Abstract
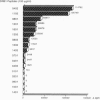
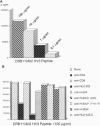
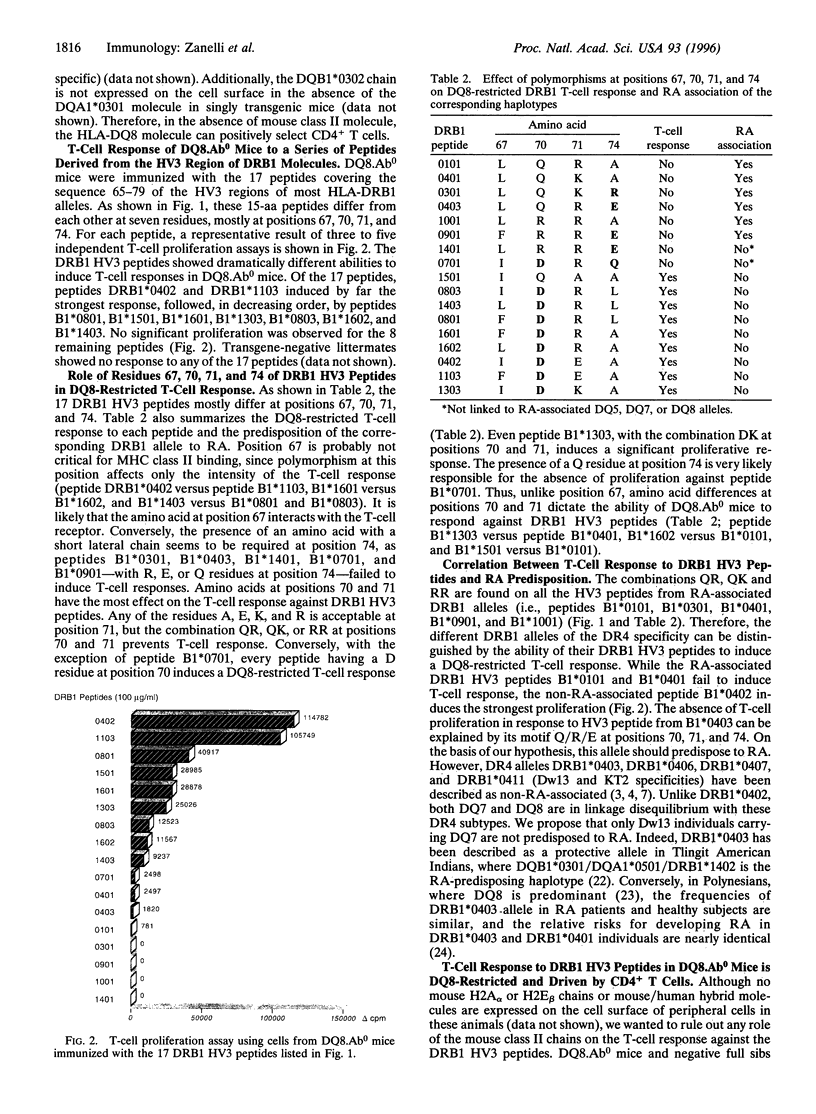
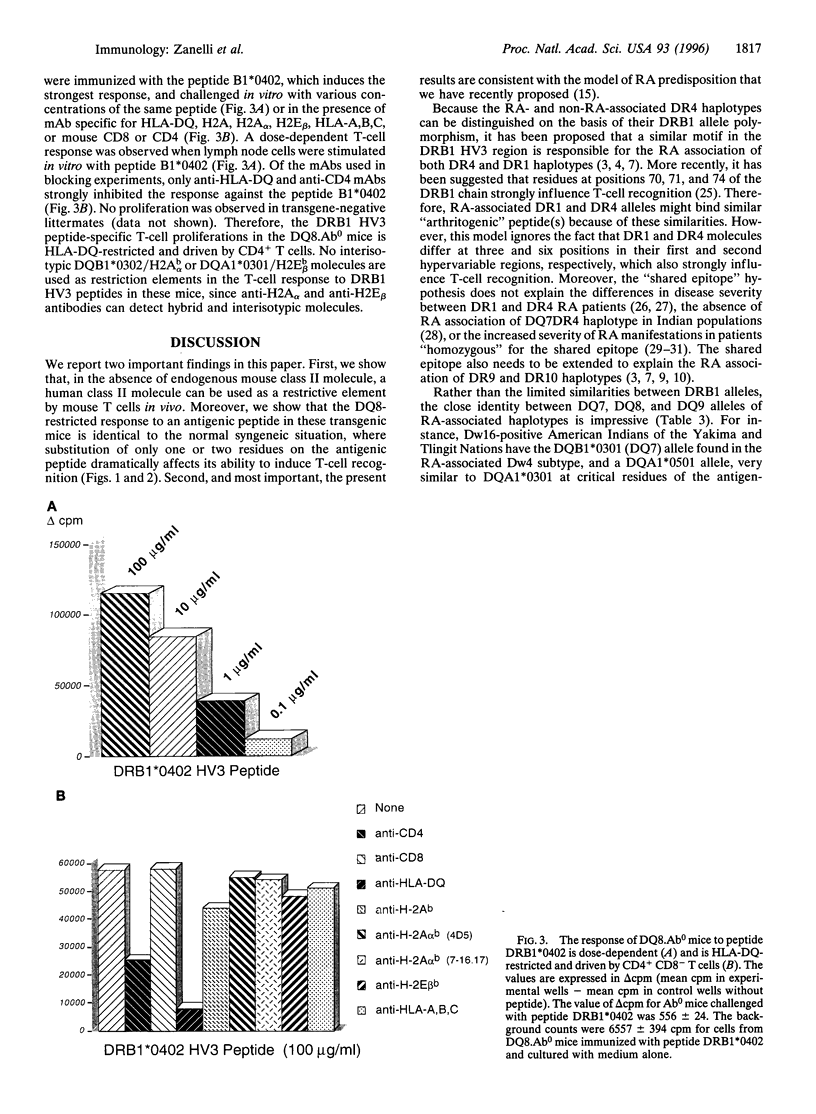
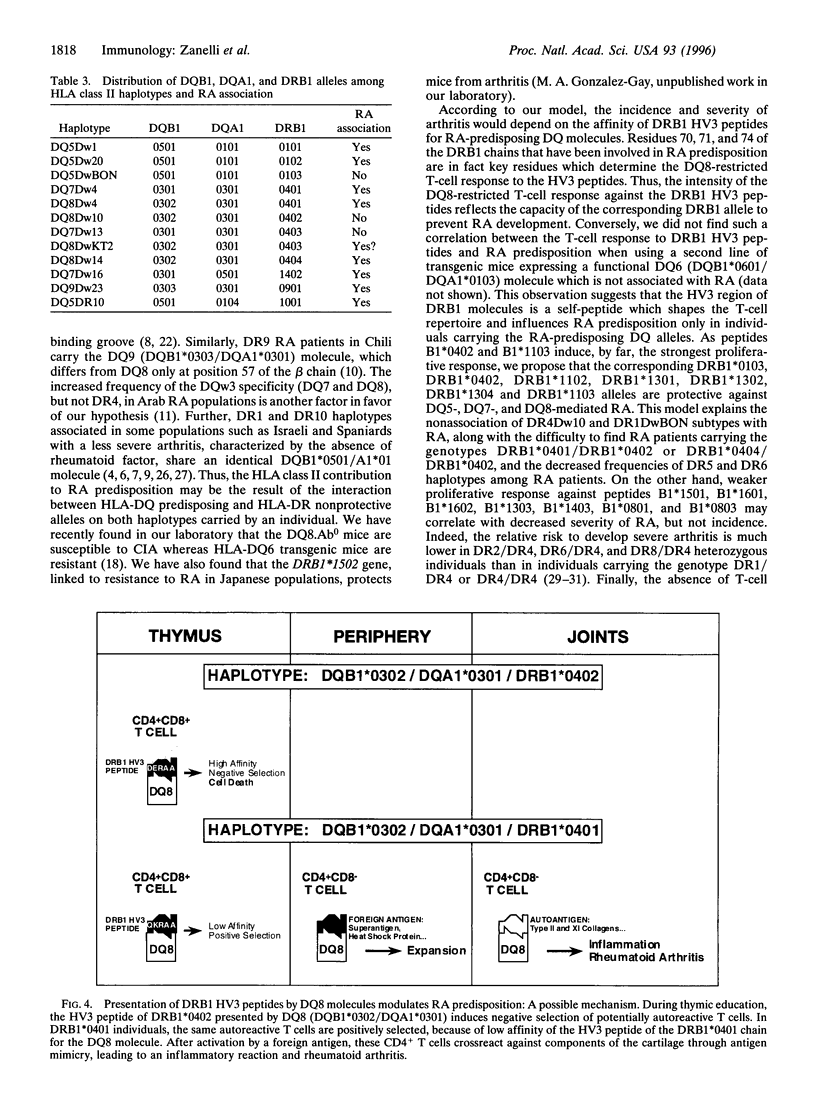
The major histocompatibility complex class II genes play an important role in the genetic predisposition to many autoimmune diseases. In the case of rheumatoid arthritis (RA), the human leukocyte antigen (HLA)-DRB1 locus has been implicated in the disease predisposition. The "shared epitope" hypothesis predicts that similar motifs within the third hypervariable (HV3) regions of some HLA-DRB1 alleles are responsible for the class II-associated predisposition to RA. Using a line of transgenic mice expressing the DQB1*0302/DQA1*0301 (DQ8) genes in the absence of endogenous mouse class II molecules, we have analyzed the antigenicity of peptides covering the HV3 regions of RA-associated and nonassociated DRB1 molecules. Our results show that a correlation exists between proliferative response to peptides derived from the HV3 regions of DRB1 chains and nonassociation of the corresponding alleles with RA predisposition. While HV3 peptides derived from nonassociated DRB1 molecules are highly immunogenic in DQ8 transgenic mice, all the HV3 peptides derived from RA-associated DRB1 alleles fail to induce a DQ8-restricted T-cell response. These data suggest that the role of the "shared epitope" in RA predisposition may be through the shaping of the T-cell repertoire.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal S., Aggarwal A., Dabadghao S., Naik S., Misra R. Compound heterozygosity of HLA-DR4 and DR1 antigens in Asian Indians increases the risk of extra-articular features in rheumatoid arthritis. Br J Rheumatol. 1995 Jan;34(1):41–44. doi: 10.1093/rheumatology/34.1.41. [DOI] [PubMed] [Google Scholar]
- Cairns J. S., Curtsinger J. M., Dahl C. A., Freeman S., Alter B. J., Bach F. H. Sequence polymorphism of HLA DR beta 1 alleles relating to T-cell-recognized determinants. Nature. 1985 Sep 12;317(6033):166–168. doi: 10.1038/317166a0. [DOI] [PubMed] [Google Scholar]
- Chicz R. M., Lane W. S., Robinson R. A., Trucco M., Strominger J. L., Gorga J. C. Self-peptides bound to the type I diabetes associated class II MHC molecules HLA-DQ1 and HLA-DQ8. Int Immunol. 1994 Nov;6(11):1639–1649. doi: 10.1093/intimm/6.11.1639. [DOI] [PubMed] [Google Scholar]
- David C. S. Genes for MHC, TCR and MIs determine susceptibility to collagen induced arthritis. APMIS. 1990 Jul;98(7):575–584. doi: 10.1111/j.1699-0463.1990.tb04974.x. [DOI] [PubMed] [Google Scholar]
- Deighton C. M., Cavanagh G., Rigby A. S., Lloyd H. L., Walker D. J. Both inherited HLA-haplotypes are important in the predisposition to rheumatoid arthritis. Br J Rheumatol. 1993 Oct;32(10):893–898. doi: 10.1093/rheumatology/32.10.893. [DOI] [PubMed] [Google Scholar]
- Dizier M. H., Eliaou J. F., Babron M. C., Combe B., Sany J., Clot J., Clerget-Darpoux F. Investigation of the HLA component involved in rheumatoid arthritis (RA) by using the marker association-segregation chi-square (MASC) method: rejection of the unifying-shared-epitope hypothesis. Am J Hum Genet. 1993 Sep;53(3):715–721. [PMC free article] [PubMed] [Google Scholar]
- Fu X. T., Bono C. P., Woulfe S. L., Swearingen C., Summers N. L., Sinigaglia F., Sette A., Schwartz B. D., Karr R. W. Pocket 4 of the HLA-DR(alpha,beta 1*0401) molecule is a major determinant of T cells recognition of peptide. J Exp Med. 1995 Mar 1;181(3):915–926. doi: 10.1084/jem.181.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X., Serjeantson S. W. Diversity in HLA-DR4-related DR,DQ haplotypes in Australia, Oceania, and China. Hum Immunol. 1991 Dec;32(4):269–276. doi: 10.1016/0198-8859(91)90090-v. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Gay M. A., Nabozny G. H., Bull M. J., Zanelli E., Douhan J., 3rd, Griffiths M. M., Glimcher L. H., Luthra H. S., David C. S. Protective role of major histocompatibility complex class II Ebd transgene on collagen-induced arthritis. J Exp Med. 1994 Oct 1;180(4):1559–1564. doi: 10.1084/jem.180.4.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Gay M. A., Zanelli E., Krco C. J., Nabozny G. H., Hanson J., Griffiths M. M., Luthra H. S., David C. S. Polymorphism of the MHC class II Eb gene determines the protection against collagen-induced arthritis. Immunogenetics. 1995;42(1):35–40. doi: 10.1007/BF00164985. [DOI] [PubMed] [Google Scholar]
- González A., Nicovani S., Massardo L., Bull P., Rodríguez L., Jacobelli S. Novel genetic markers of rheumatoid arthritis in Chilean patients, by DR serotyping and restriction fragment length polymorphism analysis. Arthritis Rheum. 1992 Mar;35(3):282–289. doi: 10.1002/art.1780350306. [DOI] [PubMed] [Google Scholar]
- Gregersen P. K., Silver J., Winchester R. J. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 1987 Nov;30(11):1205–1213. doi: 10.1002/art.1780301102. [DOI] [PubMed] [Google Scholar]
- Jönsson K., McDevitt D., McGavin M. H., Patti J. M., Hök M. Staphylococcus aureus expresses a major histocompatibility complex class II analog. J Biol Chem. 1995 Sep 15;270(37):21457–21460. doi: 10.1074/jbc.270.37.21457. [DOI] [PubMed] [Google Scholar]
- Krco C. J., Beito T. G., David C. S. Determination of tolerance to self E alpha peptides by clonal elimination of H-2E reactive T cells and antigen presentation by H-2A molecules. Transplantation. 1992 Nov;54(5):920–923. doi: 10.1097/00007890-199211000-00029. [DOI] [PubMed] [Google Scholar]
- Nelson J. L., Boyer G., Templin D., Lanier A., Barrington R., Nisperos B., Smith A., Mickelson E., Hansen J. A. HLA antigens in Tlingit Indians with rheumatoid arthritis. Tissue Antigens. 1992 Aug;40(2):57–63. doi: 10.1111/j.1399-0039.1992.tb01960.x. [DOI] [PubMed] [Google Scholar]
- Nelson J. L., Mickelson E., Masewicz S., Barrington R., Dugowson C., Koepsell T., Hansen J. A. Dw14(DRB1*0404) is a Dw4-dependent risk factor for rheumatoid arthritis. Rethinking the "shared epitope" hypothesis. Tissue Antigens. 1991 Oct;38(4):145–151. doi: 10.1111/j.1399-0039.1991.tb01888.x. [DOI] [PubMed] [Google Scholar]
- Nepom G. T., Erlich H. MHC class-II molecules and autoimmunity. Annu Rev Immunol. 1991;9:493–525. doi: 10.1146/annurev.iy.09.040191.002425. [DOI] [PubMed] [Google Scholar]
- Okada K., Boss J. M., Prentice H., Spies T., Mengler R., Auffray C., Lillie J., Grossberger D., Strominger J. L. Gene organization of DC and DX subregions of the human major histocompatibility complex. Proc Natl Acad Sci U S A. 1985 May;82(10):3410–3414. doi: 10.1073/pnas.82.10.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollier W., Thomson W. Population genetics of rheumatoid arthritis. Rheum Dis Clin North Am. 1992 Nov;18(4):741–759. [PubMed] [Google Scholar]
- Ploski R., Mellbye O. J., Rønningen K. S., Førre O., Thorsby E. Seronegative and weakly seropositive rheumatoid arthritis differ from clearly seropositive rheumatoid arthritis in HLA class II associations. J Rheumatol. 1994 Aug;21(8):1397–1402. [PubMed] [Google Scholar]
- Sattar M. A., al-Saffar M., Guindi R. T., Sugathan T. N., Behbehani K. Association between HLA-DR antigens and rheumatoid arthritis in Arabs. Ann Rheum Dis. 1990 Mar;49(3):147–149. doi: 10.1136/ard.49.3.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stastny P. Mixed lymphocyte cultures in rheumatoid arthritis. J Clin Invest. 1976 May;57(5):1148–1157. doi: 10.1172/JCI108382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan P. L., Farmiloe S., Roberts M., Geursen A., Skinner M. A. HLA-DR4 subtypes in New Zealand Polynesians. Predominance of Dw13 in the healthy population and association of Dw15 with rheumatoid arthritis. Arthritis Rheum. 1993 Jan;36(1):15–19. doi: 10.1002/art.1780360104. [DOI] [PubMed] [Google Scholar]
- Taneja V., Mehra N. K., Chandershekaran A. N., Ahuja R. K., Singh Y. N., Malaviya A. N. HLA-DR4-DQw8, but not DR4-DQw7 haplotypes occur in Indian patients with rheumatoid arthritis. Rheumatol Int. 1992;11(6):251–255. doi: 10.1007/BF00301502. [DOI] [PubMed] [Google Scholar]
- Vogt A. B., Kropshofer H., Kalbacher H., Kalbus M., Rammensee H. G., Coligan J. E., Martin R. Ligand motifs of HLA-DRB5*0101 and DRB1*1501 molecules delineated from self-peptides. J Immunol. 1994 Aug 15;153(4):1665–1673. [PubMed] [Google Scholar]
- Walser-Kuntz D. R., Weyand C. M., Weaver A. J., O'Fallon W. M., Goronzy J. J. Mechanisms underlying the formation of the T cell receptor repertoire in rheumatoid arthritis. Immunity. 1995 Jun;2(6):597–605. doi: 10.1016/1074-7613(95)90004-7. [DOI] [PubMed] [Google Scholar]
- Weyand C. M., Hicok K. C., Conn D. L., Goronzy J. J. The influence of HLA-DRB1 genes on disease severity in rheumatoid arthritis. Ann Intern Med. 1992 Nov 15;117(10):801–806. doi: 10.7326/0003-4819-117-10-801. [DOI] [PubMed] [Google Scholar]
- Willkens R. F., Nepom G. T., Marks C. R., Nettles J. W., Nepom B. S. Association of HLA-Dw16 with rheumatoid arthritis in Yakima Indians. Further evidence for the "shared epitope" hypothesis. Arthritis Rheum. 1991 Jan;34(1):43–47. doi: 10.1002/art.1780340107. [DOI] [PubMed] [Google Scholar]
- Wilson C., Ebringer A., Ahmadi K., Wrigglesworth J., Tiwana H., Fielder M., Binder A., Ettelaie C., Cunningham P., Joannou C. Shared amino acid sequences between major histocompatibility complex class II glycoproteins, type XI collagen and Proteus mirabilis in rheumatoid arthritis. Ann Rheum Dis. 1995 Mar;54(3):216–220. doi: 10.1136/ard.54.3.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winchester R., Dwyer E., Rose S. The genetic basis of rheumatoid arthritis. The shared epitope hypothesis. Rheum Dis Clin North Am. 1992 Nov;18(4):761–783. [PubMed] [Google Scholar]
- Wordsworth B. P., Lanchbury J. S., Sakkas L. I., Welsh K. I., Panayi G. S., Bell J. I. HLA-DR4 subtype frequencies in rheumatoid arthritis indicate that DRB1 is the major susceptibility locus within the HLA class II region. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10049–10053. doi: 10.1073/pnas.86.24.10049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wordsworth P., Pile K. D., Buckely J. D., Lanchbury J. S., Ollier B., Lathrop M., Bell J. I. HLA heterozygosity contributes to susceptibility to rheumatoid arthritis. Am J Hum Genet. 1992 Sep;51(3):585–591. [PMC free article] [PubMed] [Google Scholar]
- Yelamos J., Garcia-Lozano J. R., Moreno I., Aguilera I., Gonzalez M. F., Garcia A., Nuñez-Roldan A., Sanchez B. Association of HLA-DR4-Dw15 (DRB1*0405) and DR10 with rheumatoid arthritis in a Spanish population. Arthritis Rheum. 1993 Jun;36(6):811–814. doi: 10.1002/art.1780360611. [DOI] [PubMed] [Google Scholar]
- Zanelli E., Gonzalez-Gay M. A., David C. S. Could HLA-DRB1 be the protective locus in rheumatoid arthritis? Immunol Today. 1995 Jun;16(6):274–278. doi: 10.1016/0167-5699(95)80181-2. [DOI] [PubMed] [Google Scholar]