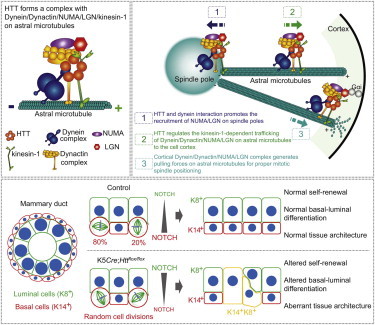
Summary
Little is known about the mechanisms of mitotic spindle orientation during mammary gland morphogenesis. Here, we report the presence of huntingtin, the protein mutated in Huntington’s disease, in mouse mammary basal and luminal cells throughout mammogenesis. Keratin 5-driven depletion of huntingtin results in a decreased pool and specification of basal and luminal progenitors, and altered mammary morphogenesis. Analysis of mitosis in huntingtin-depleted basal progenitors reveals mitotic spindle misorientation. In mammary cell culture, huntingtin regulates spindle orientation in a dynein-dependent manner. Huntingtin is targeted to spindle poles through its interaction with dynein and promotes the accumulation of NUMA and LGN. Huntingtin is also essential for the cortical localization of dynein, dynactin, NUMA, and LGN by regulating their kinesin 1-dependent trafficking along astral microtubules. We thus suggest that huntingtin is a component of the pathway regulating the orientation of mammary stem cell division, with potential implications for their self-renewal and differentiation properties.
Graphical Abstract
Highlights
-
•
HTT regulates MaSC self-renewal and cell fate specification
-
•
HTT is essential for mammary epithelial morphogenesis in vivo
-
•
HTT regulates spindle orientation in a dynein-dependent manner
-
•
HTT mediates the cortical localization of dynein/dynactin/LGN/NUMA through kinesin 1
Humbert and colleagues show that huntingtin, the protein mutated in Huntington’s disease, is required for mammary stem cell division and differentiation, with implication for mammary morphogenesis. One of the mechanisms by which huntingtin mediates its effect is the regulation of mitotic spindle orientation. In particular, huntingtin regulates spindle orientation by ensuring the cortical localization of dynein/dynactin/LGN/NUMA through kinesin 1.
Introduction
There are three distinct and differentially regulated stages in mammary gland development (embryonic, pubertal, and pregnancy/lactation), and the most substantial remodeling is postnatal (Gjorevski and Nelson, 2011). The mammary epithelium is organized into two cell layers: the luminal and basal myoepithelial layers. During pregnancy, the mammary gland completes its morphogenesis with the formation of alveolar buds where milk production is turned on at the end of pregnancy and during lactation (Silberstein, 2001). This developmental process is controlled by steroid hormones (Beleut et al., 2010). During lactation, luminal cells (LCs) produce and secrete milk, whereas basal myoepithelial cells (BCs) contract to release the milk from the nipple (Moumen et al., 2011).
Several lines of evidence indicate the existence of mammary stem cells (MaSCs) in mouse mammary tissue. These cells display the regenerative properties required for the substantial developmental changes in the adult mammary gland (Visvader and Lindeman, 2011). MaSCs have been isolated from adult mouse mammary tissue using the surface markers CD24 and β1 or α6-integrin chains (Shackleton et al., 2006). These populations are negative for steroid hormone receptors and consist of cells that express basal cell markers (Asselin-Labat et al., 2010). However, these populations appear to be composed of various subpopulations, ranging from multipotent stem cells to terminally differentiated luminal epithelial and myoepithelial cells (Visvader and Lindeman, 2011). Furthermore, the LC compartment itself is heterogeneous because progenitors of varying states of luminal differentiation and with diverse proliferative capacities can be identified (Shehata et al., 2012).
The importance of asymmetric cell divisions for stem cells/progenitors has been established in several tissues (Morin and Bellaïche, 2011, Shitamukai and Matsuzaki, 2012). In the mouse mammary gland, the reproductive cycle may alter the MaSC population by regulating the balance between symmetric and asymmetric divisions (Asselin-Labat et al., 2010, Joshi et al., 2010). Experimental perturbation of this balance results in abnormal epithelial morphogenesis and favors tumor growth (Cicalese et al., 2009, Taddei et al., 2008). Thus, MaSC divisions are important regulators of physiological and pathological stem cell biology. However, the precise molecular mechanisms underlying the division modes in mitotic MaSCs are still not understood.
The mitotic spindle is a key component of cell division. The position and orientation of the mitotic spindle are orchestrated by forces generated in the cell cortex (Grill and Hyman, 2005), where astral microtubules emanating from the mitotic spindle pole are tethered to the plasma membrane (Siller and Doe, 2009). Spindle orientation is determined by an evolutionarily conserved pathway, including cytoplasmic dynein, dynactin, the nuclear mitotic apparatus (NUMA) protein, and the G protein regulator leucine-glycine-asparagine repeat (LGN) protein (the vertebrate homolog of Caenorhabditis elegans G protein-coupled receptor (GPR-1)/GPR-2 and Drosophila protein-protein interaction networks [PINS]) (Morin and Bellaïche, 2011). During cell division, LGN is recruited to the cell cortex through glycosyl phosphatidylinositol-linked Gαi/Gαo, which binds LGN carboxy-terminal GoLoco motifs (Zheng et al., 2010). Polarity cues restrict LGN localization to specific subcortical domains, where LGN recruits NUMA (Peyre et al., 2011). NUMA in turn interacts with microtubules and with the cytoplasmic dynein/dynactin complex. The precise localization of these interactions at the cell cortex ensures the positioning of the mitotic spindle through cortical capture of astral microtubules. Although these mechanisms have been well described in the skin and neuroepithelium, their involvement in the division of MaSCs is not known.
We previously showed that huntingtin (HTT), the protein mutated in Huntington’s disease (HD), is required in murine neuronal progenitors for appropriate spindle orientation and for cell fate determination (Godin et al., 2010). Yet, the mechanisms underlying HTT function during spindle orientation remain unclear. HTT expression is not restricted to the brain: mutant HTT is detected in healthy mammary tissue and mammary tumors where it regulates tumor progression (Moreira Sousa et al., 2013). Thus, HTT may contribute to spindle orientation and cell fate choices outside the nervous system. Here, we investigated the function of HTT in mitosis of MaSCs during mouse mammary epithelium morphogenesis.
Results
In Vivo Depletion of HTT from the Basal Compartment Leads to a Decreased Epithelial Content and Alters Self-Renewal of the Basal and Luminal Progenitors
We analyzed the expression pattern of wild-type HTT in mammary glands from virgin mice by immunohistochemistry. HTT immunoreactivity was observed in the basal and luminal compartments and increased as differentiation progressed (Figure 1A). We isolated basal and luminal epithelial cells from wild-type mice using flow cytometry (Figure S1A available online; Table S1). Evaluation of basal (Krt14) and luminal (Krt18) marker expression by quantitative real-time RT-PCR confirmed that BCs and LCs were found in the CD24-low/α6-high and CD24-high/α6-low fractions, respectively (Figure S1B). HTT was detected in BCs and LCs, but the signal was strongest in the luminal fraction (Figure 1B).
Figure 1.
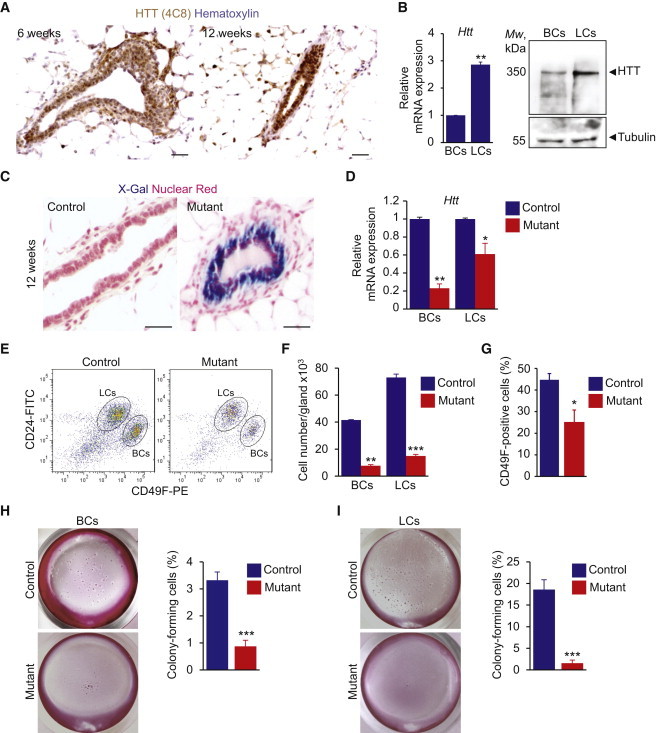
K5-Driven Loss of HTT Affects Basal and LC Populations
(A) Mammary gland sections from virgin C57Bl6/J mice stained for HTT.
(B) Quantitative real-time RT-PCR analysis of Htt gene and western blotting for HTT protein in basal and luminal mammary epithelial cells from 16-week-old virgin mice. Mw, molecular weight.
(C) LacZ-stained mammary gland sections from 12-week-old virgin control and mutant K5Cre;Httflox/flox;R26 12-week-old virgin mice.
(D) Quantitative real-time RT-PCR analysis of Htt gene expression in BCs and LCs from 16-week-old virgin mice.
(E) Representative dot plots showing separation of luminal (CD31−/CD45−/CD24+/CD49F-low) and basal (CD31−/CD45−/CD24+/CD49F-high) epithelial cells from 16-week-old virgin mouse mammary glands by flow cytometry.
(F) Number of BCs and LCs isolated per gland of 16-week-old virgin mice.
(G) Percentages of CD49F-high cells in CD45−/CD31−/CD24+ cell populations.
(H and I) Colonies formed by BCs (H) and LCs (I) isolated from mammary glands of 16-week-old virgin mice.
Scale bars, 50 μm (A and C). Error bars, SEM.∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001. See also Figure S1 and Table S1.
To test whether HTT regulates BC division and differentiation, we deleted HTT from the basal cell layer of the mammary epithelium by crossing Httflox/flox mice harboring floxed Htt alleles (Dragatsis et al., 2000) with transgenic mice expressing Cre recombinase under the control of the keratin 5 (K5) promoter (Ramirez et al., 2004). Cre expression was mostly confined to the basal cell population (Figure S1C). We analyzed the distribution of HTT-deficient cells in mutant mammary epithelium by crossing K5Cre;Httflox/flox mice with the Rosa26-LacZ reporter mouse strain (R26). At age 12 weeks, virtually all BCs were LacZ positive, whereas only 32% of LCs expressed LacZ (Figure 1C). The LacZ-negative LC population in the mutant epithelium may originate during early stages of gland development, from LacZ-negative BCs and from LacZ-negative cells committed to luminal differentiation that switched off the K5 promoter and escaped HTT deletion. Indeed, in embryonic day 18 (E18) K5Cre;Httflox/flox;R26 embryos, a majority of cells in the central part of the developing mammary ducts did not express the Cre recombinase (Figure S1D). These cells expressed keratin 8 (K8) and were negative for K5, thus displaying luminal features (Figure S1E). Alternatively, the LacZ-negative LC population in the mutant epithelium may originate during adulthood from bipotent myoepithelial and luminal stem cells (Rios et al., 2014). Htt transcript levels were 79% lower in mutant than wild-type BCs (Figure 1D). In LCs from mutant mammary epithelium, Htt expression levels were 39% lower than the control value (Figure 1D).
Fewer epithelial cells could be isolated from the mutant than control mammary glands (Figures 1E and 1F). Also, the ratio between basal and LC populations was altered in mutant mammary epithelium (Figure 1G). We then performed a functional evaluation of progenitor cell content in control and mutant BCs using colony-formation assay. Mutant BCs formed significantly less colonies than control cells (0.84% ± 0.23% versus 3.32% ± 0.3%, Figure 1H). Similarly, the HTT-depleted LCs failed to form clonal colonies as compared to the control LC population (1.55% ± 0.72% versus 18.6% ± 2.3%, Figure 1I). Thus, K5-driven depletion of HTT leads to gland hypoplasia and affects colony-forming stem/progenitor populations in basal and luminal compartments.
HTT Is Required for Basal and LC Specification
We then analyzed the transcripts of genes associated with proliferation and myoepithelial and luminal lineages (Figure 2A; Table S1). The lower than control levels of the cell proliferation marker Ki67 in BCs and LCs from mutant glands were consistent with the decrease in the overall population of epithelial cells. In the basal compartment in mutants, whereas Krt18 was upregulated, Krt14 and Trp63 were differentially regulated with Krt14 being increased and Trp63 decreased (Figure 2A). In LCs from the mutant glands, both Krt14 and Trp63 were increased, whereas Krt18 was decreased. Also, the expression levels of the epithelial-to-mesenchymal transition (EMT)-related genes (Snai1, Snai2, and Vim) were decreased in mutant BCs and increased in mutant LCs as compared to control cells (Figure 2A). We tested luminal markers: mutant LCs displayed a marked decrease in the expression levels of mature LC genes (Esr1, Pgr, and Prlr) as compared to control cells (Figure 2A). The expression levels of Elf5 and Kit transcripts that are markers of the luminal progenitor-enriched population were also significantly lower in mutant LCs than in control. This was sustained by the decreased expression of the transcription factor Gata3 in mutant BCs and LCs (Figure 2A). Finally, mutant BCs expressed higher levels of luminal markers as compared to control cells (Figure 2A). Thus, the K5-driven loss of HTT affects the proliferative potential and the identity of BC and LC populations.
Figure 2.
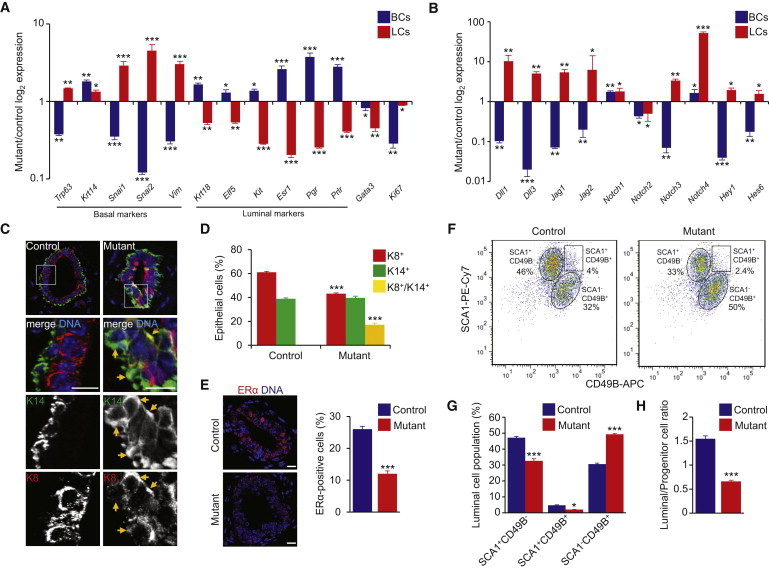
Loss of HTT Alters Basal to Luminal Specification
(A and B) Quantitative real-time RT-PCR analysis of the indicated genes in BCs and LCs from 16-week-old mice.
(C) Sections from 12-week-old mammary glands stained for K14 and K8. Arrows point to K8+K14+ epithelial cells.
(D) Percentage of K8+, K14+, and K8+K14+ cells.
(E) Sections from 12-week-old mammary glands stained for ERα. Right panel shows the percentages of ERα-positive cells.
(F) Representative dot plots showing the frequency of SCA1+ and CD49B+ cells in the LC population in 16-week-old virgin mice.
(G) Percentages of SCA1+CD49B−, SCA1+CD49B+, and SCA1−CD49B+ cells.
(H) Ratio of SCA1+CD49B−-to-SCA1−CD49B+ cells.
Scale bars, 10 μm. Error bars, SEM.∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001. See also Table S1.
NOTCH signaling is involved in cell fate determination in the mammary epithelium (Bouras et al., 2008, Yalcin-Ozuysal et al., 2010). We thus assayed the mRNAs of the Notch ligands (Dll1, Dll3, Jag1, and Jag2), the Notch1–Notch4 receptors, and the Hey1 and Hes6 target genes (Figure 2B). The NOTCH pathway was downregulated in BCs and overactivated in LCs from mutant mice, relative to controls. Immunohistochemical labeling of 12-week-old mammary glands for K14 and K8 further confirmed that basal and luminal specifications were altered in HTT-deficient mice (Figure 2C). HTT-depleted mammary ducts exhibited an unusual expansion of K14+K8+ double-positive cells (17.2% ± 1.32%, Figure 2D).
The overall increase in NOTCH signaling in the luminal compartment suggested that the absence of HTT may inhibit LC fate acquisition. Indeed, the proportion of estrogen receptor (ER) α-expressing cells decreased in mutant epithelia as compared to controls (Figure 2E). We next analyzed the luminal subpopulations by flow cytometry based on the expression of CD49B and SCA1 (Figures 2F and 2G). The SCA1+CD49B− population consists of mature LCs, whereas the SCA1−CD49B+ cells are luminal progenitor cells (Shehata et al., 2012). A high proportion of the luminal population in control epithelium was SCA1+CD49B− (47.2% ± 0.8% versus 30.6% ± 0.6% for the SCA1−CD49B+ population). In mutant conditions, the SCA1−CD49B+ luminal subpopulation was much larger (49.3% ± 0.4% versus 32.6% ± 1.3% for the SCA1+CD49B− population). Accordingly, the ratio of mature LCs to progenitors was lower in the mutant luminal fraction as compared to controls (Figure 2H). This indicates that K5-driven HTT depletion decreases the capacity of the basal and luminal compartments to properly commit to a myoepithelial or LC fate.
HTT Regulates Mammary Epithelial Morphogenesis during Pregnancy and Lactation
We investigated differentiation of mammary glands on day 18.5 of pregnancy and day 1 of lactation when HTT was strongly expressed (Figure S2A). There were fewer secretory alveoli in mutant than control glands, and the epithelial content was lower (Figures 3A and 3B). Notably, there were fewer well-developed alveoli in mutant than control glands. This was confirmed by analysis of lacZ reporter expression in K5Cre;Httflox/flox;R26 mammary glands (Figure S2B). On day 7.5 of pregnancy, most LCs in ducts and alveolus-like structures in mutant glands stained blue with X-gal, with only a few lacZ-negative cells detected. The number of lacZ-negative cells was higher during pregnancy and lactation; also, on day 1 of lactation, the well-differentiated newly formed alveoli were lacZ negative (Figure S2B).
Figure 3.
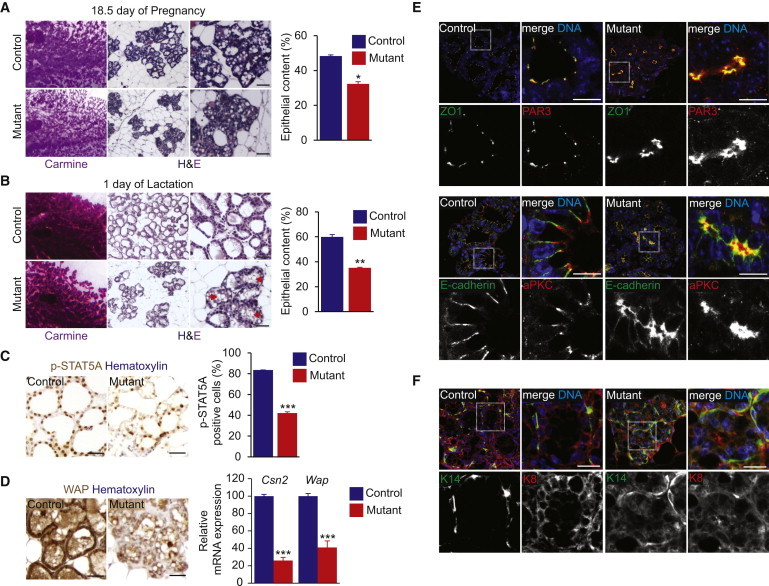
HTT Is Required for Epithelial Morphogenesis during Pregnancy and Lactation
(A and B) Carmine-stained whole mounts of mammary glands and hematoxylin and eosin staining (H&E) at low (middle) and high (right) magnifications. The histograms show the quantification of the epithelial content.
(C) Mammary gland sections stained for p-STAT5A and percentages of p-STAT5A-positive cells.
(D) Mammary gland sections from 1-day lactating mice stained for WAP and quantitative real-time RT-PCR analysis of Csn2 and Wap gene expression.
(E and F) Mammary gland sections from 18.5-day pregnant mice stained as indicated. Scale bars, 10 μm.
Scale bars, 50 μm (A–D). Error bars, SEM. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001. See also Figure S2.
On day 18.5 of pregnancy and day 1 of lactation, KI67 labeling indicated a higher rate of proliferation for mutant than control alveolar cells (Figure S2C). The formation of the lumen involves apoptosis, which was also affected by HTT depletion. On day 14.5 of pregnancy, there were more cells but less apoptosis in the lumen of mutant than control glands (data not shown). On day 18.5 of pregnancy and day 1 of lactation when the lumens are fully generated, there was no detectable apoptosis in controls (Figure S2D). In contrast, mutant alveoli still displayed cleaved caspase-3-positive cells in their lumen, suggesting a delay in lumen formation.
On day 18.5 of pregnancy, the mutant alveoli were poorly differentiated, containing few milk droplets (Figure 3A). In controls, the large cytoplasmic lipid droplets in the luminal alveolar cells on day 18.5 of pregnancy were replaced by small lipid droplets at the luminal surface on day 1 of lactation. In mutant mammary glands, the large cytoplasmic droplets remained in the alveolar cells on day 1 of lactation (Figure 3B, arrows), indicating secretory dysfunction. We investigated the subcellular localization of signal transducer and activator of transcription 5A (STAT5A) on 1 day of lactation (Figure 3C). Upon activation by prolactin, nuclear phosphorylated STAT5A (p-STAT5A) regulates the expression of genes involved in lobulo-alveolar differentiation and lactation (Jahchan et al., 2012). Mutant alveolar cells displayed less nuclear p-STAT5A than control glands. Consistent with this, the milk Whey acid protein (WAP) immunolabeling and the levels of the RNAs for the milk proteins β-casein (Csn2) and WAP (Wap) were lower in mutant than control glands (Figure 3D).
Because the overall architecture of mutant glands showed abnormalities, we tested whether epithelial cell polarity was affected. We analyzed the localization of ZO1 (zonula occludens 1), PAR3 (Partitioning Defective 3), aPKC (atypical protein kinase C), and E-cadherin in LCs on 18.5 day of pregnancy (Figure 3E). In control glands, PAR3 and ZO1 colocalized at the tight junctions of LCs, whereas in mutant alveoli, the labeling was more diffuse, and PAR3 accumulated in the cytoplasm (Figure 3E). E-cadherin, which was enriched at the lateral compartment in control LCs, accumulated with aPKC at the apical surface of mutant cells (Figure 3E). K8+ LCs were distinguishable from K14+ BCs in control alveoli, whereas both K8 and K14 immunoreactivities showed substantially abnormal patterns in mutant glands (Figure 3F). Thus, the absence of HTT alters morphological and functional differentiation, epithelial polarization, and milk production during alveologenesis.
HTT Controls Mitotic Spindle Orientation in Mouse Mammary Basal Cells
Next, we addressed potential mechanisms by which HTT may regulate mammary gland morphogenesis. HTT regulates the mitotic spindle positioning in mouse neuronal progenitors (Godin et al., 2010). We analyzed the division of BCs in ducts from the outgrowths that developed on day 7.5 of pregnancy when the stimuli of pregnancy induce basal cell division to ensure mammary gland growth (Taddei et al., 2008). HTT was observed at the spindle poles during metaphase and at the cortical area in metaphase and anaphase (Figure 4A). Similarly to what is observed in cultured cells (below) (Godin et al., 2010), HTT labeling was also present at the spindle midbody area during anaphase and telophase.
Figure 4.
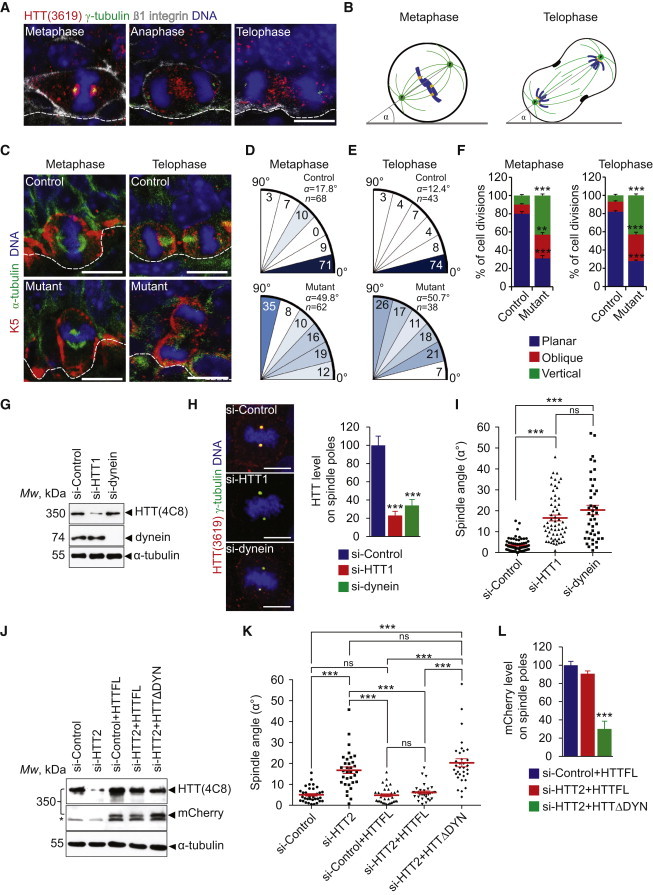
HTT Regulates Mitotic Spindle Orientation in Basal Cells in a Dynein-Dependent Manner
(A) Mammary gland sections from 7.5-day pregnant mice.
(B) Schemes illustrating measurement of the spindle angle α.
(C) Mammary gland sections from 7.5-day pregnant mice.
(D and E) Spindle angles of basal cells; values are expressed as a percentage of basal cells within each interval. Mean angle and number of measures (n) are shown.
(F) Percentages of planar (0°–30°), oblique (30°–60°), and vertical (60°–90°) divisions.
(G) Western blotting of mammary cell extracts.
(H) HTT abundance at spindle poles (right).
(I) Distribution and mean spindle angle in metaphase mammary cells.
(J) Western blotting of cell extracts. The star indicates a contaminating band.
(K) Distribution and mean spindle angles in metaphase mammary cells.
(L) HTT (mCherry) labeling at spindle poles in metaphase mammary cells.
Scale bars, 10 μm. Error bars, SEM. ns, not significant. ∗∗p < 0.01. ∗∗∗p < 0.001. See also Figure S3.
In dividing epithelial cells, the mitotic spindle undergoes a rapid phase of rotation during early metaphase until it reaches a planar orientation, followed by a longer phase of planar maintenance until anaphase onset (Peyre et al., 2011). We evaluated the orientation of the mitotic spindle during metaphase and telophase (Figures 4B and 4C). In control BCs, most metaphase and telophase cells (71% and 74%, respectively) displayed spindle angles between 0° and 15° (planar/horizontal division) (Figures 4D–4F). The spindle angles were randomized in mutant BCs, with a majority of cells displaying oblique and vertical divisions. These findings indicate that HTT is required for correct spindle orientation in dividing mammary BCs.
HTT Modulates Mitotic Spindle Orientation in Mammary Epithelial Cells in a Dynein-Dependent Manner
We used human basal-like MCF-10A mammary cells to investigate the mechanisms underlying the function of HTT in spindle orientation. Cells were transfected with si-Control, si-HTT1 targeting HTT, or si-dynein targeting the heavy chain of the dynein complex (Figure 4G). The amount of HTT at the spindle poles was affected by both small interfering RNAs (siRNAs) (Figure 4H). We analyzed the position of the spindle poles with respect to the substratum plane (Figures S3A, S3B, and 4I). In control cells, virtually all spindles were parallel to the substratum plane (3.5° ± 0.2°) (Figure 4I). In contrast, most HTT-depleted and dynein-depleted cells failed to align their spindle with the substratum plane (16.5° ± 0.4° and 20.3° ± 0.6°, respectively) (Figure 4I).
We also reduced HTT levels using si-HTT2, whose target sequence in HTT is different from that of si-HTT1 (Figure 4J). Again, a misoriented spindle was observed following the use of si-HTT2 (Figure 4K). Next, we introduced a construct encoding a full-length HTT (HTTFL; Figures 4J) (Pardo et al., 2010). si-HTT2 was designed to inhibit the expression of endogenous HTT but had no effect on the expression of the HTTFL construct (Figure 4J) (Pardo et al., 2010). Expression of the HTTFL restored the spindle orientation defect caused by si-HTT2 to the control situation (Figures 4K and S3A).
HTT interacts with dynein in neurons (Caviston et al., 2007, Gauthier et al., 2004), so HTT and dynein may act together to regulate spindle orientation. We depleted endogenous HTT using si-HTT2 and expressed a variant of HTTFL devoid of its dynein-interacting domain (HTTΔDYN) and unable to bind dynein (Pardo et al., 2010) (Figure 4J). HTTΔDYN was mislocalized from the spindle poles (Figures 4L and S3A) and failed to rescue the spindle orientation defect induced by si-HTT2 (Figures 4K and S3A).
We also investigated cell-cycle progression by video recording cells stably expressing fluorescent histone 2B (Cherry) and α-tubulin (GFP) (Figure S3C; Movies S1 and S2). The duration of mitosis was similar in si-HTT1-transfected and control cells (Figure S3C), although HTT depletion led to an alteration in spindle orientation (Figures S3C–S3E). Notably, the spindle angles measured from live-imaging data were higher than those from fixed-cell samples (Figures 4I, 4K, and S3D). Possibly, the angle can be underestimated in fixed samples due to drying effects causing flattening of the sample. These observations show that HTT regulates spindle orientation in mammary cells by a mechanism that involves its interaction with dynein.
HTT Forms a Complex with Dynein/Dynactin/NUMA/LGN in Dividing Mammary Epithelial Cells
We investigated the nature of the molecular machinery involved in the function of HTT during spindle orientation. During interphase, HTT showed a punctate distribution in the cytosol (Figure 5A). During mitosis from prophase to late anaphase, spindle poles became enriched in HTT. HTT displayed punctate staining beneath the cellular cortex reminiscent of astral microtubules plus ends at prometaphase and metaphase. HTT was also present at the spindle midzone during anaphase and telophase (Figure 5A). Other anti-HTT antibodies gave similar findings (Figure S4A).
Figure 5.
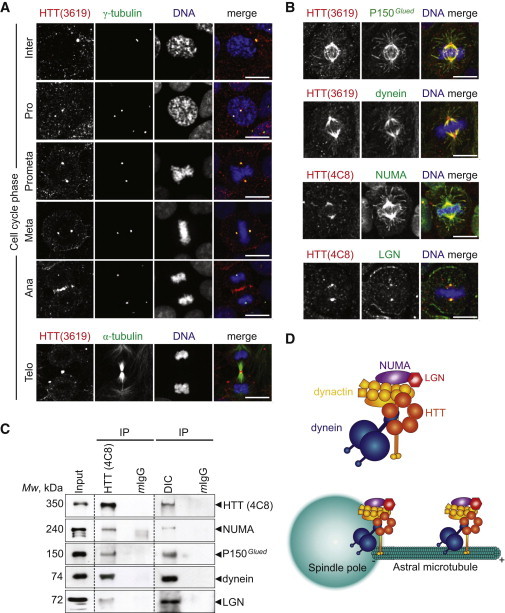
HTT Codistributes with Dynein/Dynactin/NUMA/LGN
(A and B) Mammary cells stained as indicated. Scale bars, 10 μm. Telo, telophase; Ana, anaphase; Meta, metaphase; Prometa, prometaphase; Pro, prophase; Inter, interphase.
(C) HTT/dynein/dynactin/NUMA/LGN complexes were immunoprecipitated from cells arrested in metaphase before lysis. Mouse IgG (mIgG) was used as a negative control. The immunoprecipitates (IP) were analyzed by western blotting.
(D) Cartoon showing the HTT/dynein/dynactin/NUMA/LGN complex at the spindle pole and on astral microtubules in metaphase cells.
See also Figure S4.
The dynein/dynactin complex is required for the assembly of the spindle, and it is also essential at the cell cortex to exert pulling forces on astral microtubules (Morin and Bellaïche, 2011). LGN is necessary for planar spindle orientation (Konno et al., 2008, Morin et al., 2007). To study the localization of HTT, P150Glued, dynein, NUMA, and LGN at the spindle and cellular cortex, we used a fixation procedure including incubation in anhydrous methanol containing 2% paraformaldehyde to maintain the integrity of microtubules emanating from the spindle poles (Figure 5B). We found that HTT colocalized with P150Glued, dynein, and NUMA at the spindle and spindle poles (Figure 5B). HTT, P150Glued, dynein, and NUMA were also detected on astral microtubules and microtubule plus ends. LGN formed a typical crescent shape at the cell cortex and colocalized with HTT at the spindle pole (Figure 5B). Immunoprecipitation of HTT from MCF-10A cells synchronized at metaphase led to the coimmunoprecipitation of NUMA, P150Glued, dynein, and LGN (Figure 5C). However, Gαi/Gαo did not coimmunoprecipitate with HTT (Figure S4B). Because Gαi/Gαo localization is restricted to the cell cortex, this suggests that HTT is not stably associated with the cell cortex. Thus, HTT is part of the dynein/dynactin/NUMA/LGN complex at the spindle poles and along astral microtubules and may regulate its microtubule-based delivery from the spindle poles to the cell cortex (Figure 5D).
HTT Is Required for the Cortical Localization of Dynein, Dynactin, NUMA, and LGN during Mitosis
We analyzed the influence of HTT on the mitotic localization of NUMA, P150Glued, dynein, and LGN. NUMA colocalized with P150Glued, dynactin, and LGN at the spindle poles and formed cortical crescents facing the spindle poles during metaphase (Figure 6A). HTT depletion affected the localization of P150Glued, dynein, NUMA, and LGN: these proteins relocalized from the cell cortex to accumulate at the spindle poles (Figures 6A–6D). Expression of the HTTFL restored the cortical distribution defect of P150Glued, dynein, NUMA, and LGN caused by si-HTT2 to the control situation (Figures S5A–S5D). However, HTTΔDYN failed to do so. We then analyzed the distribution of HTT partners in vivo in control and HTT-depleted dividing BCs. HTT depletion resulted in fewer BCs displaying cortical accumulation of NUMA (27% ± 5.1% versus 73% ± 2.6%) and LGN (21% ± 3.3% versus 68% ± 4.7%) during metaphase (Figure 6E). These data implicate HTT in the delivery of P150Glued, dynein, NUMA, and LGN to the cell cortex during mitosis.
Figure 6.
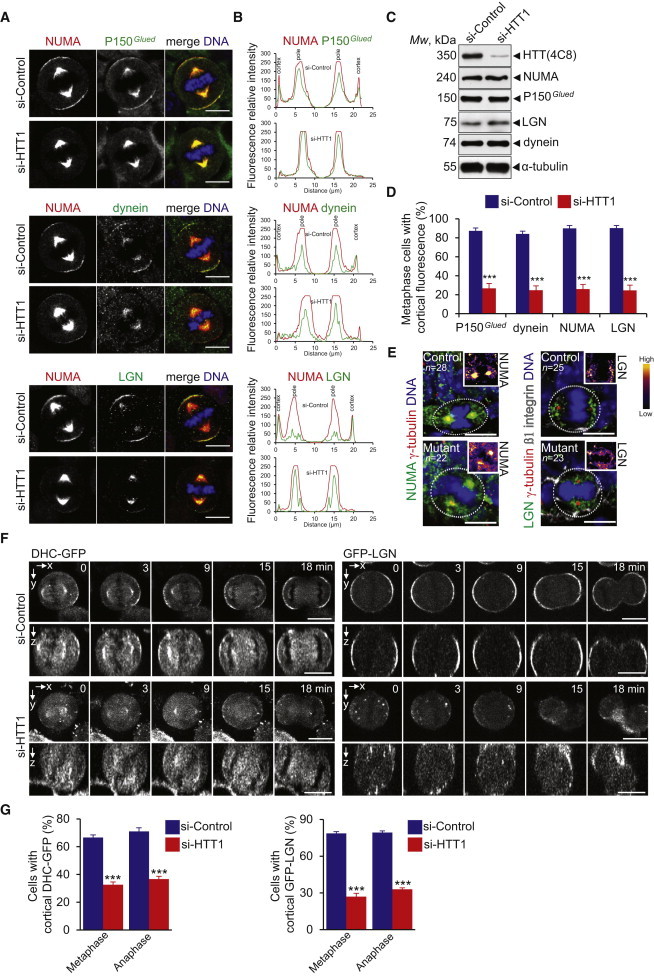
Loss of HTT Prevents Cortical Accumulation of Dynein-Dynactin-NUMA-LGN during Mitosis
(A) Mammary cells stained as indicated.
(B) Line-scan analysis (relative fluorescence intensity).
(C) Western blotting of cell extracts.
(D) Percentage of cells with cortical accumulation of dynein, P150Glued, NUMA, and LGN.
(E) Mammary gland sections from 7.5-day pregnant mice. Gradients of color intensity were applied to NUMA and LGN stainings (insets).
(F) DHC-GFP and GFP-LGN HeLa cells were video recorded. Maximum intensity and z projections are shown.
(G) Percentage of HeLa cells with cortical accumulation of DHC and LGN.
Scale bars, 10 μm. Error bars, SEM. ∗∗∗p < 0.001. See also Figure S5 and Movies S3, S4, S5, and S6.
To confirm this possibility, we performed live-cell imaging of HeLa cells stably expressing the dynein heavy chain or LGN fused to GFP (DHC-GFP and GFP-LGN; Figure 6F; Movies S3, S4, S5, and S6). During mitosis, dynein oscillates from one pole of the cell cortex to the other, generating asymmetric forces that center the spindle (Kiyomitsu and Cheeseman, 2012). In control cells, DHC-GFP was distributed asymmetrically with respect to both the cell cortex and the mitotic spindle during metaphase, and then relocalized symmetrically at anaphase onset, until telophase (Figures 6F and 6G). HTT depletion impaired the dynamics of DHC-GFP localization at the cell cortex, and the proportion of HTT-depleted cells displaying cortical DHC-GFP was low in metaphase (32.7% ± 2.6% versus 66.7% ± 1.8% for controls) and anaphase (36.7% ± 2.1% versus 71% ± 1.9%). Remarkably, cortical accumulation of GFP-LGN was decreased in HTT-depleted cells compared to control cells where GFP-LGN formed a bipolar cortical crescent from metaphase to anaphase (Figure 6F). The proportions of cells displaying cortical accumulation of GFP-LGN in metaphase and anaphase were lower in si-HTT-transfected than control cells (27% ± 2.7% versus 78.7% ± 1.4% and 33% ± 1.1% versus 79.3% ± 1.3%) (Figure 6G). These effects were confirmed by z stack analysis (Figure 6F). We conclude that HTT regulates the dynamics of the dynein/dynactin/NUMA/LGN complex, therefore ensuring its cortical localization.
HTT-Mediated Transport of the Dynein/Dynactin/NUMA/LGN Complex from the Spindle Poles to the Cell Cortex Involves Kinesin 1
HTT interacts with kinesin 1 (KIF5) through the huntingtin-associated protein 1 (HAP1) to promote microtubule-based anterograde vesicular transport in neurons (Colin et al., 2008, Gauthier et al., 2004, McGuire et al., 2006, Shirasaki et al., 2012). Furthermore, HTT transports brain-derived neurotrophic factor (BDNF) vesicles in an anterograde manner, and BDNF anterograde transport depends specifically on kinesin 1, and not kinesin 2 (Dompierre et al., 2007, Gauthier et al., 2004). We hypothesized that kinesin 1 could mediate the HTT-dependent anterograde transport of P150Glued, dynein, NUMA, and LGN from the spindle poles to the cell cortex. HTT colocalized with kinesin 1 on the spindle poles, mitotic spindle, and astral microtubules in metaphase cells (Figure 7A). Immunoprecipitation of HTT from metaphase-synchronized MCF-10A cells led to the coimmunoprecipitation of kinesin 1 (Figure 7B). HTT depletion impaired the recruitment of kinesin 1 on the spindle poles and astral microtubules (Figures 7C–7E). Conversely, si-kinesin 1 treatment altered the localization of P150Glued, dynein, and NUMA on astral microtubules and that of LGN at the cell cortex (Figures 7F–7I).
Figure 7.
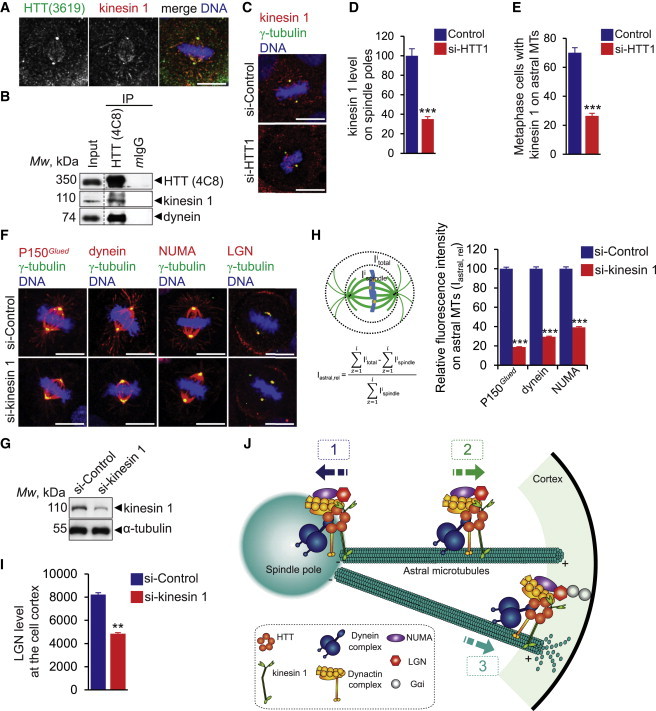
Kinesin 1 Participates in HTT-Mediated Cortical Localization of the Dynein/Dynactin/NUMA/LGN Complex during Mitosis
(A) Mammary cells stained as indicated.
(B) HTT/dynein/kinesin 1 complexes were immunoprecipitated from cells arrested in metaphase before lysis. Mouse IgG (mIgG) was used as a negative control. The immunoprecipitates were analyzed by western blotting.
(C) Mammary cells stained as indicated.
(D) Kinesin 1 abundance at spindle poles.
(E) Percentages of cells with kinesin 1 on astral microtubules.
(F) Mammary cells stained as indicated.
(G) Western blotting of cell extracts.
(H) Quantification of the relative fluorescence intensities of P150Glued, dynein, and NUMA on astral microtubules. The intensities of the spindle (Ispindle) and of the total cell (Itotal) were determined with ImageJ software. Relative intensities on astral microtubules (Iastral, rel) were calculated, and control value was set to 100.
(I) LGN abundance at the cell cortex.
(J) Model for HTT-mediated regulation of mitotic spindle orientation. During mitosis, HTT is targeted to the spindle poles through its interaction with dynein and promotes the accumulation of NUMA and LGN (1). HTT regulates the kinesin 1-dependent trafficking of dynein, dynactin, NUMA, and LGN along astral microtubules to the cell cortex (2). Once at the cell cortex, the dynein/dynactin/NUMA/LGN complex generates pulling forces on astral microtubules for mitotic spindle positioning (3).
Scale bars, 10 μm. Error bars, SEM. ∗∗p < 0.01; ∗∗∗p < 0.001. See also Figure S6.
Finally, we tested whether the recruitment of P150Glued, dynein, NUMA, and LGN to the cell cortex was microtubule dependent by treating cells with 40 nM of nocodazole to disrupt astral microtubules (data not shown). Disruption of astral microtubules altered the recruitment of HTT, P150Glued, dynein, NUMA, and LGN to the spindle poles (Figures S6A–S6D) and of P150Glued, dynein, NUMA, and LGN to the cell cortex (Figures S6C and S6E). In the majority of the cells still displaying cortical LGN, LGN was distributed more randomly on the entire cell cortex and not restricted to a bipolar cortical crescent facing the poles as in the control situation (Figures S6C and S6F). This indicates that astral microtubules are required for the establishment of the bipolar symmetrical cortical distribution of LGN during metaphase. Together, these findings support the idea that HTT regulates the dynamics of the dynein/dynactin/NUMA/LGN complex along astral microtubules through kinesin 1 (Figure 7J).
Discussion
Our studies shed light on the role of HTT as a modulator of the mammary epithelial morphogenesis and of spindle orientation in MaSCs. HTT is not only crucial for spindle pole assembly but also for the cortical localization of the dynein/dynactin/NUMA/LGN complex. HTT may be a molecular link between the dynein/dynactin and NUMA/LGN pathways ensuring the transport of the dynein/dynactin/NUMA/LGN complex from the spindle pole to the cell cortex. Indeed, we identify kinesin 1 as a molecular plus-end motor regulating the trafficking of the dynein/dynactin/NUMA/LGN complex to the cell cortex during mitosis. We propose a model in which HTT regulates MaSC division through this complex, with consequences for self-renewal and tissue cell fate specification and architecture. However, this does not exclude that HTT may also affect mammary gland morphogenesis by impairing directly luminal differentiation.
In vertebrates, a correlation between spindle orientation and the acquisition of cell fate has been proposed for the division of skin progenitors (Williams et al., 2011) and neuronal radial glial cells (reviewed in Morin and Bellaïche, 2011, Peyre and Morin, 2012, Shitamukai and Matsuzaki, 2012). This proposal is based on the observation that a loss of function of several proteins known to control spindle orientation also leads to early differentiation. However, disruption of the LGN complex causes spindle randomization with little effect on differentiation (Konno et al., 2008, Morin et al., 2007, Peyre et al., 2011), suggesting that spindle orientation and fate choices may be regulated in parallel, rather than there being a causal relationship between them. During mammary gland maturation, MaSCs amplify their pool through symmetric divisions, and then switch to a differentiation phase during which they divide asymmetrically to produce a more committed daughter cell (Joshi et al., 2010). Spindle orientation may be a mechanism controlling the balance between symmetric and asymmetric division (Joshi et al., 2010, Regan et al., 2013, Taddei et al., 2008). We observed that deletion of HTT from the basal compartment reduced the global epithelial content as a result of the decrease in the pool of MaSCs. However, the randomization of spindle orientation did not accelerate final LC fate commitment but favored an intermediate luminal progenitor cell status. Our study thus provides further support for the idea that spindle orientation is instrumental for planar division and the maintenance of the pool of cells ensuring self-renewal. The influence of spindle orientation on cell fate specification may be less clear cut and depend on different factors including the nature of the epithelium and the developmental stage.
How can mitotic spindle orientation affect cell fate acquisition in the mammary gland? A recent study showed that Aurora A kinase regulates the orientation of the mitotic spindle and the location of the postmitotic cells, thereby influencing LC fate determination (Regan et al., 2013). The authors propose that Aurora A kinase favors planar divisions through active NOTCH signaling. We show that a loss of HTT impairs NOTCH signaling during LC fate determination. HTT is essential for the cortical localization of dynein/dynactin/NUMA/LGN. In mouse embryonic skin progenitors, the loss of LGN, NUMA, and dynactin impairs asymmetric division and inhibits NOTCH signaling, leading to defects in morphogenesis (Williams et al., 2011). In addition to its role in spindle orientation, HTT may also affect NOTCH signaling through the regulation of its inhibitor NUMB that plays a role in asymmetric cell divisions (Lancaster and Knoblich, 2012). In Drosophila, NUMB interacts with the PAR3-PAR6-aPKC polarity complex promoting NOTCH activation. HTT is found in complex with PAR3 and aPKC (Shirasaki et al., 2012) and could thus regulate their cortical localization with a consequent effect on NOTCH signaling. Alternatively, HTT may act on NOTCH signaling through a mechanism involving endocytosis: HTT regulates endocytosis (Caviston et al., 2007, Velier et al., 1998), and NUMB and Adaptor Protein complex 1 and 2 (AP-1 and AP-2) are found in complex with HTT (Shirasaki et al., 2012). In fact, in Drosophila, NUMB antagonizes NOTCH signaling by influencing the recycling of NOTCH complexes via AP-1 and AP-2 (Cotton et al., 2013, Couturier et al., 2013).
HTT forms a complex with dynein and dynactin in neurons to promote axonal microtubule-based vesicular transport (Caviston et al., 2007, Gauthier et al., 2004). Here, we demonstrate that HTT regulates spindle orientation through its interaction with dynein. Dynein and dynactin are recruited to the cell cortex by the Gαi/Gαo-LGN-NUMA complex where they generate pulling forces that control spindle position and orientation (Kiyomitsu and Cheeseman, 2012, Kotak et al., 2012, Woodard et al., 2010). During metaphase, a signal comprising the spindle pole-localized polo-like kinase 1 (PLK1) regulates dynein localization by controlling the interaction between dynein/dynactin and its upstream cortical targeting factors NUMA and LGN (Kiyomitsu and Cheeseman, 2012). Dynein-dynactin movement to the astral microtubule plus ends involves CLIP-170 and LIS1 (Coquelle et al., 2002, Faulkner et al., 2000). In Drosophila neuroblasts, dynein and the plus-end motor KHC73/KIF13B act in synergy at microtubule plus ends to promote PINS-mediated spindle positioning (Lu and Prehoda, 2013). However, the microtubule-associated motors mediating dynein-dynactin transport to the astral microtubule plus ends are still unknown. We propose that LGN, NUMA, dynein, and dynactin are recruited to the cell cortex through an astral microtubule- and kinesin 1-dependent transport that is regulated by HTT. How this mechanism is coordinated in space and time with the other pathways remains to be determined.
Finally, the regulation of asymmetric/symmetric divisions is essential for the maintenance of stem cell populations, and it may also be key during tumorigenesis (Cicalese et al., 2009, Driessens et al., 2012, Quyn et al., 2010). In mammary tumoral tissues, symmetric divisions of cancer stem cells may contribute to tumor growth (Cicalese et al., 2009). Thus, our results not only open new lines of investigation for unraveling the mechanisms controlling stem cell self-renewal and cell fate specification in the mammary gland but may also have broader implications for the role of cell divisions in cancer biology.
Experimental Procedures
Constructs, siRNAs, and Antibodies
A full description of constructs, siRNAs, antibodies, and cell lines is available in Supplemental Experimental Procedures.
Immunostaining
To analyze HTT localization during mitosis, cells were prelysed 30 s in prewarmed 0.5% Triton X-100-PHEM buffer before fixation in anhydrous methanol at −20°C for 3 min and incubation with anti-HTT and anti-γ-tubulin antibodies. Alternatively, cells were fixed in anhydrous methanol at −20°C containing 2% paraformaldehyde (2 min). To visualize P150Glued, dynein, NUMA, and LGN at spindle poles and cell cortex, cells were fixed with 10% trichloracetic acid (7 min), then in cold methanol (−20°C for 10 min). Cells were immunostained with the various antibodies at 4°C (16 hr).
Pictures were captured with a 3D deconvolution imaging system or with a Leica SP5 laser-scanning confocal microscope. Images were treated with ImageJ (http://rsb.info.nih.gov/ij/; NIH).
Spindle Orientation, Quantification, and Image Analyses
Quantification of HTT, HTTFL, or HTTΔYN at spindle poles was achieved using a 3D object counter plug-in (Bolte and Cordelières, 2006). The Line Scan function of ImageJ was used to reveal the relative fluorescence intensity of NUMA, P150Glued, dynein, and LGN along a line crossing the spindle poles and the cell cortex. Spindle orientation in MCF-10A metaphase cells was determined as in Godin et al. (2010). Details can be found in Supplemental Experimental Procedures.
Live-Cell Microscopy
Imaging was performed at 37°C in 5% CO2 using an inverted microscope (Eclipse Ti; Nikon) coupled to a spinning-disk confocal system (CSU-X1; Yokogawa). Exposure times and laser power details can be found in Supplemental Experimental Procedures.
Mouse Strains
Mice were bred in a 129SV/C57BL6 genetic background. Httflox/flox mice were used as controls and K5Cre;Httflox/flox as mutants. All experiments were performed in accordance with the recommendations of the European Community (86/609/EEC) and the French National Committee (87/848) for the care and use of laboratory animals (permissions 91-448 to S.H. and 76-102 to S.E.).
Histological Analysis
Detailed information for whole-mount preparation and Carmine/X-gal staining, mammary gland processing, and immunostaining can be found in Supplemental Experimental Procedures.
Isolation of Mammary Epithelial Cells and Flow Cytometry
Mammary epithelial cells isolated from the inguinal glands of five 12-week-old virgin K5Cre mice were pooled and stained with anti-CD24-FITC, anti-CD49F-PE, anti-CD45-APC, and anti-CD31-APC antibodies (Taddei et al., 2008). CD24-low/CD49F-high (basal) and CD24-high/CD49F-low (luminal) cells were purified using FACSAria III (SORP; Becton Dickinson). CD45- and CD31-positive stromal cells were excluded from the analysis. Conjugated isotype-matching immunoglobulin Gs (IgGs) were used as negative controls. To separate luminal subpopulations, cells were stained with anti-CD49B-APC and anti-SCA1-PE-Cy7 antibodies, and the SCA1+/CD49B−, SCA1+/CD49B+, and SCA1−/CD49B+ were purified (Shehata et al., 2012).
Quantitative RT-PCR
RNA samples were retrotranscribed using the First Strand cDNA Synthesis Kit (Invitrogen). cDNAs were submitted to RT-PCR with the 7900HT Fast Real-Time PCR System using power SYBR Green PCR Master Mix (Applied Biosystems) with the oligonucleotide pairs detailed in Supplemental Experimental Procedures. Fold changes were calculated using the ddCT method. Values were normalized to those for hprt and β-actin.
Statistical Analyses
GraphPad Prism 6.0 software was used for statistical analysis. Complete statistical analyses with the number of measures are detailed in Supplemental Experimental Procedures.
Acknowledgments
We acknowledge F. Saudou for support; I. Cheeseman, Y. Fengwei, M.A. Glukhova, A. Hyman, F. Mechta-Grigoriou, and M. Piel for reagents, mice, and/or discussions; the staff of the Institut Curie imaging, histology, and animal facilities for technical help; and members of the S.H. and Saudou’s laboratories for helpful comments. This work was supported by grants from Agence Nationale pour la Recherche-Maladies Rares (ANR-09-BLAN-0080 to S.H. and ANR Blanc 2012-LIVESPIN to X.M.), Association pour la Recherche sur le Cancer (ARC subvention libre n°3188 to S.H. and ARC 2011-LIVESPIN to X.M.), Fondation pour la Recherche Médicale (équipe labellisée to S.H.), INSERM Avenir Grant (R08221JS to X.M.), CNRS (to S.H.), INSERM (to S.H.), and Institut Curie (to S.H.). S.H. is an INSERM investigator.
Published: April 3, 2014
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/3.0/).
Supplemental Information includes Supplemental Experimental Procedures, six figures, one table, and six movies and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2014.02.011.
Supplemental Information
HeLa cells stably expressing GFP-tubulin and mCherry-histone H2B were transfected with si-Control RNA for 72 hr. Images corresponding to 32 planes spaced by 0.6 μm through the cell volume were collected every 2 min, as the cells progressed through mitosis using a spinning-disk confocal microscope (CSU-X1; Yokogawa). Maximum intensity projections of tubulin (green) and histone H2B (red) are shown through time; each frame corresponds to 2 min.
HeLa cells stably expressing GFP-tubulin and mCherry-histone H2B were transfected with si-HTT1 RNA for 72 hr. Images corresponding to 32 planes spaced by 0.6 μm through the cell volume were collected every 2 min, as the cells progressed through mitosis using a spinning-disk confocal microscope (CSU-X1; Yokogawa). Maximum intensity projections of tubulin (green) and histone H2B (red) are shown through time; each frame corresponds to 2 min.
HeLa cells stably expressing DHC-GFP were transfected with si-HTT1 RNA for 72 hr. Images corresponding to 35 planes spaced by 0.6 μm through the cell volume were collected every 3 min, as the cells progressed through mitosis using a spinning-disk confocal microscope (CSU-X1; Yokogawa). Maximum intensity projections of DHC are shown through time; each frame corresponds to 3 min.
HeLa cells stably expressing DHC-GFP were transfected with si-HTT1 RNA for 72 hr. Images corresponding to 35 planes spaced by 0.6 μm through the cell volume were collected every 3 min, as the cells progressed through mitosis using a spinning-disk confocal microscope (CSU-X1; Yokogawa). Maximum intensity projections of DHC are shown through time; each frame corresponds to 3 min.
HeLa cells stably expressing GFP-LGN were transfected with si-HTT1 RNA for 72 hr. Images corresponding to 35 planes spaced by 0.6 μm through the cell volume were collected every 3 min, as the cells progressed through mitosis using a spinning-disk confocal microscope (CSU-X1; Yokogawa). Maximum intensity projections of LGN are shown through time; each frame corresponds to 3 min.
HeLa cells stably expressing GFP-LGN were transfected with si-HTT1 RNA for 72 hr. Images corresponding to 35 planes spaced by 0.6 μm through the cell volume were collected every 3 min, as the cells progressed through mitosis using a spinning-disk confocal microscope (CSU-X1; Yokogawa). Maximum intensity projections of LGN are shown through time; each frame corresponds to 3 min.
References
- Asselin-Labat M.L., Vaillant F., Sheridan J.M., Pal B., Wu D., Simpson E.R., Yasuda H., Smyth G.K., Martin T.J., Lindeman G.J., Visvader J.E. Control of mammary stem cell function by steroid hormone signalling. Nature. 2010;465:798–802. doi: 10.1038/nature09027. [DOI] [PubMed] [Google Scholar]
- Beleut M., Rajaram R.D., Caikovski M., Ayyanan A., Germano D., Choi Y., Schneider P., Brisken C. Two distinct mechanisms underlie progesterone-induced proliferation in the mammary gland. Proc. Natl. Acad. Sci. USA. 2010;107:2989–2994. doi: 10.1073/pnas.0915148107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolte S., Cordelières F.P. A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 2006;224:213–232. doi: 10.1111/j.1365-2818.2006.01706.x. [DOI] [PubMed] [Google Scholar]
- Bouras T., Pal B., Vaillant F., Harburg G., Asselin-Labat M.L., Oakes S.R., Lindeman G.J., Visvader J.E. Notch signaling regulates mammary stem cell function and luminal cell-fate commitment. Cell Stem Cell. 2008;3:429–441. doi: 10.1016/j.stem.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Caviston J.P., Ross J.L., Antony S.M., Tokito M., Holzbaur E.L. Huntingtin facilitates dynein/dynactin-mediated vesicle transport. Proc. Natl. Acad. Sci. USA. 2007;104:10045–10050. doi: 10.1073/pnas.0610628104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicalese A., Bonizzi G., Pasi C.E., Faretta M., Ronzoni S., Giulini B., Brisken C., Minucci S., Di Fiore P.P., Pelicci P.G. The tumor suppressor p53 regulates polarity of self-renewing divisions in mammary stem cells. Cell. 2009;138:1083–1095. doi: 10.1016/j.cell.2009.06.048. [DOI] [PubMed] [Google Scholar]
- Colin E., Zala D., Liot G., Rangone H., Borrell-Pagès M., Li X.J., Saudou F., Humbert S. Huntingtin phosphorylation acts as a molecular switch for anterograde/retrograde transport in neurons. EMBO J. 2008;27:2124–2134. doi: 10.1038/emboj.2008.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coquelle F.M., Caspi M., Cordelières F.P., Dompierre J.P., Dujardin D.L., Koifman C., Martin P., Hoogenraad C.C., Akhmanova A., Galjart N. LIS1, CLIP-170’s key to the dynein/dynactin pathway. Mol. Cell. Biol. 2002;22:3089–3102. doi: 10.1128/MCB.22.9.3089-3102.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton M., Benhra N., Le Borgne R. Numb inhibits the recycling of Sanpodo in Drosophila sensory organ precursor. Curr. Biol. 2013;23:581–587. doi: 10.1016/j.cub.2013.02.020. [DOI] [PubMed] [Google Scholar]
- Couturier L., Mazouni K., Schweisguth F. Numb localizes at endosomes and controls the endosomal sorting of notch after asymmetric division in Drosophila. Curr. Biol. 2013;23:588–593. doi: 10.1016/j.cub.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Dompierre J.P., Godin J.D., Charrin B.C., Cordelières F.P., King S.J., Humbert S., Saudou F. Histone deacetylase 6 inhibition compensates for the transport deficit in Huntington’s disease by increasing tubulin acetylation. J. Neurosci. 2007;27:3571–3583. doi: 10.1523/JNEUROSCI.0037-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragatsis I., Levine M.S., Zeitlin S. Inactivation of Hdh in the brain and testis results in progressive neurodegeneration and sterility in mice. Nat. Genet. 2000;26:300–306. doi: 10.1038/81593. [DOI] [PubMed] [Google Scholar]
- Driessens G., Beck B., Caauwe A., Simons B.D., Blanpain C. Defining the mode of tumour growth by clonal analysis. Nature. 2012;488:527–530. doi: 10.1038/nature11344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner N.E., Dujardin D.L., Tai C.Y., Vaughan K.T., O’Connell C.B., Wang Y., Vallee R.B. A role for the lissencephaly gene LIS1 in mitosis and cytoplasmic dynein function. Nat. Cell Biol. 2000;2:784–791. doi: 10.1038/35041020. [DOI] [PubMed] [Google Scholar]
- Gauthier L.R., Charrin B.C., Borrell-Pagès M., Dompierre J.P., Rangone H., Cordelières F.P., De Mey J., MacDonald M.E., Lessmann V., Humbert S., Saudou F. Huntingtin controls neurotrophic support and survival of neurons by enhancing BDNF vesicular transport along microtubules. Cell. 2004;118:127–138. doi: 10.1016/j.cell.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Gjorevski N., Nelson C.M. Integrated morphodynamic signalling of the mammary gland. Nat. Rev. Mol. Cell Biol. 2011;12:581–593. doi: 10.1038/nrm3168. [DOI] [PubMed] [Google Scholar]
- Godin J.D., Colombo K., Molina-Calavita M., Keryer G., Zala D., Charrin B.C., Dietrich P., Volvert M.L., Guillemot F., Dragatsis I. Huntingtin is required for mitotic spindle orientation and mammalian neurogenesis. Neuron. 2010;67:392–406. doi: 10.1016/j.neuron.2010.06.027. [DOI] [PubMed] [Google Scholar]
- Grill S.W., Hyman A.A. Spindle positioning by cortical pulling forces. Dev. Cell. 2005;8:461–465. doi: 10.1016/j.devcel.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Jahchan N.S., Wang D., Bissell M.J., Luo K. SnoN regulates mammary gland alveologenesis and onset of lactation by promoting prolactin/Stat5 signaling. Development. 2012;139:3147–3156. doi: 10.1242/dev.079616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi P.A., Jackson H.W., Beristain A.G., Di Grappa M.A., Mote P.A., Clarke C.L., Stingl J., Waterhouse P.D., Khokha R. Progesterone induces adult mammary stem cell expansion. Nature. 2010;465:803–807. doi: 10.1038/nature09091. [DOI] [PubMed] [Google Scholar]
- Kiyomitsu T., Cheeseman I.M. Chromosome- and spindle-pole-derived signals generate an intrinsic code for spindle position and orientation. Nat. Cell Biol. 2012;14:311–317. doi: 10.1038/ncb2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konno D., Shioi G., Shitamukai A., Mori A., Kiyonari H., Miyata T., Matsuzaki F. Neuroepithelial progenitors undergo LGN-dependent planar divisions to maintain self-renewability during mammalian neurogenesis. Nat. Cell Biol. 2008;10:93–101. doi: 10.1038/ncb1673. [DOI] [PubMed] [Google Scholar]
- Kotak S., Busso C., Gönczy P. Cortical dynein is critical for proper spindle positioning in human cells. J. Cell Biol. 2012;199:97–110. doi: 10.1083/jcb.201203166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster M.A., Knoblich J.A. Spindle orientation in mammalian cerebral cortical development. Curr. Opin. Neurobiol. 2012;22:737–746. doi: 10.1016/j.conb.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M.S., Prehoda K.E. A NudE/14-3-3 pathway coordinates dynein and the kinesin Khc73 to position the mitotic spindle. Dev. Cell. 2013;26:369–380. doi: 10.1016/j.devcel.2013.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire J.R., Rong J., Li S.H., Li X.J. Interaction of Huntingtin-associated protein-1 with kinesin light chain: implications in intracellular trafficking in neurons. J. Biol. Chem. 2006;281:3552–3559. doi: 10.1074/jbc.M509806200. [DOI] [PubMed] [Google Scholar]
- Moreira Sousa C., McGuire J.R., Thion M.S., Gentien D., de la Grange P., Tezenas du Montcel S., Vincent-Salomon A., Durr A., Humbert S. The Huntington disease protein accelerates breast tumour development and metastasis through ErbB2/HER2 signalling. EMBO Mol. Med. 2013;5:309–325. doi: 10.1002/emmm.201201546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin X., Bellaïche Y. Mitotic spindle orientation in asymmetric and symmetric cell divisions during animal development. Dev. Cell. 2011;21:102–119. doi: 10.1016/j.devcel.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Morin X., Jaouen F., Durbec P. Control of planar divisions by the G-protein regulator LGN maintains progenitors in the chick neuroepithelium. Nat. Neurosci. 2007;10:1440–1448. doi: 10.1038/nn1984. [DOI] [PubMed] [Google Scholar]
- Moumen M., Chiche A., Cagnet S., Petit V., Raymond K., Faraldo M.M., Deugnier M.A., Glukhova M.A. The mammary myoepithelial cell. Int. J. Dev. Biol. 2011;55:763–771. doi: 10.1387/ijdb.113385mm. [DOI] [PubMed] [Google Scholar]
- Pardo R., Molina-Calavita M., Poizat G., Keryer G., Humbert S., Saudou F. pARIS-htt: an optimised expression platform to study huntingtin reveals functional domains required for vesicular trafficking. Mol. Brain. 2010;3:17. doi: 10.1186/1756-6606-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyre E., Morin X. An oblique view on the role of spindle orientation in vertebrate neurogenesis. Dev. Growth Differ. 2012;54:287–305. doi: 10.1111/j.1440-169X.2012.01350.x. [DOI] [PubMed] [Google Scholar]
- Peyre E., Jaouen F., Saadaoui M., Haren L., Merdes A., Durbec P., Morin X. A lateral belt of cortical LGN and NuMA guides mitotic spindle movements and planar division in neuroepithelial cells. J. Cell Biol. 2011;193:141–154. doi: 10.1083/jcb.201101039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quyn A.J., Appleton P.L., Carey F.A., Steele R.J., Barker N., Clevers H., Ridgway R.A., Sansom O.J., Näthke I.S. Spindle orientation bias in gut epithelial stem cell compartments is lost in precancerous tissue. Cell Stem Cell. 2010;6:175–181. doi: 10.1016/j.stem.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Ramirez A., Page A., Gandarillas A., Zanet J., Pibre S., Vidal M., Tusell L., Genesca A., Whitaker D.A., Melton D.W., Jorcano J.L. A keratin K5Cre transgenic line appropriate for tissue-specific or generalized Cre-mediated recombination. Genesis. 2004;39:52–57. doi: 10.1002/gene.20025. [DOI] [PubMed] [Google Scholar]
- Regan J.L., Sourisseau T., Soady K., Kendrick H., McCarthy A., Tang C., Brennan K., Linardopoulos S., White D.E., Smalley M.J. Aurora A kinase regulates mammary epithelial cell fate by determining mitotic spindle orientation in a Notch-dependent manner. Cell Rep. 2013;4:110–123. doi: 10.1016/j.celrep.2013.05.044. [DOI] [PubMed] [Google Scholar]
- Rios A.C., Fu N.Y., Lindeman G.J., Visvader J.E. In situ identification of bipotent stem cells in the mammary gland. Nature. 2014;506:322–327. doi: 10.1038/nature12948. [DOI] [PubMed] [Google Scholar]
- Shackleton M., Vaillant F., Simpson K.J., Stingl J., Smyth G.K., Asselin-Labat M.L., Wu L., Lindeman G.J., Visvader J.E. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- Shehata M., Teschendorff A., Sharp G., Novcic N., Russell A., Avril S., Prater M., Eirew P., Caldas C., Watson C.J., Stingl J. Phenotypic and functional characterization of the luminal cell hierarchy of the mammary gland. Breast Cancer Res. 2012;14:R134. doi: 10.1186/bcr3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasaki D.I., Greiner E.R., Al-Ramahi I., Gray M., Boontheung P., Geschwind D.H., Botas J., Coppola G., Horvath S., Loo J.A., Yang X.W. Network organization of the huntingtin proteomic interactome in mammalian brain. Neuron. 2012;75:41–57. doi: 10.1016/j.neuron.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shitamukai A., Matsuzaki F. Control of asymmetric cell division of mammalian neural progenitors. Dev. Growth Differ. 2012;54:277–286. doi: 10.1111/j.1440-169X.2012.01345.x. [DOI] [PubMed] [Google Scholar]
- Silberstein G.B. Postnatal mammary gland morphogenesis. Microsc. Res. Tech. 2001;52:155–162. doi: 10.1002/1097-0029(20010115)52:2<155::AID-JEMT1001>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Siller K.H., Doe C.Q. Spindle orientation during asymmetric cell division. Nat. Cell Biol. 2009;11:365–374. doi: 10.1038/ncb0409-365. [DOI] [PubMed] [Google Scholar]
- Taddei I., Deugnier M.A., Faraldo M.M., Petit V., Bouvard D., Medina D., Fässler R., Thiery J.P., Glukhova M.A. Beta1 integrin deletion from the basal compartment of the mammary epithelium affects stem cells. Nat. Cell Biol. 2008;10:716–722. doi: 10.1038/ncb1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velier J., Kim M., Schwarz C., Kim T.W., Sapp E., Chase K., Aronin N., DiFiglia M. Wild-type and mutant huntingtins function in vesicle trafficking in the secretory and endocytic pathways. Exp. Neurol. 1998;152:34–40. doi: 10.1006/exnr.1998.6832. [DOI] [PubMed] [Google Scholar]
- Visvader J.E., Lindeman G.J. The unmasking of novel unipotent stem cells in the mammary gland. EMBO J. 2011;30:4858–4859. doi: 10.1038/emboj.2011.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S.E., Beronja S., Pasolli H.A., Fuchs E. Asymmetric cell divisions promote Notch-dependent epidermal differentiation. Nature. 2011;470:353–358. doi: 10.1038/nature09793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodard G.E., Huang N.N., Cho H., Miki T., Tall G.G., Kehrl J.H. Ric-8A and Gi alpha recruit LGN, NuMA, and dynein to the cell cortex to help orient the mitotic spindle. Mol. Cell. Biol. 2010;30:3519–3530. doi: 10.1128/MCB.00394-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalcin-Ozuysal O., Fiche M., Guitierrez M., Wagner K.U., Raffoul W., Brisken C. Antagonistic roles of Notch and p63 in controlling mammary epithelial cell fates. Cell Death Differ. 2010;17:1600–1612. doi: 10.1038/cdd.2010.37. [DOI] [PubMed] [Google Scholar]
- Zheng Z., Zhu H., Wan Q., Liu J., Xiao Z., Siderovski D.P., Du Q. LGN regulates mitotic spindle orientation during epithelial morphogenesis. J. Cell Biol. 2010;189:275–288. doi: 10.1083/jcb.200910021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
HeLa cells stably expressing GFP-tubulin and mCherry-histone H2B were transfected with si-Control RNA for 72 hr. Images corresponding to 32 planes spaced by 0.6 μm through the cell volume were collected every 2 min, as the cells progressed through mitosis using a spinning-disk confocal microscope (CSU-X1; Yokogawa). Maximum intensity projections of tubulin (green) and histone H2B (red) are shown through time; each frame corresponds to 2 min.
HeLa cells stably expressing GFP-tubulin and mCherry-histone H2B were transfected with si-HTT1 RNA for 72 hr. Images corresponding to 32 planes spaced by 0.6 μm through the cell volume were collected every 2 min, as the cells progressed through mitosis using a spinning-disk confocal microscope (CSU-X1; Yokogawa). Maximum intensity projections of tubulin (green) and histone H2B (red) are shown through time; each frame corresponds to 2 min.
HeLa cells stably expressing DHC-GFP were transfected with si-HTT1 RNA for 72 hr. Images corresponding to 35 planes spaced by 0.6 μm through the cell volume were collected every 3 min, as the cells progressed through mitosis using a spinning-disk confocal microscope (CSU-X1; Yokogawa). Maximum intensity projections of DHC are shown through time; each frame corresponds to 3 min.
HeLa cells stably expressing DHC-GFP were transfected with si-HTT1 RNA for 72 hr. Images corresponding to 35 planes spaced by 0.6 μm through the cell volume were collected every 3 min, as the cells progressed through mitosis using a spinning-disk confocal microscope (CSU-X1; Yokogawa). Maximum intensity projections of DHC are shown through time; each frame corresponds to 3 min.
HeLa cells stably expressing GFP-LGN were transfected with si-HTT1 RNA for 72 hr. Images corresponding to 35 planes spaced by 0.6 μm through the cell volume were collected every 3 min, as the cells progressed through mitosis using a spinning-disk confocal microscope (CSU-X1; Yokogawa). Maximum intensity projections of LGN are shown through time; each frame corresponds to 3 min.
HeLa cells stably expressing GFP-LGN were transfected with si-HTT1 RNA for 72 hr. Images corresponding to 35 planes spaced by 0.6 μm through the cell volume were collected every 3 min, as the cells progressed through mitosis using a spinning-disk confocal microscope (CSU-X1; Yokogawa). Maximum intensity projections of LGN are shown through time; each frame corresponds to 3 min.


