Abstract
Purpose
To evaluate the accuracy of ultrasonography and magnetic resonance imaging (MRI) in the diagnosis of placenta accreta and to define the most relevant specific ultrasound and MRI features that may predict placental invasion.
Material and Methods
This study was approved by the institutional review board of the French College of Obstetricians and Gynecologists. We retrospectively reviewed the medical records of all patients referred for suspected placenta accreta to two university hospitals from 01/2001 to 05/2012. Our study population included 42 pregnant women who had been investigated by both ultrasonography and MRI. Ultrasound images and MRI were blindly reassessed for each case by 2 raters in order to score features that predict abnormal placental invasion.
Results
Sensitivity in the diagnosis of placenta accreta was 100% with ultrasound and 76.9% for MRI (P = 0.03). Specificity was 37.5% with ultrasonography and 50% for MRI (P = 0.6). The features of greatest sensitivity on ultrasonography were intraplacental lacunae and loss of the normal retroplacental clear space. Increased vascularization in the uterine serosa-bladder wall interface and vascularization perpendicular to the uterine wall had the best positive predictive value (92%). At MRI, uterine bulging had the best positive predictive value (85%) and its combination with the presence of dark intraplacental bands on T2-weighted images improved the predictive value to 90%.
Conclusion
Ultrasound imaging is the mainstay of screening for placenta accreta. MRI appears to be complementary to ultrasonography, especially when there are few ultrasound signs.
Introduction
Placenta accreta is a significant cause of maternal morbidity and mortality and is presently the most common reason for emergency postpartum hysterectomy. It is an abnormal attachment of the placenta to the myometrium, and occurs when a defect of the decidua basalis allows the chorionic villi to invade the myometrium. Placenta accreta is classified on the basis of the depth of myometrial invasion. In placenta accreta vera, villi are attached to the myometrium but do not invade the muscle. In placenta increta, villi partially invade the myometrium. The most severe type is placenta percreta, in which villi penetrate through the entire myometrial thickness or beyond the serosa. Identified risk factors include surgery, placenta previa and previous cesarean section [1], [2].
Its prevalence has risen tenfold in the United States over the past 50 years due to the rising number of cesarean deliveries. Previous cesarean section increases the odds of having placenta accreta about 8.7-fold [3]. As the number of cesarean sections increases, so does the risk. Accurate prenatal identification allows optimal obstetric management, because timing and site of delivery, availability of blood products, and recruitment of a skilled anesthesia and surgical team can be organized in advance [4], [5]. Ultrasonography and magnetic resonance imaging (MRI) have been used for the diagnosis of placenta accreta, but the accuracy of these two imaging techniques remains uncertain and is dependent on the skills of the sonographer or radiologist.
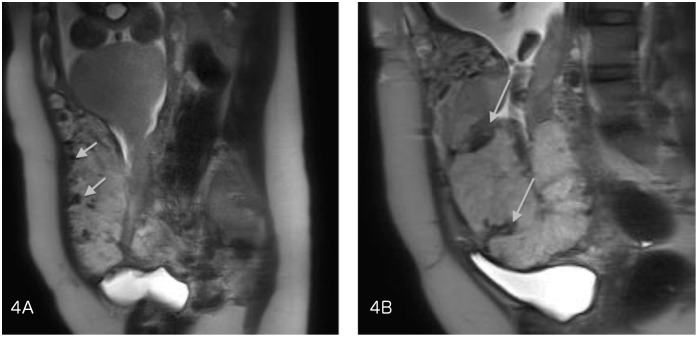
The sonographic characteristics of adherent placenta include: intraplacental lacunae, loss of the normal retroplacental clear space (Figure 1) and thinning or disruption of the hyperechogenic uterine serosa-bladder wall interface (Figure 2). Specific MRI findings in placenta accreta are: uterine bulging (Figure 3), heterogeneous signal intensity within the placenta and dark intraplacental bands on T2-weighted images (Figure 4 A–B).
Figure 1. Loss of the normal retroplacental clear space on ultrasonography.
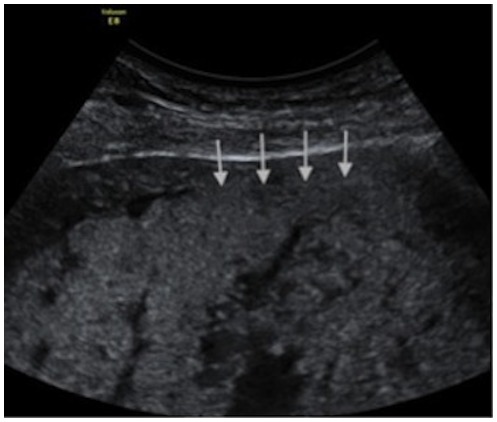
Figure 2. Uterine bulging and disruption of the hyperechogenic uterine serosa-bladder wall interface on ultrasonography.
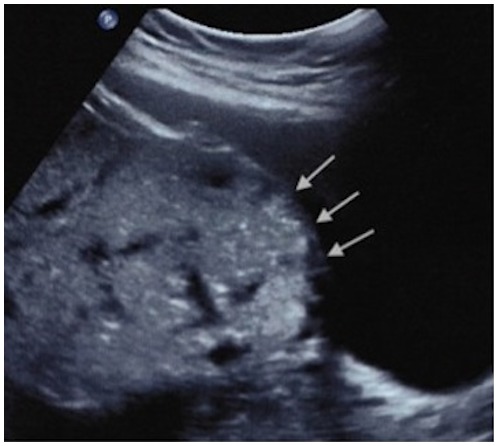
Figure 3. Uterine bulging into the bladder on MRI.
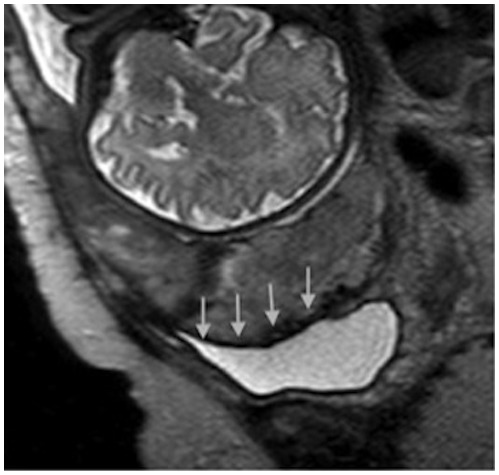
Figure 4. A–B - Dark intraplacental bands on T2-weighted images on MRI.
The purpose of this study was to evaluate the accuracy of ultrasonography and MRI in the diagnosis of placenta accreta and to define the most relevant specific ultrasound and MRI features that may predict placental invasion.
Material and Methods
We retrospectively reviewed the medical records of all patients referred for suspected placenta accreta to two university hospitals from January 2001 to May 2012. This study was approved by the institutional review board of the French College of Obstetricians and Gynecologists (Comité d’éthique de la recherche en gynécologie obstétrique [CEROG]), written informed consent was given by participants. Our study population included 42 pregnant women who had been investigated by both ultrasound and prenatal MRI. Medical chart review was used to record clinical information (Table 1).
Table 1. Clinical information.
| n = 42 | |
| Average age (in years) | 34±4.7 |
| Gravidity | 4.2±2.3 |
| Parity | 2.1±1.5 |
| Previous cesarean delivery (%) | 37 (88%) |
| Average gestational age at the time of diagnosis by ultrasonography (in weeks) | 28. 7 |
| Average gestational age at the time of MRI (in weeks) | 30.8 |
| Placental insertion (%) | |
| Previa | 32 (76.2%) |
| Anterior | 26 |
| Posterior | 7 |
| Low-lying | 5 (11.9%) |
| Anterior | 4 |
| Posterior | 2 |
| Non-low-lying | 5 (11.9%) |
| Anterior | 4 |
| Posterior | 2 |
| Final diagnosis (%) | |
| Placenta accreta/increta | 16 (38%) |
| Placenta percreta | 10 (24%) |
| Non-adherent placenta | 16 (38%) |
| Surgical management at delivery | |
| Vaginal delivery | 2 |
| Conservative management | 1 |
| Hysterectomy | 1 |
| Cesarean delivery | 40 |
| Complete delivery | 13 |
| Incomplete delivery | 3 |
| Conservative management | 14 |
| Cesarean hysterectomy | 10 |
Ultrasound and MRI were performed by obstetricians or radiologists experienced in abnormal adherent placenta. The equipment included the IU 22 system (Philips Medical Systems, Bothell, WA) and the GE Voluson 730 or E8 (GE Medical Systems, Zipf, Austria) with 4–9 MHz or 5–9 MHz transabdominal transducers, and 3–9 MHz and 4–8 MHz endovaginal transducers.
MRI was performed with a 1.5 Tesla scanner (Siemens Magnetom-Avanto, Siemens Magnetom-vision [Siemens Medical Solutions], Philips Achieva). The MRI protocols were similar in both hospitals and included T1-weighted sequences in the sagittal and axial planes, single-shot fast spin-echo T2-weighted MR sequences (HASTE, single shot TSE) and true fast imaging with steady-state precession (TrueFISP, FIESTA) in the axial, sagittal and coronal planes. 7 MRI scans were done after intravenous injection of gadolinium, 6 were MR diffusion-weighted imaging. No fetal sedation was used.
For the purpose of the study, ultrasound images and MRI were blindly reassessed by 2 raters with more than 5 years of experience in the evaluation of placentation disorders. They were blinded to the patient's diagnosis and were asked to score features previously described in the literature as useful for predicting placental invasion.
Placenta accreta was defined by clinical criteria at the time of delivery and by pathologic findings. The placenta was considered normal if it was easily removed during cesarean delivery without any bleeding complications. Ideally, the standard of reference for the diagnosis of abnormal adherent placenta is confirmation of the final histology after hysterectomy has been performed. However, hysterectomy is not always clinically indicated or possible and management should be conservative (decision to leave the placenta to involute in situ if bleeding is controlled). Therefore, in these cases pathologic examination was not available and the diagnosis was based on clinical information provided at the time of delivery and surgery. The placenta was considered as accreta when the delivery was impossible and as percreta when it was evident that the placenta had reached the uterine serosa or the adjacent organs.
Statistical analysis was performed using statistical software (Open Epi and Vassar Stats). The sensitivity (Se), specificity (Sp), positive predictive value (PPV), and negative predictive value (NPV) were calculated for both sonography and MRI. The Se and Sp values of sonography and MRI were compared by means of the McNemar test. Se, Sp, PPV and NPV were calculated for each evaluated ultrasound and MRI feature. A p value <0.05 was considered statistically significant.
Results
42 patients underwent both ultrasound and MRI to explore suspected placenta accreta. Clinical information is shown in Table 1. There were 16 cases of placenta accreta/increta, 10 cases of placenta percreta and 16 cases of non-adherent placenta. Pathologic findings were available for 27 patients. Pathologic examination was not performed in 10 cases because of conservative treatment and in 5 cases because delivery was complete and no postpartum haemorrhage occurred (the five placentas were considered normal).
40 patients had a cesarean delivery and 2 had a vaginal delivery (one medical termination of pregnancy and in one patient vaginal delivery was accepted because MRI wrongfully refuted the diagnosis of placenta accreta suspected at ultrasonography and hemostatic hysterectomy for postpartum hemorrhage had to be performed). 14 women underwent conservative treatment (4 placenta accreta/increta and 10 placenta percreta). 8 had a cesarean hysterectomy and 4 had a hysterectomy later on because of secondary complications.
Sensitivity and Specificity
Ultrasound successfully diagnosed all 26 cases of placenta accreta. In 10 of 16 women finally ascertained to have a normal placenta, ultrasound wrongfully diagnosed adherent placenta.
MRI successfully diagnosed 20 of the 26 cases of placenta accreta and wrongfully diagnosed 8 of the 16 cases of non-adherent placenta as placenta accreta. For one patient, MRI images could not be interpreted because of fetal movements. We considered this case to be wrongly interpreted negative, because there was a failure to identify placenta accreta and the exam was not useful for the clinical management of the patient.
Diagnostic sensitivity for placenta accreta was 100% for ultrasound and 76.9% for MRI (P = 0.03). Specificities were 37.5% for ultrasound and 50% for MRI (P = 0.6). The diagnosis was correct in 76.2% of cases with ultrasonography and in 66.7% with MRI. The positive predictive value was 72.2% for ultrasound and 71.4% for MRI (see Table 2).
Table 2. Sensitivity and specificity of ultrasound and MRI.
| Se | Sp | PPV | NPV | Exact diagnosis | |
| %, (CI) | %, (CI) | %, (CI) | %, (CI) | %, (CI) | |
| Ultrasound | 100 | 37.5 | 72.2 | 100 | 76.2 |
| n = 42 | (87.1–100) | (18–61) | (56–84) | (61–100) | (61–86) |
| MRI | 76.9 | 50 | 71.4 | 57 | 66.7 |
| n = 42 | (58–89) | (28–72) | (52.9–84.7) | (32.6–79) | (51–79) |
| P * McNemar test | 0.03 | 0.6 | NS |
Se = sensitivity, Sp = specificity, PPV = predictive positive value, NPV = negative predictive value.
According to placental insertion, ultrasound correctly diagnosed presence or absence of placenta accreta in 7 cases and MRI in 9 cases of the 11 posterior placenta. So there were no statistical difference between ultrasound and MRI to performe the diagnosis of placenta accreta in case of posterior localization of the placenta (p = 0,26).
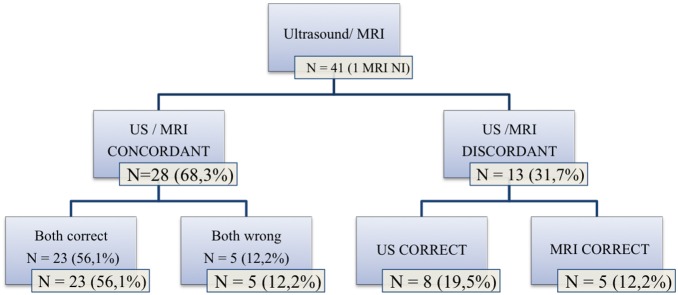
Concordance between Ultrasound and MRI
Ultrasound and MRI were concordant in 28/41 cases (68.3%). In 23 cases, both ultrasound and MRI correctly diagnosed the presence or absence of abnormal adherent placenta (without specifying the depth of the invasion), and in 5 cases both were wrong (5 false-positive diagnoses).
There was disagreement between ultrasound and MRI in 13 cases, and the sonographic diagnosis was correct in 8 of these cases. Five false-negative results given by MRI were correctly diagnosed by ultrasound. Conversely, in 5/13 cases MRI correctly invalidated a diagnosis of placenta accreta suggested by sonography. These results are shown in figure 5.
Figure 5. Concordance between ultrasound and MRI.
When ultrasound and MRI were discordant there were significantly more emergency C-sections and surgeons more ofen attempted placental delivery. However, there was no statistical increase in the rate of cesarean hysterectomy or in the number of blood transfusions. These results are shown in Table 3.
Table 3. Consequences of prenatal discordance between ultrasound and MRI.
| Concordance between ultrasound and MRI | Discordance between ultrasound and MRI | P | |
| n = 28 | n = 13 | ||
| DELIVERY | 0.02 | ||
| Vaginal delivery | 1 (4%) | 1 (8%) | 0.53 |
| Emergency C-section | 11 (39%) | 10 (77%) | 0.04 |
| Planned C-section | 16 (57%) | 2 (15%) | 0.01 |
| SURGICAL MANAGEMENT | 0.056 | ||
| Attempted placental delivery | 8 (29%) | 9 (69%) | 0.01 |
| Conservative management | 14 (50%) | 3 (23%) | NS |
| Cesarean hysterectomy | 6 (21%) | 1 (8%) | NS |
| TRANSFUSION | |||
| Number of blood transfusions | 10 (36%) | 7 (54%) | NS |
| Mean transfused blood volume (in units) | 9.5 | 8 | NS |
Ultrasound and MRI Features
In order to define the most relevant specific ultrasound and MRI features that may predict placental invasion, ultrasound and MRI images were reassessed by 2 raters with more than 5 years of experience in the evaluation of placentation disorders. All ultrasound images were reassessed (n = 42) and 39 MRI exams were reassessed (1 exam was not interpretable because of fetal movement and for 2 patients MRI images could not be retrieved).
When compared with the appearance of the normal placenta on ultrasound and MRI, 5 features were found to differ statistically significantly between patients with placental invasion and those with normal placentation. These features were loss of the normal retroplacental clear space (P = 0.0004), thinning or disappearance of the myometrium (P = 0.01), increased vascularization at the uterine serosa-bladder wall interface (P = 0.01) and vascularization perpendicular to the uterine wall (P = 0.007) on ultrasonography, and uterine bulging (P = 0.04) on MRI.
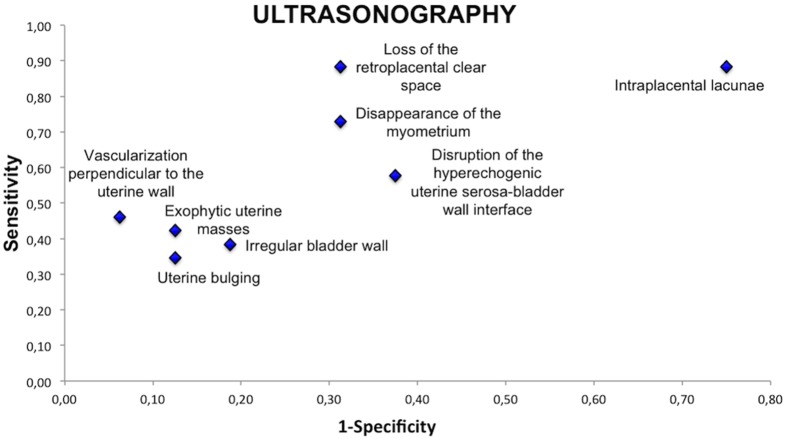
On ultrasonography, features which had better sensitivity for the detection of placental invasion were intraplacental lacunae and loss of the normal retroplacental clear space (sensitivity 88%), which respectively had a specificity of 25% and 69%. Increased vascularization in the uterine serosa-bladder wall interface and vascularization perpendicular to the uterine wall had the best PPV (92%). Loss of the normal retroplacental clear space and a pseudo-tumoral appearance of the placenta had a PPV of 82%.
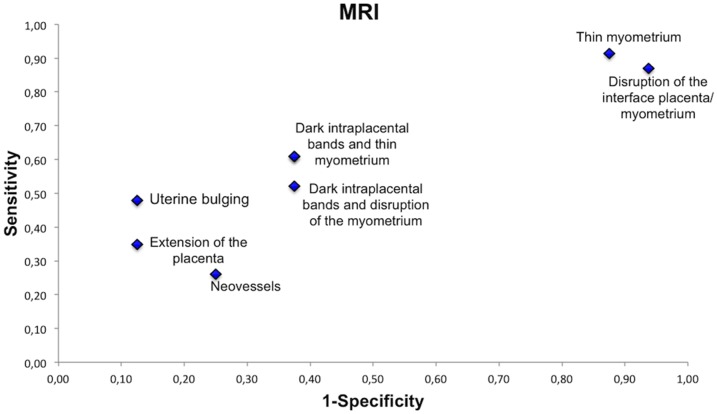
On MRI, thinning or disappearance of the myometrium had the best sensitivity (91%) but a low specificity (13%). Uterine bulging had the best positive predictive value (PPV = 85%), and its combination with the presence of dark intraplacental bands on T2-weighted images improved the predictive value to 90%. A statistically significant difference in the combination of these 2 features was seen between patients with placental invasion and those with normal placentation (P = 0.02). The sensitivity and the predictive values of ultrasound and MRI features are summarized in table 4.
Table 4. Sensitivity and predictive values of ultrasound and MRI features.
| Placentaaccreta/percreta (n = 26) | Non-adherent placenta (n = 16) | P | Se | Sp | PPV | NPV | |
| ULTRASOUND FEATURES | |||||||
| Intraplacental lacunae | 23 | 12 | 0.39 | 88% | 25% | 66% | 57% |
| Loss of the normal retroplacental clear space | 23 | 5 | 0.0004 | 88% | 69% | 82% | 79% |
| Thinning or disappearance of the myometrium | 19 | 5 | 0.01 | 73% | 69% | 79% | 61% |
| Thinning or disruption of the hyperechogenic uterine serosa-bladder wall interface | 15 | 6 | 0.34 | 58% | 63% | 71% | 48% |
| Increased vascularization at the uterine serosa-bladder wall interface | 11 | 1 | 0.01 | 42% | 94% | 92% | 50% |
| Vascularization perpendicular to the uterine wall | 12 | 1 | 0.007 | 46% | 94% | 92% | 52% |
| Exophytic uterine masses | 11 | 2 | 0.08 | 42% | 88% | 85% | 48% |
| Irregular bladder wall | 10 | 3 | 0.3 | 38% | 81% | 77% | 45% |
| Pseudo-tumoral appearance of placenta, uterine bulging | 9 | 2 | 0.15 | 35% | 88% | 82% | 45% |
| MRI FEATURES | n = 23 | n = 16 | |||||
| Uterine bulging | 11 | 2 | 0.04 | 48% | 88% | 85% | 54% |
| Dark intraplacental bands on T2-weighted images | 14 | 6 | 0.2 | 61% | 63% | 70% | 53% |
| Disruption of the interface between placenta and myometrium on T2-weighted images | 20 | 15 | 0.63 | 87% | 6% | 57% | 25% |
| Thinning or disappearance of the myometrium | 21 | 14 | 1 | 91% | 13% | 60% | 50% |
| Extension of the placenta on T2-weighted images | 8 | 2 | 0.15 | 35% | 88% | 80% | 48% |
| Presence of neovessels | 6 | 4 | 1 | 26% | 75% | 60% | 41% |
| Dark intraplacental bands and thinning or disappearance of the myometrium | 14 | 6 | 0.2 | 61% | 63% | 70% | 53% |
| Dark intraplacental bands and disruption of the interface beetwen placenta and myometrium | 12 | 6 | 0.5 | 52% | 63% | 67% | 48% |
| Uterine bulging and dark intraplacental bands | 9 | 1 | 0.02 | 39% | 94% | 90% | 52% |
In order to visualize the sensitivity and specificity of each feature, we represented these values on receiver operating characteristics curves (Figures 6–7). On ultrasonography, the most relevant features were loss of the normal retroplacental clear space, thinning or disappearance of the myometrium and vascularization perpendicular to the uterine wall. On MRI, the most relevant features were uterine bulging and the presence of dark intraplacental bands associated with thinning or disappearance of the myometrium.
Figure 6. Sensitivity and specificity of ultrasound features.
Figure 7. Sensitivity and specificity of MRI features.
7 MRI scans were done after intravenous injection of gadolinium and for 6 patients MR diffusion-weighted imaging was performed in addition to conventional sequences. There were no statistical differences in the accuracy of MRI for the diagnosis of placenta accreta when using gadolinium injection or MR diffusion-weighted imaging.
Discussion
Although ultrasound is the mainstay in the imaging of placenta accreta, MRI has been used as an adjunct in diagnosis when the ultrasound results are equivocal and/or clinical suspicion is high. Overall, in our study, the diagnosis of abnormal attachment of the placenta to the myometrium was correct in 76.2% of cases for Doppler ultrasound and in 66.7% of cases for MRI (difference not significant). In the literature, a mixed performance is observed. The sensitivity of Doppler ultrasound ranges from 33 to 100% and its specificity from 50 to 96%, depending on the study[6]–[19]; and the sensitivity of MRI ranges from 38 to 100% and its specificity from 55 to 100% [7]–[13], [15], [16], [18]–[20].
Three recently published meta-analyses have considered the accuracy of ultrasound for the diagnosis of invasive placentation [13], the use of MRI [14] and a comparison of ultrasound and MRI [18]. D’Antonio et al [13], [14] reported a sensitivity of 90.7% for ultrasound and 94.4% for MRI, and a specificity of 96.9% for ultrasound and 84% for MRI. Meng et al [18] showed that ultrasound sensitivity was 83%, and its specificity was 95%, compared with 82% and 88%, respectively, for MRI. These meta-analyses showed good accuracy of ultrasound and MRI in the diagnosis of placental invasion. They comprised several studies and a large number of patients, but also included studies that were clinically and methodologically varied, and in which ultrasound and MRI were not applied to the same population. This may represent an unavoidable source of bias. The results are only applicable to women with placenta previa and a history of a cesarean delivery or uterine surgery.
These 3 meta-analyses reported that ultrasound and MRI are equally accurate in diagnosing the presence of invasive placentation. We found a statistical difference in sensitivity between MRI and ultrasound, but no difference in specificity or in the percentage of correct diagnoses. This statistical difference might have arisen because only when the placenta was suspected to be adherent on ultrasound was the patient referred for MRI, thus increasing the specificity of MRI and decreasing its sensitivity.
Compared with the literature, we found a better sensitivity but a lower specificity of ultrasound for the diagnosis of placenta accreta, perhaps because, as in Comstock et al. [21], we considered the placenta to be accreta as soon as one feature was present. This increases the number of false positives and reduces the specificity of the test [13], [22].
Several authors found a better performance of MRI compared to ultrasound to diagnose placenta accreta when placenta have a posterior insertion [12], [23]–[25]. Our study did not found difference between these two imaging techniques in this condition.
Many authors consider the presence of intraplacental lacunae to be the best ultrasonography feature [7], [9], [11]–[13], [17], [21], [26]–[28]. In our study, we also found a good sensitivity for this feature, but its specificity and PPV were low. In the presence of this feature we must pay attention to abnormal placentation, especially in the case of low-lying anterior insertion of the placenta and history of cesarean section, but it is not pathognomonic for placenta accreta. Its combination with other features increases its PPV. Lacunae may be present even in women with placenta previa without myometrial invasion [22], [29], but their presence increases the risk of hemorrhage at delivery [30].
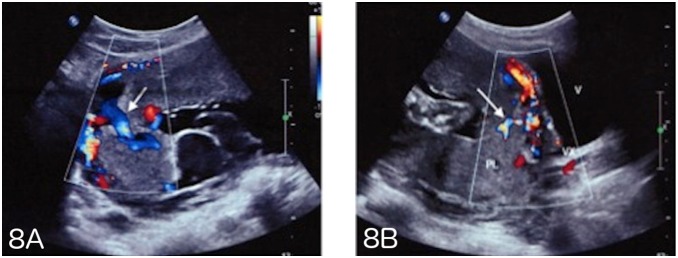
Vascularization perpendicular to the myometrium, a feature used by our teams (Figure 8), had a positive predictive value of 92% and appears to be one of the most discriminating characteristics for the diagnosis of placenta accreta. It reflects the loss of the normal architecture of the vessels of the placenta with intra-placental hypervascularization and chaotic connections. Other authors have also reported that abnormal vascularization seen by color Doppler ultrasound has the best combination of sensitivity and specificity and that its localization at the uterus-bladder interface has the best specificity in the prediction of invasive placentation [13], [15], [22].
Figure 8. A–B - Intraplacental vascularization perpendicular to the myometrium and hypervascularization on ultrasound.
The retroplacental hypoechoic clear zone represents the thickness of the decidua basalis. On ultrasonography, its disappearance literally reflects the histological observation in the case of placenta accreta. The sensitivity and PPV observed for this feature in our study are higher than those found in the literature [13], [21]. It is also the feature with the best NPV in our patient group. Cali et al. found the same results [22]. They underlined that as it had a good NPV, if the echolucent area between the placenta and the uterus is preserved, morbidly adherent placenta is unlikely to occur. It is, however, difficult to see and ideally requires a high-frequency probe oriented perpendicularly to the myometrium/placenta interface and an experienced operator. It is also interesting to measure the distance over which this zone is absent since it can be used to assess the area of abnormal adherent placenta.
As in the literature [14], [20], [22], [23], [31]–[34], we found the best PPV (90%) of MRI when dark intraplacental bands were associated with disappearance of the myometrium and uterine bulging. Lim et al. also showed that the volumes of dark intraplacental bands on T2-weighted images were significantly different in the patients with abnormal placentation and without placenta accreta (p = 0.047), and that band volumes were differed significantly between patients with accreta, increta, and percreta (p<0.0005)[12].
We have evaluated the performance of two imaging techniques used in the prenatal diagnosis of placenta accreta in the same patient population. The accreta or percreta characteristic of the placenta was based on pathological examination, which is more reliable than intraoperative surgical findings. It also specifies the diagnostic value of each feature for both imaging techniques. However, this study is retrospective, implying that the evaluation of ultrasound and MRI imaging features was done retrospectively, but without knowing the final diagnosis. With MRI this did not change the result, but we are aware that for Doppler ultrasound the absence of a dynamic study is a limitation.
We did not show an increased accuracy of MRI when using gadolinium or MR diffusion-weighted imaging. Warshak et al. [9] used gadolinium because they thought that it improved the specificity of the technique as it delineates the outer placental surface proximal to the myometrium more clearly. The European Medicines Agency recommends that contrast MRI be used with caution in pregnant women, and only if the benefits outweigh the risks [35].
Ultrasonography remains the most sensitive and commonly used imaging modality for the diagnosis of placenta accreta, because it is accurate, inexpensive, non-invasive and time-saving. MRI appears to be complementary to ultrasonography, especially when there are few ultrasound signs. In such cases, it is important to assess the value of each feature according to its PPV, but also according to the NPV of absent characteristics. In these situations MRI appears to be helpful because it can reveal signs not visible by ultrasound (dark intraplacental bands, for example) which can be used to confirm or refute the diagnosis of placenta accreta. On the other hand, if there is a strong suspicion of placenta accreta or percreta at Doppler ultrasound, with several signs present with good PPV, the result of the MRI exam should not alter the obstetric management [36]. Because of the possible burden for the patient in the case of placenta accreta, she should be referred to an appropriate institution for perpartum management and the placenta should be considered as accreta when organizing the course of delivery.
Funding Statement
The authors have no support or funding to report.
References
- 1.Bowman ZS, Eller AG, Bardsley TR, Greene T, Varner MW, et al. (2013) Risk Factors for Placenta Accreta: A Large Prospective Cohort. Am J Perinatol. doi: 10.1055/s-0033-1361833. [DOI] [PubMed]
- 2. Fitzpatrick KE, Sellers S, Spark P, Kurinczuk JJ, Brocklehurst P, et al. (2012) Incidence and risk factors for placenta accreta/increta/percreta in the UK: a national case-control study. PLOS ONE 7: e52893 10.1371/journal.pone.0052893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu S, Kocherginsky M, Hibbard JU (2005) Abnormal placentation: twenty-year analysis. Am J Obstet Gynecol 192: 1458–1461 10.1016/j.ajog.2004.12.074 [DOI] [PubMed] [Google Scholar]
- 4. Warshak CR, Ramos GA, Eskander R, Benirschke K, Saenz CC, et al. (2010) Effect of predelivery diagnosis in 99 consecutive cases of placenta accreta. Obstet Gynecol 115: 65–69 10.1097/AOG.0b013e3181c4f12a [DOI] [PubMed] [Google Scholar]
- 5. Tikkanen M, Paavonen J, Loukovaara M, Stefanovic V (2011) Antenatal diagnosis of placenta accreta leads to reduced blood loss. Acta Obstet Gynecol Scand 90: 1140–1146 10.1111/j.1600-0412.2011.01147.x [DOI] [PubMed] [Google Scholar]
- 6. Chou MM, Ho ES, Lee YH (2000) Prenatal diagnosis of placenta previa accreta by transabdominal color Doppler ultrasound. Ultrasound Obstet Gynecol 15: 28–35 10.1046/j.1469-0705.2000.00018.x [DOI] [PubMed] [Google Scholar]
- 7. Millischer-Bellaïche A-E, Grangé G, Adamsbaum C (2009) Imagerie des placentas accretas. Imagerie de la femme 19: 84–88. [Google Scholar]
- 8. Lam G, Kuller J, McMahon M (2002) Use of magnetic resonance imaging and ultrasound in the antenatal diagnosis of placenta accreta. J Soc Gynecol Investig 9: 37–40. [DOI] [PubMed] [Google Scholar]
- 9. Warshak CR, Eskander R, Hull AD, Scioscia AL, Mattrey RF, et al. (2006) Accuracy of ultrasonography and magnetic resonance imaging in the diagnosis of placenta accreta. Obstet Gynecol 108: 573–581 10.1097/01.AOG.0000233155.62906.6d [DOI] [PubMed] [Google Scholar]
- 10. Dwyer BK, Belogolovkin V, Tran L, Rao A, Carroll I, et al. (2008) Prenatal diagnosis of placenta accreta: sonography or magnetic resonance imaging? J Ultrasound Med 27: 1275–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Masselli G, Brunelli R, Casciani E, Polettini E, Piccioni MG, et al. (2008) Magnetic resonance imaging in the evaluation of placental adhesive disorders: correlation with color Doppler ultrasound. Eur Radiol 18: 1292–1299 10.1007/s00330-008-0862-8 [DOI] [PubMed] [Google Scholar]
- 12. Lim PS, Greenberg M, Edelson MI, Bell KA, Edmonds PR, et al. (2011) Utility of ultrasound and MRI in prenatal diagnosis of placenta accreta: a pilot study. AJR Am J Roentgenol 197: 1506–1513 10.2214/AJR.11.6858 [DOI] [PubMed] [Google Scholar]
- 13. D’Antonio F, Iacovella C, Bhide A (2013) Prenatal identification of invasive placentation using ultrasound: systematic review and meta-analysis. Ultrasound Obstet Gynecol 42: 509–517 10.1002/uog.13194 [DOI] [PubMed] [Google Scholar]
- 14.D’Antonio F, Iacovella C, Palacios-Jaraquemada J, Bruno CH, Manzoli L, et al. (2014) Prenatal Identification Of Invasive Placentation Using Magnetic Resonance Imaging (Mri): A Systematic Review And Meta-Analysis. Ultrasound Obstet Gynecol. doi: 10.1002/uog.13327. [DOI] [PubMed]
- 15. Elhawary TM, Dabees NL, Youssef MA (2013) Diagnostic value of ultrasonography and magnetic resonance imaging in pregnant women at risk for placenta accreta. J Matern Fetal Neonatal Med 26: 1443–1449 10.3109/14767058.2013.784740 [DOI] [PubMed] [Google Scholar]
- 16. Peker N, Turan V, Ergenoglu M, Yeniel O, Sever A, et al. (2013) Assessment of total placenta previa by magnetic resonance imaging and ultrasonography to detect placenta accreta and its variants. Ginekol Pol 84: 186–192. [DOI] [PubMed] [Google Scholar]
- 17.Bauwens J, Coulon C, Azaïs H, Bigot J, Houfflin-Debarge V (2014) [Placenta accreta: Can prenatal diagnosis be performed? Ultrasound and MRI interests. About 27 cases.]. Gynecol Obstet Fertil. doi: 10.1016/j.gyobfe.2014.01.009. [DOI] [PubMed]
- 18. Meng X, Xie L, Song W (2013) Comparing the diagnostic value of ultrasound and magnetic resonance imaging for placenta accreta: a systematic review and meta-analysis. Ultrasound Med Biol 39: 1958–1965 10.1016/j.ultrasmedbio.2013.05.017 [DOI] [PubMed] [Google Scholar]
- 19. Maher MA, Abdelaziz A, Bazeed MF (2013) Diagnostic accuracy of ultrasound and MRI in the prenatal diagnosis of placenta accreta. Acta Obstet Gynecol Scand 92: 1017–1022 10.1111/aogs.12187 [DOI] [PubMed] [Google Scholar]
- 20. Lax A, Prince MR, Mennitt KW, Schwebach JR, Budorick NE (2007) The value of specific MRI features in the evaluation of suspected placental invasion. Magn Reson Imaging 25: 87–93 10.1016/j.mri.2006.10.007 [DOI] [PubMed] [Google Scholar]
- 21. Comstock CH, Love JJ Jr, Bronsteen RA, Lee W, Vettraino IM, et al. (2004) Sonographic detection of placenta accreta in the second and third trimesters of pregnancy. Am J Obstet Gynecol 190: 1135–1140 10.1016/j.ajog.2003.11.024 [DOI] [PubMed] [Google Scholar]
- 22. Calì G, Giambanco L, Puccio G, Forlani F (2013) Morbidly adherent placenta: evaluation of ultrasound diagnostic criteria and differentiation of placenta accreta from percreta. Ultrasound Obstet Gynecol 41: 406–412 10.1002/uog.12385 [DOI] [PubMed] [Google Scholar]
- 23. Baughman WC, Corteville JE, Shah RR (2008) Placenta accreta: spectrum of US and MR imaging findings. Radiographics 28: 1905–1916 10.1148/rg.287085060 [DOI] [PubMed] [Google Scholar]
- 24. Chou MM, Tseng JJ, Ho ESC (2002) The application of three-dimensional color power Doppler ultrasound in the depiction of abnormal uteroplacental angioarchitecture in placenta previa percreta. Ultrasound Obstet Gynecol 19: 625–627 10.1046/j.1469-0705.2002.007312.x [DOI] [PubMed] [Google Scholar]
- 25. Levine D, Hulka CA, Ludmir J, Li W, Edelman RR (1997) Placenta accreta: evaluation with color Doppler US, power Doppler US, and MR imaging. Radiology 205: 773–776. [DOI] [PubMed] [Google Scholar]
- 26. Finberg HJ, Williams JW (1992) Placenta accreta: prospective sonographic diagnosis in patients with placenta previa and prior cesarean section. J Ultrasound Med 11: 333–343. [DOI] [PubMed] [Google Scholar]
- 27. Twickler DM, Lucas MJ, Balis AB, Santos-Ramos R, Martin L, et al. (2000) Color flow mapping for myometrial invasion in women with a prior cesarean delivery. J Matern Fetal Med 9: 330–335 doi:;10.1002/1520-6661(200011/12)9: 6<330::AID-MFM1002>3.0.CO;2-O [DOI] [PubMed] [Google Scholar]
- 28. Japaraj RP, Mimin TS, Mukudan K (2007) Antenatal diagnosis of placenta previa accreta in patients with previous cesarean scar. J Obstet Gynaecol Res 33: 431–437 10.1111/j.1447-0756.2007.00549.x [DOI] [PubMed] [Google Scholar]
- 29. Hamada S, Hasegawa J, Nakamura M, Matsuoka R, Ichizuka K, et al. (2011) Ultrasonographic findings of placenta lacunae and a lack of a clear zone in cases with placenta previa and normal placenta. Prenat Diagn 31: 1062–1065 10.1002/pd.2833 [DOI] [PubMed] [Google Scholar]
- 30. Yang JI, Lim YK, Kim HS, Chang KH, Lee JP, et al. (2006) Sonographic findings of placental lacunae and the prediction of adherent placenta in women with placenta previa totalis and prior Cesarean section. Ultrasound Obstet Gynecol 28: 178–182 10.1002/uog.2797 [DOI] [PubMed] [Google Scholar]
- 31. Kayem G, Grangé G, Goffinet F (2007) [Management of placenta accreta]. Gynecol Obstet Fertil 35: 186–192 10.1016/j.gyobfe.2007.01.021 [DOI] [PubMed] [Google Scholar]
- 32. Lerner JP, Deane S, Timor-Tritsch IE (1995) Characterization of placenta accreta using transvaginal sonography and color Doppler imaging. Ultrasound Obstet Gynecol 5: 198–201 10.1046/j.1469-0705.1995.05030198.x [DOI] [PubMed] [Google Scholar]
- 33. Thorp JM Jr, Councell RB, Sandridge DA, Wiest HH (1992) Antepartum diagnosis of placenta previa percreta by magnetic resonance imaging. Obstet Gynecol 80: 506–508. [PubMed] [Google Scholar]
- 34. Derman AY, Nikac V, Haberman S, Zelenko N, Opsha O, et al. (2011) MRI of placenta accreta: a new imaging perspective. AJR Am J Roentgenol 197: 1514–1521 10.2214/AJR.10.5443 [DOI] [PubMed] [Google Scholar]
- 35. Expert Panel on MR Safety, Kanal E, Barkovich AJ, Bell C, Borgstede JP, et al. (2013) ACR guidance document on MR safe practices: 2013. J Magn Reson Imaging 37: 501–530 10.1002/jmri.24011 [DOI] [PubMed] [Google Scholar]
- 36. McLean LA, Heilbrun ME, Eller AG, Kennedy AM, Woodward PJ (2011) Assessing the role of magnetic resonance imaging in the management of gravid patients at risk for placenta accreta. Acad Radiol 18: 1175–1180 10.1016/j.acra.2011.04.018 [DOI] [PubMed] [Google Scholar]