injury and facial nerve crush.
MATERIALS AND METHODS
Mouse lines and general design
The generation and characterization of the floxed CNTFRα (flxCNTFRα) mice has been previously described (Lee et al., 2008). Briefly, exons 3–5 of the CNTFRα gene (“exon1” containing start codon) were flanked by loxP sites (“floxed”) using previously described methods (Wattler et al., 1999). Cre-induced removal of the floxed CNTFRα sequence was designed to functionally inactivate the CNTFRα gene, based on both the previous work with an unconditional CNTFRα knockout mouse (DeChiara et al., 1995) and known structure-function relationships for related cytokine receptors (Bazan, 1990). Many lines of evidence all indicate that Cre activity in the floxed CNTFRα mice leads to functional inactivation of the CNTFRα gene, as predicted. Crossing the floxed mice with a “deleter” line that produces universal, Cre-dependent floxed gene excision resulted in the designed excision of the CNTFRα gene, as revealed by Southern blot analysis (Lee et al., 2008). This cross also lead to the expected perinatal death of all homozygous floxed mice, but not heterozygous or wildtype littermates (Lee et al., 2008), all as independently seen with universal CNTFRα gene disruption (DeChiara et al., 1995). Moreover, we observed the expected loss of facial MNs specific to the homozygous floxed mice (Lee et al., 2008), again as seen with universal CNTFRα gene disruption (DeChiara et al., 1995). As noted above, Cre expression in adult MNs of the floxed CNTFRα mice through infection with an AAV-Cre vector leads to the loss of MN CNTFRα and the subsequent increase in MN death over wildtype controls infected with the same AAV-Cre (Lee et al., 2008). Finally, we have also used the floxed CNTFRα mice to specifically deplete CNTFRα in neural stem cells (Lee et al., 2013a) and skeletal muscle (Lee et al., 2013b).
The Thy1-stop-YFP line 15 reporter mice (referred to here as “YFP reporter”) have also been described (Buffelli et al., 2003) and were a generous gift from Drs. Joshua Sanes (Harvard) and Robert Brownstone (Dalhousie University). The CreER inducible Cre mice (Guo et al., 2002) were purchased from Dr. Corrinne Lobe (Sunnybrook and Women’s College, Toronto). All mice (male and female) were backcrossed at least five generations onto a 129SvEvBrd background and genotyped by polymerase chain reaction (PCR) analysis of tail biopsy DNA.
The flxCNTFRα+/+ and flxCNTFRα−/− mice were generated by flxCNTFRα+/− × flxCNTFRα+/− breeding. The other gene constructs were bred as heterozygote × wildtype to control for gene dosage. CreER+/flxCNTFRα−/− mice (i.e., wildtype at the CNTFRα locus) served as primary controls, but were not distinguishable from CreER+/flxCNTFRα+/− mice. For all experiments, age-matched (generally littermate) CreER+/flxCNTFRα+/+ mice and control mice were processed in parallel through the complete procedure from tamoxifen injection through surgery, perfusion, immunohistochemistry/histology, and image analysis by individuals kept blind to genotype. This approach controlled for the many potential sources of variability including in utero and postnatal rearing environments, variation in genetic background, age, and immunohistochemistry/histology reagents. Statistical analysis consisted of appropriate Student’s t-tests or 2-way analysis of variance (ANOVA) analysis. Animal procedures were approved by the University of Cincinnati IACUC committee.
Anatomical procedures
Mice were overdosed with avertin (20 mg/ml; intraperitoneally) and perfused with saline followed by 4% paraformaldehyde. Brains were postfixed in the same solution overnight at 4°C and cryoprotected in 30% sucrose with 2.5 mM sodium azide for at least 48 hours before sectioning. Thirty-micron coronal, cryostat sections were stained with either cresyl violet, standard Xgal histology, or previously described immunohistochemistry procedures (MacLennan et al., 1996). Antibodies (Table 1) recognizing CNTFRα (R&D Systems, Minneapolis, MN) and green fluorescent protein (GFP; Millipore, Bedford, MA; the GFP antibody recognizes YFP which is a very similar protein) were visualized through either ABC amplification (Vector Laboratories, Burlingame, CA) and cyanine-3 tyramide (Perkin Elmer, Boston, MA), or AlexaFluor-conjugated secondaries (Invitrogen, La Jolla, CA).
TABLE 1.
Primary Antibodies
| Antibody | Host isotype | lmmunogen | Source | Cat. no. | Lot no. |
|---|---|---|---|---|---|
| CNTFRα | Goat IgG | Purified full-length recombinant rat CNTFRα | R&D Systems | AF-559-NA | AlN02 |
| GFP | Chicken Ig | Purified full-length recombinant GFP | Millipore | AB16901 | JBC1778223 |
Antibody characterization
The GFP antibody: 1) recognizes GFP by western blot and immunocytochemistry in tissue culture cells transfected with plasmids driving GFP expression but not those transfected with empty vector (Millipore); 2) immunohistochemically recognizes the expected pattern of brain cells expressing Cre in GFP reporter mice but not in reporter negative mice (Xu et al., 2008); and 3) recognizes MNs specifically following tamoxifen induction of Cre in a YFP reporter (this study).
The CNTFRα antibody: 1) inhibits CNTFRα function in tissue culture (R&D Systems); 2) recognizes CNTFRα by western blot in tissue culture cells transfected with CNTFRα DNA but not in untransfected control cells (Larsen et al., 2010); 3) immunohistochemically labels neurons in brain sections and the labeling is blocked by preincubation with recombinant CNTFRα (Watt et al., 2009); and 4) labels neural stem/progenitor cells in Cre expressing wildtype mice but not in Cre expressing floxed CNTFRα mice in which the CNTFRα gene has been disrupted in the stem/neural progenitor cells (Lee et al., unpubl.).
Microscopy
Images were captured with a 12 megapixel DXM1200 camera and Nikon E800 microscope with a 10× (NA = 0.45) lens. Image brightness and contrast were adjusted with Coral Draw software. Images that were compared were identically captured and adjusted.
Tamoxifen administration
The 3–4-month-old mice were injected intraperitoneally with 200 mg/kg tamoxifen (Sigma, St. Louis, MO; cat. no. T5648) made up as a 20-mg/ml solution in corn oil (Sigma, cat. no. C8267) or an equal volume of vehicle.
Facial nerve crush
Mice were anesthetized with ketamine/xylazine (100 mg/kg). Unilateral facial nerve crush lesions were conducted according to a previously published protocol (Kuzis et al., 1999). The skin and muscle were opened to expose the facial nerve by the ear. The nerve was then freed from fascia and crushed for 10 seconds with Dumont #5 Biologic Tip forceps (Fine Science Tools).
Penetrating brain injury
Mice were anesthetized with ketamine/xylazine (100 mg/kg) and received a unilateral, stereotaxic facial nuclei injection (coordinates from Bregma: AP −5.5, ML ± 1.1, DV −5.7) with a 33G Hamilton needle (Reno, NV). They received 1.5 µl of phosphate-buffered saline (PBS) at 0.25 µl/min (with 1 minute between each 0.25 µl injected and a 10-minute wait before removal). This is the same procedure used previously as the control for AAV-Cre injections (Lee et al., 2008).
Motor neuron quantification
Thirty-micron coronal, cryostat sections were collected throughout the complete rostral caudal extent of the facial motor nucleus. Facial MNs were counted in every fourth section. To correct for cells potentially split in the Z dimension, all neurons in focus at the top border of the sections were excluded (optical disector; Hyman et al., 1998; Hatton and Von Bartheld, 1999). Counts were multiplied by 4 to estimate total MNs (“fractionator”). As with all processing and analysis, MN counting was conducted by individuals unaware of experimental conditions, including genotype.
RESULTS
As described in the Materials and Methods section, the floxed CNTFRα mouse line used here has been extensively characterized. Several lines of evidence all indicate that Cre activity in the floxed CNTFRα mice leads to functional inactivation of the CNTFRα gene.
To excise the floxed CNTFRα gene in adult facial MNs without the neurotrauma and chronic Cre expression produced by AAV-Cre, we utilized a gene construct enabling temporary induction of Cre activity in the brain without neurotrauma (Guo et al., 2002). This construct drives the expression of a fusion protein consisting of Cre linked to an estrogen receptor ligand binding domain which has been mutated so that it cannot respond to endogenous ligands. This “CreER” fusion protein, which is widely used in many different mouse lines, is retained in the cytoplasm, where it is unable to act on DNA until binding to a synthetic ligand (tamoxifen) allows it to temporarily enter the nucleus and excise floxed gene sequences (Feil et al., 1996; Brocard et al., 1997; Danielian et al., 1998; Imai et al., 2001; Metzger and Chambon, 2001; Hayashi and McMahon, 2002). Many different CreER mouse lines have been generated leading to induction of Cre in different sets of cell types. The particular CreER mouse line used here (Guo et al., 2002) expresses this fusion protein such that tamoxifen treatment leads to temporally controlled Cre activity in a variety of cell types (Guo et al., 2002). The line was specifically designed to also eliminate any uninduced “leakage” of Cre activity prior to tamoxifen induction (our results below and Guo et al., 2002), which is sometimes seen with other CreER lines.
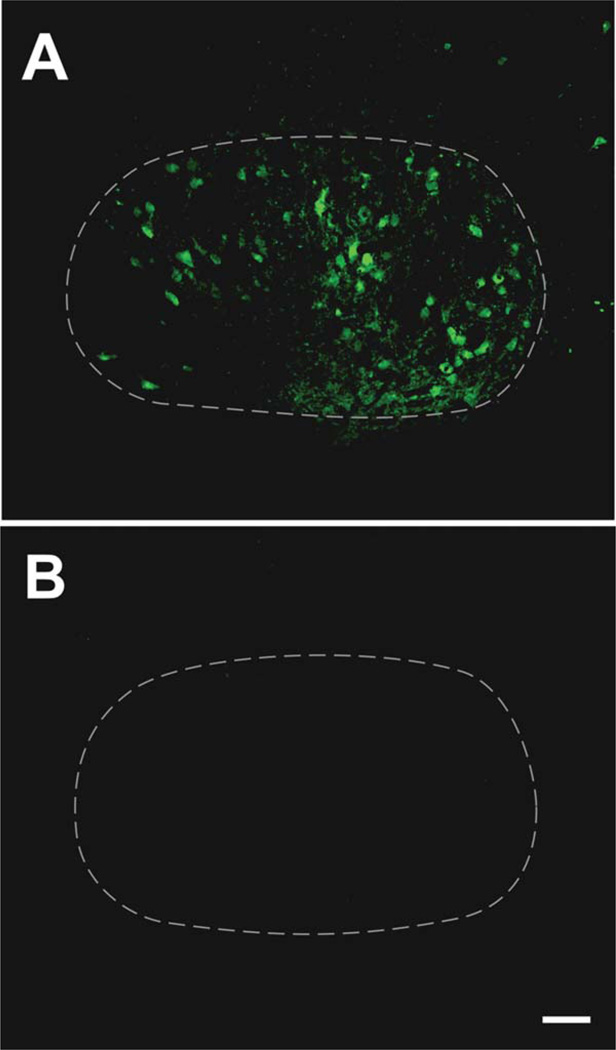
Previous studies have not determined whether Cre induction with this cell line (referred to here as simply CreER) leads to inducible floxed gene excision in adult facial MNs, as required for our study. In order to address this issue we crossed the CreER construct into a YFP reporter line (Thy1-stop-YFP line 15; Buffelli et al., 2003). The 3–4-month-old mice were injected with tamoxifen. We detected reporter signal in roughly half of the facial MNs 4 months later (Fig. 1A). The cells displayed a range of labeling intensity from strong to barely detectable, making it difficult to precisely quantify the number of positive cells (Fig. 1A). The reporter signal was not observed in age-matched vehicle-injected controls (Fig. 1B). Therefore, the data indicate that the CreER construct can be used to produce an adult onset excision of floxed genes in roughly half of facial MNs.
Figure 1.
The CreER gene construct enables inducible excision of floxed gene sequence in adult facial motor neurons. Immunohistochemical localization of YFP reporter signal in the facial motor nucleus of an adult CreER+/YFP reporter+ mouse injected with tamoxifen 4 months prior to perfusion (A) and an age-matched, vehicle-injected CreER+/YFP reporter+ control (B). The YFP signal, indicating excision of floxed gene sequence, is found in roughly half of facial motor neurons following the induction of Cre activity, with the cells displaying a range of labeling intensity (A). The reporter signal was not observed in vehicle-injected controls (B). Images in (A,B) were identically captured and adjusted. The broken white line delineates the approximate borders of the facial motor nucleus. Scale bar = 100 µm.
To excise the CNTFRα gene in adult facial MNs we crossed the CreER construct into the floxed CNTFRα mice and bred heterozygous floxed mice to produce homozygous floxed mice (referred to here as floxed mice) and wildtype littermate controls, all of which also contained the CreER construct.
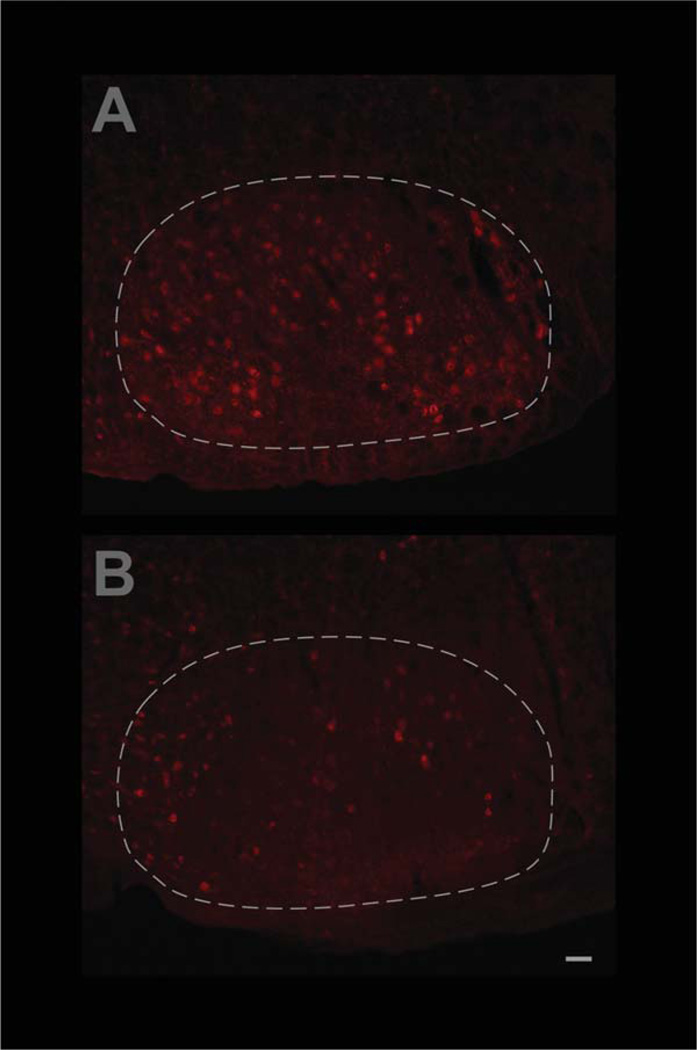
To confirm that the induced Cre activity in the facial MNs disrupts the flxCNTFRα gene as designed, control and floxed mice, all containing the CreER construct, were injected with tamoxifen at 3–4 months of age and their facial motor nuclei were examined by CNTFRα immunohistochemistry 3 months later. The floxed mice displayed only 55.9 ± 25.8% as many CNTFRα-labeled facial MNs as the littermate controls processed in parallel (Fig. 2; P < 0.05; t = 3.41; n = 4 pairs). Therefore, as with all the many other uses of this floxed line (see Materials and Methods) the Cre activity led to the expected effect on the floxed CNTFRα gene. Moreover, the data indicate that CNTFRα is depleted in the MNs by 3 months post-tamoxifen injection (i.e., well before they were challenged by insult in the below experiments).
Figure 2.
Confirmation that adult-onset CNTFRα gene excision in facial motor neurons leads to the expected loss of CNTFRα. CNTFRα-floxed mice and littermate controls, all containing the inducible Cre gene construct, CreER, were simultaneously injected with tamoxifen at 3–4 months of age and their facial motor nuclei were examined by CNTFRα immunohistochemistry 3 months later. Examples of sections from control (A) and CNTFRα-floxed (B) mice are presented. Quantification of all the data by investigators blind to genotype indicated that the floxed mice displayed only 55.9 ± 25.8% as many CNTFRα-labeled facial motor neurons as the littermate controls processed in parallel (P < 0.05; t = 3.41; n = 4 pairs). Images in (A,B) were identically captured and adjusted. The broken white line delineates the approximate borders of the facial motor nucleus. Scale bar = 50 µm.
Given the great deal of resources and time required to generate the experimental mice with a properly controlled breeding strategy (see Materials and Methods; on average only 1 in 8 mice produced is homozygous floxed and CreER+), and the approximately 1-year time frame of the experiments, rather than conducting the first experiment with na00EF;ve mice, a nerve crush challenge was incorporated into the first experiment in order to simultaneously examine the potential roles played by CNTF receptor signaling in: 1) maintaining intact MNs, and 2) promoting MN survival following nerve crush. The mice were injected with tamoxifen at 3–4 months of age and received a unilateral facial nerve crush 4 months later. Four months after that they were perfused and their facial motor nuclei sectioned to determine the number of facial MNs ipsilateral and contralateral to the lesion.
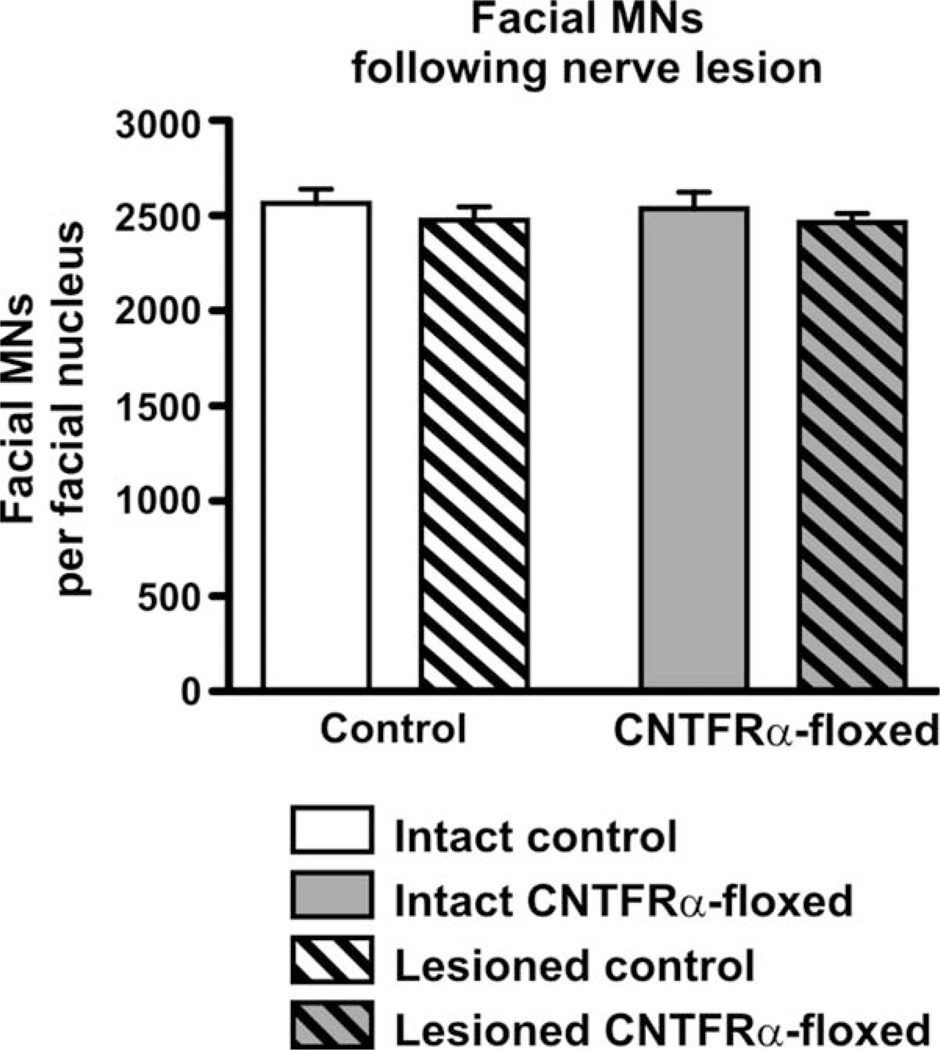
Quantification of the MNs indicated that the nerve crush had no effect on the number of MNs (Fig. 3; P > 0.05; F = 1.26; 2-way ANOVA; df = 1/36). This result was expected given that facial nerve crush in the adult has been previously shown to have no effect on MN survival in both rats and mice, in contrast to the dramatic effects it has within a week of birth (Gilad et al., 1996; Kuzis et al., 1999). Most important, the floxed mice contained the same number of MNs as the controls (Fig. 3; P > 0.05; F = 0.07; 2-way ANOVA; df = 1/36). There was also no lesion × genotype interaction, indicating that the effect of the lesion (i.e., no loss of MNs) was not changed by the disruption of CNTFRα (Fig. 3; P > 0.05; F = 0.01; 2-way ANOVA; df = 1/36). Therefore, CNTFRα is not essential for the survival of the adult MNs even if they are challenged with the nerve crush procedure.
Figure 3.
Adult-onset CNTFRα gene excision in facial motor neurons does not lead to loss of intact or axotomized motor neurons. CNTFRα-floxed mice and control mice were injected with tamoxifen at 3–4 months of age to induce Cre activity and received a unilateral facial nerve crush 4 months later. Four months after that they were perfused and the number of total (cresyl violet-stained) facial motor neurons ipsilateral and contralateral to the lesion were quantified. Two-way ANOVA analysis indicated there was no effect of lesion or CNTFRα gene excision and no interaction (P > 0.05 for all statistical comparisons). N = 9 CNTFRα-floxed mice; 11 control mice. Values are presented as mean ± SEM.
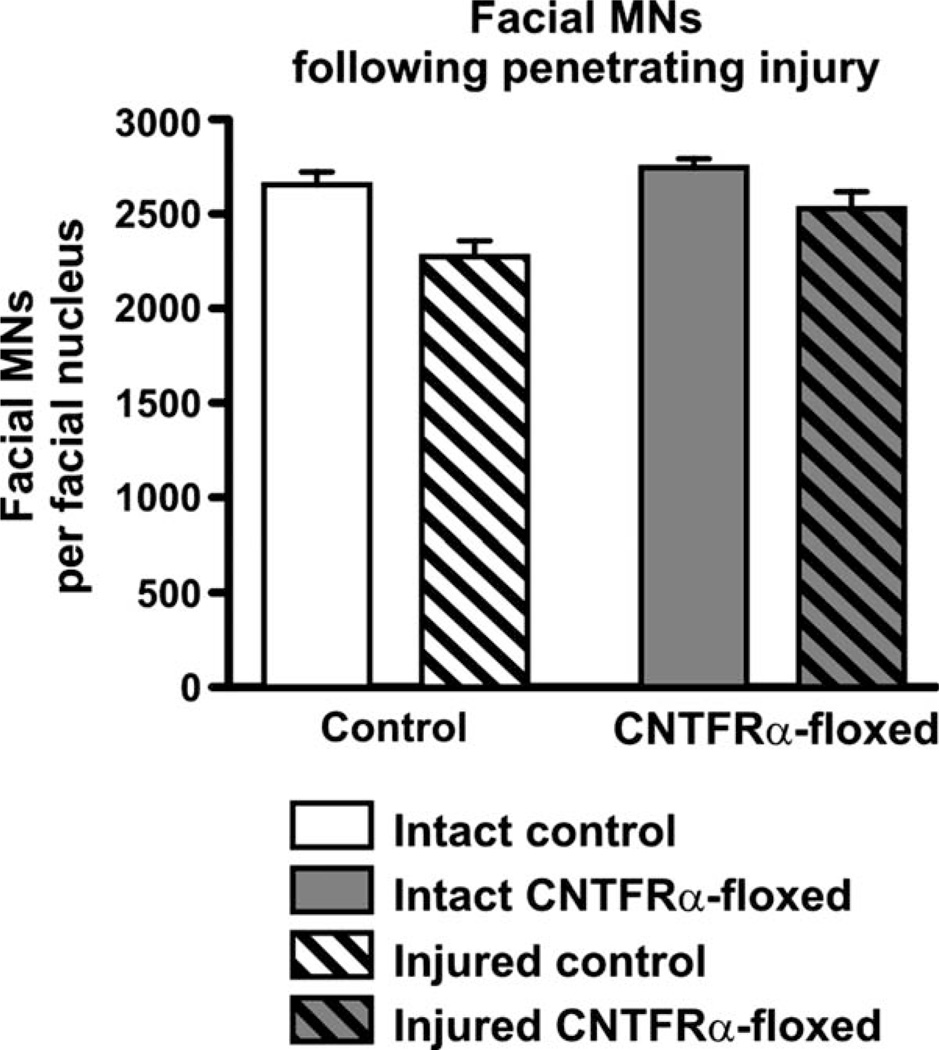
As noted in the introduction, AAV-Cre injected floxed CNTFRα mice in our previous study lost significantly more facial MNs than AAV-Cre injected controls (Lee et al., 2008). One possible explanation for this result is that CNTF receptor signaling protects the MNs from the immediate or longer-term effects of the neurotrauma associated with the injection procedure. Therefore, we next conducted an experiment analogous to the first one in which, instead of nerve crush, the mice received a unilateral penetrating injury of the facial motor nucleus using a procedure identical to that used to inject AAV-Cre in our previous study. The mice received an injection of vehicle rather than AAV-Cre to further control for any potential trauma resulting from the introduction of the fluid. Quantification of facial MNs 4 months later indicated that the injury led to an overall decrease in the number of MNs (Fig. 4). This effect was small in magnitude and presumably resulted from those MNs directly impacted by the needle. Regardless, it was highly significant (F = 16.1; P < 0.0005; 2-way ANOVA; df = 1/26), thereby demonstrating how sensitive the overall procedure was at detecting even small changes in the number of MNs. However, the injury × genotype interaction term was far from significant (Fig. 4; F = 1.2; P > 0.05 [P = 0.29]; 2-way ANOVA; df = 1/26), indicating that the effect of the lesion was not changed by the depletion of CNTFRα. Therefore, the data clearly demonstrate that the disruption of the CNTFRα gene did not increase MN death, in contrast to what would be expected if CNTFRα played an essential role in protecting the MNs following the injury. Finally, the floxed mice contained slightly more MNs overall, with the difference reaching statistical significance (F = 5.4; P < 0.05; 2-way ANOVA; df = 1/26), but this difference is not reliable given that no such difference was observed in the first experiment (Fig. 3), which utilized even more mice and should have detected the same effect if it was real.
Figure 4.
Adult-onset CNTFRα gene excision in facial motor neurons does not increase loss of motor neurons following penetrating injury of the facial motor nucleus. CNTFRα-floxed mice and control mice were injected with tamoxifen at 3–4 months of age to induce Cre activity and received a unilateral penetrating injury of the facial motor nucleus 4 months later. Four months after that they were perfused and the number of total (cresyl violet-stained) facial motor neurons ipsilateral and contralateral to the injury were quantified. The injury led to a small but significant overall decrease in the number of motor neurons. However, there was no interaction, indicating that the disruption of the CNTFRα gene did not increase motor neuron death. See text for complete description of statistical comparisons. N = 7 CNTFRα-floxed mice; 8 control mice. Values are presented as mean ± SEM.
DISCUSSION
This report is a continuation of our efforts to determine the in vivo roles played by endogenous CNTF receptor signaling in adult MNs. We used Cre/loxP technology with adult-onset targeted gene disruption to study the receptor’s adult functions independent of its developmental functions. This is a critical issue for CNTF receptor-based therapeutics involving neurodegenerative diseases or trauma because most of these conditions occur in adulthood.
We have previously disrupted the CNTFRα gene in adult facial MNs through AAV-Cre infection of the same floxed CNTFRα mouse line used here (Lee et al., 2008). We found that, as designed, this procedure led to the loss of CNTFRα in MNs of the floxed mice but not those of wildtype injected controls. Almost all MNs that lost CNTFRα died within 4 months. This finding indicated that CNTF receptors can play an essential role in the in vivo survival of adult facial MNs. However, the finding did not distinguish between significantly different roles that the receptor may perform to promote adult MN survival. The results were consistent with CNTF receptors being required for: 1) the survival of the adult MNs, even in the absence of any insult, as seen with embryonic MNs (DeChiara et al., 1995); 2) the protection of adult MNs from the traumatic insults associated with the AAV-Cre injection procedure; and 3) the protection of the adult MNs from the toxic effects of the chronic Cre activity produced by the AAV-Cre (Loonstra et al., 2001; Kaspar et al., 2002).
To address this issue, the present experiments utilized an inducible Cre gene construct which enabled us to disrupt the CNTFRα gene in adult facial MNs without the trauma or chronic Cre activity that accompanies the AAV-Cre injection. With this approach, about half of the facial MNs in the floxed mice lost all detectable CNTFRα by 3 months after the tamoxifen-induced pulse of Cre activity. MN quantification 5 months later indicated no decrease in the number of MNs in the floxed mice (i.e., those MNs without traumatic insult on the intact side; Figs. 3, 4). With about half of the MNs losing CNTFRα, one would expect to have observed at least a trend toward fewer MNs in the floxed mice if even a significant fraction of these affected MNs required CNTF receptor signaling to survive. In contrast, no such trend was seen. Moreover, the affected MNs were without CNTFRα for at least 5 months prior to quantification and consequently had an extended period to reveal any long-term requirement for CNTF receptor signaling. Therefore, the data indicate that endogenous CNTF receptor signaling is not required for the in vivo survival of adult facial MNs that have not been challenged by insult.
As noted above, our previous results (Lee et al., 2008) also raised the possibility that CNTF receptor signaling may protect MNs from the penetrating injury trauma associated with the AAV-Cre injection procedure. In the present studies we detected a very small, but statistically significant, loss of MNs from this trauma. However, CNTFRα disruption did not increase this loss, even though the MNs were given 4 months to die and reveal any potential long-term protective contribution made by CNTF receptors. Therefore, endogenous CNTF receptor signaling does not serve to protect MNs from the acute or long-term consequences of the penetrating injury trauma associated with the AAV-Cre injection procedure. Again, our previous study indicated that the CNTF receptor signaling is needed to protect MNs challenged with a combination of this trauma and the toxic effects of chronic Cre expression (Lee et al., 2008). Consequently, the present results, which indicate that the receptors are not needed to protect the MNs from the trauma, suggest that the CNTF receptor signaling is needed to protect the MNs from the toxic effects of the chronic Cre activity produced by the AAV-Cre. However, it is still theoretically possible that the receptor signaling protects the MNs from the combined insult of the trauma plus the ensuing long-term Cre expression.
Facial nerve crush in the adult has been previously shown to have no affect on MN survival in both rats and mice, in contrast to the dramatic effects it has if the lesion is administered within a week of birth (Gilad et al., 1996; Kuzis et al., 1999). A direct method to identify proteins that promote MN survival after the lesion is to genetically disrupt candidate proteins in vivo and determine if the MNs then die in the knockout mice following the lesion. As reviewed in the introduction, several lines of evidence suggest CNTF receptor signaling as a potential contributor to MN survival and therefore a good candidate for helping maintain MN survival following nerve crush in the adult. However, we found that CNTF receptor disruption in adult MNs had no affect on their survival following the lesion. This result strongly suggests that the receptor does not play an essential, nonredundant role in promoting MN survival following adult nerve crush.
The present results highlight the insult-specific nature of endogenous neuroprotective mechanisms. Clearly, it is not sufficient to label a particular endogenous mechanism as neuroprotective without also determining which forms of insults the mechanism is effective against. Current data indicate that endogenous CNTF receptor signaling promotes the survival of adult facial MNs following AAV-Cre injection of the facial motor nucleus but is not required to protect the cells from the trauma resulting from the injection or from facial nerve crush. Future studies with the same inducible Cre approach developed here should determine whether the receptor plays a protective role following other forms of trauma. Moreover, crossing the inducible Cre and floxed CNTFRα gene constructs into genetic models of MN disease should reveal roles that endogenous CNTF receptor signaling plays in protecting MNs from such insults. Notably, these genetic insults are in many ways more similar to the toxicity of chronic cellular Cre than to the acute trauma of penetrating injury or nerve lesion and may, therefore, like Cre toxicity, be more likely to interact with CNTF receptor signaling.
As discussed above, the present results indicate that CNTF receptor signaling is not required for adult facial MN survival in vivo, in the absence of insult. In contrast, neonatal MNs, including neonatal facial MNs, are reduced in number by 30%–50% in unconditional CNTFRα knockout mice (DeChiara et al., 1995). Therefore, CNTF receptor signaling plays very different roles in MNs at different stages of development. This is not only an interesting result from a basic research perspective, it also emphasizes the need for adult-onset gene disruption studies in efforts to design therapies for adult neurological disorders.
ACKNOWLEDGMENTS
We thank Drs. Joshua Sanes and Robert Brownstone for the Thy1-stop-YFP line 15 reporter mice and Glenn Doerman for assistance with the figures.
Grant sponsor: National Institutes of Health; Grant numbers: NS35224 and NS052700 (to A.J.M.).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ROLE OF AUTHORS
Study concept and design: AJM. Acquisition of data: NL, CER, RPS, RR. Analysis and interpretation of data NL, CER, RPS, RR, AJM. Drafting of article: AJM.
LITERATURE CITED
- Bazan JF. Structural design and molecular evolution of a cytokine receptor superfamily. Proc Natl Acad Sci U S A. 1990;87:6934–6938. doi: 10.1073/pnas.87.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocard J, Warot X, Wendling O, Messaddeq N, Vonesch JL, Chambon P, Metzger D. Spatio-temporally controlled site-specific somatic mutagenesis in the mouse. Proc Natl Acad Sci U S A. 1997;94:14559–14563. doi: 10.1073/pnas.94.26.14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffelli M, Burgess RW, Feng G, Lobe CG, Lichtman JW, Sanes JR. Genetic evidence that relative synaptic efficacy biases the outcome of synaptic competition. Nature. 2003;424:430–434. doi: 10.1038/nature01844. [DOI] [PubMed] [Google Scholar]
- Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol. 1998;8:1323–1326. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- Davis S, Aldrich TH, Stahl N, Pan L, Taga T, Kishimoto T, Ip NY, Yancopoulos GD. LIFRβ and gp130 as heterodimerizing signal transducers of the tripartite CNTF receptor. Science. 1993;260:1805–1808. doi: 10.1126/science.8390097. [DOI] [PubMed] [Google Scholar]
- DeChiara TM, Vejsada R, Poueymirou WT, Acheson A, Suri C, Conover JC, Friedman B, McClain J, Pan L, Stahl N, Ip NY, Kato A, Yancopoulos GD. Mice lacking the CNTF receptor, unlike mice lacking CNTF, exhibit profound motor neuron deficits at birth. Cell. 1995;83:313–322. doi: 10.1016/0092-8674(95)90172-8. [DOI] [PubMed] [Google Scholar]
- Derouet D, Rousseau F, Alfonsi F, Froger J, Hermann J, Barbier F, Perret D, Diveu C, Guillet C, Preisser L, Dumont A, Barbado M, Morel A, deLapeyriere O, Gascan H, Chevalier S. Neuropoietin, a new IL-6-related cytokine signaling through the ciliary neurotrophic factor receptor. Proc Natl Acad Sci U S A. 2004;101:4827–4832. doi: 10.1073/pnas.0306178101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson GCA, Lelievre E, Guillet C, Chevalier S, Plun-Favreau H, Froger J, Suard I, Benoit de Coignac A, Delneste Y, Bonnefoy J-Y, Gauchat J-F, Gascan H. CLF associates with CLC to form a functional heteromeric ligand for the CNTF receptor complex. Nat Neurosci. 2000;3:867–872. doi: 10.1038/78765. [DOI] [PubMed] [Google Scholar]
- Feil R, Brocard J, Mascrez B, LeMeur M, Metzger D, Chambon P. Ligand-activated site-specific recombination in mice. Proc Natl Acad Sci U S A. 1996;93:10887–10890. doi: 10.1073/pnas.93.20.10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilad VH, Tetzlaff WG, Rabey JM, Gilad GM. Accelerated recovery following polyamines and aminoguanidine treatment after facial nerve injury in rats. Brain Res. 1996;724:141–144. doi: 10.1016/0006-8993(96)00287-9. [DOI] [PubMed] [Google Scholar]
- Guo C, Yang W, Lobe CG. A cre recombinase transgene with mosaic, widespread tamoxifen-inducible action. Genesis. 2002;32:8–18. doi: 10.1002/gene.10021. [DOI] [PubMed] [Google Scholar]
- Hatton WJ, Von Bartheld CS. Analysis of cell death in the trochlear nucleus of the chick embryo: calibration of the optical dissector counting method reveals systematic bias. J Comp Neurol. 1999;409:169–186. [PubMed] [Google Scholar]
- Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol. 2002;244:305–318. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Gomez-Isla T, Irizarry MC. Stereology: a practical primer for neuropathology. J Neuropath Exp Neurol. 1998;57:305–310. doi: 10.1097/00005072-199804000-00001. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Wong V, Holmlund TH, Greene T, Cedarbaum JM, Lindsay RM, Mitsumoto H. Histometric effects of ciliary neurotrophic factor in wobbler mouse motor neuron disease. Ann Neurol. 1995;37:47–54. doi: 10.1002/ana.410370110. [DOI] [PubMed] [Google Scholar]
- Imai T, Jiang M, Chambon P, Metzger D. Impaired adipogenesis and lipolysis in the mouse upon selective ablation of the retinoid X receptor alpha mediated by a tamoxifen-inducible chimeric Cre recombinase (Cre-ERT2) in adipocytes. Proc Natl Acad Sci U S A. 2001;98:224–228. doi: 10.1073/pnas.011528898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip NY, Nye SH, Boulton TG, Davis S, Taga T, Li Y, Birren SJ, Yasukawa K, Kishimoto T, Anderson DJ, Stahl N, Yancopoulos GD. CNTF and LIF act on neuronal cells via shared signaling pathways that involve IL-6 signal transducing receptor component gp130. Cell. 1992;69:1121–1132. doi: 10.1016/0092-8674(92)90634-o. [DOI] [PubMed] [Google Scholar]
- Kaspar BK, Vissel B, Bengoechea T, Crone S, Randolph-Moore L, Muller R, Brandon EP, Schaffer D, Verma IM, Lee K-F, Heinemann SF, Gage FH. Adeno-associated virus effectively mediates conditional gene modification in the brain. Proc Natl Acad Sci U S A. 2002;99:2320–2325. doi: 10.1073/pnas.042678699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzis K, Coffin JD, Eckenstein FP. Time course and age dependence of motor neuron death following facial nerve crush injury: role of fibroblast growth factor. Exp Neurol. 1999;157:77–87. doi: 10.1006/exnr.1999.7014. [DOI] [PubMed] [Google Scholar]
- Larsen JV, Hansen M, Moller B, Madsen P, Scheller J, Nielsen M, Petersen CM. Sortilin facilitates signaling of ciliary neurotrophic factor and related helical type 1 cytokines targeting the gp130/leukemia inhibitory factor receptor β heterodimer. Mol Cell Biol. 2010;30:4175–4187. doi: 10.1128/MCB.00274-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N, Robitz R, Zurbrugg RJ, Karpman AM, Mahler AM, Cronier SA, Vesey R, Spearry RP, Zolotukhin S, MacLennan AJ. Conditional, genetic disruption of ciliary neurotrophic factor receptors reveals a role in adult motor neuron survival. Eur J Neurosci. 2008;27:2830–2837. doi: 10.1111/j.1460-9568.2008.06298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N, Batt MK, Cronier BA, Jackson MC, Bruno Garza JL, Trinh DS, Mason CO, Spearry RP, Bhattacharya S, Robitz R, Nakafuku M, MacLennan AJ. Ciliary neurotrophic factor receptor regulation of adult forebrain neurogenesis. J Neurosci. 2013a;33:1241–1258. doi: 10.1523/JNEUROSCI.3386-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N, Spearry RP, Leahy KM, Robitz R, Trinh DS, Mason CO, Zurbrugg RJ, Batt MK, Paul RJ, MacLennan AJ. Muscle ciliary neurotrophic factor receptor α promotes axonal regeneration and functional recovery following peripheral nerve lesion. J Comp Neurol. 2013b doi: 10.1002/cne.23324. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay RM, Wiegand SJ, Altar CA, DiStefano PS. Neurotrophic factors: from molecule to man. Trends Neurosci. 1994;17:182–190. doi: 10.1016/0166-2236(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Loonstra A, Vooijs M, Beverloo HB, Allak BA, van Drunen E, Kanaar R, Berns A, Jonkers J. Growth inhibition and DNA damage induced by Cre recombinase in mammalian cells. Proc Natl Acad Sci U S A. 2001;98:9209–9214. doi: 10.1073/pnas.161269798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan AJ, Vinson EN, Marks L, McLaurin DL, Pfeifer M, Lee N. Immunohistochemical localization of ciliary neurotrophic factor receptor α expression in the rat nervous system. J Neurosci. 1996;16:621–630. doi: 10.1523/JNEUROSCI.16-02-00621.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger D, Chambon P. Site- and time-specific gene targeting in the mouse. Methods. 2001;24:71–80. doi: 10.1006/meth.2001.1159. [DOI] [PubMed] [Google Scholar]
- Oppenheim RW, Prevette D, Qin-Wei Y, Collins F, MacDonald J. Control of embryonic motoneuron survival in vivo by ciliary neurotrophic factor. Science. 1991;251:1616–1618. doi: 10.1126/science.2011743. [DOI] [PubMed] [Google Scholar]
- Sagot Y, Tan SA, Baetge E, Schmalbruch H, Kato AC, Aebischer P. Polymer encapsulated cell lines genetically engineered to release ciliary neurotrophic factor can slow down progressive motor neuronopathy in the mouse. Eur J Neurosci. 1995;7:1313–22. doi: 10.1111/j.1460-9568.1995.tb01122.x. [DOI] [PubMed] [Google Scholar]
- Sendtner M, Kreutzberg GW, Thoenen H. Ciliary neurotrophic factor prevents the degeneration of motor neurons after axotomy. Nature. 1990;345:440–441. doi: 10.1038/345440a0. [DOI] [PubMed] [Google Scholar]
- Sendtner M, Schmalbruch H, Stockli KA, Carroll P, Kreutzberg GW, Thoenen H. Ciliary neurotrophic factor prevents degeneration of motor neurons in mouse mutant progressive motor neuronopathy. Nature. 1992;358:502–504. doi: 10.1038/358502a0. [DOI] [PubMed] [Google Scholar]
- Watt JA, Lo D, Cranston HJ, Paden CM. CNTF receptor alpha is expressed by magnocellular neurons and expression is upregulated in the rat supraoptic nucleus during axonal sprouting. Exp Neurol. 2009;215:135–141. doi: 10.1016/j.expneurol.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattler S, Kelly M, Nehls M. Construction of gene targeting vectors from ëKOS genomic libraries. BioTechniques. 1999;26:1150–1160. doi: 10.2144/99266rr02. [DOI] [PubMed] [Google Scholar]
- Xu Q, Tam M, Anderson SA. Fate mapping Nkx2.1-lineage cells in the mouse telencephalon. J Comp Neurol. 2008;506:16–29. doi: 10.1002/cne.21529. [DOI] [PubMed] [Google Scholar]