Abstract
Purpose
The first generation of childhood cancer survivors is now aging into their fourth and fifth decades of life, yet health risks across the aging spectrum are not well established.
Methods
Analyses included 14,359 5-year survivors from the Childhood Cancer Survivor Study, who were first diagnosed when they were younger than 21 years old and who received follow-up for a median of 24.5 years after diagnosis (range, 5.0 to 39.3 years) along with 4,301 of their siblings. Among the survivors, 5,604 were at least 35 years old (range, 35 to 62 years) at last follow-up. Severe, disabling, life-threatening, and fatal health conditions more than 5 years from diagnosis were classified using the Common Terminology Criteria for Adverse Events, grades 3 to 5 (National Cancer Institute).
Results
The cumulative incidence of a severe, disabling, life-threatening, or fatal health condition was greater among survivors than siblings (53.6%; 95% CI, 51.5 to 55.6; v 19.8%; 95% CI, 17.0 to 22.7) by age 50 years. When comparing survivors with siblings, hazard ratios (HR) were significantly increased within the age group of 5 to 19 years (HR, 6.8; 95% CI, 5.5 to 8.3), age group of 20 to 34 years (HR, 3.8; 95% CI, 3.2 to 4.5), and the ≥ 35 years group (HR, 5.0; 95% CI, 4.1 to 6.1), with the HR significantly higher among those ≥ 35 years versus those 20 to 34 years old (P = .03). Among survivors who reached age 35 years without a previous grade 3 or 4 condition, 25.9% experienced a subsequent grade 3 to 5 condition within 10 years, compared with 6.0% of siblings (P < .001).
Conclusion
Elevated risk for morbidity and mortality among survivors increases further beyond the fourth decade of life, which affects the future clinical demands of this population relative to ongoing surveillance and interventions.
INTRODUCTION
Improvement in survival rates of childhood cancer over the last half-century is one of the major achievements of modern medicine. The relative 5-year survival for children with cancer, which was less than 30% in 1960, is now over 80%.1 There are currently more than 363,000 survivors of childhood cancer in the United States.1 The impact of this growing number of cancer survivors is particularly apparent when one considers that one in 680 people between the ages of 20 and 50 years in the United States is a survivor of childhood cancer.2
Previous research has documented that childhood cancer survivors are at increased risk for a broad range of serious health conditions.3–6 However, it is not clear what the prevalence of adverse health conditions will be as this at-risk population ages into middle and late adulthood. Our current analysis was undertaken to address two questions that are important for the clinical care of aging childhood cancer survivors: what is the risk of future serious health problems for survivors who developed at least one serious health condition during their childhood or young adult years, and do survivors who have reached their third decade of life without developing a serious condition have an elevated risk of serious morbidity? The Childhood Cancer Survivor Study (CCSS) performed follow-up for more than 7,600 survivors and siblings older than 35 years, providing the opportunity to address these important questions in aging survivors of childhood cancer.7,8
METHODS
Population
The CCSS is a multi-institutional retrospective cohort study, with longitudinal follow-up of survivors of childhood cancer who were treated at 26 institutions in the United States and Canada. Study eligibility included diagnosis of cancer before age 21 years; initial treatment between January 1, 1970, and December 31, 1986; and survivors had to be alive at 5 years after diagnosis of leukemia, CNS malignancy, Hodgkin lymphoma, non-Hodgkin lymphoma, Wilms tumor, neuroblastoma, soft-tissue sarcoma, or a bone tumor. A random sample of siblings of CCSS participants served as a comparison population (n = 4,031). The cohort methodology and study design have been previously described in detail.7,9 The CCSS was approved by the institutional review boards at the 26 participating centers. Participants provided informed consent.
All participants completed a baseline survey (administered between 1994 and 1999) that included demographics, personal/family medical history, and chronic health conditions, including assessment of subsequent malignant neoplasms. A surrogate (parent, spouse, or next of kin) completed the baseline survey for survivors who died more than 5 years after diagnosis, who were under age 18 years, or who were unable to complete the survey. Sex and race/ethnicity characteristics were available from the surveys. Subsequently, there have been four additional follow-up surveys (Appendix Fig A1 [online-only]). Study surveys can be viewed at http://ccss.stjude.org.
Health Condition Outcomes
At baseline and at subsequent follow-up evaluations, participants completed a multi-item survey, which included participant's age at onset of organ-based health conditions. Severity scoring was applied based on the Common Terminology Criteria for Adverse Events (version 4.0, National Cancer Institute), which is used to score both acute and chronic conditions in patients and survivors of cancer.10 This system grades conditions as mild (grade 1), moderate (grade 2), severe or disabling (grade 3), life-threatening (grade 4), or fatal (grade 5). Our current analysis was restricted to conditions grades 3 to 5 that developed at least 5 years after the cancer diagnosis (thus, conditions that developed during therapy or shortly thereafter were not included). When not enough information was available to distinguish between grades, the lower grade was assigned. Subsequent malignant neoplasms, not including recurrence of the primary childhood malignancy, were initially ascertained through self- or proxy-report questionnaires and/or death certificates and were confirmed by pathology report or, when not available, other medical records. Only subsequent malignant neoplasms occurring 5 or more years after childhood cancer diagnoses were evaluated.11,12 For assessment of mortality, the CCSS cohort was linked with the National Death Index to ascertain date and cause of death.
Cancer diagnosis and treatment data (including chemotherapy and radiotherapy) were abstracted from medical records at the treating institutions for the 12,593 patients who provided a signed medical release.8,13
Statistical Analysis
Cox proportional hazards models were used to compare severe, disabling, life-threatening, or fatal health conditions among survivors, versus siblings, and were reported as hazard ratios (HR) and 95% CI. Age was used as the time scale to account for the increasing risk of severe, disabling, life-threatening, or fatal health conditions owing to age. Survivors entered the analysis at an age equivalent to 5 years postdiagnosis; siblings entered at age 5 years. Because participants could have reported multiple grade 3 to 5 conditions, the models used a counting-process approach, using all reported unique conditions for each participant, and accounting for intraparticipant correlations using sandwich SE estimates.14,15 Separate analyses were conducted to compare overall grade 3 to 5 conditions, plus each of 13 organ systems of interest between survivors and siblings. Additional analyses compared rates of grade 3 to 5 health conditions for subgroups of survivors who received specific treatments or combinations of treatments with siblings. Finally, a supplemental multivariable analysis was undertaken among survivors to evaluate how treatment factors affected the hazards of developing grade 3 to 5 health conditions after the age of 35 years. All analyses were adjusted for sex and race/ethnicity.
To compare the HRs between age periods, age was added as a time-varying covariate, categorized in three periods (participants ages 5 to 19 years, 20 to 34 years, and 35 years and older). The interaction between age period and group provided HRs for survivors versus siblings within each age interval. Late onset of specific severe, disabling, life-threatening, or fatal health conditions was compared in additional Cox proportional hazards models, with both survivors and siblings entering at age 35 years. We completed a sensitivity analysis to evaluate the potential impact of participants dropping out from the CCSS before age 35 years and analyzed participants who, based on birth and death dates, survived past age 35 years using the Inverse Probability Censored Weighting method to weight analyses to account for differences between participants who dropped out of our study before age 35 years versus those who did not.16
Person-years and number of events were calculated for survivors and siblings within each age period and across all ages. To calculate incidence rates (per 1,000 person-years) of severe, disabling, life-threatening, or fatal health conditions for survivors and siblings, person-years and number of events were fit with Poisson regression models. Models based on all ages included a restricted cubic spline for age,17 and parameter estimates from these models were used to calculate predicted incidence rates per 1,000 person-years at a median age of 31 years.
Cumulative incidence rates for each of the primary outcomes were calculated separately for survivors and siblings, with deaths other than those as a result of fatal health conditions of interest (ie, recurrence of primary cancer or external causes such as accidents, injuries, or suicide) treated as competing risk events.18 Similar to the Cox models, age was used as the time scale with appropriately staggered age of entry to the cohort.19 For each outcome, the cumulative incidence was computed based on time to the earliest reported age of the event of interest. In addition to unconditional curves, conditional cumulative incidence curves were evaluated, conditioning for survival free of a grade 3-5 condition up to ages 26, 35, and 45 years. P values for comparisons between cumulative incidence for survivors and siblings at specific ages were calculated using Wald tests. Data were analyzed with SAS software, version 9.3 (SAS Institute, Cary, NC) and Stata/SE 12.1 (StataCorp, College Station, TX).
RESULTS
As listed in Table 1 and Appendix Figures A1A and A1B, 14,359 5-year survivors and 4,031 siblings completed the baseline survey. Demographic and treatment characteristics for the entire cohort and for those participants who were at least age 35 years at last follow-up are included. For survivors who were 35 years or older at the last follow-up, longitudinal recruitment and retention rates (Appendix Fig A1C) and a description of nonparticipation are also included (Appendix Table A1). For survivors, the median age at last follow-up was 31 years (range, 5 to 58; interquartile range, 13 years), at a median of 24.5 years from primary cancer diagnosis (range, 5 to 39.3; interquartile range, 9.6 years). Of survivors, 5,604 were 35 years old or older at last follow-up. The median age at last follow-up of siblings was 34 years (range, 3 to 62; interquartile range, 14 years).
Table 1.
Demographic and Treatment Characteristics for Survivors and Siblings
| Characteristic | Total Cohort |
Age 35 or Older at Last Follow-Up |
||||||
|---|---|---|---|---|---|---|---|---|
| Survivors |
Siblings |
Survivors |
Siblings |
|||||
| No. of Participants (n = 14,359) | % | No. of Participants (n = 4,031) | % | No. of Participants (n = 5,604) | % | No. of Participants (n = 1,969) | % | |
| Sex | ||||||||
| Female | 6,645 | 46.3 | 2,088 | 51.8 | 2,660 | 47.5 | 1,074 | 54.5 |
| Male | 7,714 | 53.7 | 1,943 | 48.2 | 2,944 | 52.5 | 895 | 45.5 |
| Race/ethnicity* | ||||||||
| Non-Hispanic white | 12,397 | 86.6 | 3,509 | 90.4 | 5,063 | 90.6 | 1,768 | 92.8 |
| Hispanic | 749 | 5.2 | 149 | 3.8 | 219 | 3.9 | 55 | 2.9 |
| Non-Hispanic black | 733 | 5.1 | 123 | 3.2 | 192 | 3.4 | 49 | 2.6 |
| Other | 429 | 3.0 | 101 | 2.6 | 114 | 2.0 | 34 | 1.8 |
| Age at last contact, years | ||||||||
| 0-19 | 1,524 | 10.6 | 267 | 6.6 | — | — | — | — |
| 20-29 | 4,493 | 31.3 | 1,136 | 28.2 | — | — | — | — |
| 30-39 | 5,226 | 36.4 | 1,396 | 34.6 | 2,488 | 44.4 | 737 | 37.4 |
| 40-49 | 2,747 | 19.1 | 979 | 24.3 | 2,747 | 49.0 | 979 | 49.7 |
| ≥ 50 | 369 | 2.5 | 253 | 6.3 | 369 | 6.6 | 253 | 12.8 |
| Age at primary diagnosis, years | ||||||||
| 0-4 | 5,754 | 40.1 | — | 686 | 12.2 | |||
| 5-9 | 3,201 | 22.3 | 1,052 | 18.8 | ||||
| 10-14 | 2,913 | 20.3 | 1,887 | 33.7 | ||||
| 15-20 | 2,491 | 17.3 | 1,979 | 35.3 | ||||
| Primary diagnosis | ||||||||
| Acute lymphoblastic leukemia | 4,329 | 30.1 | — | 1,195 | 21.3 | |||
| Acute myeloid leukemia | 356 | 2.5 | 128 | 2.3 | ||||
| Other leukemia | 145 | 1.0 | 46 | 0.8 | ||||
| CNS tumors | 1,876 | 13.1 | 654 | 11.7 | ||||
| Hodgkin lymphoma | 1,927 | 13.4 | 1,341 | 23.9 | ||||
| Non-Hodgkin lymphoma | 1,080 | 7.5 | 557 | 9.9 | ||||
| Kidney tumors | 1,256 | 8.7 | 221 | 3.9 | ||||
| Neuroblastoma | 955 | 6.7 | 120 | 2.1 | ||||
| Soft tissue sarcoma | 1,246 | 8.7 | 579 | 10.3 | ||||
| Bone tumors | 1,189 | 8.3 | 763 | 13.6 | ||||
| Therapy for primary diagnosis | ||||||||
| Surgery* | ||||||||
| Any surgery | 10,234 | 81.3 | 4,368 | 86.5 | ||||
| Nephrectomy | 1,091 | 8.7 | 203 | 4.0 | ||||
| Splenectomy | 1,257 | 10.0 | 915 | 18.1 | ||||
| Surgery only | 909 | 7.3 | 372 | 7.4 | ||||
| Chemotherapy* | ||||||||
| Any chemotherapy | 10,137 | 80.6 | — | 3,813 | 75.5 | |||
| Alkylator | 6,826 | 54.3 | — | 2,749 | 54.4 | |||
| Anthracycline | 5,190 | 41.3 | — | 1,841 | 36.5 | |||
| Bleomycin | 756 | 6.0 | — | 425 | 8.4 | |||
| Cisplatin | 738 | 5.9 | — | 193 | 3.8 | |||
| Methotrexate | 5,676 | 45.1 | — | 2,042 | 40.4 | |||
| Radiotherapy* | ||||||||
| Any radiotherapy | 8,547 | 68.0 | — | 3,709 | 73.4 | |||
| Brain | 4,010 | 32.6 | — | 1,393 | 28.1 | |||
| Chest | 2,467 | 20.1 | — | 1,458 | 29.4 | |||
| Abdomen | 2,200 | 17.9 | — | 1,126 | 22.7 | |||
| Pelvis | 1,863 | 15.2 | — | 887 | 17.9 | |||
| Combinations* | ||||||||
| Chest RT plus bleomycin | 339 | 2.8 | — | 188 | 3.8 | |||
| Chest RT plus abdominal/pelvic RT | 1,435 | 11.7 | — | 851 | 17.2 | |||
| Alkylator plus abdominal/pelvic RT | 1,636 | 13.3 | — | 762 | 15.4 | |||
| Chest RT plus anthracyclines | 966 | 7.9 | — | 395 | 8.0 | |||
| Anthracyclines plus an alkylator | 5,190 | 41.3 | — | 1,841 | 36.5 | |||
Abbreviations: RT, radiotherapy.
Percentages calculated on total No. of participants on whom information was available.
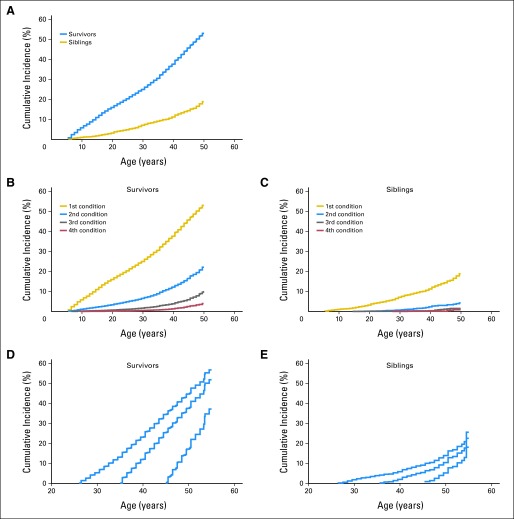
Compared with siblings, survivors had an increased cumulative incidence of severe, disabling, life-threatening, or fatal health conditions (Fig 1 [overall]; Appendix Fig A2, Appendix Table A2 [by primary childhood cancer diagnosis]). The increase in cumulative incidence between survivors and siblings evident at 20 years (survivors: 16.0%; 95% CI, 14.9 to 17.2; v siblings: 3.3%; 95% CI, 2.8 to 3.9; Fig 1A) increased with age (at 50 years: 53.6% of survivors; 95% CI, 51.5 to 55.6; v 19.8% of siblings; 95% CI, 17.0 to 22.7). Notably, 24-year-old survivors of childhood cancer had the same cumulative incidence of grade 3 to 5 health conditions (19.6%) as the 50-year-old siblings. Multivariable analysis confirmed that the HR for a severe, disabling, life-threatening, or fatal health condition increased for survivors 35 years old and older compared with survivors 20 to 34 years old (HR, 5.0 v 3.8; P = .03; Table 2).
Fig 1.
Cumulative incidence of chronic health conditions for (A) grades 3 to 5 chronic health conditions, (B) multiple grade 3 to 5 conditions in survivors, (C) multiple grade 3 to 5 conditions in siblings, (D) conditioned based on no previous grade 3 to 5 conditions among survivors by ages 25, 35, or 45, and (E) conditioned based on no previous grade 3 to 5 conditions among siblings by ages 25, 35, or 45.
Table 2.
Incidence Rates and Hazard Ratios by Organ System for Severe, Disabling, Life-Threatening, or Fatal Health Conditions for Survivors Compared With Siblings Among All Survivors and Age-Specific Strata of Survivors
| Organ System* | All Participants |
Ages 5-19 Years |
Ages 20-34 Years |
Ages ≥ 35 Years |
P† | ||||
|---|---|---|---|---|---|---|---|---|---|
| Survivors (n = 14,359) | Siblings (n = 4,031) | Survivors (n = 11,868) | Siblings (n = 4,031) | Survivors (n = 12,835) | Siblings (n = 3,764) | Survivors (n = 5,604) | Siblings (n = 1,969) | ||
| All conditions | |||||||||
| PY | 271,148 | 117,251 | 101,644 | 59,370 | 13,5061 | 43,161 | 34,443 | 14,719 | |
| No. of events | 5,588 | 490 | 1,509 | 134 | 2,510 | 209 | 1,569 | 147 | |
| IR | 15.4 | 3.0 | 12.7 | 2.1 | 15.9 | 4.6 | 38.9 | 9.3 | |
| 95% CI | 14.7 to 16.1 | 2.5 to 3.5 | 11.8 to 13.6 | 1.6 to 2.6 | 15.0 to 16.9 | 3.8 to 5.6 | 36.2 to 41.8 | 7.4 to 11.7 | |
| HR | 4.9 | 1.0 | 6.8 | 1.0 | 3.8 | 1.0 | 5.0 | 1.0 | .03 |
| 95% CI | 4.3 to 5.5 | 5.5 to 8.3 | 3.2 to 4.5 | 4.1 to 6.1 | |||||
| Subsequent malignancy | |||||||||
| PY | 270,609 | 117,248 | 101,619 | 59,370 | 134,791 | 43,161 | 34,200 | 14,717 | |
| No. of events | 999 | 47 | 172 | 3 | 460 | 22 | 367 | 22 | |
| IR | 3.9 | 0.5 | 1.3 | 0.1 | 2.6 | 0.5 | 8.1 | 1.3 | |
| 95% CI | 3.5 to 4.3 | 0.3 to 0.9 | 1.1 to 1.5 | 0.0 to 0.2 | 2.3 to 2.9 | 0.3 to 0.8 | 7.2 to 9.9 | 0.8 to 2.3 | |
| HR | 8.5 | 1.0 | 29.5 | 1.0 | 6.7 | 1.0 | 7.5 | 1.0 | .71 |
| 95% CI | 6.4 to 11.4 | 9.3 to 93.1 | 4.3 to 10.2 | 4.9 to 11.5 | |||||
| Hearing | |||||||||
| PY | 270,173 | 117,248 | 101,578 | 59,370 | 134,617 | 43,161 | 33,979 | 14,717 | |
| No. of events | 394 | 44 | 150 | 16 | 164 | 17 | 80 | 11 | |
| IR | 0.7 | 0.1 | 1.5 | 0.4 | 1.3 | 0.6 | 2.4 | 1.2 | |
| 95% CI | 0.6 to 0.9 | 0.1 to 0.2 | 1.3 to 1.8 | 0.3 to 0.7 | 1.0 to 1.5 | 0.3 to 1.0 | 1.9 to 3.1 | 0.6 to 2.3 | |
| HR | 4.3 | 1.0 | 6.5 | 1.0 | 3.1 | 1.0 | 3.2 | 1.0 | .95 |
| 95% CI | 3.1 to 5.9 | 3.8 to 11.0 | 1.9 to 5.2 | 1.7 to 6.0 | |||||
| Vision | |||||||||
| PY | 270,173 | 117,248 | 101,578 | 59,370 | 134,617 | 43,161 | 33,979 | 14,717 | |
| No. of events | 333 | 61 | 152 | 35 | 133 | 18 | 48 | 8 | |
| IR | 0.6 | 0.1 | 1.4 | 0.6 | 0.9 | 0.4 | 1.3 | 0.5 | |
| 95% CI | 0.4 to 0.7 | 0.1 to 0.2 | 1.1 to 1.7 | 0.3 to 0.9 | 0.7 to 1.1 | 0.2 to 0.7 | 0.9 to 1.7 | 0.2 to 1.3 | |
| HR | 2.6 | 1.0 | 2.8 | 1.0 | 2.3 | 1.0 | 2.8 | 1.0 | .68 |
| 95% CI | 2.0 to 3.5 | 1.8 to 4.1 | 1.4 to 3.8 | 1.2 to 7.0 | |||||
| Endocrine | |||||||||
| PY | 270,186 | 117,248 | 101,581 | 59,370 | 134,622 | 43,161 | 33,984 | 14,717 | |
| No. of events | 976 | 89 | 308 | 31 | 478 | 36 | 190 | 22 | |
| IR | 3.7 | 0.9 | 1.7 | 0.2 | 2.0 | 0.4 | 3.2 | 0.6 | |
| 95% CI | 3.3 to 4.1 | 0.6 to 1.3 | 1.5 to 2.0 | 0.1 to 0.4 | 1.8 to 2.3 | 0.2 to 0.6 | 2.7 to 3.7 | 0.3 to 1.2 | |
| HR | 4.5 | 1.0 | 5.3 | 1.0 | 4.3 | 1.0 | 3.8 | 1.0 | .65 |
| 95% CI | 3.6 to 5.6 | 3.6 to 5.6 | 3.1 to 6.0 | 2.4 to 5.8 | |||||
| Respiratory | |||||||||
| PY | 270,291 | 117,248 | 101,582 | 59,370 | 134,668 | 43,161 | 34,036 | 14,716 | |
| No. of events | 212 | 17 | 39 | 3 | 98 | 7 | 75 | 7 | |
| IR | 0.7 | 0.1 | 0.3 | 0.1 | 0.6 | 0.2 | 1.9 | 0.5 | |
| 95% CI | 0.5 to 0.9 | 0.0 to 0.3 | 0.2 to 0.5 | 0.0 to 0.2 | 0.5 to 0.8 | 0.1 to 0.4 | 1.5 to 2.5 | 0.2 to 1.1 | |
| HR | 5.0 | 1.0 | 6.6 | 1.0 | 4.4 | 1.0 | 5.0 | 1.0 | .81 |
| 95% CI | 3.0 to 8.2 | 2.1 to 21.2 | 2.1 to 9.4 | 2.3 to 10.8 | |||||
| Cardiac | |||||||||
| PY | 270,407 | 117,251 | 101,585 | 59,370 | 134,718 | 43,161 | 34,105 | 14,718 | |
| No. of events | 1,158 | 73 | 189 | 13 | 463 | 28 | 506 | 32 | |
| IR | 3.1 | 0.3 | 1.7 | 0.3 | 3.2 | 0.7 | 14.0 | 2.5 | |
| 95% CI | 2.8 to 3.5 | 0.2 to 0.5 | 1.5 to 2.0 | 0.1 to 0.5 | 2.8 to 3.7 | 0.4 to 1.2 | 12.3 to 16.0 | 1.6 to 4.0 | |
| HR | 6.9 | 1.0 | 8.6 | 1.0 | 5.2 | 1.0 | 7.8 | 1.0 | .15 |
| 95% CI | 5.2 to 9.1 | 4.2 to 17.3 | 3.4 to 7.9 | 5.4 to 11.5 | |||||
| GI | |||||||||
| PY | 270,233 | 117,248 | 101,583 | 59,370 | 134,645 | 43,161 | 34,005 | 14,717 | |
| No. of events | 285 | 14 | 91 | 3 | 133 | 6 | 61 | 5 | |
| IR | 0.6 | 0.1 | 0.9 | 0.1 | 1.0 | 0.2 | 1.8 | 0.4 | |
| 95% CI | 0.5 to 0.8 | 0.0 to 0.2 | 0.7 to 1.2 | 0.0 to 0.3 | 0.8 to 1.2 | 0.1 to 0.5 | 1.3 to 2.4 | 0.1 to 1.1 | |
| HR | 9.3 | 1.0 | 18.3 | 1.0 | 6.9 | 1.0 | 6.7 | 1.0 | .96 |
| 95% CI | 5.3 to 16.5 | 5.7 to 59.1 | 3.0 to 15.6 | 2.4 to 18.5 | |||||
| Renal | |||||||||
| PY | 270,183 | 117,248 | 101,578 | 59,370 | 134,625 | 43,161 | 33,981 | 14,717 | |
| No. of events | 112 | 10 | 46 | 3 | 50 | 6 | 16 | 1 | |
| IR | 0.2 | 0.1 | 0.5 | 0.0 | 0.4 | 0.1 | 0.5 | 0.1 | |
| 95% CI | 0.1 to 0.3 | 0.0 to 0.3 | 0.4 to 0.7 | 0.0 to 0.2 | 0.3 to 0.6 | 0.0 to 0.3 | 0.3 to 0.9 | 0.0 to 0.7 | |
| HR | 5.3 | 1.0 | 13.1 | 1.0 | 2.6 | 1.0 | 7.5 | 1.0 | .35 |
| 95% CI | 2.6 to 10.8 | 3.2 to 54.3 | 1.1 to 6.0 | 1.0 to 58.4 | |||||
| Musculoskeletal | |||||||||
| PY | 270,182 | 117,248 | 101,578 | 59,370 | 134,626 | 43,162 | 33,979 | 14,717 | |
| No. of events | 217 | 17 | 59 | 2 | 116 | 8 | 42 | 7 | |
| IR | 0.5 | 0.1 | 0.7 | 0.0 | 1.0 | 0.2 | 1.5 | 0.6 | |
| 95% CI | 0.4 to 0.6 | 0.0 to 0.1 | 0.6 to 1.0 | 0.0 to 0.2 | 0.8 to 1.3 | 0.1 to 0.6 | 1.1 to 2.1 | 0.3 to 1.3 | |
| HR | 4.9 | 1.0 | 14.9 | 1.0 | 4.4 | 1.0 | 2.8 | 1.0 | .38 |
| 95% CI | 3.0 to 8.1 | 3.5 to 63.0 | 2.2 to 9.1 | 1.3 to 5.9 | |||||
| Neurologic | |||||||||
| PY | 270,193 | 117,248 | 101,578 | 59,370 | 134,628 | 43,162 | 33,986 | 14,717 | |
| No. of events | 479 | 60 | 190 | 12 | 199 | 31 | 90 | 17 | |
| IR | 0.9 | 0.5 | 1.8 | 0.2 | 1.5 | 0.7 | 2.6 | 1.1 | |
| 95% CI | 0.7 to 1.0 | 0.3 to 0.8 | 1.5 to 2.2 | 0.1 to 0.4 | 1.2 to 1.7 | 0.4 to 1.1 | 2.0 to 3.2 | 0.6 to 2.1 | |
| HR | 3.5 | 1.0 | 10.6 | 1.0 | 2.0 | 1.0 | 2.2 | 1.0 | .78 |
| 95% CI | 2.6 to 4.8 | 5.8 to 19.3 | 1.3 to 3.0 | 1.2 to 3.8 | |||||
| Other hematologic | |||||||||
| PY | 270,178 | 117,248 | 101,578 | 59,370 | 134,619 | 43,162 | 33,982 | 14,717 | |
| No. of events | 374 | 58 | 103 | 13 | 188 | 30 | 83 | 15 | |
| IR | 1.1 | 0.5 | 0.8 | 0.2 | 1.1 | 0.6 | 2.0 | 0.8 | |
| 95% CI | 0.9 to 1.3 | 0.3 to 0.9 | 0.7 to 1.0 | 0.1 to 0.3 | 0.9 to 1.4 | 0.4 to 1.0 | 1.5 to 2.5 | 0.4 to 1.5 | |
| HR | 2.7 | 1.0 | 4.8 | 1.0 | 2.0 | 1.0 | 2.6 | 1.0 | .43 |
| 95% CI | 2.0 to 3.6 | 2.6 to 8.9 | 1.3 to 2.9 | 1.5 to 4.5 | |||||
| Other infectious/immunologic | |||||||||
| PY | 270,238 | 117,248 | 101,580 | 59,370 | 134,665 | 43,162 | 33,994 | 14,717 | — |
| No. of events | 48 | 0 | 10 | 0 | 27 | 0 | 11 | 0 | |
| IR | 0.2 | — | 0.1 | — | 0.2 | — | 0.3 | — | |
| 95% CI | 0.1 to 0.3 | 0.0 to 0.2 | 0.1 to 0.3 | 0.1 to 0.5 | |||||
| HR | — | — | — | — | |||||
| 95% CI | |||||||||
NOTE. Incidence rates are per 1,000 person-years. Em-dashes represent not enough events to analyze.
Abbreviations: HR, hazard ratio; IR, incidence rate; PY, person-years.
Each organ system represents a multivariable model, with separate models for all survivors and age-specific strata. Models allow for repeated events across age categories and are adjusted for race and sex, and age is used as a time-dependent variable.
P value for difference in HR between ages 20-34 and ages ≥ 35 years.
By age 50 years, 22.5% of survivors (95% CI, 20.7 to 24.4) had two or more severe, disabling, life-threatening, or fatal health conditions compared with 4.3% of siblings (95% CI, 3.0 to 5.8; Figs 1B and 1C). Among survivors who reached age 35 years without a previous severe, disabling, life-threatening, or fatal health condition, 25.9% experienced a new grade 3 to 5 condition within 10 years, compared with 6.0% of healthy siblings (P < .001; Figs 1D and 1E; Appendix Table A3).
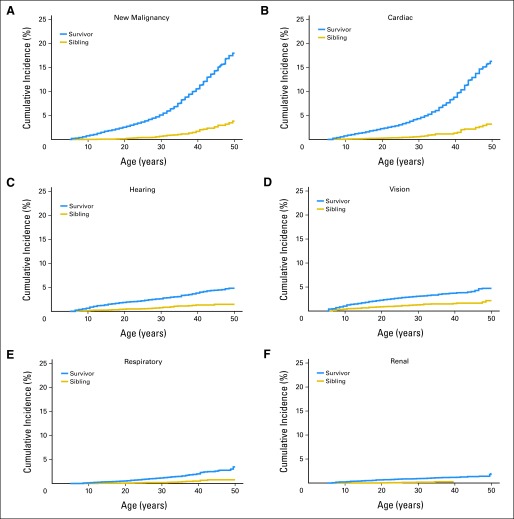
Beyond age 35 years, survivors experienced a marked increase in cumulative incidence (Fig 2; Appendix Fig A3) and incidence rates of grade 3 to 5 cardiac events (Table 2) and the development of malignant neoplasms that were not observed in other organ systems. This observation remained consistent even when restricting analyses to grade 3 to 4 events (ie, excluding mortality, data not shown). Notably, at age 50 years, certain organ systems had a low cumulative incidence of severe, disabling, life-threatening, or fatal health conditions (renal: 1.8%; 95% CI, 1.3 to 2.5; pulmonary: 3.8%; 95% CI, 3.0 to 4.7).
Fig 2.
Cumulative incidence of selected grade 3 to 5 conditions by organ system. (A) New malignancy, (B) cardiac, (C) hearing, (D) vision, (E) respiratory, and (F) renal.
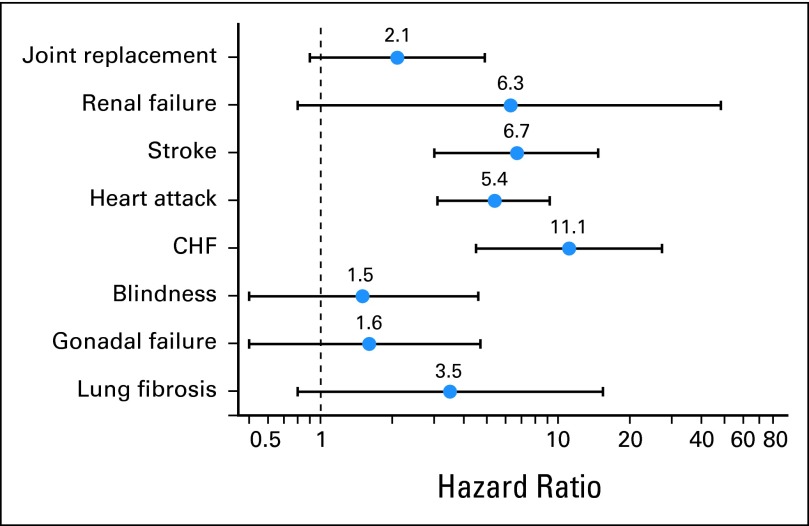
Specific chronic conditions were assessed (Fig 3, Appendix Fig A4). Survivors 35 years and older experienced increased risk for first occurrence of stroke, heart attack, and congestive heart failure compared with siblings (Fig 3). Evidence for an increase in risk for new onset joint replacement, renal failure, blindness, gonadal failure, and lung fibrosis did not achieve statistical significance. Even beyond age 35 years, primary cancer therapy was associated with a new onset of severe, disabling, life-threatening, or fatal health conditions (any radiotherapy: HR, 5.7; 95% CI, 4.6 to 7.0; any chemotherapy: HR, 4.9; 95% CI, 4.0 to 6.0; surgery alone: HR, 1.8; 95% CI, 1.2 to 2.7; Table 3). In addition, survivors exposed to radiation to the chest or neck (HR, 2.8; 95% CI, 2.3 to 3.4), brain (HR, 2.1; 95% CI, 1.6 to 2.7), or total body (HR, 3.9; 95% CI, 2.3 to 6.7) and survivors who were exposed to bleomycin (HR, 1.4; 95% CI, 1.1 to 1.9) or ≥ 300 mg/m2 of anthracycline chemotherapy were at increased risk for grade 3 to 5 conditions compared with survivors not exposed to those therapies (Appendix Table A4).
Fig 3.
Hazard ratios and 95% CIs of survivors versus siblings for specific chronic conditions that first occurred at or after age 35 years, adjusted for age, race, and sex. CHF, congestive heart failure.
Table 3.
Hazard Ratios and 95% CIs for Development of Grade 3-5 Health Conditions at or Beyond Age 35 Years, After Specific Therapy for Primary Cancer, Compared With Siblings
| Therapy for Treatment of Primary Cancer | HR | 95% CI |
|---|---|---|
| Surgery | ||
| Any surgery | 5.0 | 4.1 to 6.1 |
| Surgery only | 1.8 | 1.2 to 2.7 |
| Nephrectomy | 3.1 | 1.9 to 5.1 |
| Splenectomy | 7.4 | 6.0 to 9.1 |
| Radiation | ||
| Any radiation | 5.7 | 4.6 to 7.0 |
| Chest RT | 7.0 | 5.7 to 8.6 |
| CNS RT | 4.9 | 3.8 to 6.3 |
| Abdominal RT | 7.1 | 5.7 to 8.7 |
| Pelvic RT | 6.6 | 5.3 to 8.2 |
| No radiation | 2.6 | 2.0 to 3.3 |
| Chemotherapy | ||
| Any chemotherapy | 4.9 | 4.0 to 6.0 |
| Cisplatin | 4.5 | 2.9 to 6.9 |
| Alkylator | 5.5 | 4.4 to 6.7 |
| Anthracycline | 4.6 | 3.7 to 5.8 |
| Methotrexate | 3.6 | 2.9 to 4.6 |
| Bleomycin | 6.9 | 5.1 to 9.2 |
| Combinations | ||
| Chest RT plus bleomycin | 9.1 | 6.5 to 12.7 |
| Chest RT plus abdominal or pelvic RT | 7.7 | 6.2 to 9.5 |
| Abdominal or pelvic RT plus alkylator | 6.9 | 5.5 to 8.6 |
| Chest RT plus anthracyclines | 7.3 | 5.5 to 9.7 |
| Anthracyclines plus an alkylator | 4.6 | 3.7 to 5.8 |
NOTE. Each row represents a separate multivariable model adjusted for sex and race. Age is used as the time scale. Models allow for multiple events and participants may have had another grade 3-4 event before age 35 years.
Abbreviations: HR, hazard ratio; RT, radiation therapy.
Using the Inverse Probability Censored Weighting analysis method, we replicated our analyses for grade 3 to 5 conditions in participants who were at least 35 years old. We calculated HRs to compare survivors with siblings, for the complete age ≥ 35 years cohort (Appendix Table A5), and subgroups defined by treatment categories (Appendix Table A6), and we also calculated cumulative incidence estimates after the age 35 years. Differences from our original results were generally less than a tenth of a decimal place for both HRs and cumulative incidence (these were virtually superimposable on each other).
DISCUSSION
Health outcomes research conducted over the last three decades has established that survivors of childhood cancer are at increased risk for morbidity and mortality during their childhood and young adult years, largely as a result of adverse effects of the therapies that cured their primary malignancies.3–6,20,21 We previously reported that for survivors of childhood cancer at a mean age of 26 years, 62% had at least one chronic health condition with about one-quarter of survivors having a severe, life-threatening, or disabling condition.4 Others subsequently confirmed our observation of the substantial burden of morbidity experienced among young adults who had survived a childhood cancer.20,21 We now identify that elevated risk for severe, disabling, life-threatening, or fatal health conditions extends across the aging spectrum into the fourth and fifth decades of life, increasing significantly beyond age 35 years versus a sibling comparison population. After assessing more than 18,000 participants contributing over 388,000 person-years of time, survivors 35 years old and older were five-fold more likely than same-age, same-sex siblings to experience a new onset of a severe, disabling, life-threatening, or fatal health condition. Indeed, even as the general population ages and accumulates age-related chronic health conditions, there seems to be no point in time where the morbidity and mortality seen in a noncancer population is equivalent to that of survivors of childhood cancer.
In addition, the absolute magnitude of this burden of morbidity in middle age is striking. By age 50 years, 50% of survivors of childhood cancer will have experienced severe, disabling, or life-threatening morbidity or death, most commonly as a result of cardiovascular, pulmonary, hepatic, renal, and gonadal dysfunction, along with the development of subsequent malignant neoplasms. Furthermore, those who survive a first new condition remain at risk for developing additional conditions, such that by 50 years old, 22.5% have had at least two and 10.1% have had three or more grade 3 to 5 events. The National Academies' Institute of Medicine (Washington, DC) has previously recommended that these survivors receive “risk-based” care.22 Our current findings provide a compelling rationale for continuation of risk-based health care through adulthood.
These findings have important implications for cancer screening and prevention. Though the cumulative incidence of events increased across all organ systems, after age 35 years survivors had a disproportionate increase in the incidence of subsequent malignant neoplasms and cardiac events. Although the occurrence of new malignancies has been well documented,6,23 we now demonstrate that this profound rise in incidence occurs during an important window of vulnerability, before the age-threshold when general population screening guidelines recommend screenings to start (eg, breast cancer at age 40 years; colon cancer at age 50 years).24,25 This finding highlights the importance of disseminating established guidelines for follow-up care from the Children's Oncology Group.26 Similarly, early detection of cardiomyopathy and medical intervention may mitigate progression to heart failure in survivors exposed to anthracycline chemotherapy or chest-directed radiotherapy.27–29 Finally, the risk for stroke and myocardial infarction, identified in this study to have increased more than five-fold in survivors older than 35 years, may be modified by promoting a healthy lifestyle that can reduce rates of certain traditional cardiovascular risk factors (obesity, dyslipidemia, hypertension, diabetes, and smoking).
In our study, 24-year-old survivors of childhood cancer have the same cumulative incidence of grade 3 to 5 health conditions as 50-year-old siblings. In addition, survivors are known to have higher rates of physical impairment and fatigue30–32 and early onset of age-related memory impairment,33 which raises the question of whether exposure to systemic chemotherapy or even focal radiotherapy at a young age may accelerate the aging process. In our study, even healthy survivors (ie, no previous health condition before age 45 years) had a significant increase in risk for future conditions, indicating an underlying predisposition for early onset of poor outcomes. Mechanisms for aging such as telomere shortening, accumulation of free-radical mediated injury, and cellular senescence have been hypothesized to be operative in cancer survivors, but this has yet to be established.34
The CCSS has uniquely provided the first systematic, longitudinal characterization of overall physical health in a large, geographically diverse population of survivors aging through middle adulthood with comparison to a sibling population. Despite these strengths, a number of limitations should be considered. First, all outcomes were self-reported, with validation of only the subsequent malignant neoplasms. Therefore, we excluded mild and moderate events from this analysis (grades 1 and 2), expecting improved validity from higher grade outcomes. Second, therapies for many childhood malignancies have evolved over time and, thus, results from this study may not be directly generalizable to more recently treated populations. However, traditional chemotherapeutics and radiotherapy continue to be the backbone of cancer treatment for most childhood malignancies.35,36 Finally, though analysis of a subset of the CCSS cohort that is now at least 35 years old has the potential for participation bias, the similarity between the unweighted and weighted analyses accounting for differences between participants and nonparticipants provide strong evidence that drop-out after the age 35 years has little impact on our analytic results. Comparisons of demographic distributions and cancer-related characteristics between overall participants (n = 14,359) and nonparticipants have been previously published.9 In general, participant and nonparticipants were similar regarding sex, cancer diagnosis, age at diagnosis, age at contact, and type of cancer treatment. However, the rate of nonparticipation was significantly higher among the next-of-kin of survivors who were deceased at the baseline evaluation compared with next of kin of survivors who were alive at baseline. Comparisons between those who completed baseline and those who were lost to follow-up (ie, could not be located for baseline completion) were not statistically significant except for vital status.
In summary, we have identified that risk for morbidity and mortality continues across the life span for cancer survivors and actually increases beyond age 35 years when compared with a population of siblings. By age 50 years, more than half of survivors have experienced a severe, disabling, or life-threatening event, including death. These data raise concerns for acceleration of the aging process in this population; highlight the need for longitudinal, risk-based follow-up; and identify the increasing health burden on this population as they age.
Appendix
Table A1.
CCSS Participants Expected to Be Age ≥ 35 Years at Time of Last Attempted Follow-Up Survey
| Characteristic | Completed Baseline |
Died Before Age 35 |
Alive at Age 35 Years and Eligible for Follow-Up |
Completed Follow-Up ≥ Age 35 Years |
Did Not Have Follow-Up by Age 35 Years |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of Participants (n = 7,809) | % | No. of Participants (n = 980) | % | No. of Participants (n = 6,829) | % | No. of Participants (n = 5,604) | % | No. of Participants (n = 1,225) | % | |
| Sex* | ||||||||||
| Female | 3,565 | 45.7 | 393 | 40.1 | 3,172 | 46.4 | 2,660 | 47.5 | 512 | 41.8 |
| Male | 4,244 | 54.3 | 587 | 59.9 | 3,657 | 53.6 | 2,944 | 52.5 | 713 | 58.2 |
| Race/ethnicity*† | ||||||||||
| Non-Hispanic white | 6,924 | 88.9 | 860 | 87.8 | 6,064 | 89.1 | 5,063 | 90.6 | 1,001 | 82.2 |
| Hispanic | 347 | 4.5 | 41 | 4.2 | 306 | 4.5 | 219 | 3.9 | 87 | 7.1 |
| Non-Hispanic black | 326 | 4.2 | 52 | 5.3 | 274 | 4.0 | 192 | 3.4 | 82 | 6.7 |
| Other | 188 | 2.4 | 26 | 2.7 | 162 | 2.4 | 114 | 2.0 | 48 | 3.9 |
| Age at first diagnosis, years* | ||||||||||
| 0-4 | 1,123 | 14.4 | 142 | 14.5 | 981 | 14.4 | 686 | 12.2 | 295 | 24.1 |
| 5-9 | 1,590 | 20.4 | 208 | 21.2 | 1,382 | 20.2 | 1,052 | 18.8 | 330 | 26.9 |
| 10-14 | 2,605 | 33.4 | 314 | 32.0 | 2,291 | 33.5 | 1,887 | 33.7 | 404 | 33.0 |
| 15-20 | 2,491 | 31.9 | 316 | 32.2 | 2,175 | 31.8 | 1,979 | 35.3 | 196 | 16.0 |
| Primary diagnosis* | ||||||||||
| Acute lymphoblastic leukemia | 1,748 | 22.4 | 221 | 22.6 | 1,527 | 22.4 | 1,195 | 21.3 | 332 | 27.1 |
| Acute myeloid leukemia | 178 | 2.3 | 29 | 3.0 | 149 | 2.2 | 128 | 2.3 | 21 | 1.7 |
| Other leukemia | 89 | 1.1 | 28 | 2.9 | 61 | 0.9 | 46 | 0.8 | 15 | 1.2 |
| CNS tumors | 979 | 12.5 | 175 | 17.9 | 804 | 11.8 | 654 | 11.7 | 150 | 12.2 |
| Hodgkin lymphoma | 1,757 | 22.5 | 208 | 21.2 | 1,549 | 22.7 | 1,341 | 23.9 | 208 | 17.0 |
| Non-Hodgkin lymphoma | 734 | 9.4 | 50 | 5.1 | 684 | 10.0 | 557 | 9.9 | 127 | 10.4 |
| Kidney tumors | 330 | 4.2 | 29 | 3.0 | 301 | 4.4 | 221 | 3.9 | 80 | 6.5 |
| Neuroblastoma | 191 | 2.4 | 21 | 2.1 | 170 | 2.5 | 120 | 2.1 | 50 | 4.1 |
| Soft tissue sarcoma | 778 | 10.0 | 91 | 9.3 | 687 | 10.1 | 579 | 10.3 | 108 | 8.8 |
| Bone tumors | 1,025 | 13.1 | 128 | 13.1 | 897 | 13.1 | 763 | 13.6 | 134 | 10.9 |
| Therapy for primary diagnosis | ||||||||||
| Surgery† | ||||||||||
| Any surgery* | 5,890 | 86.3 | 745 | 89.2 | 5,145 | 85.9 | 4,368 | 86.5 | 777 | 82.3 |
| Nephrectomy* | 300 | 4.4 | 29 | 3.5 | 271 | 4.5 | 203 | 4.0 | 68 | 7.2 |
| Splenectomy* | 1,159 | 17.0 | 133 | 15.9 | 1,026 | 17.1 | 915 | 18.1 | 111 | 11.8 |
| Surgery alone | 455 | 6.7 | 19 | 2.3 | 436 | 7.3 | 372 | 7.4 | 64 | 6.8 |
| Chemotherapy† | ||||||||||
| Any chemotherapy* | 5,310 | 77.7 | 726 | 86.9 | 4,584 | 76.4 | 3,813 | 75.5 | 771 | 81.5 |
| Alkylator | 3,897 | 57.1 | 628 | 75.5 | 3,269 | 54.5 | 2,749 | 54.4 | 520 | 55.1 |
| Anthracycline | 2,676 | 39.2 | 474 | 57.0 | 2,202 | 36.7 | 1,841 | 36.5 | 361 | 38.2 |
| Bleomycin | 622 | 9.1 | 123 | 14.8 | 499 | 8.3 | 425 | 8.4 | 74 | 7.8 |
| Cisplatin* | 367 | 5.4 | 126 | 15.1 | 241 | 4.0 | 193 | 3.8 | 48 | 5.1 |
| Methotrexate* | 2,820 | 41.3 | 348 | 41.7 | 2,472 | 41.2 | 2,042 | 40.4 | 430 | 45.5 |
| Radiotherapy† | ||||||||||
| Any RT | 5,127 | 75.0 | 720 | 86.1 | 4,407 | 73.5 | 3,709 | 73.4 | 698 | 74.1 |
| Brain* | 2,020 | 30.2 | 331 | 40.9 | 1,689 | 28.7 | 1,393 | 28.1 | 296 | 32.3 |
| Chest* | 1,953 | 29.2 | 287 | 35.5 | 1,666 | 28.3 | 1,458 | 29.4 | 208 | 22.7 |
| Abdomen | 1,515 | 22.7 | 205 | 25.3 | 1,310 | 22.3 | 1,126 | 22.7 | 184 | 20.1 |
| Pelvis* | 1,234 | 18.5 | 216 | 26.7 | 1,018 | 17.3 | 887 | 17.9 | 131 | 14.3 |
| Chest RT + bleomycin | 297 | 4.5 | 85 | 10.5 | 212 | 3.6 | 188 | 3.8 | 24 | 2.6 |
| Chest RT + abdominal/pelvic RT* | 1,152 | 17.2 | 194 | 24.0 | 958 | 16.3 | 851 | 17.2 | 107 | 11.7 |
| Alkylator + abdominal/pelvic RT | 1,121 | 16.8 | 232 | 28.7 | 889 | 15.1 | 762 | 15.4 | 127 | 13.9 |
| Chest RT + anthracyclines | 647 | 9.7 | 171 | 21.2 | 476 | 8.1 | 395 | 8.0 | 81 | 8.9 |
| Anthracyclines + alkylator | 2,676 | 39.2 | 474 | 57.0 | 2,202 | 36.7 | 1,841 | 36.5 | 361 | 38.2 |
| Age at baseline, years* | ||||||||||
| 0-19 | 287 | 3.7 | 281 | 29.0 | 6 | 0.1 | 6 | 0.1 | 0 | 0.0 |
| 20-29 | 4,276 | 54.8 | 580 | 59.8 | 3,696 | 54.1 | 2,733 | 48.8 | 963 | 78.6 |
| 30-39 | 2,904 | 37.2 | 109 | 11.2 | 2,795 | 40.9 | 2,533 | 45.2 | 262 | 21.4 |
| 40-49 | 332 | 4.3 | 0 | 0.0 | 332 | 4.9 | 332 | 5.9 | 0 | 0.0 |
| Health status at baseline* | ||||||||||
| Excellent | 1,265 | 18.3 | 16 | 8.8 | 1,249 | 18.6 | 1,012 | 18.4 | 237 | 19.6 |
| Very good | 2,633 | 38.1 | 45 | 24.7 | 2,588 | 38.5 | 2,176 | 39.5 | 412 | 34.1 |
| Good | 2,204 | 31.9 | 64 | 35.2 | 2,140 | 31.8 | 1,747 | 31.7 | 393 | 32.5 |
| Fair | 677 | 9.8 | 42 | 23.1 | 635 | 9.4 | 494 | 9.0 | 141 | 11.7 |
| Poor | 123 | 1.8 | 15 | 8.2 | 108 | 1.6 | 82 | 1.5 | 26 | 2.2 |
| Education at baseline* | ||||||||||
| Patient age < 18 years | 217 | 2.9 | 217 | 23.3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| High school or less | 1,989 | 27.0 | 314 | 33.8 | 1,675 | 26.0 | 1,257 | 23.8 | 418 | 36.4 |
| Completed high school/some college | 2,536 | 34.4 | 355 | 38.2 | 2,181 | 33.9 | 1,769 | 33.4 | 412 | 35.9 |
| College graduate | 2,627 | 35.6 | 44 | 4.7 | 2,583 | 40.1 | 2,264 | 42.8 | 319 | 27.8 |
NOTE. For this analysis, we identified the eligible population as individuals who reached age 35 years after entering our cohort and before the last follow-up questionnaire was administered (2007). Among these, the participant population included individuals who contributed data to our analysis after age 35 years via a questionnaire or with cause of death information. Prediction models were built separately for survivors and siblings to calculate predicted probabilities of participation. The prediction model for survivors used the 6,829 alive (as of age 35) and eligible survivors (5,604 participants) and was based on the following covariates: age at diagnosis, race, sex, diagnosis group, any surgery, any chemotherapy, any brain radiation, any chest RT plus abdomen or pelvis RT, age at baseline, education at baseline, and self-reported health status at baseline. The prediction model for siblings used the 2,270 alive and eligible siblings (1,969 participants) and was based on the following covariates: race, sex, age at baseline, education at baseline, and self-reported health status at baseline.
Abbreviations: CCSS, Childhood Cancer Survivor Study; RT, radiotherapy.
P < .05 for comparison of participants who completed follow-up at age ≥ 35 years with those who did not complete follow up, based on χ2 test.
Percentages calculated on total No. of patients on whom information was available.
Table A2.
Grade 3-5 Conditions
| Category | Grade | Condition | Occurring More Than 5 Years After Diagnosis |
Occurring at or After Age 35 Years |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Survivors (n = 14,359) |
Siblings (n = 4,031) |
Survivors (n = 5,604) |
Siblings (n = 1,969) |
|||||||
| No. of Participants | % | No. of Participants | % | No. of Participants | % | No. of Participants | % | |||
| Subsequent neoplasms | 3 | Benign meningioma with surgery, thyroid cancer | 153 | 1.1 | 10 | 0.2 | 29 | 0.5 | 4 | 0.2 |
| 4 | Breast carcinoma-in-situ | 63 | 0.4 | 3 | 0.1 | 51 | 0.9 | 3 | 0.2 | |
| Malignancy other than non-melanoma skin cancer or thyroid cancer | 657 | 4.6 | 35 | 0.9 | 229 | 4.1 | 15 | 0.8 | ||
| 5 | Malignancy, death | 360 | 2.5 | 5 | 0.1 | 139 | 2.5 | 2 | 0.1 | |
| Total | 1,233 | 8.6 | 53 | 1.3 | 448 | 8.0 | 24 | 1.2 | ||
| Hearing | 3 | Hearing loss requiring a hearing aid | 387 | 2.7 | 42 | 1.0 | 77 | 1.4 | 10 | 0.5 |
| 4 | Deafness in both ears not corrected by hearing aid | 45 | 0.3 | 3 | 0.1 | 9 | 0.2 | 2 | 0.1 | |
| Total | 432 | 3.0 | 45 | 1.1 | 86 | 1.5 | 12 | 0.6 | ||
| Vision | 3 | Cataracts, requiring surgery | 142 | 1.0 | 8 | 0.2 | 33 | 0.6 | 4 | 0.2 |
| Legally blind in one eye | 150 | 1.0 | 38 | 0.9 | 9 | 0.2 | 3 | 0.2 | ||
| Moderate, severe and profound impairment in one eye | 5 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | ||
| 4 | Legally blind in both eyes or loss of an eye | 36 | 0.3 | 12 | 0.3 | 6 | 0.1 | 1 | 0.1 | |
| Moderate, severe and profound impairment in both eyes | 4 | 0.0 | 3 | 0.1 | 0 | 0.0 | 0 | 0.0 | ||
| Total | 337 | 2.3 | 61 | 1.5 | 48 | 0.9 | 8 | 0.4 | ||
| Speech | 3 | Aphonia | 1 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Total | 1 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | ||
| Endocrine | 3 | Corticoadrenal insufficiency | 4 | 0.0 | 0 | 0.0 | 1 | 0.0 | 0 | 0.0 |
| Panhypopituitarism | 4 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | ||
| Diabetes insipidus | 9 | 0.1 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | ||
| Diabetes, requiring insulin therapy and/or diabetic end organ disease | 129 | 0.9 | 22 | 0.5 | 34 | 0.6 | 8 | 0.4 | ||
| Ovarian failure | 334 | 2.3 | 45 | 1.1 | 14 | 0.2 | 3 | 0.2 | ||
| Testicular hypofunction | 78 | 0.5 | 4 | 0.1 | 4 | 0.1 | 1 | 0.1 | ||
| Thyroid nodules, requiring surgery | 480 | 3.3 | 25 | 0.6 | 139 | 2.5 | 11 | 0.6 | ||
| 5 | Diabetes with ketoacidosis, death | 4 | 0.0 | 0 | 0.0 | 1 | 0.0 | 0 | 0.0 | |
| Metabolic death | 3 | 0.0 | 0 | 0.0 | 1 | 0.0 | 0 | 0.0 | ||
| Total | 1,045 | 7.3 | 96 | 2.4 | 194 | 3.5 | 23 | 1.2 | ||
| Respiratory | 3 | Emphysema | 24 | 0.2 | 2 | 0.0 | 11 | 0.1 | 0 | 0.0 |
| Emphysema, requiring medication | 15 | 0.1 | 1 | 0.0 | 9 | 0.2 | 1 | 0.1 | ||
| Lung fibrosis, requiring oxygen | 39 | 0.3 | 6 | 0.1 | 16 | 0.3 | 2 | 0.1 | ||
| Pulmonary hypertension | 8 | 0.1 | 0 | 0.0 | 3 | 0.1 | 0 | 0.0 | ||
| 4 | Other diseases of lung | 41 | 0.3 | 2 | 0.0 | 8 | 0.1 | 1 | 0.1 | |
| Pulmonary embolism | 13 | 0.1 | 4 | 0.1 | 4 | 0.1 | 3 | 0.2 | ||
| Respiratory arrest | 9 | 0.1 | 3 | 0.1 | 2 | 0.0 | 0 | 0.0 | ||
| 5 | Pulmonary death | 73 | 0.5 | 0 | 0.0 | 27 | 0.5 | 0 | 0.0 | |
| Total | 222 | 1.5 | 18 | 0.4 | 80 | 1.4 | 7 | 0.4 | ||
| Cardiac | 3 | Arrhythmia, requiring pacemaker | 83 | 0.6 | 11 | 0.3 | 41 | 0.7 | 3 | 0.2 |
| Cerebral embolism | 5 | 0.0 | 0 | 0.0 | 1 | 0.0 | 0 | 0.0 | ||
| Congestive heart failure requiring medication | 302 | 2.1 | 11 | 0.3 | 120 | 2.1 | 5 | 0.3 | ||
| Heart attack, angina or coronary heart disease not requiring a cardiac catheterization | 184 | 1.3 | 16 | 0.4 | 103 | 1.8 | 13 | 0.7 | ||
| Hypertension, severe | 8 | 0.1 | 2 | 0.0 | 0 | 0.0 | 0 | 0.0 | ||
| Hypotension | 18 | 0.1 | 3 | 0.1 | 3 | 0.1 | 0 | 0.0 | ||
| Pericardial disease requiring surgical intervention | 22 | 0.2 | 0 | 0.0 | 13 | 0.2 | 0 | 0.0 | ||
| 4 | Endocarditis | 14 | 0.1 | 1 | 0.0 | 5 | 0.1 | 0 | 0.0 | |
| Heart attack requiring cardiac catheterization or angioplasty or CABG | 169 | 1.2 | 20 | 0.5 | 101 | 1.8 | 12 | 0.6 | ||
| Heart transplantation | 30 | 0.2 | 0 | 0.0 | 3 | 0.1 | 0 | 0.0 | ||
| Heart valve replacement | 59 | 0.4 | 3 | 0.1 | 48 | 0.9 | 0 | 0.0 | ||
| Stroke/CVA | 302 | 2.1 | 18 | 0.4 | 89 | 1.6 | 7 | 0.4 | ||
| Ventricular fibrillation/flutter | 1 | 0.0 | 1 | 0.0 | 1 | 0.0 | 1 | 0.1 | ||
| 5 | Cardiovascular death | 156 | 1.1 | 2 | 0.0 | 73 | 1.3 | 2 | 0.1 | |
| Total | 1,353 | 9.4 | 88 | 2.2 | 601 | 10.7 | 43 | 2.2 | ||
| GI | 3 | Cirrhosis of liver | 70 | 0.5 | 2 | 0.0 | 19 | 0.3 | 1 | 0.1 |
| Surgery for intestinal obstruction | 185 | 1.3 | 12 | 0.3 | 30 | 0.5 | 4 | 0.2 | ||
| 4 | Liver transplantation | 2 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |
| 5 | Gastrointestinal death | 30 | 0.2 | 0 | 0.0 | 13 | 0.2 | 0 | 0.0 | |
| Total | 287 | 2.0 | 14 | 0.3 | 62 | 1.1 | 5 | 0.3 | ||
| Renal | 3 | Acute renal failure | 3 | 0.0 | 1 | 0.0 | 2 | 0.0 | 0 | 0.0 |
| Nephrotic syndrome | 3 | 0.0 | 3 | 0.1 | 1 | 0.0 | 0 | 0.0 | ||
| Neurogenic bladder | 6 | 0.0 | 1 | 0.0 | 2 | 0.0 | 0 | 0.0 | ||
| 4 | Chronic renal failure | 41 | 0.3 | 2 | 0.0 | 3 | 0.1 | 1 | 0.1 | |
| Dialysis or kidney transplantation | 81 | 0.6 | 4 | 0.1 | 9 | 0.2 | 0 | 0.0 | ||
| End-stage renal disease | 2 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | ||
| 5 | Death as a result of renal failure | 10 | 0.1 | 0 | 0.0 | 1 | 0.0 | 0 | 0.0 | |
| Total | 146 | 1.0 | 11 | 0.3 | 18 | 0.3 | 1 | 0.1 | ||
| Musculoskeletal | 3 | Amputation | 102 | 0.7 | 3 | 0.1 | 12 | 0.2 | 0 | 0.0 |
| Joint replacement | 187 | 1.3 | 14 | 0.3 | 54 | 1.0 | 7 | 0.4 | ||
| 5 | Musculoskeletal death | 8 | 0.1 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |
| Total | 297 | 2.1 | 17 | 0.4 | 66 | 1.2 | 7 | 0.4 | ||
| Neurologic | 3 | Multiple sclerosis | 6 | 0.0 | 11 | 0.3 | 1 | 0.0 | 3 | 0.2 |
| Paralysis | 16 | 0.1 | 0 | 0.0 | 1 | 0.0 | 0 | 0.0 | ||
| Problems with balance or ability to manipulate objects, severe | 51 | 0.4 | 6 | 0.1 | 19 | 0.3 | 3 | 0.2 | ||
| Problems with learning or memory, severe | 40 | 0.3 | 3 | 0.1 | 7 | 0.1 | 2 | 0.1 | ||
| 4 | Coma and stupor | 22 | 0.2 | 1 | 0.0 | 2 | 0.0 | 0 | 0.0 | |
| Mental retardation, problems with memory or learning, disabling | 36 | 0.3 | 3 | 0.1 | 3 | 0.1 | 0 | 0.0 | ||
| Paralysis, severe | 321 | 2.2 | 36 | 0.9 | 49 | 0.9 | 10 | 0.5 | ||
| Problems with balance or ability to manipulate objects, disabling | 14 | 0.1 | 4 | 0.1 | 5 | 0.1 | 1 | 0.1 | ||
| 5 | Neurologic death | 25 | 0.2 | 0 | 0.00 | 5 | 0.1 | 0 | 0.0 | |
| Total | 531 | 3.7 | 64 | 1.6 | 92 | 1.6 | 19 | 1.0 | ||
| Hematologic | 3 | Blood clot in head, lung, arm, leg, or pelvis | 361 | 2.5 | 60 | 1.5 | 80 | 1.4 | 16 | 0.8 |
| 4 | Aplastic anemia NOS | 1 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |
| 5 | Hematologic death | 8 | 0.1 | 0 | 0.0 | 1 | 0.0 | 0 | 0.0 | |
| Thromboembolic death | 6 | 0.0 | 0 | 0.0 | 2 | 0.0 | 0 | 0.0 | ||
| Total | 376 | 2.6 | 60 | 1.5 | 83 | 1.5 | 16 | 0.8 | ||
| Infectious disease | 5 | Death as a result of infection | 47 | 0.3 | 0 | 0.0 | 10 | 0.2 | 0 | 0.0 |
| Total | 47 | 0.3 | 0 | 0.0 | 10 | 0.2 | 0 | 0.0 | ||
Abbreviations: CABG, coronary artery bypass grafting; CVA, stroke; NOS, not otherwise specified.
Table A3.
No. of Survivors With Multiple Conditions, Based On Having No Previous Conditions Before Age Listed
| Age Reached With No Previous Conditions (years) | No. of Survivors | No. of Chronic Health Conditions |
||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ≥ 4 | ||
| 26 | 9,348 | 7,578 | 1,259 | 327 | 113 | 71 |
| 35 | 4,483 | 3,657 | 611 | 137 | 45 | 33 |
| 45 | 842 | 715 | 104 | 14 | 5 | 4 |
Table A4.
Multivariable HRs and 95% CIs for Development of Grade 3-5 Health Conditions at or After Age 35 Years, Within the Survivor Population After Specific Therapy for Primary Cancer
| Therapy for Treatment of Primary Cancer | HR* | 95% CI |
|---|---|---|
| Surgery | ||
| Any surgery | 1.1 | 0.8 to 1.5 |
| Radiation | ||
| Chest/neck RT | 2.8 | 2.3 to 3.4 |
| CNS RT | 2.1 | 1.6 to 2.7 |
| Abdominal RT | 1.1 | 0.7 to 1.6 |
| Total body RT | 3.9 | 2.3 to 6.7 |
| Other RT | 1.7 | 1.7 to 2.3 |
| Chemotherapy | ||
| Anthracycline, 1-299 mg/m2† | 0.9 | 0.7 to 1.2 |
| Anthracycline, ≥ 300 mg/m2† | 1.2 | 1.0 to 1.5 |
| Methotrexate | 0.7 | 0.6 to 0.9 |
| Bleomycin | 1.4 | 1.1 to 1.9 |
Abbreviations: HR, hazard ratio; RT, radiation therapy.
From a multivariable model including all covariates listed, plus sex and race, with age as the time scale. Model allows for multiple events and participants may have had a different grade 3-4 event before age 35 years.
Referent group received no exposure to anthracycline.
Table A5.
HRs by Organ System and for Specific Severe, Disabling, Life-Threatening, or Fatal Health Conditions at or After Age 35 Years for Survivors Versus Siblings With Weighting Based on Nonparticipation Rates, and Without Weighting (for comparison to Table 2)
| Condition | With Weights |
Without Weights |
||
|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |
| Organ system | ||||
| All conditions | 5.0 | 4.1 to 6.1 | 5.0 | 4.1 to 6.1 |
| Subsequent malignancy | 7.7 | 5.0 to 11.7 | 7.5 | 4.9 to 11.5 |
| Hearing | 3.3 | 1.8 to 6.3 | 3.2 | 1.7 to 6.0 |
| Vision | 2.8 | 1.2 to 6.9 | 2.8 | 1.2 to 7.0 |
| Endocrine | 3.8 | 2.4 to 5.9 | 3.8 | 2.4 to 5.8 |
| Respiratory | 5.3 | 2.5 to 11.5 | 5.0 | 2.3 to 10.8 |
| Cardiac | 7.9 | 5.4 to 11.6 | 7.8 | 5.4 to 11.5 |
| GI | 7.0 | 2.5 to 19.5 | 6.7 | 2.4 to 18.5 |
| Renal | 8.6 | 1.1 to 67.4 | 7.5 | 1.0 to 58.3 |
| Musculoskeletal | 2.8 | 1.3 to 6.0 | 2.8 | 1.3 to 5.9 |
| Neurologic | 2.2 | 1.2 to 3.8 | 2.2 | 1.2 to 3.8 |
| Other hematologic | 2.6 | 1.4 to 4.5 | 2.6 | 1.5 to 4.5 |
| Specific conditions | ||||
| Joint replacement | 2.2 | 1.0 to 4.6 | 2.2 | 1.0 to 4.6 |
| Renal failure | 6.9 | 0.9 to 55.3 | 6.0 | 0.6 to 47.7 |
| Stroke | 7.0 | 3.3 to 14.8 | 6.6 | 3.1 to 14.1 |
| Heart attack | 5.0 | 3.0 to 8.3 | 5.0 | 3.1 to 8.3 |
| Congestive heart failure | 11.4 | 4.7 to 27.3 | 10.9 | 4.5 to 26.0 |
| Blindness | 1.6 | 0.5 to 4.5 | 1.6 | 0.5 to 4.6 |
| Gonadal failure | 1.3 | 0.4 to 4.3 | 1.5 | 0.5 to 4.5 |
| Lung fibrosis | 3.6 | 0.8 to 16.0 | 3.5 | 0.8 to 15.7 |
NOTE. Each row represents a separate multivariable model, adjusted for sex and race, with age as the time scale. Models allow for multiple events and participants may have had a grade 3-4 event before age 35 years.
Abbreviation: HR, hazard ratio.
Table A6.
HRs and 95% CIs for Development of Grade 3-5 Health Conditions at or After Age 35 Years, After Specific Therapy for Primary Cancer, Versus Siblings Both With and Without Weighting for Nonparticipation
| Therapy for Treatment of Primary Cancer | With Weights |
Without Weights |
||
|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |
| Surgery | ||||
| Any surgery | 5.1 | 4.2 to 6.2 | 5.0 | 4.1 to 6.1 |
| Surgery only | 1.8 | 1.2 to 2.7 | 1.8 | 1.2 to 2.7 |
| Nephrectomy | 3.3 | 2.0 to 5.3 | 3.1 | 1.9 to 5.1 |
| Splenectomy | 7.5 | 6.1 to 9.3 | 7.4 | 6.0 to 9.1 |
| Radiation | ||||
| Any radiation | 5.8 | 4.7 to 7.0 | 5.7 | 4.6 to 7.0 |
| Chest RT | 7.1 | 5.8 to 8.7 | 7.0 | 5.7 to 8.6 |
| CNS RT | 5.0 | 3.9 to 6.4 | 4.9 | 3.8 to 6.3 |
| Abdominal RT | 7.1 | 5.7 to 8.8 | 7.1 | 5.7 to 8.7 |
| Pelvic RT | 6.6 | 5.3 to 8.2 | 6.6 | 5.3 to 8.2 |
| No radiation | 2.6 | 2.0 to 3.3 | 2.6 | 2.0 to 3.3 |
| Chemotherapy | ||||
| Any chemotherapy | 4.9 | 4.0 to 6.1 | 4.9 | 4.0 to 6.0 |
| Cisplatin | 4.8 | 3.1 to 7.5 | 4.5 | 2.9 to 6.9 |
| Alkylator | 5.5 | 4.5 to 6.8 | 5.5 | 4.4 to 6.7 |
| Anthracycline | 4.7 | 3.7 to 5.9 | 4.6 | 3.7 to 5.8 |
| Methotrexate | 3.7 | 2.9 to 4.7 | 3.6 | 2.9 to 4.6 |
| Bleomycin | 7.0 | 5.3 to 9.4 | 6.9 | 5.1 to 9.2 |
| Combinations | ||||
| Chest RT + bleomycin | 9.3 | 6.7 to 13.0 | 9.1 | 6.5 to 12.7 |
| Chest RT + abdominal or pelvic RT | 7.8 | 6.3 to 9.6 | 7.7 | 6.2 to 9.5 |
| Abdominal or pelvic RT + alkylator | 6.9 | 5.5 to 8.7 | 6.9 | 5.5 to 8.6 |
| Chest RT + anthracyclines | 7.4 | 5.6 to 9.9 | 7.3 | 5.5 to 9.7 |
| Anthracyclines + an alkylator | 4.7 | 3.7 to 5.9 | 4.6 | 3.7 to 5.8 |
NOTE. Each row represents a separate multivariable model adjusted for sex and race, with age as the time scale. Models allow for multiple events and participants may have had a grade 3-4 event before age 35 years.
Abbreviations: HR, hazard ratio; RT, radiation therapy.
Fig A1.
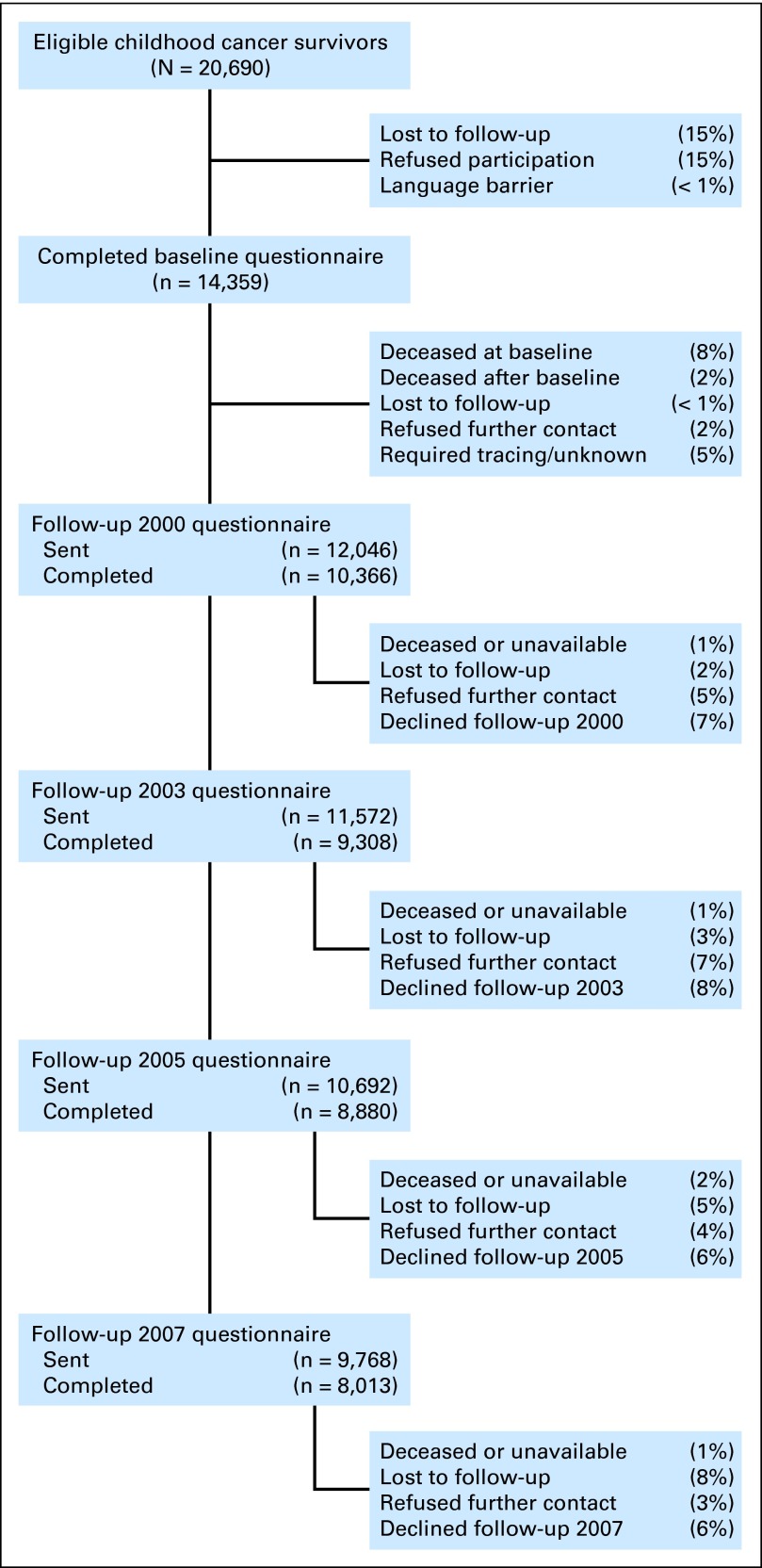
(A) Study population: survivor recruitment and longitudinal participation among those ages 35 years or older as of last follow-up in 2007.(B) Study population: sibling recruitment and longitudinal participation among those ages 35 years or older as of last follow-up in 2007. (C) Study population: survivor recruitment and longitudinal participation among those ages 35 years or older as of last follow-up in 2007. (*) For 228 participants, age at baseline ≥ 35 years and baseline was the last follow-up. Median follow-up time since age 35 years was 3 years, with a maximum of 18 years. (†) For 322 participants, age at follow-up in 2000 was ≥ 35 years and follow-up 2000 was the last follow-up; median follow-up time since age 35 years was 3 years, with a maximum of 15 years. (‡) For 596 participants, age at follow-up in 2003 was ≥ 35 years and follow-up in 2003 was the last follow-up; median follow-up time since age 35 years was 5 years, with a maximum of 20 years. (§) For 4,458 participants, age at follow-up in 2007 was ≥ 35 years and follow-up in 2007 was the last follow-up; median follow-up time since age 35 years was 6 years, with a maximum of 23 years. Overall, for the 5,604 participants in the subcohort, median follow-up time since age 35 years was 5 years.
Fig A2.
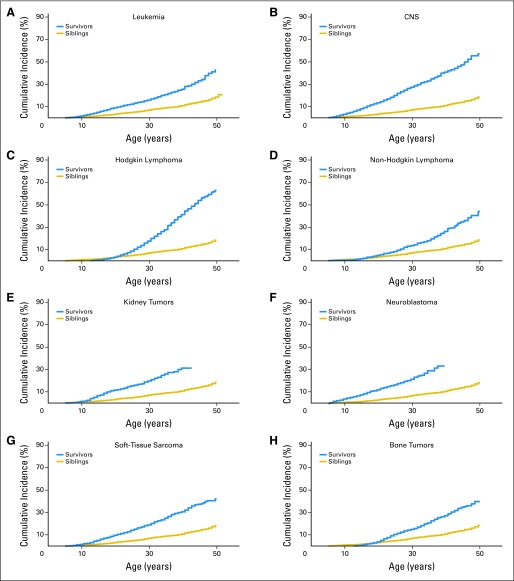
Cumulative incidence of chronic health conditions for severe, disabling, life-threatening, or fatal health conditions, by primary childhood cancer diagnosis. (A) leukemia, (B) CNS tumors, (C) Hodgkin lymphoma, (D) non-Hodgkin lymphoma, (E) kidney tumors, (F) neuroblastoma, (G) soft-tissue sarcoma, and (H) bone tumors.
Fig A3.
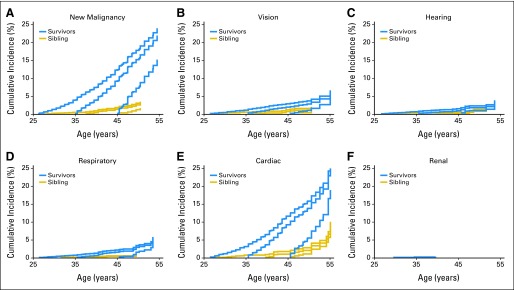
Cumulative incidence of selected grade 3 to 5 conditions by organ system, based on having no previous grade 3 to 5 events among survivors and siblings. (A) New malignancy, (B) vision, (C) hearing, (D) respiratory, (E) cardiac, and (F) renal.
Fig A4.
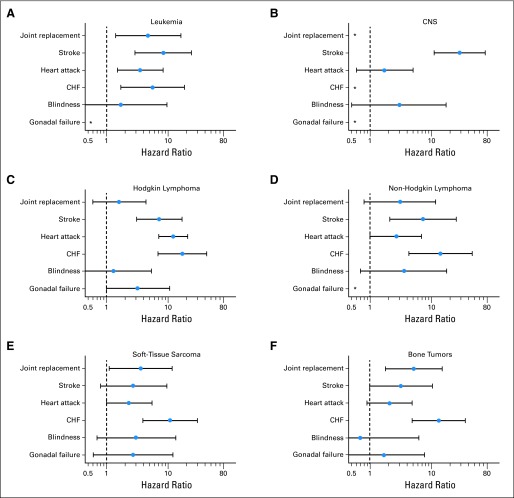
Hazard ratios and 95% CIs for specific chronic conditions that first occurred on or after age 35 years, adjusted for age and sex for survivors of (A) leukemia, (B) CNS tumors, (C) Hodgkin lymphoma, (D) non-Hodgkin lymphoma, (E) soft-tissue sarcomas, and (F) bone tumors. CHF, congestive heart failure. (*) No events.
Footnotes
Supported by Grant No. CA55727 from the National Cancer Institute (L.L.R.); support to St Jude Children's Research Hospital was also provided by Grant No. CA21765 from the Cancer Center Support (R. Gilbertson, Principal Investigator) and the American Lebanese-Syrian Associated Charities.
Presented at the 48th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 1-5, 2012.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Gregory T. Armstrong, Toana Kawashima, Wendy Leisenring, Marilyn Stovall, Melissa M. Hudson, Charles A. Sklar, Leslie L Robison, Kevin C. Oeffinger
Financial support: Leslie L Robison
Provision of study materials or patients: Gregory T. Armstrong, Leslie L Robison
Collection and assembly of data: Gregory T. Armstrong, Toana Kawashima, Wendy Leisenring, Kayla Stratton, Marilyn Stovall, Leslie L Robison, Kevin C. Oeffinger
Data analysis and interpretation: Gregory T. Armstrong, Toana Kawashima, Wendy Leisenring, Kayla Stratton, Melissa M. Hudson, Charles A. Sklar, Leslie L Robison, Kevin C. Oeffinger
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Howlader N, Noone AM, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975-2008. Bethesda, MD: National Cancer Institute; 2012. [Google Scholar]
- 2.Mariotto AB, Rowland JH, Yabroff KR, et al. Long-term survivors of childhood cancers in the United States. Cancer Epidemiol Biomarkers Prev. 2009;18:1033–1040. doi: 10.1158/1055-9965.EPI-08-0988. [DOI] [PubMed] [Google Scholar]
- 3.Hudson MM, Ness KK, Gurney JG, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309:2371–2381. doi: 10.1001/jama.2013.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 5.Mertens AC, Liu Q, Neglia JP, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: The Childhood Cancer Survivor Study. J Natl Cancer Inst. 2008;100:1368–1379. doi: 10.1093/jnci/djn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedman DL, Whitton J, Leisenring W, et al. Subsequent neoplasms in 5-year survivors of childhood cancer: The Childhood Cancer Survivor Study. J Natl Cancer Inst. 2010;102:1083–1095. doi: 10.1093/jnci/djq238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: A National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27:2308–2318. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leisenring WM, Mertens AC, Armstrong GT, et al. Pediatric cancer survivorship research: Experience of the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2319–2327. doi: 10.1200/JCO.2008.21.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robison LL, Mertens AC, Boice JD, et al. Study design and cohort characteristics of the Childhood Cancer Survivor Study: A multi-institutional collaborative project. Med Pediatr Oncol. 2002;38:229–239. doi: 10.1002/mpo.1316. [DOI] [PubMed] [Google Scholar]
- 10.Cancer Therapy Evaluation Program. Bethesda, MD: National Cancer Institute; Common terminology criteria for adverse events, version 3.0. http://ctep.cancer.gov. [Google Scholar]
- 11.Neglia JP, Friedman DL, Yasui Y, et al. Second malignant neoplasms in five-year survivors of childhood cancer: Childhood cancer survivor study. J Natl Cancer Inst. 2001;93:618–629. doi: 10.1093/jnci/93.8.618. [DOI] [PubMed] [Google Scholar]
- 12.Meadows AT, Friedman DL, Neglia JP, et al. Second neoplasms in survivors of childhood cancer: Findings from the Childhood Cancer Survivor Study cohort. J Clin Oncol. 2009;27:2356–2362. doi: 10.1200/JCO.2008.21.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stovall M, Weathers R, Kasper C, et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: Use in epidemiological studies. Radiat Res. 2006;166:141–157. doi: 10.1667/RR3525.1. [DOI] [PubMed] [Google Scholar]
- 14.Andersen PK, Gill RD. Cox's regression model for counting processes: A large sample study. Ann Stat. 1982;10:1100–1120. [Google Scholar]
- 15.Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. J Am Stat Assoc. 1989;84:1074–1078. [Google Scholar]
- 16.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Stone CJ, Koo CY. Proceedings of the Statistical Computing Section ASA. Washington, DC: American Statistical Association; 1985. p. 45. [Google Scholar]
- 18.Gooley TA, Leisenring W, Crowley J, et al. Estimation of failure probabilities in the presence of competing risks: New representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 19.Heisey DM, Patterson BR. A review of methods to estimate cause-specific mortality in presence of competing risks. J Wildl Manage. 2006;70:1544–1555. [Google Scholar]
- 20.Geenen MM, Cardous-Ubbink MC, Kremer LC, et al. Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. JAMA. 2007;297:2705–2715. doi: 10.1001/jama.297.24.2705. [DOI] [PubMed] [Google Scholar]
- 21.Landier W, Armenian SH, Lee J, et al. Yield of screening for long-term complications using the Children's Oncology Group long-term follow-up guidelines. J Clin Oncol. 2012;30:4401–4408. doi: 10.1200/JCO.2012.43.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hewitt M, Weiner SL, Simone JV, et al., editors. Childhood Cancer Survivorship: Improving Care and Quality of Life. Washington, DC: National Academy Press; 2003. [PubMed] [Google Scholar]
- 23.Armstrong GT, Liu W, Leisenring W, et al. Occurrence of multiple subsequent neoplasms in long-term survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. J Clin Oncol. 2011;29:3056–3064. doi: 10.1200/JCO.2011.34.6585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oeffinger KC, Ford JS, Moskowitz CS, et al. Breast cancer surveillance practices among women previously treated with chest radiation for a childhood cancer. JAMA. 2009;301:404–414. doi: 10.1001/jama.2008.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henderson TO, Oeffinger KC, Whitton J, et al. Secondary gastrointestinal cancer in childhood cancer survivors: A cohort study. Ann Intern Med. 2012;156:757–766. doi: 10.1059/0003-4819-156-11-201206050-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Landier W, Bhatia S, Eshelman DA, et al. Development of risk-based guidelines for pediatric cancer survivors: The Children's Oncology Group long-term follow-up guidelines from the Children's Oncology Group Late Effects Committee and Nursing Discipline. J Clin Oncol. 2004;22:4979–4990. doi: 10.1200/JCO.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 27.Lipshultz SE, Lipsitz SR, Sallan SE, et al. Long-term enalapril therapy for left ventricular dysfunction in doxorubicin-treated survivors of childhood cancer. J Clin Oncol. 2002;20:4517–4522. doi: 10.1200/JCO.2002.12.102. [DOI] [PubMed] [Google Scholar]
- 28.Armstrong GT, Plana JC, Zhang N, et al. Screening adult survivors of childhood cancer for cardiomyopathy: Comparison of echocardiography and cardiac magnetic resonance imaging. J Clin Oncol. 2012;30:2876–2884. doi: 10.1200/JCO.2011.40.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cardinale D, Colombo A, Lamantia G, et al. Anthracycline-induced cardiomyopathy: Clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol. 2010;55:213–220. doi: 10.1016/j.jacc.2009.03.095. [DOI] [PubMed] [Google Scholar]
- 30.Ness KK, Hudson MM, Ginsberg JP, et al. Physical performance limitations in the Childhood Cancer Survivor Study cohort. J Clin Oncol. 2009;27:2382–2389. doi: 10.1200/JCO.2008.21.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mulrooney DA, Ness KK, Neglia JP, et al. Fatigue and sleep disturbance in adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study (CCSS) Sleep. 2008;31:271–281. doi: 10.1093/sleep/31.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ness KK, Krull KR, Jones KE, et al. Physiologic frailty as a sign of accelerated aging among adult survivors of childhood cancer: A report from the St Jude lifetime cohort study. J Clin Oncol. 2013;31:4496–4503. doi: 10.1200/JCO.2013.52.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Armstrong GT, Reddick WE, Petersen RC, et al. Evaluation of memory impairment in aging adult survivors of childhood acute lymphoblastic leukemia treated with cranial radiotherapy. J Natl Cancer Inst. 2013;105:899–907. doi: 10.1093/jnci/djt089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maccormick RE. Possible acceleration of aging by adjuvant chemotherapy: A cause of early onset frailty? Med Hypotheses. 2006;67:212–215. doi: 10.1016/j.mehy.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 35.Hudson MM, Neglia JP, Woods WG, et al. Lessons from the past: Opportunities to improve childhood cancer survivor care through outcomes investigations of historical therapeutic approaches for pediatric hematological malignancies. Pediatr Blood Cancer. 2012;58:334–343. doi: 10.1002/pbc.23385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Green DM, Kun LE, Matthay KK, et al. Relevance of historical therapeutic approaches to the contemporary treatment of pediatric solid tumors. Pediatr Blood Cancer. 2013;60:1083–1094. doi: 10.1002/pbc.24487. [DOI] [PMC free article] [PubMed] [Google Scholar]