Supplemental Digital Content is Available in the Text.
Key Words: antiretroviral therapy, HIV care, costing, integration, Vietnam
Abstract
Background:
Vietnam achieved rapid scale-up of antiretroviral therapy (ART), although external funds are declining sharply. To achieve and sustain universal access to HIV services, evidence-based planning is essential. To date, there had been limited HIV treatment and care cost data available in Vietnam.
Methods:
Cost data of outpatient and inpatient HIV care were extracted at 21 sentinel facilities (17 adult and 4 pediatric) that epitomize the national program. Step-down costing for administration costs and bottom-up resource costing for drugs, diagnostics, and labor were used. Records of 1401 adults and 527 pediatric patients were reviewed.
Results:
Median outpatient care costs per patient-year for pre-ART, first year ART, later year ART, and second-line ART were US $100, US $316, US $303, and US $1557 for adults; and US $171, US $387, US $320, and US $1069 for children, respectively. Median inpatient care cost per episode was US $162 for adults and US $142 for children. Non-antiretroviral (ARV) costs in adults at stand-alone facilities were 44% (first year ART) and 24% (later year ART) higher than those at integrated facilities. Adults who started ART with CD4 count ≤100 cells per cubic millimeter had 47% higher non-ARV costs in the first year ART than those with CD4 count >100 cells per cubic millimeter. Adult ARV drug costs at government sites were from 66% to 85% higher than those at donor-supported sites in the first year ART.
Conclusions:
The study found that HIV treatment and care costs in Vietnam are economical, yet there is potential to further promote efficiency through strengthening competitive procurement, integrating HIV services, and promoting earlier ART initiation.
INTRODUCTION
Vietnam has a concentrated HIV epidemic with estimated HIV prevalence in the general population (age, 15–49 years) at 0.45% and the prevalence in people who inject drugs at 13.4% in 2011.1 An estimated 248,500 people were living with HIV in 20111 and 60,924 adults and children were receiving antiretroviral therapy (ART) at the end of 2011.1 However, ART coverage compared with estimated needs was still limited to 54%,1 and further scale-up is critical.
Vietnam's national HIV response has relied on external funds, such as President's Emergency Funds for AIDS Relief (PEPFAR) and Global Fund to fight AIDS, Tuberculosis, and Malaria (GF). The National AIDS spending assessment reported that 14.5% of total AIDS expenditure in 2009–2010 was financed by domestic sources, 11.9% by private, and 73.7% by international sources.1 Furthermore, Vietnam is transitioning from a low-income country to a lower middle-income country as per capita income reached US $1130 by the end of 2010.2 With this transition, external funds for Vietnam's HIV response started to decline sharply. Careful planning is increasingly important to achieve further scale-up and sustain access to essential HIV services. Although it is important to mobilize additional domestic and international funds, evidence-guided policy decisions to optimize resource allocations and promote cost-effective service delivery are critical. However, there have been very limited data on HIV treatment and care costs in Vietnam.
The purpose of this study was to provide the latest data on HIV treatment and care costs in Vietnam from the health system perspective to inform development of the national strategy, forecast resource needs, and optimize service delivery models.
METHODS
Sites, Sampling, and Costing Period
Data were collected at 21 sentinel facilities (17 for adult care and 4 for pediatric care) included in the annual national HIV facility survey3 (see Table S1, Supplemental Digital Content, http://links.lww.com/QAI/A441). The facilities were chosen to epitomize the program in Vietnam and to reflect the diverse range of HIV treatment and care facilities. Among adult care facilities, pre-ART and first-line ART services were available at 16 facilities, whereas second- line ART was available at only 5 facilities and inpatient care services at 8 facilities. Twelve adult outpatient clinics were integrated facilities established within existing health facilities, that is, hospitals and health centers, where HIV services were delivered along with other non-HIV health services. Four adult outpatient clinics were stand-alone facilities providing only HIV services, that is, provincial AIDS centers and some clinics in Ho Chi Minh City (see Table S12, Supplemental Digital Content, http://links.lww.com/QAI/A441).
At each adult site, the sample design required systematic sampling of the medical records of 20 patients receiving pre-ART or first-line ART services, 30 patients receiving second-line ART, and 40 patients receiving inpatient care. At the 4 pediatric sites, the medical records of 30 children were systematically sampled for each care phase. Sampling frames consisted of the following: For pre-ART, a list of patients in pre-ART status at the end of February 2010 who have had at least 1 visit in the last 12 months; for the first year of first-line ART (ART year 1), a list of patients who started first-line ART during the period from April 1, 2008, to March 31, 2009, excluding transfer-in cases; for the second and later years of ART (ART year 2+), a list of patients who started first-line ART before April 1, 2008, and who were neither lost to follow-up nor dead before May 1, 2009 (ie, receiving at least 1 month of treatment during the period from April 1, 2009, to March 31, 2010); for inpatient care, a list of inpatients with confirmed HIV infection who were discharged between April 1, 2009, and March 31, 2010.
Costs for pre-ART, ART year 2+, and second-line ART phases were analyzed cross-sectionally, and the costs incurred in the period from April 1, 2009, to March 31, 2010, were estimated. For patients who entered into each phase after April 1, 2009, and those who left that care phase before March 31, 2010, cost information was collected only for the period they were followed, but their cost estimates were annualized. ART year 1 costs were analyzed as a cohort, for respective 12-month periods (or until the patient died or was classified as lost) from the date ART was initiated for each patient. The cohort-based analysis was necessary for this phase because there is a greater concentration of costs toward the first few months until treatment is stabilized.4 The costing reference period of ART year 1 was from April 2008 to March 2010, and approximately half of the patient-months of treatment were in the preceding 12 months of the costing period of the other phases; however, no obvious difference was identified in diagnostic tests or opportunistic infection (OI) drugs used between earlier and later intake patients in this phase. Inpatient costs covered the whole period of hospitalization.
During the costing period, stavudine (d4T) + lamivudine (3TC) + nevirapine was the most commonly prescribed ART regimen in Vietnam (Table 2), and the commonly performed laboratory tests included CD4 lymphocyte count, liver function test, complete blood count, and serological tests for hepatitis B and hepatitis C.
TABLE 2.
Cost Components of Adult Outpatient Care Costs Per Patient-Year, ARV Regimens, and Reliance on External Funds Disaggregated by Donors
Analysis of Costs and Treatment Outcomes
A hybrid of top-down and bottom-up costing approaches was used, similar to the approach previously documented,5,6 to overcome information constraints at facilities in resource-limited settings. For antiretroviral (ARV) drugs, drugs to prevent or treat OI drugs and diagnostic tests, quantities were extracted from patient records and relevant prices were applied. For labor and overhead costs (including annual capital costs), we estimated total costs for the ward, clinic, or facility and divided by total products to obtain unit costs, which were then applied to patients based on the number of visits, inpatient days, or diagnostic services used. For cases where health staff worked on a mix of outpatient HIV treatment and other services, regardless of whether working at integrated or stand-alone facilities, the approximate share of time spent on different tasks was obtained, and payroll costs were prorated accordingly. The overhead costs included administrative and operational costs, for example, utilities, office supplies, rentals, repairs, and annual capital costs. For the annual capital costs, the purchase value of equipment was multiplied by a straight-line depreciation rate based on asset life of 3–10 years following government regulations to obtain rough estimates of equipment depreciation. All the costs were estimated as year-2009 values, by using the annual payroll, overhead budgets, service fees, and drug prices from 2009 as the unit costs, which were then multiplied by the quantities obtained. This constituted an implicit adjustment to 2009 prices for all care phases, including ART year 1. Economic costs were not estimated because relevant information needed to calculate the opportunity costs of the resources used for HIV treatment, that is, labor, equipment, land, and buildings, was not available. Costs were converted using an average exchange rate over the costing period of 18,462 Vietnam dong per United States dollar.7
The mean costs per patient-year or per episode were weighted so that the proportion of cases in each facility type (donor and health system level) in our sample was adjusted to be proportional to total patients in these facility types nationwide (details in the Appendix, see Tables S7 and S9, Supplemental Digital Content, http://links.lww.com/QAI/A441). For adult patients, association between costs and facility or patient characteristics was examined, including facility types (stand-alone and integrated facilities), level of immunodeficiency, donor support, and health system levels. Sensitivity analysis was performed for the following scenarios: (1) Alternative approaches to annualize adult pre-ART costs (many patients had less than 12-month follow-up in pre-ART care); (2) effects of d4T phase-out on ART year 1 costs (half of patient-months costed as ART year 1 phase lies in the previous 12 months of other care phases); (3) potential effects of incomplete recording of OI drugs prescribed. Statistical analysis was performed using Wald test for 2-group comparison, following a natural log transformation of cost values.
To assess potential association between the costs and treatment outcomes at adult ART sites, retention rates on ART at 12 months among patients who started ART in 2008 were obtained from the annual national HIV facility survey. Data were extracted from the ART register at each facility, and retention rate was analyzed based on the month in which ART was started and the month of attrition if it occurred, following the procedure proposed by World Health Organization.8
Data Extraction and Ethics
Data were collected by a team of trained data extractors using standardized data entry templates. A data set containing detailed labor and overhead data was compiled in MS Excel. Patient record information was also compiled into databases for each care phase. Data were analyzed using STATA Version 11.0. To ensure confidentiality, data did not include any names or addresses of the patients but only a code on the sample frame. The study protocol was reviewed and approved by the ethical review committee of the Hanoi School of Public Health.
RESULTS
Size and Characteristics of Sampled Patients
The total number of people receiving ART at sampled facilities at the end of September 2009 (midpoint of costing period) was 8110 adults and 937 children, respectively, which corresponds to 24% and 50% of total adults and children receiving ART nationwide at that time. At the sampled facilities, medical records of 1401 adult patients, consisting of 305 in pre-ART, 332 in ART year 1, 323 in ART year 2+, 122 in second-line ART, and 319 in inpatient care; and the records of 527 children, consisting of 104 in pre-ART, 104 in ART year 1, 120 in ART year 2+, 79 in second-line ART, and 439 in inpatient care, were reviewed. From 48% to 83% of sampled adults and from 51% to 61% of sampled children were males, and the median ages of sampled adults and children were in the range from 31 to 34 and from 3 to 8, respectively, depending on the care phase.
Total Costs and Cost Components in Adults and Children
In adults, median outpatient care cost per patient-year was US $100 for pre-ART care, US $316 for ART year 1, US $303 for ART year 2+, and US $1557 for second-line ART (Table 1). In children, median outpatient care cost per patient-year was US $171 for pre-ART care, US $387 for ART year 1, US $320 for ART year 2+, and US $1069 for second-line ART (Table 1). Median inpatient care costs per episode were US $162 for adults and US $142 for children.
TABLE 1.
Total Costs and the Breakdown by Cost Components for Different Phases of Adult and Pediatric HIV Care and Treatment in Vietnam
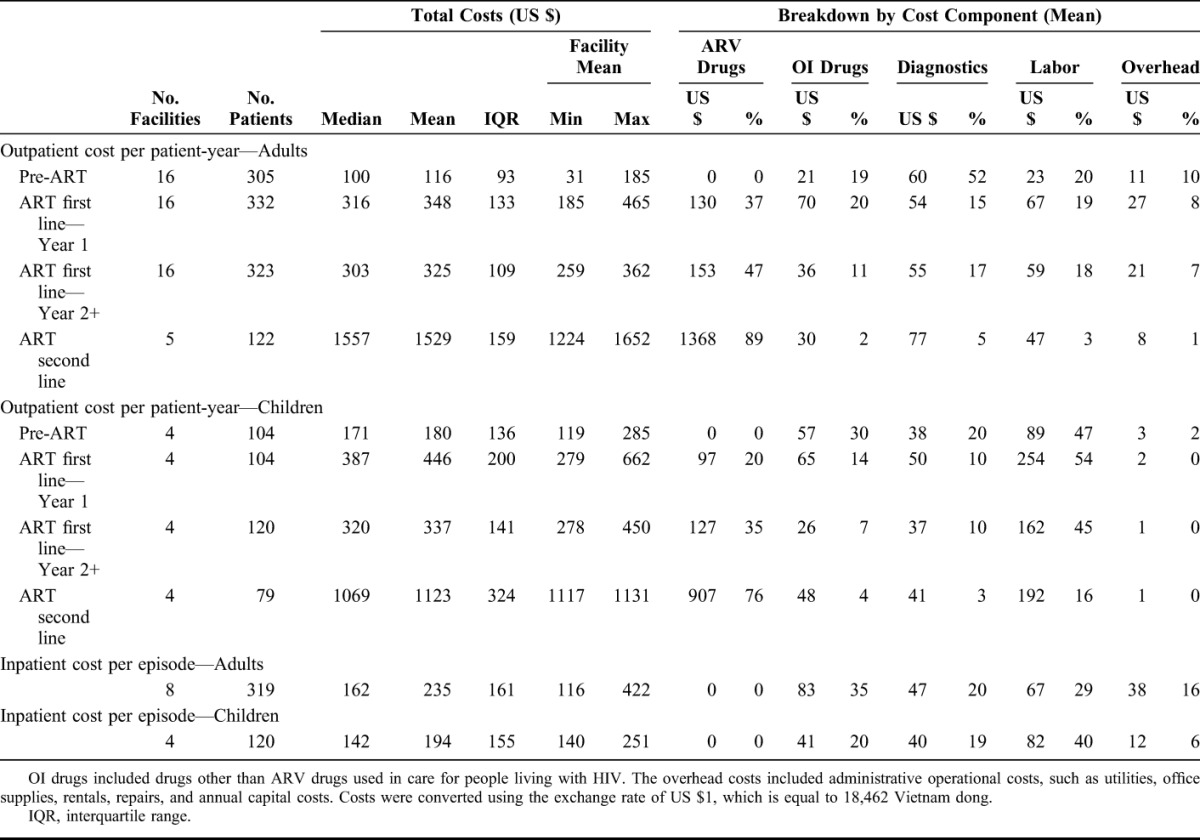
In adults, during pre-ART care, the largest cost component was diagnostics, which comprised 52% of the total costs per patient-year. Once ART was initiated, the largest cost component was ARV drugs, which accounted for 37%, 47%, and 89% of the total costs per patient-year in ART year 1, ART year 2+, and second-line ART (Table 1). OI drugs, diagnostics, and labor costs, respectively, consisted of 10%–20% of total costs in each ART phase. In contrast, in pediatric care, the largest cost component was labor costs both in pre-ART and ART phases, accounting for 45%–54% of total costs per patient-year, except in second-line ART, for which ARV drug cost was the largest component.
Factors Affecting Total Costs and Cost Components in Adults
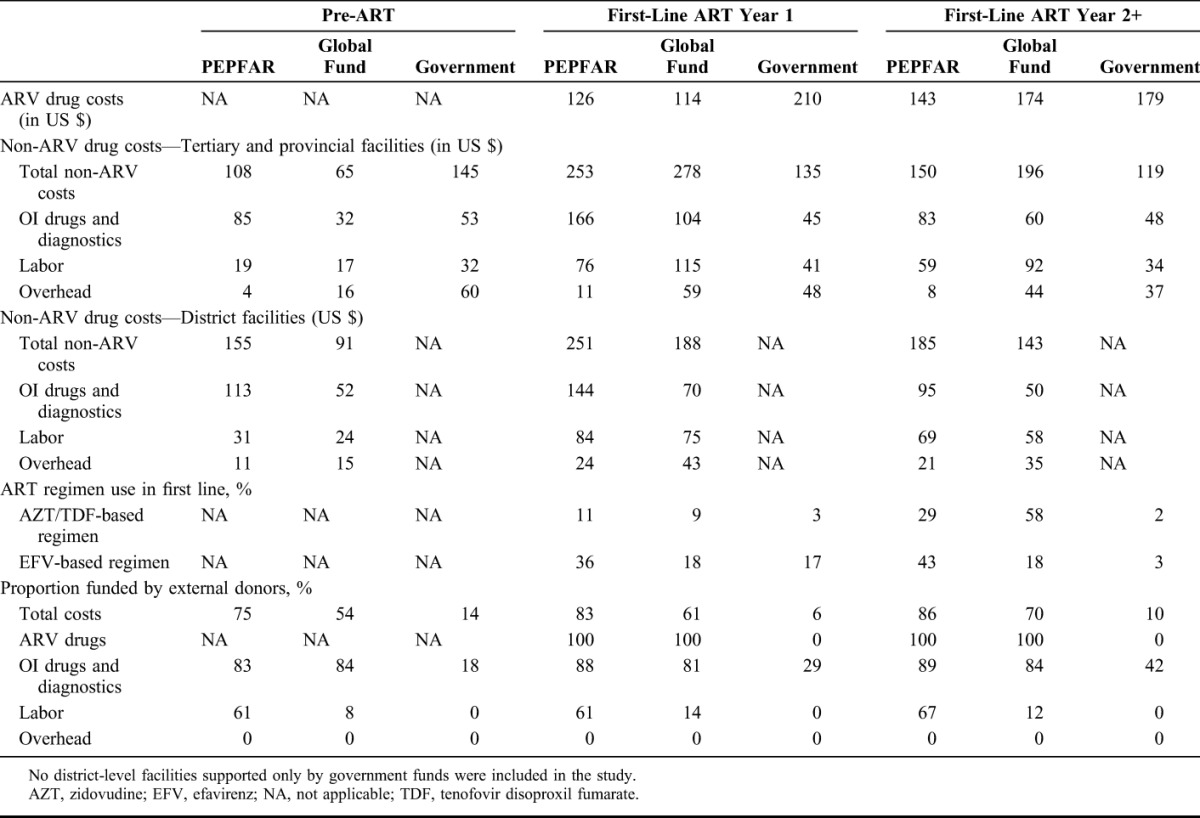
In adult ART year 1, ARV cost per patient-year was US $210 at government sites, whereas it was only US $126 and US $114 per patient-year at PEPFAR and GF-supported sites, respectively (Table 2). For adult ART year 2+, ARV costs were highest at government sites, but the difference among the donors was smaller (Table 2). These differences are due to different unit costs and prescription patterns among different donors. The price of d4T + 3 TC + nevirapine per patient-year procured by government sites was US $148, which was 1.6 and 1.5 times higher than the price paid by GF and PEPFAR, respectively. The price of d4T + 3 TC+ efavirenz per patient-year was US $355, which was 2.9 and 2.3 times higher than the price paid by GF and PEPFAR projects. Zidovudine, tenofovir disoproxil fumrate, and efavirenz were used more commonly at PEPFAR and GF–supported sites than at Government sites, especially in ART year 2+ (Table 2).
For tertiary and provincial facilities, GF sites had the highest non-ARV costs in all ART phases (eg, ART year 1 cost was US $253, US $278, and US $135 at PEPFAR, GF, and government sites, respectively); but for the district facilities, PEPFAR sites had higher non-ARV costs than GF sites (US $251 and US $188 for ART year 1 at PEPFAR and GF sites, respectively) (Table 2). In ART year 1 and year 2+, OI drug and diagnostic costs were the largest non-ARV cost components at PEPFAR and government sites, although labor cost was the largest non-ARV cost component at GF sites (Table 2). Overhead costs were much lower in PEPFAR sites compared with GF and government sites (Table 2). The proportion of total costs funded by external donors was highest at PEPFAR sites followed by GF sites, for example, for adult ART year 1, 83%, 61%, and 6% of the total costs were funded by donors at PEPFAR, GF, and government sites, respectively.
Mean non-ARV cost per patient-year at 4 stand-alone sites was US $298 for year 1, which was 44% higher than that at 12 integrated sites (P = 0.010), and was US $207 for year 2+, which was also 24% higher (P = 0.065) (Fig. 1). Labor and overhead costs were major causes of the higher costs at stand-alone sites. In ART year 1, at stand-alone and integrated sites, labor costs were US $111 and US $61, respectively (P = 0.010), and overhead costs were US $76 and US $21, respectively (P < 0.001) (Fig. 1). The various parameters comparing stand-alone and integrated sites are available in Table S12 (see Supplemental Digital Content, http://links.lww.com/QAI/A441).
FIGURE 1.
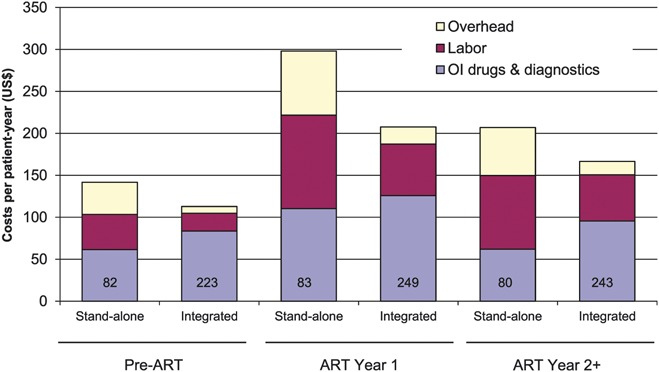
Adult non-ARV costs per patient-year for outpatient services at stand-alone and integrated facilities. Stand-alone facilities (n = 4) are facilities that deliver only HIV services, and integrated facilities (n = 12) are hospitals and district health centers where HIV services are delivered along with other health services. Sample sizes are shown inside the columns. The following costs were significantly different between the 2 groups: Total non-ARV costs in ART year 1 (P = 0.010); labor costs in pre-ART (P = 0.003), ART year 1 (P = 0.010), and ART year 2+ (P = 0.057); overhead costs in pre-ART (P = 0.003), ART year 1 (P < 0.001), and ART year 2+ (P < 0.001). OI drug and diagnostics costs were not significantly different between 2 groups in any phase.
Mean non-ARV costs in adult patients who had low CD4 (≤100 cells/mm3) at initiation of ART were US $256 per patient-year in ART year 1, which was 47% higher than those who had higher CD4 count (>100 cells/mm3) (P = 0.027) (Fig. 2). In each care phase, OI drug costs were from 2.0 to 3.9 times higher in patients with CD4 ≤100 cells per cubic millimeter compared with those with CD4 >100 cells per cubic millimeter (P < 0.001, pre-ART and ART year 1; P = 0.706, ART year 2+; P = 0.151, inpatient care) (Fig. 2).
FIGURE 2.
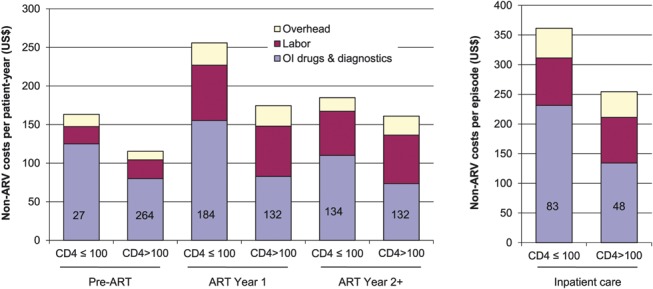
Adult non-ARV costs per patient-year for outpatient and inpatient care services disaggregated by CD4 levels. Patients were stratified according to the latest CD4 value for pre-ART and inpatient care, and by the CD4 at ART initiation for ART year 1 and ART year 2+ phases. The sample sizes are shown inside each column. The following costs were significantly different between the 2 groups: Total non-ARV costs in ART year 1 (P = 0.027); OI drug costs in pre-ART (P < 0.001), ART year 1 (P < 0.001), and inpatient care (P = 0.151). Labor, diagnostics, and overhead costs in all phases were not significantly different between the 2 groups.
The results of the sensitivity analysis are shown in Table S11 (see Supplemental Digital Content, http://links.lww.com/QAI/A441). Fifty-four percent of sampled adult pre-ART patients had follow-up less than 12 month; when pre-ART costs were calculated based on the actual follow-up period instead of annualizing individual patient costs, median costs per patient-year were 21% higher. When ART regimens prescribed in year 1 were assumed to be the same as those prescribed for ART year 2+ when the use of less-expensive d4T was reduced, median costs per patient-year were 6% higher. If it was assumed that facilities having the OI drug costs in the lowest quartile had average OI drug costs of all the facilities to address potential incomplete recording, the total costs increased only 3% for pre-ART and 1% for ART year 1 and ART year 2+ phases.
The average retention rate on ART at 12 months after ART initiation among adult patients at the 16 studied outpatient clinics was 86.3%. There was no correlation between the ART year 1 costs and the retention rate at 12 months after ART initiation (Fig. 3), indicating that lower cost per patient-year is not necessarily leading to poorer treatment outcomes.
FIGURE 3.
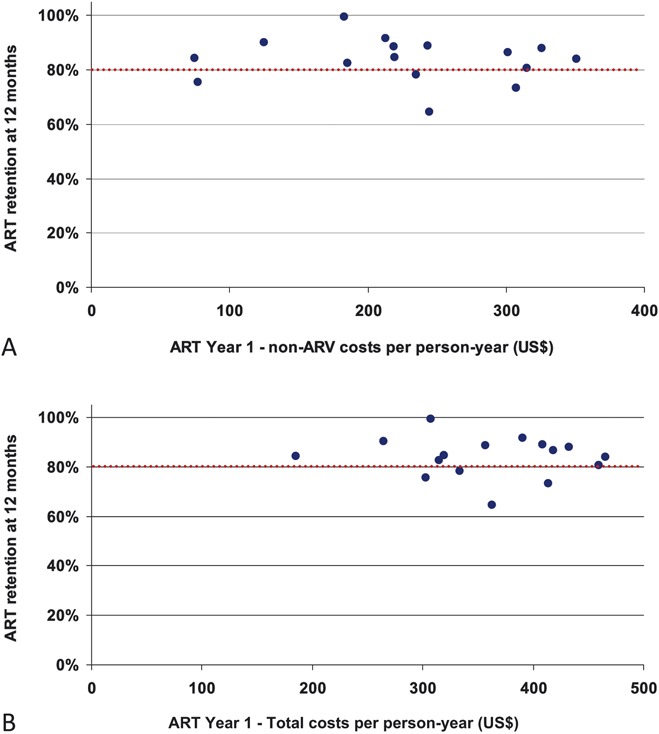
ART retention at 12 months and costs per patient-year (A, non-ARV costs; B, Total costs) at 16 adult outpatient services. The ART retention rate at 12 months among those started ART in 2008 was calculated following WHO patient monitoring guidelines. The ART year 1 costs were estimated for those starting ART between April 2008 and March 2009, through sampling the patients as described in the text.
DISCUSSION
This study provides the first-ever evidence base for nationwide HIV treatment and care costs in Vietnam. The analysis showed that costs of HIV treatment and care delivery in Vietnam are reasonable; however, it also suggests some potential to further promote efficiency.
The present analysis suggests that HIV treatment and care costs for adults in Vietnam are lower than most of those reported in other published studies. According to a recent systematic review, the median HIV treatment and care cost per patient-year in low-income, lower middle-income, and upper middle-income countries were US $ 792, US $932, and US $1454, respectively.9 Cost analysis conducted in 2006–2007 at 43 PEPFAR-supported outpatient clinics providing HIV treatment reported that the median ART cost was at US $880.10 In contrast, the present study showed that the median cost per patient-year of first-line ART was US $316 for the first year and US $303 for later years. Comparison of the costing study results is often complex because of the methodological differences. The costs are also affected significantly by the year of studies because of factors such as the decline in ARV drug price and growth in the number of treated patients. Nevertheless, the data suggest that delivery of HIV treatment and care in Vietnam is at least moderately economical. Few studies have reported pediatric HIV treatment and care costs from low- or middle-income countries. A study from Brazil reported the cost of per child per year as US $2039, and in Ethiopia yearly costs were US $961 for new patients and US $933 for established patients.9 In contrast, the present study found that median outpatient ART cost per pediatric patient-year was US $412 for the first year and US $341 for later years, which are considerably lower than those previously reported values.
Most ARV drugs procured by the government had higher costs than the same drugs procured with external funds. In Vietnam, ARV drugs funded by PEPFAR and GF are procured through bidding by international procurement agencies. The government also procures ARV drugs through bidding, but international suppliers may not be fully involved. As the government aims to increase reliance on domestic funding, it seems critical for the government to strengthen ARV drug procurement to obtain lower prices through international competition. As Vietnam aims to further scale-up HIV treatment access, these findings suggest the importance of rationalizing resource allocation and strengthening care standards to deliver quality services across different donors.
A recent systematic review reported that studies comparing costs at stand-alone and integrated services are scarce, and thus at present no firm conclusion can be drawn whether there are any economic benefits of integrating HIV treatment into general health services.11 Our results suggest that non-ARV costs are higher at stand-alone facilities than at integrated facilities, and that labor costs and other indirect costs are the major factors leading to these higher costs. These results are probably because of the fact that facility capacity utilization is higher at integrated facilities, as people attend facilities for various health services, whereas only HIV patients attend stand-alone facilities, and thus the unit indirect costs are smaller at integrated sites. A potential explanation for lower labor costs at integrated facilities is that health-care workers tend to have multiple roles and spend part of their time delivering non-HIV health services, resulting in lower labor unit cost per patient.
Our results confirmed that the care costs are higher in patients with severe immunodeficiency. Higher care costs associated with HIV disease progression have been documented by other studies.12–14 A review of 9 costing studies showed that the costs are substantially higher at CD4 below 100 cells per cubic millimeter.13 It has also been shown that early ART initiation before patients progress to advanced immunodeficiency is associated with lower morbidity and mortality15–18 and greater cost-effectiveness compared with ART initiation at lower CD4 count.19 Furthermore, a recent mathematical modeling study in Vietnam estimated that earlier initiation of ART will prevent a substantial number of new HIV infections and markedly reduce the need for ART in the future.20 These lines of evidence suggest promoting earlier ART initiation could potentially be cost-effective and cost saving. Currently, late ART initiation is very common in Vietnam with 52.7% of those who started ART in 2010 having a CD4 count <100 cells per cubic millimeter.1 Promoting earlier uptake of HIV diagnosis and treatment through interventions and policy changes seems critical to achieve better therapeutic, preventive, and economic outcomes.
Our study did not find a correlation between costs and treatment outcomes measured by retention rate at 12 months, suggesting some sites had good treatment outcomes (retention rate >80% at 12 months) with relatively low costs (<US $300 per patient-year). It would be worthwhile to further investigate how these sites were able to achieve decent treatment outcomes with relatively low financial inputs, which may inform efficient service delivery models.
Our study has several limitations. First, some program costs were not included, for example, costs related to training of health care providers and technical assistance. Second, the present study was conducted from a health system perspective, and costs related to community-based services were not included, although it was recognized that community-based services play important roles in facilitating early access to HIV treatment, and supporting adherence and retention. Third, some sites had incomplete recording of clinical or financial data, which posed challenges in the analysis, but efforts were made to obtain more complete and accurate data through repeatedly contacting the sites. Fourth, our study design did not allow estimation of the aggregated costs of outpatient and inpatient care, nor analysis of whether investment in outpatient care might have any effects on inpatients costs. Fifth, sampling of facilities was not completely random, thus our sample was not representative of all facilities in the country. However, the sampled facilities were selected to epitomize the national program by including a diverse range of facilities across several dimensions. Moreover, the total of sampled facilities accounted for a substantial proportion of patients receiving ART nationwide (24% of adults and 50% of children) and, consequently, we consider the findings of the study fairly robust. Sixth, the costing reference period of ART year 1 only overlaps for half of the person-months with other phases. This slightly reduces comparability if there were any changes in patterns of service inputs, whereas prices and unit costs applied to ART year 1 cases were the same as for other phases.
Despite the limitations, the study provides the most comprehensive evidence base of the costs of delivering HIV treatment and care for adults and children in Vietnam. Although the study found that HIV treatment and care delivery in Vietnam are already economical, it also found potential to further improve efficiency; for example, through strengthening competitive procurement of ARV drugs, integrating HIV treatment and care into general health facilities, and facilitating earlier ART initiation. The importance of reducing reliance on external funds in some donor sites was also highlighted. These findings are being used to inform the Ministry of Health to mobilize alternative resources and to optimize the policy and program to establish efficient and sustainable service delivery.
ACKNOWLEDGMENTS
The authors would like to express their sincere appreciation to Dr Hoang Van Minh, Dr Pham Huy Tuan Kiet, and Dr Kieu Huu Hanh for supervising the data collection and to the staff of Hanoi School of Public Health and the health care workers at the provincial AIDS centers and facilities in undertaking the data extraction. The authors are also grateful to Dr Nick Medland, Dr Nguyen Bui Duc, Dr Bui Duc Duong, Dr Tran Xuan Bach, Dr Masami Fujita, Dr Fabio Mesquita, Dr Nguyen Thi Thuy Van, and Mrs Pham Hong Thuy for their important technical inputs and information. The authors also would like to thank Ms Bianca Juca, Ms Camila Oliveira Mulhall, and Ms Nguyen Hang for their contribution to data analysis and Ms Nguyen Thi Vinh for her secretarial support.
Footnotes
Supported by World Health Organization Vietnam Country Office and US Center for Disease Control and Prevention Vietnam Country Office. The authors alone are responsible for the views expressed in this [article] and they do not necessarily represent the views, decisions or policies of the institutions with which they are affiliated.
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jaids.com).
REFERENCES
- 1.Government of Vietnam. Vietnam AIDS Response Progress Report 2012. Hanoi, Vietnam: National Committee for AIDS, Drugs and Prostitution Prevention and Control; 2012 [Google Scholar]
- 2.World Bank. Vietnam overview. Available at: http://www.worldbank.org/en/country/vietnam/overview. Accessed December 27, 2012
- 3.Do TN, Nguyen TM, Do MH, et al. Combining cohort analysis and monitoring of HIV early-warning indicators of drug resistance to assess antiretroviral therapy services in Vietnam. Clin Infect Dis. 2012;54(suppl 4):S306–S312 [DOI] [PubMed] [Google Scholar]
- 4.Leisegang R, Cleary S, Hislop M, et al. Early and late direct costs in a Southern African antiretroviral treatment programme: a retrospective cohort analysis. PLoS Med. 2009;6:e1000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewis MA, La Forgia GM, Sulvetta MB. Measuring public hospital costs: empirical evidence from the Dominican Republic. Soc Sci Med. 1996;43:221–234 [DOI] [PubMed] [Google Scholar]
- 6.Shepard DS, Hodgkin D, Anthony YE. Analysis of Hospital Costs: A Manual for Managers. Geneva, Switzerland: World Health Organization; 2000 [Google Scholar]
- 7.Governemnt Statistic Office of Vietnam. Key indicators on National Accounts. 2012. Available at: http://www.gso.gov.vn/default_en.aspx?tabid=468&idmid=3&ItemID=12981. Accessed October 16, 2012
- 8.WHO. Patient Monitoring Guidelines for HIV Care and Antiretroviral Therapy (ART). Geneva, Switzerland: World Health Organization; 2006 [Google Scholar]
- 9.Galarraga O, Wirtz VJ, Figueroa-Lara A, et al. Unit costs for delivery of antiretroviral treatment and prevention of mother-to-child transmission of HIV: a systematic review for low- and middle-income countries. Pharmacoeconomics. 2011;29:579–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menzies NA, Berruti AA, Berzon R, et al. The cost of providing comprehensive HIV treatment in PEPFAR-supported programs. AIDS. 2011;25:1753–1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sweeney S, Obure CD, Maier CB, et al. Costs and efficiency of integrating HIV/AIDS services with other health services: a systematic review of evidence and experience. Sex Transm Infect. 2012;88:85–99 [DOI] [PubMed] [Google Scholar]
- 12.Fleishman JA, Yehia BR, Moore RD, et al. The economic burden of late entry into medical care for patients with HIV infection. Med Care. 2010;48:1071–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levy A, Johnston K, Annemans L, et al. The impact of disease stage on direct medical costs of HIV management: a review of the international literature. Pharmacoeconomics. 2010;28(suppl 1):35–47 [DOI] [PubMed] [Google Scholar]
- 14.Chen RY, Accortt NA, Westfall AO, et al. Distribution of health care expenditures for HIV-infected patients. Clin Infect Dis. 2006;42:1003–1010 [DOI] [PubMed] [Google Scholar]
- 15.Hogg RS, Yip B, Chan KJ, et al. Rates of disease progression by baseline CD4 cell count and viral load after initiating triple-drug therapy. JAMA. 2001;286:2568–2577 [DOI] [PubMed] [Google Scholar]
- 16.Severe P, Juste MA, Ambroise A, et al. Early versus standard antiretroviral therapy for HIV-infected adults in Haiti. N Engl J Med. 2010;363:257–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sterne JA, May M, Costagliola D, et al. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet. 2009;373:1352–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen DB, Do NT, Shiraishi RW, et al. Outcomes of antiretroviral therapy in Vietnam: results from a national evaluation. PLoS ONE. 2013;8:e55750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walensky RP, Wood R, Ciaranello AL, et al. Scaling up the 2010 World Health Organization HIV treatment guidelines in resource-limited settings: a model-based analysis. PLoS Med. 2010;7:e1000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kato M, Granich R, Bui DD, et al. The potential impact of expanding antiretroviral therapy and combination prevention in Vietnam: towards elimination of HIV transmission. J Acquir Immunodefic Syndr. 2013;63:e142–e149 [DOI] [PMC free article] [PubMed] [Google Scholar]


