Abstract
Purpose
Stereotactic body radiation therapy (SBRT) is a technically demanding prostate cancer treatment that may be less expensive than intensity-modulated radiation therapy (IMRT). Because SBRT may deliver a greater biologic dose of radiation than IMRT, toxicity could be increased. Studies comparing treatment cost to the Medicare program and toxicity are needed.
Methods
We performed a retrospective study by using a national sample of Medicare beneficiaries age ≥ 66 years who received SBRT or IMRT as primary treatment for prostate cancer from 2008 to 2011. Each SBRT patient was matched to two IMRT patients with similar follow-up (6, 12, or 24 months). We calculated the cost of radiation therapy treatment to the Medicare program and toxicity as measured by Medicare claims; we used a random effects model to compare genitourinary (GU), GI, and other toxicity between matched patients.
Results
The study sample consisted of 1,335 SBRT patients matched to 2,670 IMRT patients. The mean treatment cost was $13,645 for SBRT versus $21,023 for IMRT. In the 6 months after treatment initiation, 15.6% of SBRT versus 12.6% of IMRT patients experienced GU toxicity (odds ratio [OR], 1.29; 95% CI, 1.05 to 1.53; P = .009). At 24 months after treatment initiation, 43.9% of SBRT versus 36.3% of IMRT patients had GU toxicity (OR, 1.38; 95% CI, 1.12 to 1.63; P = .001). The increase in GU toxicity was due to claims indicative of urethritis, urinary incontinence, and/or obstruction.
Conclusion
Although SBRT was associated with lower treatment costs, there appears to be a greater rate of GU toxicity for patients undergoing SBRT compared with IMRT, and prospective correlation with randomized trials is needed.
INTRODUCTION
Stereotactic body radiation therapy (SBRT) is an innovative and aggressively marketed1 form of radiation therapy that is disseminating into national practice for the treatment of prostate cancer. Compared with the more standard intensity-modulated radiation therapy (IMRT), SBRT is technologically more intensive2,3 and delivers higher doses of radiation per treatment, with an entire course of treatment delivered in up to five visits. In comparison, since IMRT typically delivers a complete course of radiation in 7 to 9 weeks, SBRT may be less expensive overall. The accelerated radiation therapy course associated with SBRT may also mean higher radiobiologic doses than standard fractionated IMRT,4 which may lead to greater local cancer control.5
Given the large doses of radiation per treatment and complexity of SBRT compared with IMRT, there is concern regarding its safety,6 particularly because SBRT has disseminated nationally beyond the originating institutions. Early reports from pioneering institutions suggest that SBRT has acceptable acute toxicity and has cancer control outcomes similar to IMRT.7–9 However, although these reports have shown SBRT to be generally safe, late urinary symptom flares have led some investigators at these leading institutions to alter their SBRT technique.10 Given that toxicity from prostate radiation therapy can include symptoms that vary from rectal bleeding to increased urinary frequency to erectile dysfunction, it is unclear whether reports from pioneering institutions accurately reflect differences in toxicity outcomes between SBRT and IMRT as practiced nationally.
Given the cost of IMRT for prostate cancer, there has been interest in SBRT as a potentially more cost-effective treatment.11 This interest is fueled in part by institutional estimates of what is typically charged for SBRT in relation to IMRT.2,3 However, an assessment of Medicare payments for delivery, planning, and management of SBRT has not been performed. Measuring the cost and outcomes of radiation therapy treatment for Medicare beneficiaries is particularly important, given the significant costs associated with prostate cancer treatment and the impact of new treatment technologies in prostate cancer care12 in men older than age 65 years.13 As SBRT disseminates nationally, and in the absence of comparative effectiveness research, it is important to understand what Medicare pays for SBRT treatment, and the comparative toxicity of SBRT and IMRT for prostate cancer as practiced nationally. To fill this knowledge gap, we compared the treatment cost and toxicity outcomes among patients receiving IMRT or SBRT for primary treatment of prostate cancer by using a comprehensive, population-based analysis of a national sample of Medicare beneficiaries with prostate cancer.
METHODS
Data Source and Study Sample
We used the Chronic Conditions Warehouse (CCW), which is a comprehensive database of 100% of Medicare fee-for-service claims for patients with specific conditions, including prostate cancer.14 The Human Investigation Committee of the Yale School of Medicine determined that this study did not constitute human patients research.
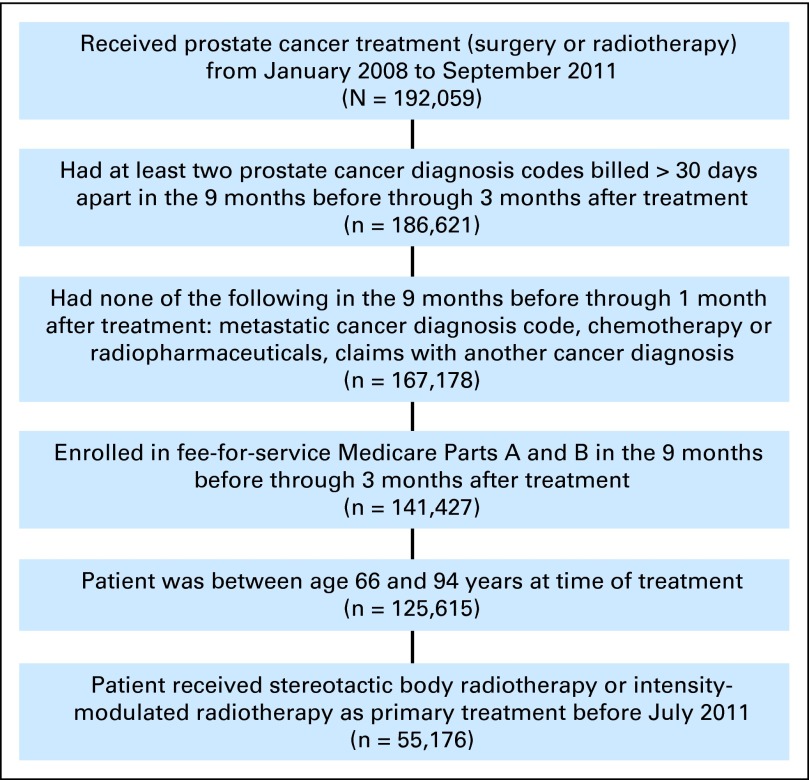
We identified early-stage patients with prostate cancer age 66 to 94 years from Medicare claims submitted from January 2008 through June 201115 (Appendix Figure A1, online only) who received either IMRT or SBRT as primary treatment. We assigned treatment date as the date of first radiation treatment. Patients who did not have Medicare Parts A and B fee-for-service coverage in the 9 months before treatment date were excluded. For the analysis of toxicity at 6, 12, and 24 months, we included only those patients who had sufficient follow-up time and Medicare Parts A and B fee-for-service coverage during follow-up.
Construction of Variables
We identified type of radiation therapy by using Healthcare Common Procedure Coding System (HCPCS) codes. Patients were assigned to the SBRT group if there were any codes for SBRT delivery,15 and patients were assigned to the IMRT group if there were four or more codes for IMRT treatment delivery or if they had the IMRT treatment planning code in addition to four or more generic external beam treatment delivery codes. Although requiring a greater number of treatments would have ensured a more uniform group of patients, we included patients with four or more treatments to err on the side of including all patients who underwent IMRT, regardless of whether they completed treatment or not. In this sense, requiring only four delivery codes for external beam treatment is akin to performing an intention-to-treat analysis with administrative claims. Patients with three or fewer codes for IMRT treatment delivery (1.3% of the sample) were excluded a priori because this number of treatment delivery claims was below what was felt to be reasonably indicative of a full course of treatment.
To be consistent with prior work,15 we searched claims for HCPCS or International Classification of Diseases, ninth revision (ICD-9) procedure or diagnosis codes associated with the following a priori categories of toxicity: genitourinary (GU), GI, and other toxicity (Appendix Table A1, online only). Our primary outcome was whether a patient had a complication from 0 to 6, 0 to 12, or 0 to 24 months after start of treatment. We also investigated whether patients had a complication in the 13- to 24-month time period, given that late (occurring > 1 year after initiation of treatment) toxicity from radiation therapy may be distinct from early toxicity.
Patient characteristics included age, race, residence in a metropolitan county, and median household income at the ZIP Code level. Receipt of an influenza vaccination or visit to a primary care provider between 9 months and 1 month before treatment was recorded to account for access to primary care, which is an important factor in the development and recording of toxicity. Androgen deprivation therapy (ADT) in the 6 months before through 3 months after initiation of radiation therapy was assessed15 by adapting algorithms used in prior studies.16 Comorbidities were identified by searching Medicare claims billed between 9 months and 1 month before treatment date for specific ICD-9 diagnoses that appeared on at least one inpatient or two or more outpatient/physician claims billed more than 30 days apart. We looked for comorbid conditions that we had previously found were statistically significantly associated with survival in a sample of patients without cancer.17
Statistical Analysis
We examined characteristics among all SBRT and IMRT patients and used the χ2 test to assess differences in the distributions between groups. To adjust for treatment selection bias from known confounders, we used Mahalanobis matching18 using age, race, residence in a metropolitan county, comorbidity, receipt of ADT, prior influenza vaccination, prior visit to a primary care provider, and income. We matched two IMRT patients to each SBRT patient; when two or more SBRT patients matched the same IMRT patient, one SBRT patient was randomly selected as a match, and this process was repeated until all SBRT patients had two matched IMRT patients. Matching was done separately for patients with 6, 12, and 24 months of follow-up. We assessed the validity of the matching by using χ2 tests.
To estimate the effect of SBRT on different categories of toxicity, we estimated an adjusted random effects logit model for each of the follow-up periods that included an indicator for whether the patient was treated with SBRT or IMRT and specified the match group as a random effect. Although Mahalanobis matching takes into account all variables simultaneously to match appropriate IMRT patients to SBRT patients, there were slight imbalances between individual variables after the matching. Inclusion of all matching variables in the models resulted in overspecification; therefore, each model was adjusted for age, comorbidity, and use of ADT, which were the variables thought most likely to influence outcomes. For comparisons of the three main categories of toxicity, we used P < .05 to determine statistical significance; however, for the subcategories of toxicity, we assumed a more rigorous level of statistical significance by the Šidák correction.
Calculation of Costs
The per-patient payment by Medicare for SBRT and IMRT was estimated for patients in the 6-month matched sample by summing all radiation therapy planning, management, and delivery codes in the 3 months following treatment initiation. Costs were adjusted to 2011 dollars by using the Consumer Price Index. To assess cancer-related costs, we calculated the difference in total Medicare expenditures between the year before and the year following treatment. We estimated the cost in the year before treatment by tallying the costs in the 12 to 6 months before treatment and multiplying by two. We did this to avoid including any costs associated with the cancer diagnostic and treatment planning phase. To reduce the influence of outliers, all costs were winsorized by setting the cost of all patients above the 97.5 percentile to the value of the 97.5 percentile. Costs of treatment were compared by using the Wilcoxon Mann-Whitney U test. Analysis was performed by using SAS v9.2 (SAS Institute, Cary, NC) and STATA v13 (STATA, College Station, TX).
RESULTS
We identified 53,841 patients who received IMRT and 1,335 patients who received SBRT during the study period with at least 6 months of follow-up (Table 1). SBRT patients were more likely to be white, younger, healthier, from higher income areas, and less likely to undergo ADT, which may indicate less aggressive disease (Table 1). The median number of IMRT treatments was 42 (interquartile range, 40 to 43 treatments). For our analysis of toxicity from 0 to 6 months after initiation of treatment, we successfully matched 1,335 SBRT patients to 2,670 IMRT patients (Table 1). We successfully matched 1,126 SBRT patients with 12 months of follow-up to 2,252 IMRT patients (of 46,295) and 696 SBRT patients with at least 24 months of follow-up to 1,392 IMRT patients (of 29,757; data not shown).
Table 1.
Distribution of Overall and Matched Sample Characteristics Among Patients Who Received SBRT or IMRT
| Characteristic | Overall Sample |
Matched Sample |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SBRT |
IMRT |
P* | SBRT |
IMRT |
P* | |||||
| No. | % | No. | % | No. | % | No. | % | |||
| Total No. | 1,335 | 100.0 | 53,841 | 100.0 | 1,335 | 100.0 | 2,670 | 100.0 | ||
| Age at diagnosis, years | < .01 | 1.00 | ||||||||
| 67-69 | 397 | 29.7 | 12,447 | 23.1 | 397 | 29.7 | 792 | 29.7 | ||
| 70-74 | 464 | 34.8 | 19,021 | 35.3 | 464 | 34.8 | 934 | 35.0 | ||
| 75-79 | 340 | 25.5 | 15,148 | 28.1 | 340 | 25.5 | 676 | 25.3 | ||
| 80-84 | 112 | 8.4 | 5,954 | 11.1 | 112 | 8.4 | 224 | 8.4 | ||
| 85-94 | 22 | 1.6 | 1,271 | 2.4 | 22 | 1.6 | 44 | 1.6 | ||
| Race | < .01 | 1.00 | ||||||||
| White | 1,177 | 88.2 | 45,658 | 84.8 | 1,177 | 88.2 | 2,354 | 88.2 | ||
| Black | 114 | 8.5 | 5,803 | 10.8 | 114 | 8.5 | 228 | 8.5 | ||
| Other | 44 | 3.3 | 2,380 | 4.4 | 44 | 3.3 | 88 | 3.3 | ||
| Metro residence | < .01 | .99 | ||||||||
| Metro | > 1,149† | > 86.1 | 42,337 | 78.6 | > 1,149† | > 86.1 | > 2,311† | > 86.5 | ||
| Nonmetro | 175 | 13.1 | 11,430 | 21.2 | 175 | 13.1 | 347 | 13.0 | ||
| Unknown | < 11† | < 0.8 | 74 | 0.1 | < 11† | < 0.8 | < 11† | < 0.4 | ||
| Comorbidity score (No. of conditions) | < .01 | .99 | ||||||||
| None | 801 | 60.0 | 30,171 | 56.0 | 801 | 60.0 | 1,606 | 60.1 | ||
| 1-2 | 430 | 32.2 | 19,466 | 36.2 | 430 | 32.2 | 856 | 32.1 | ||
| ≥ 3 | 104 | 7.8 | 4,204 | 7.8 | 104 | 7.8 | 208 | 7.8 | ||
| Pretreatment influenza vaccination‡ | 500 | 37.5 | 19,095 | 35.5 | .13 | 500 | 37.5 | 1,000 | 37.5 | 1.00 |
| Pretreatment primary care provider visit‡ | 1,311 | 98.2 | 52,612 | 97.7 | .24 | 1,311 | 98.2 | 2,622 | 98.2 | 1.00 |
| Concomitant androgen deprivation therapy | 148 | 11.1 | 23,789 | 44.2 | < .01 | 148 | 11.1 | 299 | 11.2 | .91 |
| Median household income ($) | < .01 | 1.00 | ||||||||
| ≤ 32,145 | 170 | 12.7 | 10,752 | 20.0 | 170 | 12.7 | 340 | 12.7 | ||
| 32,146-38,374 | 175 | 13.1 | 10,347 | 19.2 | 175 | 13.1 | 351 | 13.1 | ||
| 38,375-46,125 | 215 | 16.1 | 10,407 | 19.3 | 215 | 16.1 | 430 | 16.1 | ||
| 46,126-58,861 | 226 | 16.9 | 10,167 | 18.9 | 226 | 16.9 | 452 | 16.9 | ||
| ≥ 58,862 | 499 | 37.4 | 9,799 | 18.2 | 499 | 37.4 | 997 | 37.3 | ||
| Unknown | 50 | 3.7 | 2,369 | 4.4 | 50 | 3.7 | 100 | 3.7 | ||
NOTE. Matched sample is of patients with at least 6 months of follow-up.
Abbreviations: IMRT, intensity-modulated radiation therapy; SBRT, stereotactic body radiation therapy.
P value calculated by using χ2. All P values are two-sided.
Actual value is not provided because the Centers for Medicare and Medicaid Services prohibits publication of cell sizes < 11.
In the 9 months through 1 month prior to treatment.
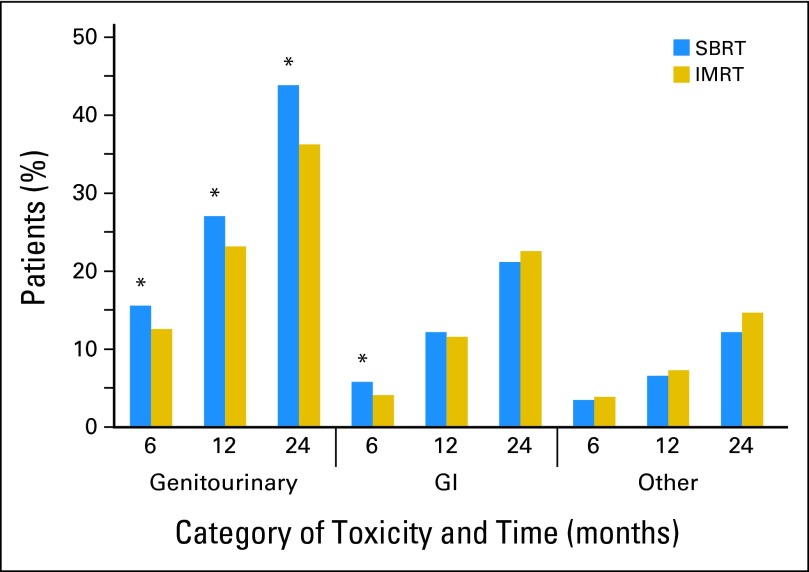
There was increased GU toxicity for SBRT versus IMRT for all time periods (Table 2 and Fig 1). By 6 months after treatment initiation, 15.6% of SBRT patients had a claim indicative of treatment-related GU toxicity versus 12.6% of IMRT patients (odds ratio [OR], 1.29; 95% CI, 1.05 to 1.53; P = .009). By 12 months after treatment initiation, 27.1% of SBRT versus 23.2% of IMRT patients had a claim indicative of GU toxicity (OR, 1.23; 95% CI, 1.03 to 1.43; P = .01), and by 24 months after treatment initiation, 43.9% of SBRT versus 36.3% of IMRT patients had a claim indicative of GU toxicity (OR, 1.38; 95% CI, 1.12 to 1.63; P = .001).
Table 2.
Adjusted Random Effects Logit Model of Categories of Toxicity
| Toxicity Type and Follow-Up Interval (months) | Adjusted Random Effects Logit Model* |
||
|---|---|---|---|
| OR† | 95% CI | P | |
| GU | |||
| 6 | 1.29 | 1.05 to 1.53 | .009 |
| 12 | 1.23 | 1.03 to 1.43 | .01 |
| 24 | 1.38 | 1.12 to 1.63 | .001 |
| GI | |||
| 6 | 1.42 | 1.00 to 1.85 | .02 |
| 12 | 1.06 | 0.82 to 1.29 | .62 |
| 24 | 0.92 | 0.71 to 1.12 | .49 |
| Other | |||
| 6 | 0.89 | 0.57 to 1.20 | .51 |
| 12 | 0.89 | 0.63 to 1.15 | .44 |
| 24 | 0.80 | 0.58 to 1.02 | .11 |
| Any | |||
| 6 | 1.22 | 1.02 to 1.41 | .02 |
| 12 | 1.12 | 0.96 to 1.29 | .13 |
| 24 | 1.16 | 0.94 to 1.37 | .12 |
Abbreviations: GU, genitourinary; OR, odds ratio.
Random effects logit model specified the match group (stereotactic body radiation therapy or intensity-modulated radiation therapy) as a random effect and adjusted for age, comorbidity, and use of androgen deprivation therapy.
OR is for stereotactic body radiation therapy compared with intensity-modulated radiation therapy.
Fig 1.
Rate of treatment-related toxicity by time period for patients receiving stereotactic body radiation therapy (SBRT) versus intensity-modulated radiation therapy (IMRT). (*) Statistically significant difference between SBRT and IMRT.
The increased GU toxicity associated with SBRT was largely due to increased urethra- and bladder-related toxicity (Table 3). When examining subcategories of toxicity, patients who underwent SBRT had significantly more claims indicative of diagnostic procedures for incontinence and obstruction at 6, 12, and 24 months after treatment compared with patients who underwent IMRT, and more claims indicative of a diagnosis of or procedures to correct or investigate urethritis, urethral strictures, and obstruction at 12 and 24 months after treatment. These comparisons were all significant by the level defined by the Šidák correction of P < .003. In addition to the time periods above, there was increased late GU toxicity when examining only those toxicities reported 13 to 24 months after initiation of treatment for SBRT versus IMRT (OR, 1.33; 95% CI, 1.06 to 1.59; P = .005).
Table 3.
Adjusted Random Effects Logit Model of Subcategories of Genitourinary Toxicity
| Toxicity | Duration of Follow-Up |
|||||
|---|---|---|---|---|---|---|
| 6 Months |
12 Months |
24 Months |
||||
| OR* | P† | OR* | P† | OR* | P† | |
| Diagnostic procedures to investigate incontinence or obstruction | 1.80 | < .001 | 1.64 | < .001 | 2.23 | < .001 |
| Urethritis, urethral strictures, and bladder outlet obstruction | 1.25 | .14 | 1.45 | .002 | 1.78 | < .001 |
| Therapeutic procedures to correct urinary incontinence | 0.71 | .22 | 1.00 | 1.00 | 1.33 | .09 |
| Other genitourinary toxicity | 0.77 | .45 | 1.14 | .58 | 0.73 | .23 |
| Infections | 1.01 | .99 | 2.30 | .11 | 2.42 | .15 |
| Erectile dysfunction | 1.46 | .03 | 1.15 | .28 | 1.13 | .35 |
Abbreviation: OR, odds ratio.
Stereotactic body radiation therapy compared with intensity-modulated radiation therapy.
P values are from adjusted random effects logit model based on the Šidák correction for multiple comparisons; P < .003 is significant (significant values shown in bold).
There was increased GI toxicity for SBRT versus IMRT at 6 months, with 5.8% of SBRT patients having had a claim indicative of GI toxicity versus 4.1% of IMRT patients (OR, 1.42; 95% CI, 1.00 to 1.85; P = .02; Table 2). However, by 12 months, there was no difference, with 12.2% of SBRT versus 11.6% of IMRT patients having had GI toxicity (OR, 1.06; 95% CI, 0.82 to 1.29; P = .62). There was also no difference at 24 months, with 21.2% of SBRT versus 22.6% of IMRT patients having had GI toxicity (OR, 0.92; 95% CI, 0.71 to 1.12; P = .49). There were no subcategories of GI complications that were significantly different for SBRT versus IMRT.
There was no difference in other (ie, non-GU and non-GI) toxicity at any time point, with 12.2% of SBRT patients and 14.7% of IMRT patients having another complication within 24 months post-treatment (OR, 0.80; 95% CI, 0.58 to 1.02; P = .11). The overall toxicity rate was significantly increased for SBRT versus IMRT at 6 months but was not significantly different at 12 and 24 months.
The mean per-patient cost to Medicare for a full course of SBRT was $13,645 (95% CI, $13,370 to $13,921), whereas the cost of a course of IMRT was $21,023 (95% CI, $20,780 to $21,265; P < .001; Table 4). The mean cost of the two subcategories of complications (diagnostic procedures to investigate incontinence or obstruction, urethritis, urethral strictures, and bladder outlet obstructions) was $145 (95% CI, $69 to $221) for SBRT and $69 (95% CI, $44 to $95; P < .001) for IMRT. The mean cost of nonradiation cancer-related care in the year following SBRT treatment was $2,963 (95% CI, $2,295 to $3,630) versus $1,978 (95% CI, $1,535 to $2,420; P < .001) for IMRT.
Table 4.
Cancer-Related, Radiation, Nonradiation Cancer-Related, and Complication Costs ($)
| Treatment | Mean Cancer-Related Cost ($)* | 95% CI ($) | Mean Radiation Cost ($)* | 95% CI ($) | Mean Nonradiation Cancer-Related Cost ($)* | 95% CI ($) | Mean Complication Cost ($)* | 95% CI ($) |
|---|---|---|---|---|---|---|---|---|
| SBRT | 16,608 | 15,878 to 17,338 | 13,645 | 13,370 to 13,921 | 2,963 | 2,295 to 3,630 | 145 | 69 to 221 |
| IMRT | 23,000 | 22,505 to 23,496 | 21,023 | 20,780 to 21,265 | 1,978 | 1,535 to 2,420 | 69 | 44 to 95 |
Abbreviations: IMRT, intensity-modulated radiation therapy; SBRT, stereotactic body radiation therapy.
Wilcoxon Mann-Whitney U test P < .001.
DISCUSSION
In a national sample of men receiving radiation therapy for treatment of prostate cancer, SBRT was associated with increased GU toxicity compared with IMRT in the first 2 years following treatment. The increased GU toxicity was present at all time points and indicated an increase in both acute and later toxicity. This increase was largely associated with a higher rate of urethritis, urethral strictures, urinary incontinence, and obstruction among patients receiving SBRT. Although randomized trials comparing different fractionation schema are currently underway,19–22 it may be several years before the insights from these clinical trials are known, although it is possible that data may be available within 2 years. As we await the results of these forthcoming trials, patients are still in need of comparative data. Furthermore, complementary to randomized trial data, observational study data explores clinical outcomes in community practice.
The finding of increased GU toxicity for SBRT in comparison to IMRT is plausible and is consistent with some clinical reports to date.8,10,23 Although current SBRT technique may be able to minimize radiation dose to adjacent normal tissue through the use of prostate immobilization and targeting, the typical technique in use during the study period did not avoid irradiating the urethra, given concerns of local recurrence.10,23 Clinical reports have hinted at increased late urinary complications for SBRT; King et al23 noted urethral strictures requiring dilation and cystoscopy in 3.5% (two of 57) of patients who underwent prostate SBRT, which is higher than what has been reported for IMRT (0.5%).24 In contrast, although Chen et al10 noted a late urinary symptom flare occurring at a median of 9 months (range, 5 to 22 months) post-SBRT, the majority of symptoms resolved with conservative management, and other investigators have noted low toxicity overall for SBRT.25 Despite conflicting reports, given the potentially high doses of radiation delivered to the urethra and bladder neck by SBRT, our finding of greater GU toxicities for SBRT remains plausible.
Despite our finding of increased toxicity, it is still possible that SBRT may be preferable to IMRT for both insurers and patients. Given that a late risk of fistula is a concern for SBRT, it is important to note that we found no statistically significant difference in the incidence of fistulas between IMRT and SBRT at any time point. First, SBRT is a more convenient treatment given its shorter treatment length. Second, we found that despite an increased cost of complications and medical care, SBRT was still less expensive than IMRT overall. Third, SBRT may be more effective than IMRT in terms of cancer cure and so may be preferable in the long-term when accounting for the morbidity of cancer recurrence.2 Other studies support this possibility—that despite increased toxicity of SBRT in comparison to IMRT, SBRT still may be more cost-effective.3,26
Our study has several limitations. First, although we were able to measure the relative frequency of Medicare claims of treatment-related toxicity, the grading of toxicity was not possible. Second, although we detected toxicities that required intervention or medical diagnosis, we could not detect milder toxicities that did not require medical intervention. Third, we included diagnostic procedures such as cystourethroscopy and complex uroflometry as indicative of treatment-related toxicity. This was done because we felt that an increased rate of these claims in a matched sample of patients indicated a potentially clinically significant finding. Fourth, there may have been unmeasured confounders, in particular, differences in baseline GU and GI function, prostate gland volume, cancer histology, and stage that we could not adjust for; however, we believe that these unknown confounders would likely have been biased in favor of SBRT because, during the period of study, SBRT was initially adopted for low-risk disease.23 For example, since patients who underwent IMRT were more likely to undergo ADT, it is likely that patients in the IMRT group had higher-risk disease. Therefore, patients with IMRT were probably more likely to undergo pelvic radiation therapy, a more toxic technique. Fifth, we lacked treatment-related information such as the actual dose of radiation therapy and fields used (including the use of pelvic radiation therapy) and dose volume constraints used in treatment. Furthermore, it is possible that not all SBRT techniques are the same and that there may be differences in toxicity between treatment platforms that we were not able to take into account.10 Finally, it is unclear whether 24 months is sufficient to capture all late toxicities. Although current literature indicates that patients treated with at least 5 years of follow-up are unlikely to have new increments in patient toxicity between 2 and 5 years after treatment,27 late radiation toxicity can still arise during this time,28 necessitating longer follow-up before definite conclusions can be made.
Given the rapid pace of innovation and the relative novelty of prostate SBRT, SBRT technique may have improved in recent years, potentially reducing the difference in toxicity between SBRT and IMRT. For example, Chen et al10 have already taken steps to reduce GU toxicity related to SBRT by recommending that instrumentation of the urethra be limited as much as possible and by modifying their institutional protocol to deliver a maximum of 110% of the prescription dose to the urethra. Others have also demonstrated that it is theoretically possible to reduce urethral doses with careful dosimetry,29 although concerns about reducing cancer control remain.30 In addition, given the technical complexity of SBRT, there may be both a physician and an institutional learning curve for using SBRT for the treatment of prostate cancer, with improved outcomes that can come only from direct clinical experience. Regardless, as radiation therapy techniques improve, and as practitioners become more experienced with SBRT, the comparison between SBRT and IMRT is a moving target. Therefore, up-to-date prospective quality-of-life comparisons are needed.
In conclusion, although SBRT is less expensive, we found increased GU toxicity for men undergoing SBRT compared with standard IMRT for treatment of prostate cancer in a national sample of Medicare beneficiaries. Prospective studies are urgently needed to investigate the risks and benefits of SBRT relative to its lower cost compared with IMRT.
Acknowledgment
We thank Brigette A. Davis, Cancer Outcomes, Public Policy, and Effectiveness Research Center at Yale, for her assistance in preparing the manuscript. We thank General Dynamics Information Technology (Buccaneer Computer Systems and Service) and Chronic Conditions Data Warehouse (under contract with the Centers for Medicare and Medicaid Services).
Glossary Terms
- comparative effectiveness research:
the generation and synthesis of evidence that compares the benefits and harms of alternative methods to prevent, diagnose, treat, and monitor a clinical condition or to improve the delivery of care. The purpose of comparative effectiveness research is to assist consumers, clinicians, purchasers, and policy makers to make informed decisions that will improve health care at the individual and population levels.
- intensity-modulated radiation therapy:
radiation treatment using beams with nonuniform fluence profiles that shape the dose distribution in the target volume and adjacent normal structures. Beam modulation is typically achieved via multileaf collimators or custom-milled compensators to achieve the appropriate fluence profiles calculated by inverse optimization algorithms. The radiation beam is divided into beamlets of varying intensity such that the sum from multiple beams via inverse planning results in improved tumor targeting and normal tissue sparing. A technique of radiation therapy delivery in which the intensity of each beamlet of radiation coming from a specific angle can be adjusted to provide a desired dose distribution when the doses delivered from all beamlets are added from a single angle and from all dose delivery angles. An advanced type of high-precision radiotherapy, which aims to improve the coverage of the radiotherapy target and/or minimize radiation dose to surrounding normal tissue.
Appendix
Table A1.
Procedure and Diagnosis Codes Used to Assess Toxicity
| Toxicity | Healthcare Common Procedure Coding System | International Classification of Diseases, 9th revision |
|
|---|---|---|---|
| Procedure | Diagnosis | ||
| Genitourinary complications | |||
| Infection | 59.1x, 59.2, 59.8x, 59.9, 604.0 | ||
| Upper urinary tract dysfunction (hydronephrosis, lymphocele, ureter or kidney dysfunction) | 50900, 50940, 50120, 50395, 50600, 50605, 50700, 50715, 50760, 50770, 50780, 50782, 50783, 50785, 50800, 50810, 50815, 50820, 50825, 50840, 52290, 52332, 52334 | 55.02, 55.03, 55.12, 55.93, 55.94, 56.1, 56.41, 56.74, 56.75, 56.81, 56.86, 56.89, 56.91, 59.93, 97.61, 97.62 | 591, 593.3, 593.4, 596.6, 997.5, 593.5,* 457.8, 593.8x* |
| Urethral stricture, obstruction | 50040, 50125, 50390, 50392, 50393, 50394, 50398, 50572, 50951, 50953, 50970, 50972, 51010, 51040, 51520, 51600, 51610, 51705, 51800, 51820, 51880, 52000, 52005, 52214, 52275, 52276, 52281, 52282, 52283, 52310, 52335, 52450, 52500, 52510, 52601, 52612, 52614, 52620, 52630, 52640, 53000, 53010, 53020, 53400, 53405, 53410, 53415, 53420, 53425, 53600, 53601, 53605, 53620, 53621, 53640 | 56.31, 57.0, 57.1, 57.12, 57.17, 57.18, 57.19, 57.32, 57.85, 57.89, 57.91, 57.92, 57.93, 57.94, 57.95, 58.0, 58.1, 58.44, 58.46, 58.47, 58.5, 58.6, 59.8, 59.94, 6.95, 97.63, 97.64, 97.69, 97.65, 57.2x, 57.4x, 58.2x, 58.3x | 596.0, 598.x |
| Incontinence/obstruction, diagnostic | 51725, 51726, 51736, 51741, 51772, 51784, 51785, 51792, 51795, 51797 | 89.21, 89.22, 89.23, 89.24, 89.25 | |
| Incontinence, therapeutic procedures or diagnosis codes | 11950, 11951, 11952, 11954, 51715, 51840, 51841, 53440, 53442, 53443, 53444, 53445, 53446, 53447, 53449, 90911, A4335,* A5149,* E0740, L8603 | 58.93, 58.99, 59.3, 59.4, 59.5, 59.6, 59.7x | 599.82,* 788.3x* |
| Erectile dysfunction, treatment or diagnosis codes | 54400, 54401, 54405, C1813, C2622, C3500, L7900, J0270, J0275, J2440, J2760 | 64.95, 64.97 | 607.84* |
| GI complications | |||
| Fistula | 45800, 45805, 45820, 45825, 50920, 50930, 53520 | 48.73, 48.93, 56.84, 57.82, 57.83, 57.84, 58.43 | 569.81, 596.1, 596.2, 599.1, 998.6, 565.x |
| Rectal repair | 45562, 45563 | 48.31, 48.32, 48.33 | |
| Stenosis | 45500, 45905, 45910, 46700 | 96.22, 96.23 | |
| Colectomy/rectal resection | 44140, 44141, 44143, 44144, 44145, 44146, 44147, 44150, 44151, 44152, 44153, 44155, 44156, 44157, 44158, 44160, 44204, 44205, 44206, 44207, 44208, 44209, 44210, 44211, 44212, 45110, 45111, 45112, 45113, 45114, 45116, 45119, 45123 | 46.03, 46.04, 49.6, 48.4x, 48.5x, 48.6x, 48.7x, 46.1x, 49.7x | |
| Other | 56.1,* 56.9,* 569.41,* 997.4,* 555.x,* 557.x,* 558.x,* 56.8x,* 569.x* | ||
| Other complications | |||
| Wound complication/damage to skeletal muscle, connective tissue, bone | 26990, 27120, 27122, 27125, 27126, 27130, 27132, 27134, 27137, 27138, 27234, 27242, 99183 | 54.0, 54.91, 59.19, 93.95, 54.1x, | 457.1,* 457.8,* 595.89, 682.2, 998.83, 998.3x, |
| RBC transfusion | P9021 | 99.0x | |
| Systemic derangements | 584.x | ||
| Infection potentially related to treatment | 45000, 45005, 45020, 49060, 51080 | 79.7, 998.59, 566.x, 567.x | |
| Nerve lesions/injuries | 353,* 353.1,* 353.4,* 353.5,* 353.8,* 353.9,* 355.0,* 355.2,* 355.79,* 953.x, 956.x | ||
| Fractures | 27187, 27193, 27194, 27200, 27202, 27210, 27214, 27215, 27216, 27217, 27218, 27220, 27222, 27224, 27225, 27226, 27227, 27228, 27230, 27232, 27235, 27236, 27238, 27240, 27244, 27245, 27246, 27248, 27255, 27256, 27257, 27258, 27259, 27495 | ||
Indicates this code could be for a potentially preexisting condition. If a patient had this code during the complication assessment period and also had this code during the 9 months prior to start of treatment, it was not considered a complication.
Fig A1.
Sample selection.
Footnotes
See accompanying editorial on page 1183
Supported by Grant No. R01CA149045 from the National Cancer Institute, National Institutes of Health (NIH), by Clinical and Translational Science Award Program Grant No. KL2 RR024138 from the National Center for Advancing Translational Science (J.B.Y.), and by the NIH Roadmap for Medical Research.
The study sponsor (National Institutes of Health) did not play a role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: James B. Yu, 21st Century Oncology; Cary P. Gross, 21st Century Oncology, Medtronic Expert Testimony: None Patents, Royalties, and Licenses: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: James B. Yu, Cary P. Gross
Administrative support: Pamela R. Soulos, Cary P. Gross
Collection and assembly of data: Laura D. Cramer, Pamela R. Soulos
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Bentzen SM, Wasserman TH. Balancing on a knife's edge: Evidence-based medicine and the marketing of health technology. Int J Radiat Oncol Biol Phys. 2008;72:12–14. doi: 10.1016/j.ijrobp.2008.05.044. [DOI] [PubMed] [Google Scholar]
- 2.Hodges JC, Lotan Y, Boike TP, et al. Cost-effectiveness analysis of stereotactic body radiation therapy versus intensity-modulated radiation therapy: An emerging initial radiation treatment option for organ-confined prostate cancer. J Oncol Pract. 2012;8:e31s–e37s. doi: 10.1200/JOP.2012.000548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sher DJ, Parikh R, Mays-Jackson S, et al. Cost-effectiveness analysis of SBRT versus IMRT for low-risk prostate cancer. Am J Clin Oncol. doi: 10.1097/COC.0b013e31827a7d2a. [epub ahead of print on December 27, 2012] [DOI] [PubMed] [Google Scholar]
- 4.Cho LC, Timmerman R, Kavanagh B. Hypofractionated external-beam radiotherapy for prostate cancer. Prostate Cancer. 2013;2013:103547. doi: 10.1155/2013/103547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jabbari S, Weinberg VK, Shinohara K, et al. Equivalent biochemical control and improved prostate-specific antigen nadir after permanent prostate seed implant brachytherapy versus high-dose three-dimensional conformal radiotherapy and high-dose conformal proton beam radiotherapy boost. Int J Radiat Oncol Biol Phys. 2010;76:36–42. doi: 10.1016/j.ijrobp.2009.01.029. [DOI] [PubMed] [Google Scholar]
- 6.Zaorsky NG, Studenski MT, Dicker AP, et al. Stereotactic body radiation therapy for prostate cancer: Is the technology ready to be the standard of care? Cancer Treat Rev. 2013;39:212–218. doi: 10.1016/j.ctrv.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Webb S. Conformal intensity-modulated radiotherapy (IMRT) delivered by robotic linac: Testing IMRT to the limit? Phys Med Biol. 1999;44:1639–1654. doi: 10.1088/0031-9155/44/7/305. [DOI] [PubMed] [Google Scholar]
- 8.King CR, Brooks JD, Gill H, et al. Stereotactic body radiotherapy for localized prostate cancer: Interim results of a prospective phase II clinical trial. Int J Radiat Oncol Biol Phys. 2009;73:1043–1048. doi: 10.1016/j.ijrobp.2008.05.059. [DOI] [PubMed] [Google Scholar]
- 9.Freeman DE, King CR. Stereotactic body radiotherapy for low-risk prostate cancer: Five-year outcomes. Radiat Oncol. 2011;6:3. doi: 10.1186/1748-717X-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen LN, Suy S, Uhm S, et al. Stereotactic body radiation therapy (SBRT) for clinically localized prostate cancer: The Georgetown University experience. Radiat Oncol. 2013;8:58. doi: 10.1186/1748-717X-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aneja S, Pratiwadi RR, Yu JB. Hypofractionated radiation therapy for prostate cancer: Risks and potential benefits in a fiscally conservative health care system. Oncology (Williston Park) 2012;26:512–518. [PubMed] [Google Scholar]
- 12.Makarov DV, Yu JB, Desai RA, et al. The association between diffusion of the surgical robot and radical prostatectomy rates. Med Care. 2011;49:333–339. doi: 10.1097/MLR.0b013e318202adb9. [DOI] [PubMed] [Google Scholar]
- 13.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 14.The National Association for Proton Therapy. 2012. http://www.proton-therapy.org.
- 15.Yu JB, Soulos PR, Herrin J, et al. Proton versus intensity-modulated radiotherapy for prostate cancer: Patterns of care and early toxicity. J Natl Cancer Inst. 2013;105:25–32. doi: 10.1093/jnci/djs463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shahinian VB, Kuo YF, Gilbert SM. Reimbursement policy and androgen-deprivation therapy for prostate cancer. N Engl J Med. 2010;363:1822–1832. doi: 10.1056/NEJMsa0910784. [DOI] [PubMed] [Google Scholar]
- 17.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Rubin DB. Bias reduction using Mahalanobis-metric matching. Biometrics. 1980;36:293–298. [Google Scholar]
- 19.Clinicaltrials.gov. Radiation Therapy in Treating Patients With Prostate Cancer ( NCT01434290) 2013. http://clinicaltrials.gov/show/NCT01434290.
- 20.Clinicaltrials.gov. Radiation Hypofractionation Via Extended Versus Accelerated Therapy (HEAT) For Prostate Cancer ( NCT01794403) 2013. http://clinicaltrials.gov/ct2/show/NCT01794403.
- 21.Clinicaltrials.gov. Prostate Advances in Comparative Evidence ( NCT01584258) 2013. http://www.clinicaltrials.gov/ct2/show/NCT01584258.
- 22.Current Controlled Trials. Phase III Study of Hypofractionated Radiotherapy of Intermediate Risk Localized Prostate Cancer (ISRCTN45905321) 2013. http://www.controlled-trials.com/ISRCTN45905321.
- 23.King CR, Brooks JD, Gill H, et al. Long-term outcomes from a prospective trial of stereotactic body radiotherapy for low-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2012;82:877–882. doi: 10.1016/j.ijrobp.2010.11.054. [DOI] [PubMed] [Google Scholar]
- 24.Zelefsky MJ, Fuks Z, Hunt M, et al. High-dose intensity modulated radiation therapy for prostate cancer: Early toxicity and biochemical outcome in 772 patients. Int J Radiat Oncol Biol Phys. 2002;53:1111–1116. doi: 10.1016/s0360-3016(02)02857-2. [DOI] [PubMed] [Google Scholar]
- 25.Loblaw A, Cheung P, D'Alimonte L, et al. Prostate stereotactic ablative body radiotherapy using a standard linear accelerator: Toxicity, biochemical, and pathological outcomes. Radiother Oncol. 2013;107:153–158. doi: 10.1016/j.radonc.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 26.Gray PJ, Paly JJ, Yeap BY, et al. Patient-reported outcomes after 3-dimensional conformal, intensity-modulated, or proton beam radiotherapy for localized prostate cancer. Cancer. 2013;119:1729–1735. doi: 10.1002/cncr.27956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.King CR, Collins S, Fuller D, et al. Health-related quality of life after stereotactic body radiation therapy for localized prostate cancer: Results from a multi-institutional consortium of prospective trials. Int J Radiat Oncol Biol Phys. 2013;87:939–945. doi: 10.1016/j.ijrobp.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 28.Odrazka K, Dolezel M, Vanasek J, et al. Time course of late rectal toxicity after radiation therapy for prostate cancer. Prostate Cancer Prostatic Dis. 2010;13:138–143. doi: 10.1038/pcan.2009.56. [DOI] [PubMed] [Google Scholar]
- 29.Fuller DB, Naitoh J, Lee C, et al. Virtual HDR CyberKnife treatment for localized prostatic carcinoma: Dosimetry comparison with HDR brachytherapy and preliminary clinical observations. Int J Radiat Oncol Biol Phys. 2008;70:1588–1597. doi: 10.1016/j.ijrobp.2007.11.067. [DOI] [PubMed] [Google Scholar]
- 30.Vainshtein J, Abu-Isa E, Olson KB, et al. Randomized phase II trial of urethral sparing intensity modulated radiation therapy in low-risk prostate cancer: Implications for focal therapy. Radiat Oncol. 2012;7:82. doi: 10.1186/1748-717X-7-82. [DOI] [PMC free article] [PubMed] [Google Scholar]