Abstract
Background
Non-motor symptoms (NMS) in Parkinson's disease (PD) differ from those in essential tremor (ET), even before a definitive diagnosis is made. It is not clear whether patient's knowledge of the diagnosis and treatment influence their subsequent reporting of NMS.
Methods
1 year after a clinical and instrumental diagnosis, we compared the motor impairment (Movement Disorders Society (MDS)-Unified Parkinson's Disease Rating Scale-III) and non-motor symptoms (NMSQuest) in PD (n = 31) and ET (n = 21) patients.
Results
PD patients reported more NMS than did the ET patients (p = 0.002). When compared to their baseline report, at follow-up, PD patients reported less nocturia (p = 0.02), sadness (p = 0.01), insomnia (p = 0.02), and restless legs (p = 0.04) and more nausea (p = 0.024), unexplained pain (p = 0.03), weight change (p = 0.009), and daytime sleepiness (p = 0.03). When compared to their baseline report, ET patients reported less loss of interest (p = 0.03), anxiety (p = 0.006), and insomnia (p = 0.02). Differences in reported weight change (p<0.0001) and anxiety (p = 0.001) between PD and ET patients were related to pharmacological side effects or to a reduction in the ET individuals.
Discussion
The reporting of NMS is influenced by subjective factors, and might vary with the patient's knowledge of the diagnosis or the effectiveness of treatment.
Keywords: Non-motor symptoms, NMSQuest, Parkinson's disease, essential tremor, follow-up
Introduction
Parkinson's disease (PD) is a neurodegenerative disease characterized by motor signs (bradykinesia plus tremor, rigidity, or postural instability, according to the UK PD Brain Bank [UKPDBB] criteria)1 and non-motor symptoms (NMS). NMS in PD include constipation,2 orthostatic hypotension,3 hyposmia,4 rapid eye movement sleep behavior disorder (RBD),5 neuropsychiatric complaints (anxiety, depression),6 and cognitive defects.7 Essential tremor (ET) is a movement disorder characterized by a rhythmic tremor affecting the hands and forearms.8 ET is associated with relevant NMS, such as frontal dysfunction, dementia, anxiety, depression, poor sleep quality, and subjective reduction of hearing.9 We previously demonstrated that, with respect to their NMS, PD and ET patients are suggestible,10 but despite this, NMS do exist and have been reported by affected patients who are not aware of their diagnosis. The aim of this study was to examine self-reports of NMS by PD and ET patients 1 year after learning their diagnosis, and to compare these reports to those at baseline.
Methods
Study design
The PD and ET patients included in this study were described in our recently published paper, which notes the inclusion and exclusion criteria.10 The participants were selected from individuals visiting the Operative Unit for Nuclear Medicine of the General Hospital of Barletta, and who had received [123I] β-CIT (2β-carbomethoxy-3β-[4-iodophenyl]tropane) single photon emission computed tomography (SPECT) for diagnostic purposes over a 6-month period (from March 2012 to September 2012). All patients provided written informed consent. The patients were interviewed for clinical history, evaluated with the NMSQuest,11 answered a 30-item self-administered questionnaire evaluating NMS, and scored for motor symptoms (Unified Parkinson's Disease Rating Scale [UPDRS]-III). A diagnosis of PD or ET was made by neurologists who were not involved in this study, and were made on the basis of the clinical and instrumental data (imaging and [123I] β-CIT SPECT). A diagnosis was assigned according to the established diagnostic criteria,1,8 and the diagnosing neurologists were responsible for determining the treatment. One year after the first evaluation, the selected individuals (n = 76) were followed-up to assess the diagnosis of PD based on the response to dopaminergic therapy and the other UKPDBB supportive and exclusion criteria. Twenty-three individuals were excluded from the final study sample because of the appearance of signs or symptoms suggesting a diagnosis other than PD or ET (namely vascular or iatrogenic parkinsonism, progressive supranuclear paralysis, Lewy body dementia, or psychogenic movement disorder). One additional ET patient was lost at follow-up because of a change of residence. The 24 excluded individuals did not differ from the included study sample with respect to their age (69.5±7 years old), gender distribution (16 men), or duration of symptoms. A diagnosis of PD was confirmed in 31 individuals (9 females, 22 males) 1 year after the first evaluation according to the following criteria: 1) the presence of a parkinsonian syndrome, 2) the absence of any exclusion criteria according to the UKPDBB diagnostic criteria, 3) reduced representation of striatal dopaminergic nerve terminals according to the Dopamine Transporter (DAT) scan results, and 4) the response to dopaminergic therapy. Twenty-two patients (10 females, 12 males) with ET were selected from the included participants according to published diagnostic criteria.8 The NMS and UPDRS score were re-assessed at the 1-year follow-up visit.
Statistical analysis
Unpaired t-tests were used to compare the groups with respect to age, the number of NMS, and UPDRS scores. Fisher's exact test was used to compare the frequency of NMS between the PD and ET groups. The data are expressed as the mean±SD. InStat software (GraphPad, La Jolla, CA, USA) was used to perform the statistical analysis.
Results
Patient demographics, UPDRS, and NMS characteristics at follow-up
There were no significant differences between the PD (n = 31) and ET (n = 21) patients with regards to the mean age or the male/female sex distribution. All of the PD patients were taking dopaminergic drugs at the time of the evaluations. Twenty-five PD patients were taking l-dopa alone or associated with either a monoamine oxidase B inhibitor (iMAO-B), a dopamine agonist, or both, and six were taking iMAO-Bs plus agonists. None of the ET patients was taking tremor-directed symptomatic treatments; they had been discouraged from doing so by their neurologists because of the mildness of their tremors (maximal amplitude less than 3 cm) relative to the possible eventual occurrence of drug-related side effects. The UPDRS scores were higher in the PD than in ET patients (PD 16.5±8, ET 3.43±2, p<0.0001). The PD patients complained of more NMS (10.16±6.9) than the ET patients (5±2.9, p = 0.002). The results are described in Table 1
Table 1. Group Comparison of Patients Demographics, UPDRS, and NMS Characteristics at Follow-up.
| Parkinson's Disease | Essential Tremor | PD vs ET at Follow-up | |||||
|---|---|---|---|---|---|---|---|
| Baseline n = 31 (%) | Follow-up n = 31 (%) | p Value | Baseline n = 22 (%) | Follow-up n = 21 (%) | p Value | p Value | |
| Age | 69.1±8.2 | 70.2±6.8 | 0.5 | 67.6±9.1 | 68.6±7.1 | 0.7 | 0.4 |
| Men | 22 (71) | 22 (71) | 1.0 | 12 (54) | 11 (52) | 1.0 | 1.0 |
| UPDRS-III | 27.0±12 | 16.5±8 | 0.0001 | 4.7±3 | 3.4±2 | 0.1 | <0.0001 |
| H & Y | ≤2 | ≤2 | - | - | - | - | - |
| Tremor | 22 (71) | 22 (71) | 1.0 | 22 (100) | 21 (100) | 1.0 | 0.007 |
| Dopaminergic therapy | 8 (26) | 31 (100) | <0.0001 | 1 (4) | 0 (0) | 1.0 | <0.0001 |
| Total LEDD (mg/day) | NA | 412±76 | - | 0 | 0 | - | - |
| l-dopa | 8 (26) | 25 (81) | <0.0001 | 1 (4) | 0 (0) | 1.0 | <0.0001 |
| NMS | 10.4±4.9 | 10.2±6.9 | 0.1 | 8.4±3.3 | 5±2.9 | 0.0009 | 0.002 |
Abbreviations: ET, Essential Tremor; H & Y: Hoehn & Yahr score; LEDD, l-dopa-equivalent Daily Dosage; NA, Not Available; NMS, Non-motor Symptoms; PD, Parkinson's Disease; UPDRS-III, Unified Parkinson's Disease Rating Scale.
Numbers in brackets indicate the percentage of patients in that subgroup. Significant p values are shown in bold.
Complaints of specific NMS in the PD and ET patients at follow-up
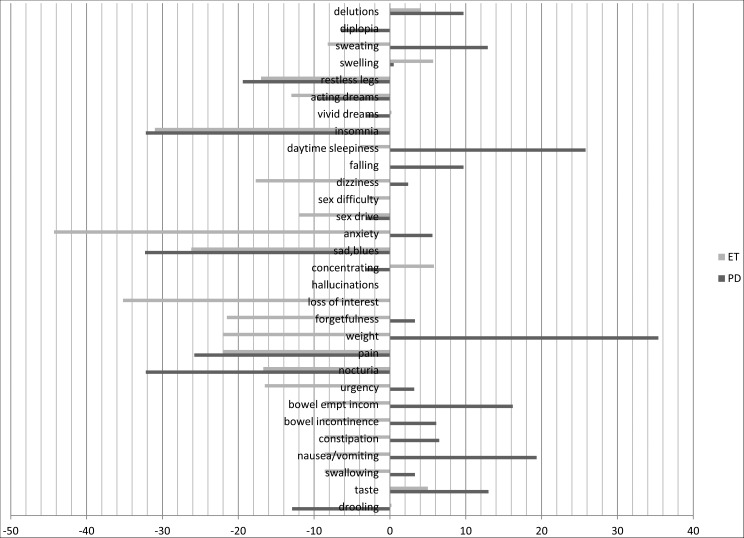
Changes from baseline (1 year earlier) were observed in the percentage of PD patients who complained of NMS. The following NMS decreased: drooling (−12.9%), nocturia (−32.2%), pain (−25.8%), sadness (−32.3%), insomnia (−32.2%), and restless legs syndrome (RLS) (−19.4%); the following NMS increased: daytime sleepiness (+25.8%), taste (+13%), incomplete bowel emptying (+16.2%), change in weight (+35.4%), and sweating (+12.9%). ET patients reported the following changes in NMS: urgency (−16.5%), nocturia (−16.7%), pain (−22%), weight change (−22%), forgetfulness (−21.5%), loss of interest (−35.2%), sadness (−26.2%), anxiety (−44.3%), reduced sex drive (−12%), orthostatic dizziness (−17.7%), and RLS (−17%). PD patients exhibited a higher frequency of the following NMS compared with ET patients: hyposmia, weight change, loss of interest, hallucinations, anxiety, orthostatic dizziness, falling, vivid dreams, and acting out during dreams (REM behavior disorder, RBD). The increased complaints of weight change and orthostatic dizziness by the PD patients compared with the ET patients was the most significant results (p<0.0001). None of the ET patients who reported vomiting were actively consuming central nervous system-active drugs. See Table 2 and Figure 1 for results.
Table 2. Frequency of Non-motor Symptoms in Parkinson's Disease and Essential Tremor Patients at Follow-up.
| Parkinson's Disease | Essential Tremor | PD vs ET at Follow- up | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NMS | Baseline | Follow-up | p Value | Baseline | Follow-up | p Value | p Value | ||||
| n = 31 | % | n = 31 | % | n = 22 | % | n = 21 | % | ||||
| Drooling | 9/31 | 29 | 5/31 | 16.1 | 0.4 | 1/22 | 4.5 | 1/21 | 4.7 | 1.0 | 0.4 |
| Taste/smell | 10/31 | 32.2 | 14/31 | 45.2 | 0.4 | 1/22 | 4.5 | 2/21 | 9.5 | 0.6 | 0.007*** |
| Swallowing | 6/31 | 19.3 | 7/31 | 22.6 | 1.0 | 4/22 | 18.1 | 2/21 | 9.5 | 0.6 | 0.3 |
| Nausea/vomiting | 0/31 | 0 | 6/31 | 19.3 | 0.02 | 4/22 | 18.1 | 2/21 | 9.5 | 0.6 | 0.4 |
| Constipation | 6/31 | 19.3 | 8/31 | 25.8 | 0.8 | 4/22 | 18.1 | 2/21 | 9.5 | 0.6 | 0.2 |
| Bowel incontinence | 3/31 | 10 | 5/31 | 16.1 | 0.7 | 3/22 | 13.6 | 1/21 | 4.7 | 0.6 | 0.7 |
| Incomplete bowel emptying | 6/31 | 9.6 | 8/31 | 25.8 | 0.7 | 4/22 | 18.1 | 2/21 | 9.5 | 0.6 | 0.2 |
| Urgency | 17/31 | 54.8 | 18/31 | 58 | 1.0 | 12/22 | 54.5 | 8/21 | 38.0 | 0.4 | 0.3 |
| Nocturia | 19/31 | 61.2 | 9/31 | 29 | 0.02 | 11/22 | 50 | 7/21 | 33.3 | 0.3 | 0.8 |
| Pains | 11/31 | 35.4 | 3/31 | 9.6 | 0.03 | 9/22 | 41 | 4/21 | 19.0 | 0.2 | 0.4 |
| Weight | 13/31 | 42 | 24/31 | 77.4 | 0.009 | 9/22 | 41 | 4/21 | 19.0 | 0.2 | <0.0001** |
| Forgetfulness, memory | 15/31 | 48.3 | 16/31 | 51.6 | 1.0 | 11/22 | 50 | 6/21 | 28.5 | 0.2 | 0.2 |
| Loss of interest | 19/31 | 61.2 | 19/31 | 61.2 | 1.0 | 13/22 | 59 | 5/21 | 23.8 | 0.03 | 0.01** |
| Hallucinations | 6/31 | 19.3 | 7/31 | 19.3 | 1.0 | 0/22 | 0 | 0/21 | 0 | 1.0 | 0.03**** |
| Concentrating | 16/31 | 51.6 | 15/31 | 48.4 | 1.0 | 5/22 | 22.7 | 6/21 | 28.5 | 0.7 | 0.3 |
| Sad, blues | 16/31 | 51.6 | 6/31 | 19.3 | 0.01 | 11/22 | 50 | 5/21 | 23.8 | 0.1 | 1.0 |
| Anxiety | 20/31 | 64.5 | 22/31 | 70.1 | 0.8 | 15/22 | 68.1 | 5/21 | 23.8 | 0.006 | 0.001**** |
| Sex drive | 16/31 | 51.6 | 15/31 | 48.4 | 1.0 | 11/22 | 50 | 8/21 | 38.0 | 0.5 | 0.1 |
| Sex difficulty | 9/31 | 29 | 9/31 | 29 | 1.2 | 8/22 | 36.3 | 7/21 | 33.3 | 1.0 | 0.8 |
| Orthostatic dizziness | 21/31 | 67.7 | 22/31 | 70.1 | 1.0 | 6/22 | 27.2 | 2/21 | 9.5 | 0.2 | <0.0001** |
| Falling | 6/31 | 19.3 | 9/31 | 29 | 0.5 | 0/22 | 0 | 0/21 | 0 | 1.0 | 0.0073*** |
| Daytime sleepiness | 3/31 | 9.6 | 11/31 | 35.4 | 0.03 | 4/22 | 18.2 | 3/21 | 14.3 | 1.0 | 0.1 |
| Insomnia | 18/31 | 58 | 8/31 | 25.8 | 0.02 | 12/22 | 50 | 4/21 | 19.0 | 0.02 | 0.7 |
| Vivid dreams | 10/31 | 32.2 | 9/31 | 29 | 1.0 | 1/22 | 4.5 | 1/21 | 4.7 | 1.0 | 0.036**** |
| Acting out during dreams | 18/31 | 58 | 15/31 | 48.4 | 0.6 | 6/22 | 27.3 | 3/21 | 14.3 | 0.4 | 0.017**** |
| RLS | 9/31 | 29 | 2/31 | 9.6 | 0.04 | 8/22 | 36 | 4/21 | 19.0 | 0.3 | 0.2 |
| Swelling | 7/31 | 22 | 7/31 | 22.5 | 1.0 | 5/22 | 18.1 | 5/21 | 23.8 | 1.0 | 1.0 |
| Sweating | 4/31 | 12.9 | 5/31 | 25.8 | 0.3 | 7/22 | 32 | 5/21 | 23.8 | 0.7 | 0.5 |
| Diplopia | 7/31 | 22.5 | 5/31 | 16.1 | 0.7 | 0/22 | 0 | 0/21 | 0 | 1.0 | 0.1 |
| Delusions | 3/31 | 9.6 | 6/31 | 19.3 | 0.5 | 0/22 | 0 | 1/21 | 4.1 | 0.5 | 0.2 |
Abbreviations: ET, Essential Tremor; NMS, Non-motor Symptoms; PD, Parkinson's Disease RLS, restless legs syndrome.
Fisher's exact test was used for all statistical comparisons.
Significant values are expressed in bold. *p<0.0001; **p<0.001; ***p<0.01; ****p<0.05.
Figure 1. Percentage of Changes in the Declaration of Specific Non-motor Symptoms in both Parkinson's Disease and Essential Tremor Patients at Follow-up.
Columns represent the percentage variation in the number of declared non-motor symptoms at follow-up with respect to baseline (intended as 100% the global number of either Parkinson's disease or essential tremor patients).
Discussion
The fundamental finding of our study is that ET patients may experience spontaneous relief of NMS. We observed a significant and spontaneous reduction in the number of complaints of several NMS in these patients, and these patients were not receiving pharmacological therapy at the time of the follow-up visit. The reduction included NMS known to frequently affect ET patients, such as loss of interest, anxiety, and insomnia.10,12,13 As we have previously suggested,10 we hypothesize that a patient's perception of symptoms might change after learning about the diagnosis and the expected symptoms through mass media, the Internet, and printed information. Although increased reports of dementia, anxiety, executive deficit, depression, and poor sleep quality have been associated with ET,9 our findings should not be interpreted as variant results. The early follow-up (after 1 year from the first assessment) might only have affected the subjective declaration of NMS, whereas the appearance of specific NMS due to disease-associated physiopathological mechanisms would be expected later in the course of ET.
The total number of NMS declared by the PD patients did not change at the follow-up visit; however, there were changes in some of the specific symptoms. The number of patients reporting daytime sleepiness, weight change, and nausea/vomiting increased at 1 year from the initial evaluation. This finding was in accordance with results from a previous study14 and is possibly related to the pharmacological side effects of dopaminergic therapy. Conversely, the same report described a significant reduction in the number of complaints of drooling, nocturia, unexplained pain, sadness, insomnia, and RLS in the identical PD patient sample.14 Although it would be tempting to suggest that at least some of the reported improvements might be related to suggestion or adaptive mechanisms, as observed in ET, the responsiveness of specific NMS to pharmacological therapy has been extensively described.14,15 Drooling improves due to the dopaminergic-induced reduction of akinesia, nocturia positively responds to rotigotine,16 and l-Dopa and dopamine agonists have a specific antidepressant effect through limbic dopamine D3 receptor agonism.17 Sleep-associated disorders, such as insomnia and RLS, typically improve following dopaminergic therapy.18,19
Due to the decrease in NMS in the ET patients, the total number of NMS in the PD patients was significantly different from those observed in the ET patients at follow-up. The PD patients reported the same number of NMS before and 1-year after diagnosis. The UPDRS part III score significantly decreased upon treatment initiation, providing a diagnostic clue suggestive of PD (as encoded by the UKPDBB criteria). This finding was partly in accordance with previous studies that reported no significant changes in the number of NMS in patients with PD after 2 years of follow-up14 and a direct correlation between the total number of NMS and therapy-induced amelioration of the UPDRS score.20
Our work demonstrates that reports of NMS by patients with movement disorders are common; however, some NMS in PD appear to be highly reproducible and responsive to treatment. The major weakness of our investigation is the small study sample. Although this reduced the power of the statistical analyses, we do not expect that this shortcoming biased our findings.
Footnotes
Funding: None.
Financial Disclosures: None.
Conflict of Interests: The authors report no conflict of interest.
References
- 1.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abbott RD, Petrovitch H, White LR, et al. Frequency of bowel movements and the future risk of Parkinson's disease. Neurology. 2001;57:456–462. doi: 10.1212/WNL.57.3.456. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein DS. Orthostatic hypotension as an early finding in Parkinson's disease. Clin Auton Res. 2006;16:46–54. doi: 10.1007/s10286-006-0317-8. [DOI] [PubMed] [Google Scholar]
- 4.Ponsen MM, Stoffers D, Booij J, van Eck-Smit BL, Wolters E, Berendse HW. Idiopathic hyposmia as a preclinical sign of Parkinson's disease. Ann Neurol. 2004;56:173–181. doi: 10.1002/ana.20160. [DOI] [PubMed] [Google Scholar]
- 5.Schenck CH, Bundlie SR, Mahowald MW. Delayed emergence of a parkinsonian disorder in 38% of 29 older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder. Neurology. 1996;46:388–393. doi: 10.1212/WNL.46.2.388. [DOI] [PubMed] [Google Scholar]
- 6.Shiba M, Bower JH, Maraganore DM, et al. Anxiety disorders and depressive disorders preceding Parkinson's disease: a case-control study. Mov Disord. 2000;15:669–677. doi: 10.1002/1531-8257(200007)15:4<669::AID-MDS1011>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 7.Ishihara L, Brayne C. A systematic review of depression and mental illness preceding Parkinson's disease. Acta Neurol Scand. 2006;113:211–220. doi: 10.1111/j.1600-0404.2006.00579.x. [DOI] [PubMed] [Google Scholar]
- 8.Deuschl G, Bain P, Brin M, an ad hoc Scientific Committee Consensus Statement of the Movement Disorder Society on Tremor. Mov Disord. 1998;13(Suppl 3):2–23. doi: 10.1002/mds.870131303. [DOI] [PubMed] [Google Scholar]
- 9.Chandran V, Kumar Pal P. Essential tremor: beyond the motor features. Parkinsonism Relat Disord. 2012;18:407–413. doi: 10.1016/j.parkreldis.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Giorelli M, Bagnoli J, Consiglio L, et al. Do non-motor symptoms in Parkinson's disease differ from essential tremor before initial diagnosis? A clinical and scintigraphic study. Parkinsonism Relat Disord. 2014;20:17–21. doi: 10.1016/j.parkreldis.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Chaudhuri KR, Martinez-Martin P, Schapira AHV, et al. International multicenter pilot study of the first comprehensive self-completed nonmotor symptoms questionnaire for Parkinson's disease: the NMSQuest study. Mov Disord. 2006;21:916–923. doi: 10.1002/mds.20844. [DOI] [PubMed] [Google Scholar]
- 12.Gasparini M, Bonifati V, Fabrizio E, et al. Frontal lobe dysfunction in essential tremor: a preliminary study. J Neurol. 2001;248:399–402. doi: 10.1007/s004150170181. [DOI] [PubMed] [Google Scholar]
- 13.Benito-León J, Luois ED, Bermejo-Pareja F. Neurological Disorders in Central Spain (NEDICES) Study Group. Population-based case control study of cognitive function in essential tremor. Neurology. 2006;66:69–74. doi: 10.1212/01.wnl.0000192393.05850.ec. [DOI] [PubMed] [Google Scholar]
- 14.Erro R, Picillo M, Vitale C, et al. Non-motor symptoms in early Parkinson's disease: a 2-year follow-up study on previously untreated patients. J Neurol Neurosurg Psychiatry. 2013;84:14–17. doi: 10.1136/jnnp-2012-303419. [DOI] [PubMed] [Google Scholar]
- 15.Chaudhuri KR, Schapira AHV. Non-motor symptoms of Parkinson's disease: dopaminergic pathophysiology and treatment. Lancet Neurol. 2009;8:464–74. doi: 10.1016/S1474-4422(09)70068-7. [DOI] [PubMed] [Google Scholar]
- 16.Metta V, Naidu Y, Muzerengi S, et al. The beneficial effect of rotigotine transdermal patch on nocturia in Parkinson's disease. 6th International Congress on Mental Dysfunctions and Other Non Motor Features in Parkinson's Disease. Parkinsonism Relat Disord. 2008:51. abstract P01. [Google Scholar]
- 17.Rektorova I, Rektor I, Bares M, et al. Pramipexole and pergolide in the treatment of depression in Parkinson's disease: a national multicentre prospective randomized study. Eur J Neurol. 2003;10:399–406. doi: 10.1046/j.1468-1331.2003.00612.x. [DOI] [PubMed] [Google Scholar]
- 18.Leeman AL, O’Neill CJ, Nicholson PW, et al. Parkinson's disease in the elderly: response to and optimal spacing of night time dosing with levodopa. Br J Clin Pharmacol. 1987;24:637–43. doi: 10.1111/j.1365-2125.1987.tb03223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muzerengi S, Lewis H, Chaudhuri KR. Restless legs syndrome: a review of diagnosis and management. Int J Sleep Disord. 2006;1:34–46. [Google Scholar]
- 20.Antonini A, Barone P, Marconi R, et al. The progression of non-motor symptoms in Parkinson's disease and their contribution to motor disability and quality of life. J Neurol. 2012;259:2621–31. doi: 10.1007/s00415-012-6557-8. [DOI] [PubMed] [Google Scholar]