Abstract
Recent reports suggest that polymorphisms in the carbonic anhydrase gene CA6 (also known as gustin) may explain additional variation in the bitterness of 6-n-propylthiouracil beyond that explained by variation in the bitter receptor gene TAS2R38. CA6 (gustin) has been implicated in taste bud function and salivary buffer capacity. In the present study we examined associations between polymorphisms in the CA6 gene with salt and bitter taste perception, and oral anatomy. 243 subjects (146 female) aged 18–45 rated the intensity of five concentrations of 6-n-propylthiouracil and NaCl on a generalized Labeled Magnitude Scale (gLMS) in duplicate and one concentration of potassium chloride (KCl). Using salivary DNA, we examined 12 SNPs within CA6 in relation to taste intensity and number of fungiform papillae. We observed no difference in bitter taste perception from 6-n-propylthiouracil (PROP) or from potassium chloride for any of the SNPs examined. Perceived saltiness of NaCl on the other hand was significantly associated with a number of CA6 polymorphisms, and particularly rs3737665. Nonetheless, FP density did not vary between alleles of rs3737665, nor with any of the other CA6 SNPs. Also, we fail to find any evidence that CA6 effects on taste perception are due to differences in fungiform papilla number. Additional work is needed to confirm whether variations within the CA6 gene may be responsible for differences in salt taste perception.
Keywords: Salt perception, Bitter perception, Carbonic anhydrase, Polymorphisms, Fungiform papillae
1. Introduction
Taste perception has long been known to vary across individuals. Since the discovery of ‘taste blindness’ to phenylthiocarbamide (PTC) in the early 1930s [1,2], substantial research has been carried out to understand the biological basis of individual differences in taste perception. The perception of the bitter-tasting compounds PTC and 6-n-propylthiouracil (PROP) have received particular attention as they are members of a specific class of thiourea-containing compounds that also includes naturally occurring compounds found in brassica vegetables [3,4]. Since glucosinolates in Brassica vegetables are hydrolyzed to isothiocyanantes, molecules with recognized beneficial effects (e.g. [5]), the juxtaposition of aversive taste sensations and lower vegetable intake (e.g. [6,7]) with intake of beneficial phytonutrients (e.g. [8]) has attracted substantial interest. However, only in the last decade have we been able to examine the molecular genetics underlying this dimorphism. The TAS2R38 gene (née PTC; HGNC:9584) was identified in 2003 [9], and was found to encode a receptor, hT2R38, which responds to PTC and PROP in vitro and in vivo [10,11]. Because polymorphisms in TAS2R38 associate with differences in taste perception [10–12] and vegetable intake [13], this genetic variation may have broader impact on food choice and nutritional status [14,15], although not all data support this view [16,17].
Subsequent work on TAS2R38 haplotypes and taste perception indicated other additional unknown genetic factors might also be involved in PROP bitterness perception [11,12,18,19], potentially located on chromosome 16 [20]. Differences in the number of fungiform papillae (FP) are often discussed as being involved in PROP perception, as FP density is thought to be a rough indicator of taste nerve innervation [21], and indeed, correlations between the number of FP and perceived bitterness [22,23] and sweetness [24] have been reported. Further, number of FP reportedly correlates with PROP taste intensity independently of TAS2R38, as number of FP does not differ with diplotype [12], suggesting FP number may be an anatomical marker of overall taste intensity. However, when the link between PROP and FP was explored within genetically homogenous individuals, the expected relationship between PROP and FP was absent in the TAS2R38 heterozygotes, demonstrating that the association between FP and taste perception is not straightforward. Moreover, not all reports support the finding that number of FP is directly correlated with PROP: a recent epidemiological study found no association between PROP intensity and FP number [25]. Indeed, it has been suggested that FP number is a more accurate predictor of taste intensity perception in small areas of the anterior tongue than for whole-mouth stimulation [26].
The term ‘supertaster’ was first coined by Linda Bartoshuk following observations in her laboratory that PROP tasters (defined via threshold) were more varied in their perception than nontasters [27]. Using suprathreshold methods, they found that descriptions of PROP from ‘tasters’ ranged from mildly to intensely bitter. Traditionally, PTC/PROP tasters had been separated from nontasters using detection thresholds, or response to an antimodal concentration (see [28]). This separation agreed with the prevailing theory at the time; i.e. that the ability to taste thiourea compounds at low concentrations was a simple Mendelian-inherited dominant trait, with T indicating the taster allele and t indicating the nontaster allele. Thus, Tt and TT individuals would phenotypically be tasters, and tt individuals nontasters (although other modes of inheritance were occasionally suggested (cf. [29–31]; see [32] for a detailed review).
In 1994, Bartoshuk, Duffy and Miller published the first peer reviewed paper on supertasting, subdividing tasters into ‘supertasters’ and ‘medium tasters’ via multiple PROP and sodium chloride solutions as whole-mouth stimuli, which were rated for intensity using magnitude estimation [22]. They speculated that ‘supertasters’, those reporting intense bitterness from PROP, might be homozygous dominant (e.g. TT) with ‘medium tasters’ being heterozygous (e.g. Tt) [22], although molecular data later disproved this (e.g. [11,12]). The concept of supertasting has evolved over time, and a variety of phenotyping methods now exist to determine who is or is not a supertaster (see [33] for a review). In the original 1994 paper, a ratio of PROP intensity to salt intensity was used to categorize individuals: a ratio below 0.8 were defined as nontasters, and above 1.2 were considered supertasters [22]. Using similar logic, a graphical variant of this method uses three concentrations of salt and PROP solutions [34]. In the graphical method, the psychophysical function is plotted for each individual, and they are categorized into super, medium and nontasters depending on whether the PROP ratings are higher, the same, or lower than those for salt, respectively [34]. This can be reduced to a one solution test, which still shows high test–retest reliability [34]. It was later demonstrated that using sound rather than NaCl as a reference against which to normalize the PROP ratings resulted in greater effect sizes [35], since the intensity of a wide range of taste stimuli, including salt, increase with increased PROP perception [35–37]. Given this limitation in salt-based classification methods, other researchers have normalized PROP ratings to other modalities like tones [19] or weights [38] or remembered sensations like the brightness of the sun [39] prior to classification, while other reports use raw (unnormalized) PROP ratings and classify individuals either on the basis of a priori cutoffs [6,40] or the observed distribution in the data [41,42]. Notably, both the observed distribution method, and the a-priori cut-off method are typically based on the early assumption that a given population should have a 25/50/25 split, with the lower quarter being nontasters, the upper quarter being supertasters and the remainder being medium tasters. Simple Mendelian genetics would dictate that the population should have the proportions 25% tt, 50% Tt, and 25% TT. However, this assumption may not be valid, given newer data.
Other work suggests PROP bitterness is a continuous variable and should be treated as such (e.g. [21], although traditional trimodal classification schemes may reflect the underlying distribution of larger populations [43]. Most work uses liquid stimuli, although PROP has also been delivered via filter paper discs [44] or dissolving strips [45] placed on the tongue; such methods are particularly useful for conducting large-scale studies outside of the laboratory environment (e.g. [17,46].
In addition to variability from different classification schemes, choice of scaling methods may hinder comparisons across research groups and populations. Although the field has largely settled on the general Labeled Magnitude Scale (gLMS) [47] or its predecessor, the Labeled Magnitude Scale (LMS)[48], use of other psychophysical scales complicates interpretation of earlier reports (e.g. [49–52].
Regardless of the classification method, it has often been shown that PROP bitterness perception correlates with greater intensity ratings from other taste stimuli. However, Lim et al. [53], state that other taste stimuli may be better markers of general taste ability, due to the bimodal nature of PROP taste perception. Using NaCl, sucrose, citric acid and quinine as the taste stimuli, they found that while the four taste stimuli correlated to each other, PROP correlated only to the bitterness of quinine. Rather than the typical suggestion that number of taste buds or FP density could explain covariation between PROP and other tastants, Lim and colleagues suggested that correlated intensities might be due to a central gain mechanism; whether this mechanism might have a simple genetic correlate is unknown.
The role of taste bud density in generalized supertasting (hypergeusia) [33] has been revisited recently, with a report which suggested PROP taste intensity also associates variations in the CA6 (gustin) gene [54]. The CA6 gene encodes the carbonic anhydrase VI protein, an enzyme that catalyzes the hydration of carbon hydroxide in saliva, [55] and is thought to have an important role in taste bud function. A SNP in CA6, rs2274327 (Thr55Arg) results in different variations, which have been implicated in salivary buffer capacity; in individuals with the highest buffer capacity, those with two thymine nucleotides (i.e. TT allele carriers) were significantly lower than expected by chance [55]. A range of other SNPs within CA6 were examined in that report, but rs2274327 appeared to be the only functional SNP, at least with regard to buffer capacity.
Padiglia and colleagues [14] examined the rs2274333 SNP within CA6 and observed that ‘A’ (adenine) alleles were more frequent in supertasters (as defined by a graphical PROP:salt ratio). The authors speculated the differences in taste intensity might have been due to varying FP density, although they did not measure FP in their report. Therefore, an open question remains as to whether SNPs within the CA6 gene are related to taste intensity for other taste stimuli. Also, it is unknown whether number of FP varies with polymorphisms within CA6. Thus, the goals of the present study were to: a) examine putatively functional SNPs in the CA6 gene as predictors of variation in suprathreshold taste intensity for salty and bitter tastants, and b) assess potential relationships between CA6 SNPs and number of fungiform papillae.
2. Materials and methods
2.1. Participants
243 reportedly healthy participants (146 women), aged 18–45 were recruited from the Pennsylvania State University campus and surrounding area. Written, informed consent was obtained from each participant and participants were reimbursed for their time. Individual test sessions with the participant and experimenter took approximately 60 min to complete, of which 5–10 min was spent photographing the tongue.
2.2. Stimuli and psychophysical measurement
As reported elsewhere, the first day of testing included taste stimuli in two different blocks as part of a larger, ongoing study into the genetics of oral sensation (e.g. [56]). Subjects rated their perceived intensity of stimuli on a generalized Labeled Magnitude Scale (gLMS) with a top anchor of ‘strongest imaginable sensation of any kind’. (Snyder et al. [47], found that the modifier ‘imaginable’ is not needed, but we included it for comparability with previous reports). All participants completed a short orientation session on the use of the scale and an onscreen test to rate a range of remembered and imagined taste and non-taste stimuli before proceeding to the taste tests (e.g. [57]).
In the first block of stimuli, participants received: sucrose (0.5 M), potassium chloride (0.56 M), quinine hydrochloride (0.41 mM), capsaicin (0.25 uM) and a mixture of monosodium glutamate/inosine monophosphate (100 mM/50 mM). (Adding IMP to MSG intensifies the perception of umami in a superadditive fashion [58]). After being instructed that the presented stimuli may elicit multiple sensations, participants were asked to consider all sensations on all trials, and rate the intensity sweet, sour, salty, savory/umami, bitter, and burning sensations on separate generalized Labeled Magnitude Scales.
PROP phenotype was collected using standard methods, as described elsewhere [6]. Briefly, participants received ten 10 mL PROP solutions (3.2, 1, 0.32, 0.1, and 0.032 mM, in duplicate), ten 10 mL salt solutions (0.01, 0.032, 0.1, 0.32, and 1 M, in duplicate) and 25 1000 Hz tones in fixed blocks (i.e. 5 tones, 5 salt solutions, 5 tones, 5 salt solutions, 5 tones, 5 PROP solutions, 5 tones, 5 PROP solutions, and 5 tones). Within a block, stimulus order was counterbalanced. Tones (50 to 90 dB in 10 dB steps) were generated with a Maico MA39 audiometer calibrated to deliver the specified sound pressure level binaurally. The tastants, USP grade 6-n-propylthiouracil, potassium chloride (both Sigma) and kosher salt, were prepared using reverse osmosis (RO) water. Participants rinsed with room temperature RO water between each sample, with minimum interstimulus interval of 30 s enforced between each sample. Overall intensity was rated on a gLMS.
2.3. DNA Collection and Genotyping
DNA was obtained using salivary collection kits (Genotek, Ontario, Canada) according to manufacturers directions, and genotyped using Sequenom MassARRAY (Sequenom, San Diego, CA) technology. We examined 12 SNPs within CA6: rs12748400, rs17032907, rs2274327, rs2274328, rs2274333, rs2274334, rs3737665, rs3765964, rs3765965, rs3765967, rs3765968 and rs7545200. Within TAS2R38, one SNP, A49P (rs713598) was examined in the present analyses. TAS2R38 contains three SNPs (A49P, A262V and V296I) which form two common haplotypes, designated P–A–V and A–V–I. These associate differentially with PROP bitterness perception. Since these SNPs show strong linkage disequilibrium, the A49P SNP alone was deemed a sufficient indicator of haplotype for the present analyses. Genotypes were assigned automatically via MassARRAY software (Sequenom) and independently inspected by 2 technicians. As a standard procedure, 15% of samples are rerun to ensure reliability.
2.4. Fungiform papilla counting
The researcher stained the front section of participants’ tongues using blue coloring on sterile cotton swab, allowing FP to be visualized. The participant rested his or her chin on a table-mounted chin rest, and held their tongue between a specially-constructed transparent holder. This consisted of two clear plastic slides (VWR International), 25 mm × 77 mm in dimension, fastened together with a screw and bolt, and containing a 6 mm diameter white adhesive circle. 3–5 photographs were taken of the tongue in a flat plane. Photographs were taken with a Canon EOS Rebel T3i camera used in manual mode with the following settings: ISO 800, Aperture: 29 (F29), shutter speed: 1/200. The focus on the lens was set to automatic focus (AV mode). The procedure was carried out in a darkened interior room without windows or other ambient light, using a macro ring light attachment, Canon MR-14EX, which was set in ETTL mode at −1. Pictures were transferred to a computer and using Adobe Photoshop CS5.1, the images were enlarged and the number of FP within a circle of 6 mm in diameter was counted, for two areas, on the left and right of the midline of the tongue, near the tip. Previous research has found this to be the optimum area for counting FP. An average for both areas was obtained and this number converted to FP per cm2. Two researchers independently counted each tongue.
2.5. Statistical analysis
Repeated-measures ANOVA was performed separately for each CA6 SNP to examine the effect of genotype on salt and PROP perception, with concentration as the repeated measure. To control for TAS2R38 genotype, allelic variation at the A49P SNP was entered in the model. Prior reports have implicated the carbonic anhydrase VI (CA6) rs2274333 SNP in categorical PROP taster status [14], so we tested the association between rs2274333 diplotype and PROP taster status via chi-square, using two different classification methods for PROP taster groups. Bi-variate Pearson’s correlation was used to check for correlation between FP density and PROP intensity. One-way ANOVA was performed to test whether the perceived intensity of KCl and whether the number of FP varied in the different CA6 diplotype groups. Statistical analyses were carried out in SPSS, Version 21.
3. Results
3.1. SNPs within CA6 did not associate with PROP intensity, irrespective of TAS2R38
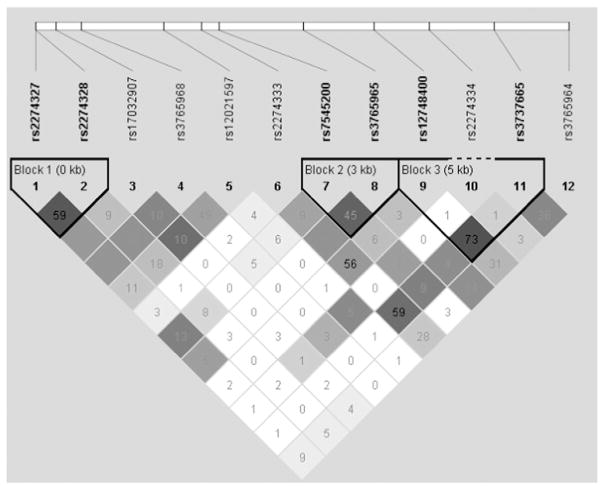
We failed to find any evidence that the Ser90Gly SNP (rs2274333; chr 1) explained variation in PROP intensity [F(2,238) = 0.119; p = 0.888), in contrast to prior reports [54]. This SNP was not in linkage disequilibrium with any of the SNPs in CA6; the LD plot for the 12 SNPs we tested is shown in Fig. 1. No differences in PROP intensity were observed for any of the other SNPs.
Fig. 1.
LD plot of SNPs examined within CA6. SNPs are listed in the order on which they lie on the chromosome. Black lines indicate LD blocks.
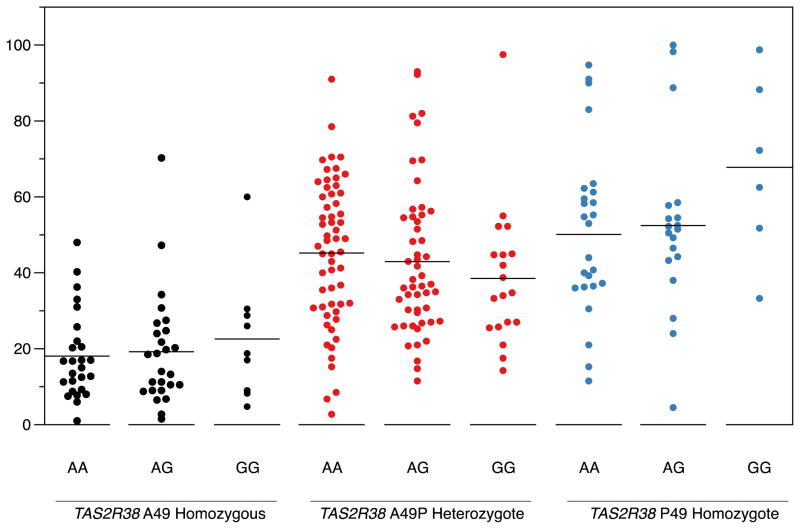
To ensure that any potential association between CA6 and PROP intensity was not obscured by the effect of TAS2R38 genotype, we repeated the analysis, controlling for TAS2R38 variation. In repeated measures ANOVA testing for simultaneous effects of CA6 and TAS2R38 SNPs on PROP intensity, there was a main effect of the TAS2R38 A49P SNP [F(1,236) = 112.74; p < 0.001] as expected. However, the Ser90Gly rs2274333 SNP was still not a significant predictor of PROP bitterness [F(2, 236) = 0.122; p = 0.886]. Nor were similar models for the other CA6 SNPs significant (all p’s > .05). Fig. 2 shows the mean bitterness intensity ratings in the different SNPs of rs2274333 in the A49P SNPs of TAS2R38 for the highest concentration of PROP (3.2 mM, where any difference in perception between genotypes would be expected to be greatest).
Fig. 2.
Perceived bitterness for 3.2 mM PROP on a gLMS. Dots represent individuals with varying alleles for the CA6 rs2274333 Ser90Gly SNP, nested within TAS2R38 allele. The horizontal black lines are group means. As expected, PROP bitterness differed by TAS2R38 (p<.0001; see text). Within TAS2R38 groups, AA individuals for the CA6 rs2274333 SNP did not show elevated bitterness (p>.8; see text for details).
3.2. SNPs within CA6 associated with NaCl intensity
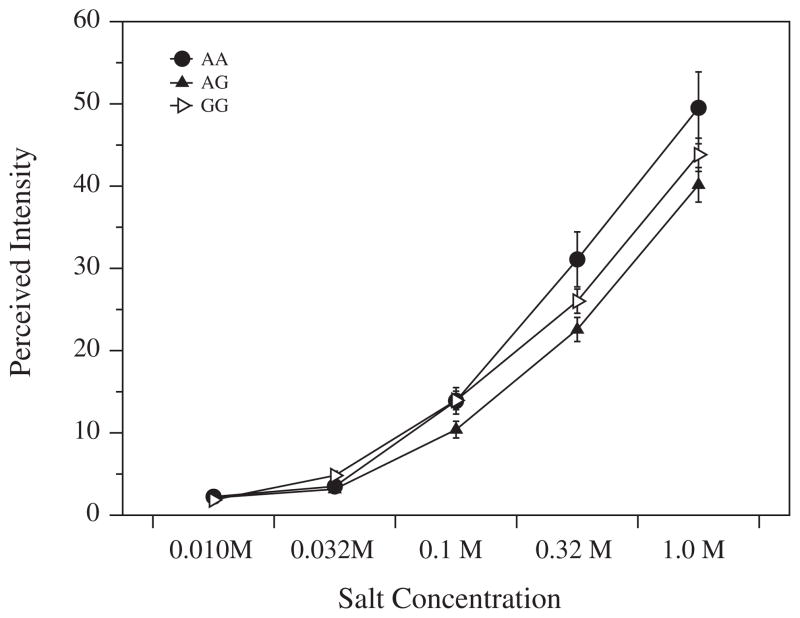
Since the SNPs that we tested in CA6 did not associate with PROP intensity, we examined perceived NaCl intensity to determine whether this could explain the association with taster status in previous reports, as salt ratings are half of the ratio used when the phenotype is defined in this way. We observed that two of the CA6 SNPs were significantly associated with salt intensity via one-way repeated measures ANOVA. The synonymous Asn256 SNP (rs3737665; chr 1) associated with differences in the perceived intensity of NaCl (Fig. 3) [F(2,237) = 4.54; p = 0.012].
Fig. 3.
Perceived Salt Intensity with alleles of rs3737665. Salt intensity for a range of salt solutions was rated on the gLMS. Error bars depict standard error.
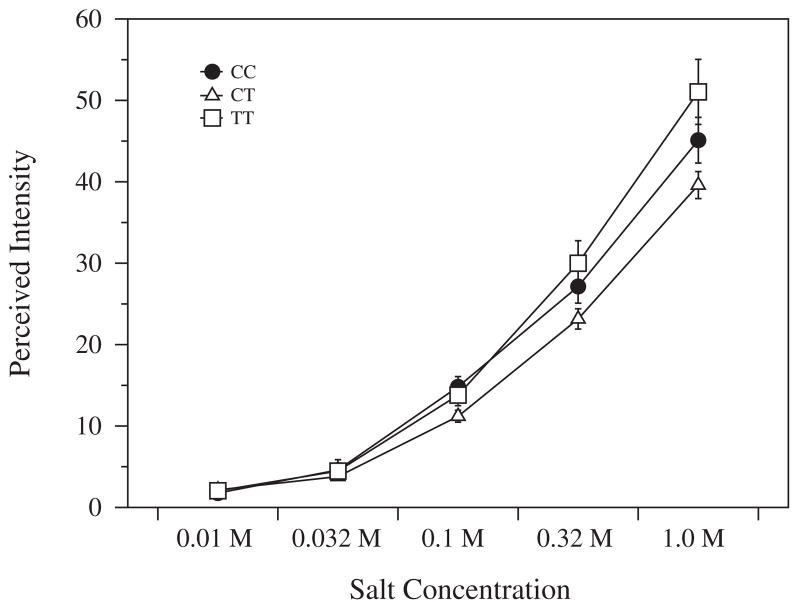
The Gly287Glu SNP (rs3765964; chr 1) also associated with differences (Fig. 4) in the perceived intensity of NaCl [F(2,240) =6.25; p = 0.002].
Fig. 4.
Perceived Salt Intensity with alleles of rs3765964. Salt intensity for a range of salt solutions was rated on the gLMS. Error bars depict standard error.
3.3. Effects of CA6 SNPs on the saltiness and bitterness of KCL
One-way ANOVA was used to examine the saltiness and bitterness ratings for 0.56 M KCl for each CA6 SNP; bitterness did not vary as a function of any of these SNPs. Regarding saltiness, two SNPs were significantly associated with KCl saltiness. Again, the synonymous Asn256 SNP (rs3737665; chr 1) associated with differences in KCl saltiness [F(2,237) = 3.645; p = 0.028]. The Thr55Met SNP (rs2274327; chr 1) also associated with differences in KCl saltiness [F(2,236) = 3.83; p = 0.023].
3.4. Taster status and PROP ratio
Using the ratio method to trichotomously classify individuals as non-, medium and supertasters (NT, MT, ST) [22], there was no evidence of distributional differences for the Ser90Gly SNP (rs2274333) across groups, in conflict with earlier reports. However, our data were skewed towards ‘supertasters’ when using this classification scheme. Because of reports that supertasting is more common in women than in men [59], we checked that this over representation was not due to the greater proportion of women: in our data, the proportion of supertasters (ST) using cut-off values of 0.8 and 1.2, did not differ in men and women: 66.7% ST, 12.1% MT and 21.2% NT, versus 61.2% ST, 15.0 % MT and 23.8% NT, respectively (χ2 (2) = 1.565, p = 0.45). This proportion of ST is still much larger than would typically be expected (assuming 20–30% supertasters, a dataset of this size would be expected to have 50–75 supertasters). This is likely partly due to the arbitrary nature of the 1.2 cut-off for medium and supertasters. Modifying the criterion upward to a value of 2.25 gave more expected proportions.
To further investigate the previously reported link between taster status and alleles of CA6, a PROP:salt ratio was created and cut-offs of 1.2 and 0.8 for the supertaster and nontaster groups were applied per Bartoshuk et al. [22]. As shown in Table 1, there was no relationship between PROP group (as determined via PROP ratio) and rs2274333 diplotype (χ2 (4) = 9.325, p = 0.053).
Table 1.
Chi-squared table of rs2274333 diplotype by PROP taster status (as determined by traditional PROP:salt ratio cut-off values).
| rs2274333 (Ser90Gly) | Supertaster (63% of total) | Medium taster (14% of total) | Nontaster (23% of total) |
|---|---|---|---|
| Ser90 (n 109) | 74 | 10 | 25 |
| Ser90Gly (n 98) | 62 | 14 | 22 |
| Gly90 (n 34) | 16 | 10 | 8 |
We also adjusted the ratio for the ST:MT cutoff, to 2.25, to obtain more typical ST:MT:NT proportions, (29.85, 47.3% and 22.9%), and repeated the chi-squared analysis on these adjusted ratios (Table 2). Again, we found no evidence of a relationship between PROP group and rs2274333 diplotype (χ2 (4) = 1.02, p = 0.91), in contrast to that reported previously for rs2274333 by Padiglia et al. [14], and Calo et al. [54].
Table 2.
PROP taster status by rs2274333 diplotype (taster status was determined by adjusted PROP:salt ratio cut-offs to obtain the expected ST:MT:NT distribution).
| rs2274333 (Ser90Gly) | Supertaster | Medium taster | Nontaster |
|---|---|---|---|
| Ser90 (n 109) | 30 | 54 | 25 |
| Ser90Gly (n 98) | 33 | 43 | 22 |
| Gly90 (n 34) | 10 | 16 | 8 |
3.5. PROP intensity, FP density and variations in CA6
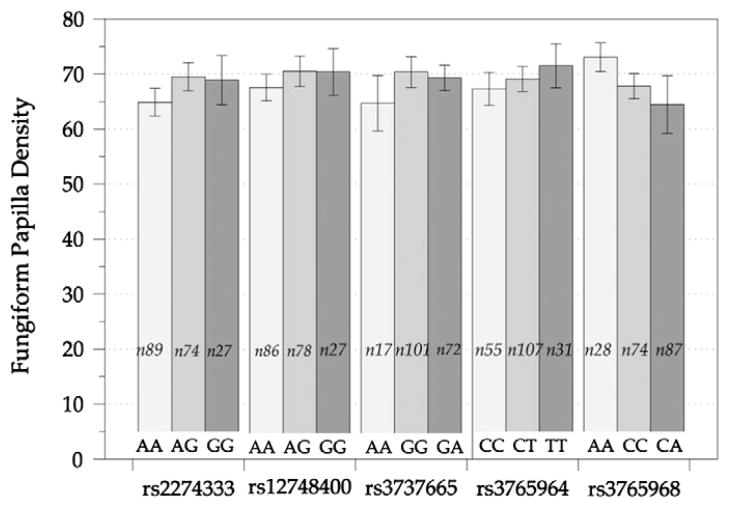
Fungiform papillae (FP) densities ranged from 22.99 to 128.22 FP per cm2, and the average density was 69.71/cm2 (1.62 S.E.M). Here, there was no correlation observed between FP density and PROP bitter intensity. Further, FP density did not differ by any of the SNPs in CA6 that were shown above to associate with salt intensity (Fig. 5). Controlling for age and gender did not change this conclusion.
Fig. 5.
FP density in different allele groups for a range of CA6 SNPs. Shown are FP densities per cm2 for all the CA6 SNPs suggested in one-way ANOVA analysis to associate with one or more concentrations of salt intensity. The n in each bar represents the number of participants for that allele group and error bars represent the standard error.
4. Discussion
Here we find evidence that polymorphisms in the carbonic anhydrase gene CA6 associate with the perception of salt, but not PROP. Recently, Calò and colleagues (2011) reported that mean bitterness from PROP differed by the rs2274333 SNP in CA6 in 72 Sardinian individuals: bitterness from 3.2 mM PROP was greater in individuals with the AA diplotype (Ser90 homozygotes) compared to the GG diplotype (Gly90 homozygotes). Here, we failed to replicate this result in a larger mixed ancestry North American cohort. Previously, Padiglia and coworkers [14] reported that the frequency of rs2274333 AA diplotypes was overrepresented in supertasters (defined via a salt ratio). Again, we failed to replicate this finding in present data using supertaster groups defined via salt ratios. Additionally, we failed to find any association between CA6 polymorphisms and number of FP.
A recent report by Bae et al. (2012) examined perception of the closely-related thiourea-containing compound PTC in relation to three SNPs (rs2274327, rs2274333 and rs2274328) within the carbonic anhydrase gene in Korean participants, with taste disorders and in a control group with normal taste function. Although they found that for one of the SNPs they examined (rs2274328) the frequency of AA individuals was greater than expected in the taste disorder subjects, they found no association with PTC sensitivity for any of the SNPs examined. It is unclear how the CA6 gene (which does not encode a specific receptor for PROP but rather is thought to exert an effect via differential zinc binding) could explain variation in PROP bitterness observed by Calò et al. (2011) but not PTC bitterness, when variation in the hT2R38 receptor correlates strongly with perception of both of these compounds. Further, research conducted around the time that the chromosomal location for the TAS2R38 gene was determined, suggested a region of chromosome 16 might be associated with PTC taste perception [20]. However, CA6 is located on chromosome 1.
The finding of Bae and colleagues [60], in conjunction with present data, suggests that the observation by Padiglia and colleagues [14] regarding the allele frequencies in supertasters may arise from the manner in which taster status in those studies was determined. That is, the use of the PROP:salt ratio to define taste phenotype may have suggested an association with PROP intensity when in fact the underlying association was with salt taste intensity. However this would not explain the differences observed in absolute bitterness intensity later reported for the same SNP [54].
Nonetheless, a number of studies have shown that salt intensity increases as a function of PROP intensity, which taken in consideration with our findings, suggest that the use of a PROP:salt ratio may not be the most appropriate choice to define PROP supertaster status as we now know that salt intensity ratings are confounded with PROP intensity ratings. Further, the underlying SNP responsible for this perceptual difference may in fact be rs3737665 rather than rs2274333 as previously reported, as we observed LD between these SNPs (Fig. 1) and found more differences across rs3737665 alleles compared to rs2274333 between the five concentrations of salt tested.
Carbonic anhydrase has been described both as a salivary zinc metalloprotein, and as a trophic factor for taste bud development. Meanwhile, PROP sensitivity is often but not always reported to associate with fungiform papillae density [22,23,61], c.f. Prutkin et al. [62] (in women) and Garneau and Derr [25]. Prutkin and colleagues reported a correlation between FP and PROP intensity, but only in women. Within the studies that have observed a correlation between FP density and PROP bitterness, the relationship is not absolute; Yackinous and Guinard [61] found that the distribution of FP overlapped PROP taster groups. Other work indicates FP number is independent of the TAS2R38 gene [12]. Since variation in the CA6 gene reportedly explains an additional variation beyond TAS2R38 with regard to PROP, the logical conclusion was thus that fungiform papilla densities might differ with SNPs of CA6, e.g. [14,54]. However, we fail to find evidence to support this speculation here, as we did not observe any significant difference in fungiform papillae density with any of the CA6 SNPs examined. Essick and colleagues [63] showed that nontasters tended to have larger and fewer FP than medium and supertasters of PROP. Considering that the taste buds lie within the FP, it is not unreasonable to assume that larger FP would have more taste buds within them. Thus, the link between FP and taste intensity that is observed in some studies remains unclear, and is a topic that deserves further attention. One possible factor may be that the measurement of one area of the tongue does not take into account differences in total tongue size, meaning that smaller people could appear to have a greater FP density. This might explain some of the observations of greater FP numbers in females.
Padiglia et al. [14] reported that salivary zinc concentrations were lower in PROP supertasters, and suggested that the rs2274333 SNP may influence the binding between zinc and gustin, which could then reduce the taste function. Bae and colleagues [60] observed that the number of participants who had a taste disorder was higher in AA individuals for rs2274328, yet they also showed that rs2274333 was not in LD with rs2274328. Therefore it is possible that both of these SNPs separately influence zinc binding. Alternatively, other SNPs within the CA6 gene may influence other aspects of taste perception such as saliva content and/or volume. Recently, salivary flow has been shown to influence the perception of salty, but not bitter tastes, [64], thus it remains possible that other SNPs within CA6 differentially affect salivary flow which might explain why these SNPs associate with salt but not bitter, taste intensity. More effective salivary buffering action is associated with increased concentrations of total protein and calcium, sodium and chloride, all of which tend to be higher during high flow rates [65,66]. Peres et al. [55] showed that in those with high salivary buffer capacity, the frequency of C alleles (one or more) for the rs2274327 SNP was greater than expected. As this SNP was reported to be in strong LD with rs2274328 [60], it is therefore possible that the differential taste function observed may be due, at least in part, to differences in salivary buffer capacity and/or salivary flow. Here, however, we did not find a significant difference in the ratings for either NaCl or KCl for either of those particular SNPs. Further work is needed in this area to pinpoint the causal regions within CA6 for these difference and to further explore the interplay between these two factors and the range of known SNPs within the CA6 gene.
5. Conclusion
In this large laboratory study of individuals with mixed ancestry, neither PROP intensity ratings nor fungiform papillae density differed with genetic variations in CA6. These results imply that a previous report [14] suggesting supertasters were more common in individuals homozygous for the ‘A’ allele at SNP rs2274333 might have stemmed from a difference in salt perception rather than perceived bitterness, since the authors had used a ratio of PROP and salt perception to classify individuals. However, the individual PROP bitterness scores in the Italian population were later shown to vary with CA6 differences [54]. It is unclear why we were unable to replicate that finding in this sample, but one possibility is that although not a ‘genetically isolated’ population, the participants from that study were primarily Italian Europeans, and the cohort has been previously described as “ethnically homogenous” [67], whereas the participants from the present sample were more ethnically diverse, in addition to the much larger sample size presented here. The finding that salt perception varies with some variations in CA6 may further our understanding of the transduction of the taste of salt, and future work will further investigate this using a variety of other salty stimuli.
HIGHLIGHTS.
246 subjects rated multiple bitter and salty stimuli on a gLMS.
Polymorphisms (SNPs) within the CA6 gene were examined.
Number of fungiform papilla (FP) were quantified.
Several SNPs associated with saltiness, but not bitterness.
There was no association with CA6 SNPs and FP number.
Acknowledgments
Funding
This work was supported by the Pennsylvania State University and NIH grant DC010904.
References
- 1.Fox AL. Six in ten “tasteblind” to bitter chemical. Sci Newsl. 1931;9:249. [Google Scholar]
- 2.Blakeslee AF. Genetics of sensory thresholds: taste for phenyl thio carbamide. Proc Natl Acad Sci U S A. 1932;18:120–30. doi: 10.1073/pnas.18.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnicot NA, Harris H, Kalmus H. Taste thresholds of further eighteen compounds and their correlation with P.T. C thresholds. Ann Eugen. 1951;16:119–28. doi: 10.1111/j.1469-1809.1951.tb02464.x. [DOI] [PubMed] [Google Scholar]
- 4.Boyd WC. Taste reactions to antithyroid substances. Science. 1950;112:153. doi: 10.1126/science.112.2901.153. [DOI] [PubMed] [Google Scholar]
- 5.Traka M, Mithen R. Glucosinolates, isothiocyanates and human health. Phytochem Rev. 2009;8:269–82. [Google Scholar]
- 6.Dinehart ME, Hayes JE, Bartoshuk LM, Lanier SL, Duffy VB. Bitter taste markers explain variability in vegetable sweetness, bitterness, and intake. Physiol Behav. 2006;87:304–13. doi: 10.1016/j.physbeh.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 7.Basson MD, Bartoshuk LM, Dichello SZ, Panzini L, Weiffenbach JM. Association between 6-n-propylthiouracil (PROP) bitterness and colonic neoplasms. Dig Dis Sci. 2005;50:483–9. doi: 10.1007/s10620-005-2462-7. [DOI] [PubMed] [Google Scholar]
- 8.Drewnowski A, Gomez-Carneros C. Bitter taste, phytonutrients, and the consumer: a review. Am J Clin Nutr. 2000;72:1424–35. doi: 10.1093/ajcn/72.6.1424. [DOI] [PubMed] [Google Scholar]
- 9.Kim U-K, Jorgenson E, Coon H, Leppert M, Risch N. Positional cloning of the human quantitative trait locus underlying taste sensitivity to phenylthiocarbamide. Science. 2003;299:1221–5. doi: 10.1126/science.1080190. [DOI] [PubMed] [Google Scholar]
- 10.Bufe B, Breslin PAS, Kuhn C, Reed DR, Tharp CD, Slack JP, et al. The Molecular Basis of Individual Differences in Phenylthiocarbamide and Propylthiouracil Bitterness Perception. Curr Biol. 2005;15:322–7. doi: 10.1016/j.cub.2005.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duffy VB, Davidson AC, Kidd JR, Kidd KK, Speed WC. Bitter receptor gene (TAS2R38), 6-n-propylthiouracil (PROP) bitterness and alcohol intake. Alcohol Clin Exp Res. 2004;28:1629–37. doi: 10.1097/01.ALC.0000145789.55183.D4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayes JE, Bartoshuk LM, Kidd JR, Duffy VB. Supertasting and PROP bitterness depends on more than the TAS2R38 gene. Chem Senses. 2008;33:255–65. doi: 10.1093/chemse/bjm084. [DOI] [PubMed] [Google Scholar]
- 13.Colares-Bento FCJ, Souza VC, Toledo JO, Moraes CF, Alho CS, Lima RM, et al. Implication of the G145C polymorphism (rs713598) of the TAS2r38 gene on food consumption by Brazilian older women. Arch Gerontol Geriatr. 2012;54:e13–8. doi: 10.1016/j.archger.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 14.Padiglia A, Zonza A, Atzori E, Chillotti C, Calo C, Tepper BJ, et al. Sensitivity to 6-n-propylthiouracil is associated with gustin (carbonic anhydrase VI) gene polymorphism, salivary zinc, and body mass index in humans. Am J Clin Nutr. 2010;92:539–45. doi: 10.3945/ajcn.2010.29418. [DOI] [PubMed] [Google Scholar]
- 15.Tepper BJ. Nutritional implications of genetic taste variation: the role of PROP sensitivity and other taste phenotypes. Annu Rev Nutr. 2008;28:367–88. doi: 10.1146/annurev.nutr.28.061807.155458. [DOI] [PubMed] [Google Scholar]
- 16.Timpson NJ, Christensen M, Lawlor DA, Gaunt TR, Day IN. TAS2R38 (phenylthiocarbamide) haplotypes, coronary heart disease traits, and eating behavior in the British Women’s Heart and Health Study. Am J Clin Nutr. 2005;81:1005–11. doi: 10.1093/ajcn/81.5.1005. [DOI] [PubMed] [Google Scholar]
- 17.O’Brien SA, Feeney EL, Scannell AGM, Markey A, Gibney ER. Bitter Taste Perception and Dietary Intake Patterns in Irish Children. J Nutrigenet Nutrigenomics. 2013;6:43–58. doi: 10.1159/000348442. [DOI] [PubMed] [Google Scholar]
- 18.Kim U-K, Wooding S, Ricci D, Jorde LB, Drayna D. Worldwide haplotype diversity and coding sequence variation at human bitter taste receptor loci. Hum Mutat. 2005;26:199–204. doi: 10.1002/humu.20203. [DOI] [PubMed] [Google Scholar]
- 19.Duffy VB, Peterson JM, Bartoshuk LM. Associations between taste genetics, oral sensation and alcohol intake. Physiol Behav. 2004;82:435–45. doi: 10.1016/j.physbeh.2004.04.060. [DOI] [PubMed] [Google Scholar]
- 20.Drayna D, Coon H, Kim U-K, Elsner T, Cromer K. Genetic analysis of a complex trait in the Utah Genetic Reference Project: a major locus for PTC taste ability on chromosome 7q and a secondary locus on chromosome 16p. Hum Genet. 2003;112:567–72. doi: 10.1007/s00439-003-0911-y. [DOI] [PubMed] [Google Scholar]
- 21.Hayes JE, Duffy VB. Revisiting sugar-fat mixtures: sweetness and creaminess vary with phenotypic markers of oral sensation. Chemical Senses. 2007;32:225–36. doi: 10.1093/chemse/bjl050. [DOI] [PubMed] [Google Scholar]
- 22.Bartoshuk LM, Duffy VB, Miller IJ. PTC/PROP tasting: anatomy, psychophysics, and sex effects. Physiol Behav. 1994;56:1165–71. doi: 10.1016/0031-9384(94)90361-1. [DOI] [PubMed] [Google Scholar]
- 23.Delwiche JF, Buletic Z, Breslin PA. Relationship of papillae number to bitter intensity of quinine and PROP within and between individuals. Physiol Behav. 2001;74:329–37. doi: 10.1016/s0031-9384(01)00568-6. [DOI] [PubMed] [Google Scholar]
- 24.Zhang G, Zhang H, Wang X, Zhan Y, Deng S, Qin Y. The relationship between fungiform papillae density and detection threshold for sucrose in the young males. Chem Senses. 2009;34:93–9. doi: 10.1093/chemse/bjn059. [DOI] [PubMed] [Google Scholar]
- 25.Garneau NL, Derr T. Statistical Analysis of Factors Previously Described as Significant in the Ability to Taste Propylthiouracil Yields Roles for Age, Sex, and TAS2R38 Haplotype, but not Fungiform Papillae Density. AChems. 2013;XXXV:235. [Google Scholar]
- 26.Bajec M, Pickering G. Thermal taste, PROP responsiveness, and perception of oral sensations. Physiol Behav. 2008;95:581–90. doi: 10.1016/j.physbeh.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 27.Bartoshuk LM. Sweetness: history, preference and genetic variability. Food Technol. 1991;45:108–13. [Google Scholar]
- 28.Lawless H. A comparison of different methods used to assess sensitivity to the taste of phenylthiocarbamide (PTC) Chem Senses. 1980;5:247–56. [Google Scholar]
- 29.Blakeslee AF, Salmon TN. Genetics of sensory thresholds: individual taste reactions for different substances. Proc Natl Acad Sci U S A. 1935;21:84–90. doi: 10.1073/pnas.21.2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reddy BM, Rao DC. Phenylthiocarbamide taste sensitivity revisited: complete sorting test supports residual family resemblance. Genet Epidemiol. 1989;6:413–21. doi: 10.1002/gepi.1370060304. [DOI] [PubMed] [Google Scholar]
- 31.Olson J, Boehnke M, Neiswanger K, Roche A, Siervogel R. Alternative genetic models for the inheritance of the phenylthiocarbamide taste deficiency. Genet Epidemiol. 1989;6:423–34. doi: 10.1002/gepi.1370060305. [DOI] [PubMed] [Google Scholar]
- 32.Guo SW, Reed DR. The genetics of phenylthiocarbamide perception. Ann Hum Biol. 2001;28:111–42. doi: 10.1080/03014460151056310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hayes JE, Keast RSJ. Two decades of supertasting: where do we stand? Physiol Behav. 2011;104:1072–4. doi: 10.1016/j.physbeh.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tepper BJ, Christensen CM, Cao J. Development of brief methods to classify individuals by PROP taster status. Physiol Behav. 2001;73:571–7. doi: 10.1016/s0031-9384(01)00500-5. [DOI] [PubMed] [Google Scholar]
- 35.Bartoshuk LM, Duffy VB, Lucchina LA, Prutkin J, Fast K. PROP (6-n-propylthiouracil) supertasters and the saltiness of NaCl. Ann N Y Acad Sci. 1998;855:793–6. doi: 10.1111/j.1749-6632.1998.tb10660.x. [DOI] [PubMed] [Google Scholar]
- 36.Ko CW, Hoffman HJ, Lucchina LA, Snyder DJ, Weiffenbach JM, Bartoshuk LM. Differential perceptions of intensity for the four basic taste qualities in PROP supertasters versus nontasters. Chem Senses. 2000;25:639–40. [Google Scholar]
- 37.Hayes JE, Sullivan BS, Duffy VB. Explaining variability in sodium intake through oral sensory phenotype, salt sensation and liking. Physiol Behav. 2010;100:369–80. doi: 10.1016/j.physbeh.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Delwiche JF, Buletic Z, Breslin PAS. Covariation in individuals’ sensitivities to bitter compounds: evidence supporting multiple receptor/transduction mechanisms. Percept Psychophys. 2001;63:761–76. doi: 10.3758/bf03194436. [DOI] [PubMed] [Google Scholar]
- 39.Porubcan AR, Vickers ZM. Characterizing milk aftertaste: the effects of salivation rate, PROP taster status, or small changes in acidity, fat, or sucrose on acceptability of milk to milk dislikers. Food Qual Prefer. 2005;16:608–20. [Google Scholar]
- 40.Lanier SA, Hayes JE, Duffy VB. Sweet and bitter tastes of alcoholic beverages mediate alcohol intake in of-age undergraduates. Physiol Behav. 2005;83:821–31. doi: 10.1016/j.physbeh.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 41.Prescott J, Swain-Campbell N. Responses to Repeated Oral Irritation by Capsaicin, Cinnamaldehyde and Ethanol in PROP Tasters and Non-tasters. Chem Senses. 2000;25:239–46. doi: 10.1093/chemse/25.3.239. [DOI] [PubMed] [Google Scholar]
- 42.Delwiche JF, Buletic Z, Breslin PA. Relationship of papillae number to bitter intensity of quinine and PROP within and between individuals. Physiol Behav. 2001;74:329–37. doi: 10.1016/s0031-9384(01)00568-6. [DOI] [PubMed] [Google Scholar]
- 43.Hayes JE, Pickering GJ. Wine Expertise Predicts Taste Phenotype. 2012;63:80–4. doi: 10.5344/ajev.2011.11050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao L, Kirkmeyer SV, Tepper BJ. A paper screening test to assess genetic taste sensitivity to 6-n-propylthiouracil. Physiol Behav. 2003;78:625–33. doi: 10.1016/s0031-9384(03)00057-x. [DOI] [PubMed] [Google Scholar]
- 45.Smutzer G, Desai H, Coldwell SE, Griffith JW. Validation of Edible Taste Strips for Assessing PROP Taste Perception. Chem Senses. 2013;38:529–39. doi: 10.1093/chemse/bjt023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feeney E, O’Brien S, Markey A, Scannell A, Gibney E. Genetic variation in taste perception - does it have a role in healthy eating? Proc Nutr Soc. 2011;70:135–43. doi: 10.1017/S0029665110003976. [DOI] [PubMed] [Google Scholar]
- 47.Snyder DJ, Fast K, Bartoshuk LM. Valid comparisons of suprathreshold sensations. J Conscious Stud. 2004;11:96–112. [Google Scholar]
- 48.Green BG, Shaffer GS, Gilmore MM. Derivation and Evaluation of a Semantic Scale of Oral Sensation Magnitude with Apparent Ratio Properties. Chem Senses. 1993;18:683–702. [Google Scholar]
- 49.Drewnowski A, Henderson SA, Shore AB. Genetic sensitivity to 6-n-propylthiouracil (PROP) and hedonic responses to bitter and sweet tastes. Chem Senses. 1997;22:27–37. doi: 10.1093/chemse/22.1.27. [DOI] [PubMed] [Google Scholar]
- 50.Drewnowski A, Henderson SA, Barratt-Fornell A. Genetic sensitivity to 6-n-propylthiouracil and sensory responses to sugar and fat mixtures. Physiol Behav. 1998;63:771–7. doi: 10.1016/s0031-9384(97)00540-4. [DOI] [PubMed] [Google Scholar]
- 51.Tepper BJ, Nurse RJ. Fat Perception is Related to PROP Taster Status. Physiol Behav. 1997;61:949–54. doi: 10.1016/s0031-9384(96)00608-7. [DOI] [PubMed] [Google Scholar]
- 52.Yackinous CA, Guinard JX. Relation between PROP taster status and fat perception, touch, and olfaction. Physiol Behav. 2001;72:427–37. doi: 10.1016/s0031-9384(00)00430-3. [DOI] [PubMed] [Google Scholar]
- 53.Lim J, Urban L, Green BG. Measures of Individual Differences in Taste and Creaminess Perception. Chem Senses. 2008;33:493–501. doi: 10.1093/chemse/bjn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Calò C, Padiglia A, Zonza A, Corrias L, Contu P, Tepper BJ, et al. Polymorphisms in TAS2R38 and the taste bud trophic factor, gustin gene co-operate in modulating PROP taste phenotype. Physiol Behav. 2011;104:1065–71. doi: 10.1016/j.physbeh.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 55.Peres RC, Camargo G, Mofatto LS, Cortellazzi KL, Santos MC, Nobre-dos-Santos M, et al. Association of polymorphisms in the carbonic anhydrase 6 gene with salivary buffer capacity, dental plaque pH, and caries index in children aged 7–9 years. Pharmacogenomics J. 2010;10:114–9. doi: 10.1038/tpj.2009.37. [DOI] [PubMed] [Google Scholar]
- 56.Allen AL, McGeary JE, Knopik VS, Hayes JE. Bitterness of the Non-nutritive Sweetener Acesulfame Potassium Varies With Polymorphisms in TAS2R9 and TAS2R31. Chem Senses. 2013;38:379–89. doi: 10.1093/chemse/bjt017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hayes JE, Allen AL, Bennett SM. Direct comparison of the generalized visual analog scale (gVAS) and general labeled magnitude scale (gLMS) Food Qual Prefer. 2013;28:36–44. doi: 10.1016/j.foodqual.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamaguchi S. Synergistic taste effect of monosodium glutamate and disodium 5′-inosinate. J Food Sci. 1967;32:473–8. [Google Scholar]
- 59.Bartoshuk LM, Duffy VB, Reed D, Williams A. Supertasting, earaches and head injury: genetics and pathology alter our taste worlds Neurosci. Biobehav Rev. 1996;20:79–87. doi: 10.1016/0149-7634(95)00042-d. [DOI] [PubMed] [Google Scholar]
- 60.Bae J-W, Kim U-K, Oh S-k, Rhyu MR, Shin SH, Ye M-K. The Relationship between Single Nucleotide Polymorphisms of the Carbonic Anhydrase 6 Gene and Phenylthiocarbamide Taste Sensitivity, Taste Disorder. Korean J Otorhinolaryngol Head Neck Surg. 2012;55:155–60. [Google Scholar]
- 61.Yackinous CA, Guinard JX. Relation between PROP (6-n-propylthiouracil) taster status, taste anatomy and dietary intake measures for young men and women. Appetite. 2002;38:201–9. doi: 10.1006/appe.2001.0481. [DOI] [PubMed] [Google Scholar]
- 62.Prutkin J, Duffy V, Etter L, Fast K, Gardner E, Lucchina L, et al. Genetic variation and inferences about perceived taste intensity in mice and men. Physiol Behav. 2000;69:161–73. doi: 10.1016/s0031-9384(00)00199-2. [DOI] [PubMed] [Google Scholar]
- 63.Essick GK, Chopra A, Guest S, McGlone F. Lingual tactile acuity, taste perception, and the density and diameter of fungiform papillae in female subjects. Physiol Behav. 2003;80:289–302. doi: 10.1016/j.physbeh.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 64.Heinzerling CI, Stieger M, Bult JHF, Smit G. Individually Modified Saliva Delivery Changes the Perceived Intensity of Saltiness and Sourness. Chemosens Percept. 2011;4:145–53. doi: 10.1007/s12078-011-9099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pedersen AM, Bardow A, Jensen SB, Nauntofte B. Saliva and gastrointestinal functions of taste, mastication, swallowing and digestion. Oral Dis. 2002;8:117–29. doi: 10.1034/j.1601-0825.2002.02851.x. [DOI] [PubMed] [Google Scholar]
- 66.Humphrey SP, Williamson RT. A review of saliva: normal composition, flow, and function. J Prosthet Dent. 2001;85:162–9. doi: 10.1067/mpr.2001.113778. [DOI] [PubMed] [Google Scholar]
- 67.Barbarossa IT, Carta G, Murru E, Melis M, Zonza A, Vacca C, et al. Taste sensitivity to 6-n-propylthiouracil is associated with endocannabinoid plasma levels in normal-weight individuals. Nutrition. 2013;29:531–6. doi: 10.1016/j.nut.2012.09.018. [DOI] [PubMed] [Google Scholar]