Abstract
Increased understanding of cancer pathogenesis has identified several pathways that serve as potential targets for novel targeted agents in development. The selection of targeted cancer therapy based on biomarkers has instigated a new era of personalized medicine and changed the way we practice oncology. Many targeted agents are approved for treatment of gastrointestinal malignancies most targeting tumor angiogenesis, and many more are in different phases of development. Here we briefly summarize nine different targeted agents that are approved currently in the U.S. and several other agents currently being studied in various gastrointestinal cancers.
Keywords: Gastrointestinal cancers, monoclonal antibodies, targeted drugs, tyrosine kinase inhibitors
INTRODUCTION
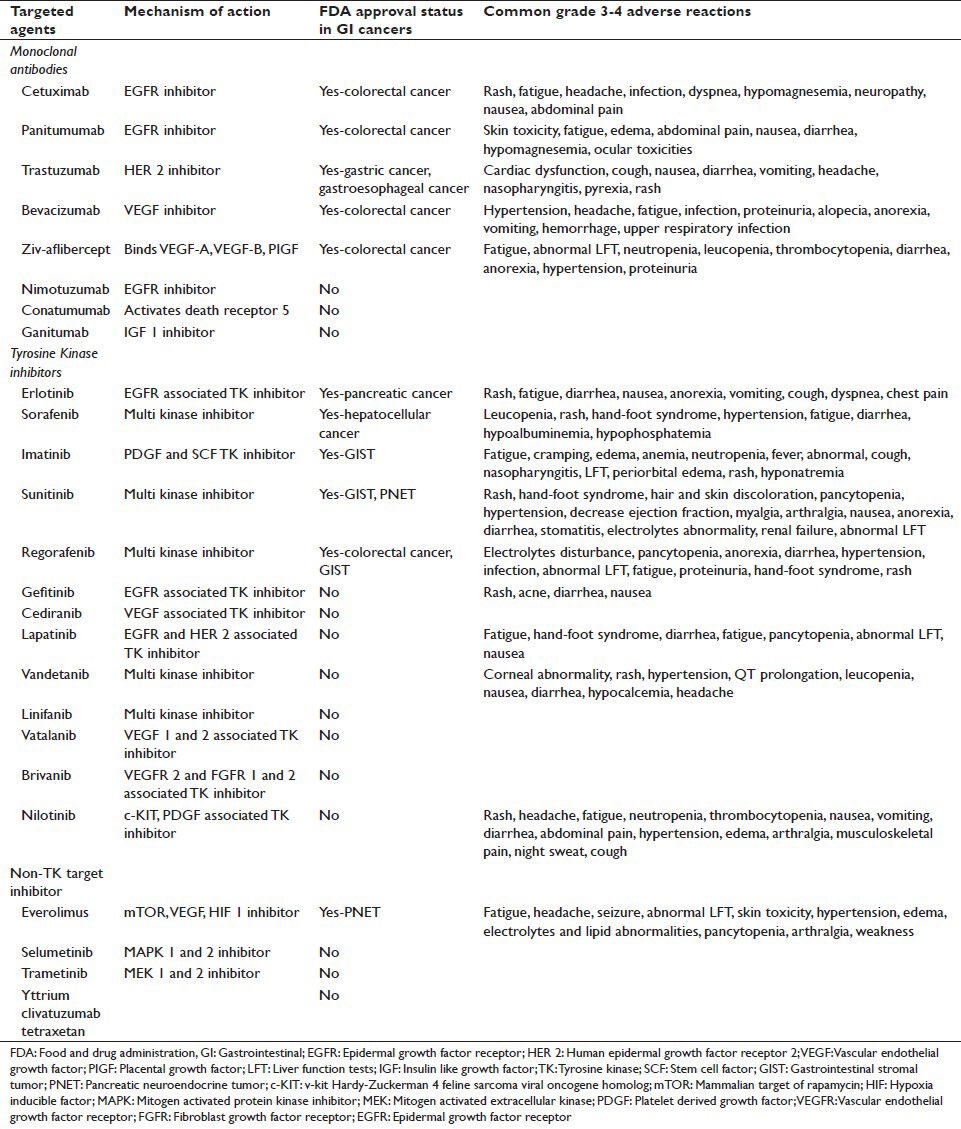
With improved understanding of cancer genomics, proteomics and molecular events associated with cell growth, proliferation, angiogenesis, apoptosis and signal transduction, along with biotechnological advancements such as immunohistochemical and hybridization techniques, our knowledge of cancer pathogenesis has increased exponentially. Though incomplete, we have identified several key molecular events involved in carcinogenesis and targeting them offers survival benefit in several cancers such as breast cancer, colon cancer and leukemia. The concept of targeted therapy was born more than a century ago but its practical application in cancer therapy took several decades. Today we have developed several agents designed to target specific molecule such as cell receptors, enzymes various cytokines and signaling pathways. The National Cancer Institute defines targeted therapy as “a type of treatment that uses drugs or other substances, such as monoclonal antibodies, to identify and attack specific cancer cells”.[1] Over the last 2 decades, the development of drugs targeted at specific molecular pathway/receptor has led us one step closer to individualized cancer therapy. Many targeted therapies have been approved by the United States Food and Drug Administration (FDA) for clinical use in humans while as many are in various phases of development as well. For the scope of this review article, we will discuss only the relevant trials in gastrointestinal cancers with regard to each targeted agent. Figure 1 shows the conceptual diagram of several molecular targets and signaling pathways involved in carcinogenesis and Table 1 describes targeted agents studied in gastrointestinal (GI) cancers and their FDA approval status.
Figure 1.
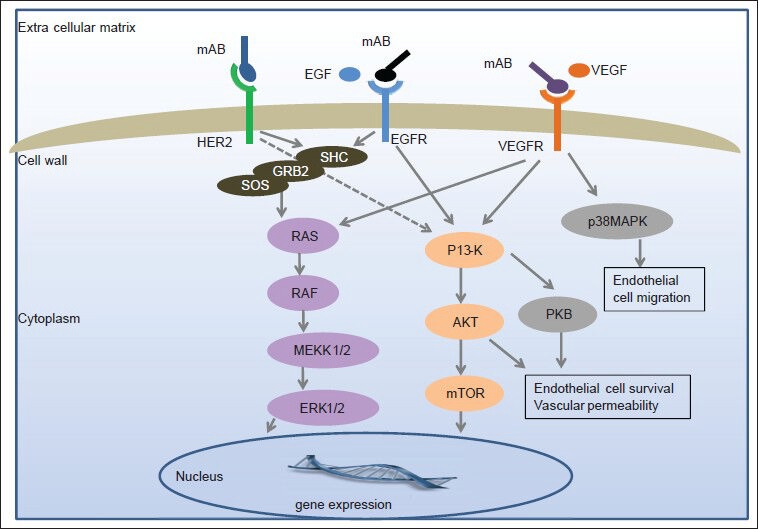
Molecular targets and signaling pathways of various targeted therapies used in the treatment of gastrointestinal cancers
Table 1.
Various targeted agents, their molecular targets, FDA approval status and common toxicities
MONOCLONAL ANTIBODIES
Cetuximab (Erbitux®)
Cetuximab, a partially humanized murine IgG1 monoclonal antibody, has been approved in combination with the chemotherapy regimen 5-fluorouracil (5-FU), leucovorin and irinotecan (FOLFIRI) as the first line treatment for KRAS mutation negative and anti-epidermal growth factor receptor (EGFR) expressing metastatic colorectal cancer based on a randomized, multicenter, open-label, phase III trial (CRYSTAL trial) where each group had 599 patients (cetuximab + FOLFIRI vs. FOLFIRI alone).[2] The addition of cetuximab to FOLFIRI reduced the risk of progression by 15%, increased the median progression free survival (PFS) from 8 to 8.9 months (mo) (hazard ratio [HR]: 0.85, 95% confidence interval [CI]: 0.72-0.99, P = 0.048), increased the median overall survival (OS) from 18.6 to 19.9 mo (HR: 0.93, 95% CI: 0.81-1.07, P = 0.31). The adjusted odds ratio for a tumor response to cetuximab group was 1.40 (95% CI: 1.12-1.77, P = 0.004). Median OS in cetuximab + FOLFIRI and FOLFIRI groups were respectively 24.9 and 21.0 mo in the wild type-KRAS population as well as 17.5 and 17.7 mo, in the mutant-KRAS population.
Cetuximab has been studied in the gastroesophageal cancer with limited success. Cetuximab in combination with chemoradiation containing different regimen has shown up to 40% objective response rate in locally advanced disease and up to 16.6 mo of OS in metastatic disease.[3,4] The Southwest Oncology Group directed (S0205) phase III trial evaluated the role of cetuximab in metastatic or locally advanced unresectable pancreatic adenocarcinoma, where 371 patients were treated with gemcitabine alone versus 372 patients with gemcitabine + cetuximab.[5] The addition of cetuximab to gemcitabine failed to improve median OS and PFS significantly (one-sided P = 0.19 for OS and P = 0.18 for PFS). Moreover, EGFR expression had no impact on median survival (HR: 0.98, 95% CI: 0.83-1.17, P = 0.42). The efficacy of gemcitabine + oxaliplatin + cetuximab was assessed in 45 unresectable or advanced hepatocellular carcinoma (HCC) patients in a multicenter phase II trial. Median PFS of 4.7 mo (95% CI: 2.6-9.5) and median OS of 9.5 mo (95% CI: 7.8-11) were observed. The 1-year survival rate was 40%.[6] An interim analysis of the BINGO trial, an open label phase II study comparing gemcitabine + oxaliplatin with or without cetuximab was presented at the 2009 American Society of Clinical Oncology meeting. At 4 mo, PFS rate was 61% (95% CI: 36-83) in cetuximab group compared to 44% (95% CI: 20-70) in the other group.[7]
Panitumumab (Vectibix®)
Panitumumab is the first fully human IgG2 kappa monoclonal antibody directed to EGFR. Panitumumab is approved for the treatment of EGFR expressing, metastatic colorectal cancer with disease progression on or following fluoropyrimidine, oxaliplatin and irinotecan containing chemotherapy regimen. The approval was based on an open label phase III trial where 231 patients were assigned to panitumumab + best supportive care and 232 patients received only best supportive care. Panitumumab significantly prolonged median PFS (8 weeks vs. 7.3 week, HR: 0.54; 95% CI: 0.44-0.66, P < 0.0001).[8] Panitumumab lacks activity in mutant KRAS expressing metastatic colorectal cancer.[9] Post hoc analysis of the original phase III trial found other clinically important findings as well such as (1) median OS of 6.4 mo in all KRAS-evaluable patients randomized to panitumumab versus 4.4 mo in patients with mutant-KRAS tumors randomized to supportive care (HR: 0.76, 95% CI: 0.60-0.98); (2) median OS of 8.1 mo in wild-KRAS tumors randomized to panitumumab versus 4.4 mo in patients with mutant-KRAS tumors randomized to supportive care (HR: 0.56, 95% CI: 0.49-0.87); (3) median OS of 7.9 mo in wild-KRAS group versus 4.7 mo in all patients with mutant-KRAS tumors, regardless of treatment group assignment.[10] Panitumumab as a first line agent in metastatic colorectal cancer was evaluated in a phase III randomized trial (PRIME) in combination with 5-fluorouracil, leucovorin and oxaliplatin (FOLFOX4). In wild-KRAS tumors, the addition of panitumumab to FOLFOX4 significantly improved PFS compared with FOLFOX4 alone (9.6 mo vs. 8.0 mo, respectively; HR: 0.80, 95% CI: 0.66-0.97, P = 0.02). However, overall response rate was not significantly different (55% vs. 48% respectively, P = 0.068).[11] Adding panitumumab to irinotecan alone does not improve OS in wild-KRAS tumors.[12] Combination of panitumumab and FOLFIRI as a second line agent showed significant improvement in PFS compared to FOLFIRI alone (HR: 0.73, 95% CI: 0.59-0.9, P = 0.004).[13] Combination targeted therapies (panitumumab + bevacizumab) when combined with standard chemotherapy (oxaliplatin and irinotecan based) as a first line agent in metastatic colorectal cancer decreases PFS and increases toxicity.[14]
Panitumumab, when studied with chemotherapy (gemcitabine + oxaliplatin + capecitabine) as a first line therapy for wild-KRAS expressing unresectable biliary cancer, median PFS of 8.3 mo (95% CI: 6.7-8.7) and median OS of 10 mo (95% CI: 7.4-12.7) were observed.
Trastuzumab (Herceptin®)
Trastuzumab, a humanized IgG1 kappa monoclonal antibody, selectively binds with extracellular domain of EGFR2 (HER2) receptor preventing ligand binding which otherwise would initiate signal transduction pathways involved in cell proliferation, differentiation, migration, adhesion and apoptosis.[15] It is approved in combination with cisplatin + capecitabine or 5-FU, for the treatment of patients with HER2 over expressing metastatic gastric or gastroesophageal junction adenocarcinoma, who have not received prior treatment for metastatic disease based on results of the ToGA trial.[16] The ToGA trial recruited 594 HER2 positive gastric or gastroesophageal junction cancer patients, randomized to trastuzumab + chemotherapy (n = 294); orchemotherapy (cisplatin + capacitabine or 5-FU) alone (n = 290). Median OS was 13.8 mo (95% CI: 12-16) in trastuzumab group versus 11.1 mo (95% CI: 10-13) in chemotherapy alone group (HR: 0.74, 95% CI: 0.60-0.91, P = 0.0046). The addition of trastuzumab improved median survival by 2.5 mo. Median PFS was 6.7 mo in trastuzumab group versus 5.5 mo in chemotherapy alone group (P = 0.0002). The overall response rate was favorable for trastuzumab (47.3% vs. 34.5%, P = 0.0017). In HER-2 over expressing metastatic pancreatic cancer, addition of trastuzumab to capecitabine does not offer added benefit compared with standard chemotherapy.[17]
Bevacizumab (Avastin®)
Bevacizumab, an angiogenesis inhibitor, is a humanized monoclonal antibody that inhibits vascular endothelial growth factor (VEGF). Bevacizumab is approved as the first or second line treatment for metastatic colorectal cancer in combination with 5-FU based on a phase III, randomized, placebo controlled trial.[18] The trial included 411 and 402 patients suffering from metastatic colon cancer to irinotecan + 5-FU + leucovorin + placebo group and irinotecan + 5-FU + leucovorin + bevacizumab group, respectively. Median OS as well as PFS were significantly longer in bevacizumab group (20.3 mo vs. 15.6 mo for OS, HR: 0.66, P < 0.001 and 10.6 mo vs. 6.2 mo for PFS, HR: 0.54, P < 0.001). Median survival in bevacizumab group was 74.3% versus 63.4% in the other group (P < 0.001). Recently, a systematic review of all trials comprising infusional bolus of 5-FU based chemotherapy was performed. Median PFS and OS were 10.8 mo (95% CI, 8.9-12.8) and 23.7 mo (95% CI, 18.1-31.6), respectively.[19] Bevacizumab is also studied in the adjuvant setting in an open label, randomized phase III trial of stage II and III colon cancer. The FOLFOX group and FOLFOX + bevacizumab groups had respectively 1338 and 1334 evaluable subjects. Addition of bevacizumab to FOLFOX did not increase disease free survival significantly (HR: 0.89, CI: 0.76-1.04, P = 0.15). The lack of benefit persist even when analysis was performed in stage II and III disease separately. However, time dependent favorable effect was observed when exploratory analysis was performed at 15 mo landmark. The HR before the 15 mo landmark strongly favored bevacizumab (HR: 0.61, CI: 0.48-0.78, P < 0.001), whereas this benefit was lost subsequently (HR: 1.22, CI: 0.98-1.52, P = 0.076).[20] In a phase II study, bevacizumab was studied in metastatic colorectal cancer as first line agent in combination with capecitabine + oxaliplatin (n = 127) and capecitabine + irinotecan (n = 120). Median PFS and OS were respectively 10.4 mo (95% CI: 9.0-12.0) and 24.4 mo (95% CI: 19.3-30.7) in capecitabine + oxaliplatin + bevacizumab group and 12.1 mo (95% CI: 10.8-13.2) and 25.5 mo (95% CI: 21.0-31.0) incapacitabine + irinotecan + bevacizumab.[21]
The AVAGAST trial was a large multinational, randomized trial designed to evaluate the efficacy of adding bevacizumab to capecitabine + cisplatin as a first-line treatment of advanced gastric cancer. Each arm had 387 patients. Median OS was 12.1 mo in bevacizumab arm versus 10.1 mo in placebo arm (HR: 0.87, 95% CI: 0.73-1.03, P = 0.1002). Both median PFS (6.7 mo vs. 5.3 mo; HR: 0.80, 95% CI: 0.68-0.93, P = 0.0037) and overall response rate (46.0% vs. 37.4%, P = 0.0315) were significantly improved with bevacizumab. Bevacizumab in combination with capecitabine + oxaliplatin showed median PFS of 7.2 mo, median OS of 10.8 mo, and 51.4% response rate in a phase II trial of metastatic gastroesophageal cancer.[22] In another trial, bevacizumab when administered with cisplatin + irinotecan in 47 patients with metastatic/unresectable gastric or gastroesophageal junction tumor, 65% overall response rate (95% CI: 46-80) and median survival of 12.3 mo (95% CI: 11.3-17.2) were observed.[23] A systematic review of phase II trials of bevacizumab in advanced HCC as a monotherapy or combination chemotherapy reported median PFS and OS ranging 5.3-9.0 mo and 5.9-13.7 mo, respectively. The disease control rate was 51.1-76.9%.[24] GI perforation is a serious adverse event associated with bevacizumab. A systematic review and meta-analysis of published randomized controlled trial found that bevacizumab had a significantly increased risk of GI perforation compared with patients treated with control medication, with a relative risk of 2.14 (95% CI: 1.19-3.85, P = 0.011). Risk varied with bevacizumab dose and tumor type. Relative risks for patients receiving bevacizumab at 5 and 2.5 mg/kg/week were 2.67 (95% CI: 1.14-6.26) and 1.61 (95% CI: 0.76-3.38), respectively. Higher risks were observed in patients with colorectal cancer (relative risk 3.10, 95% CI: 1.26-7.63).[25]
Ziv-aflibercept (Zaltrap®)
Ziv-aflibercept, a fusion protein directed towards VEGF-A, VEGF-B and a placental growth factor. Ziv-aflibercept is approved in combination with FOLFIRI for metastatic colorectal cancer that is resistant to or has progressed following oxaliplatin based regimen. A randomized, phase III trial of FOLFIRI + Ziv-aflibercept (n = 612) versus FOLFIRI + placebo (n = 614) was conducted in metastatic colorectal cancer patients who were previously treated with oxaliplatin including those who were treated with bevacizumab.[26] Median OS and PFS were significantly longer in Ziv-aflibercept group compared with the placebo group (13.5 mo. vs. 12.06 mo for OS, HR: 0.817, 95% CI: 0.713-0.913, P = 0.0032 and 6.9 mo. vs. 4.67 mo for PFS, HR: 0.758, 95% CI: 0.661-0.869; P < 0.0001). This survival benefit of Ziv-aflibercept persisted even when subgroup analysis was performed for bevacizumab pretreated patients. Response rate was 19.8% (95% CI: 16.4-23.2) in Ziv-aflibercept group compared with 11.1% (95% CI: 8.5-13.8) in placebo group (P = 0.0001).
A randomized, double-blind, placebo controlled, phase III trial evaluated the addition of aflibercept to gemcitabine in patients with advanced pancreatic cancer. The study was stopped for futility following a planned interim analysis.[27]
Nimotuzumab (Theracim®, h-R3®)
Nimotuzumab, a humanized monoclonal antibody, binds to extracellular domain of EGFR, and inhibits downstream signal conduction. It is approved in more than 20 countries but not U.S. for various indications (i.e., gliomas, head and neck cancer, esophageal cancer, etc.) with very favorable side-effect profile. A retrospective study evaluated 66 esophageal squamous cell cancer patients treated with Nimotuzumab and radiation/chemoradiation. Median OS and PFS were 26.0 mo and 16.7 mo, respectively. OS, PFS and locoregional control at 2 years were 54%, 37% and 80%, respectively.[28] A phase II trial evaluated nimotuzumab 200 mg weekly as a second line agent in advanced pancreatic cancer patients. For 36 evaluable patients, median PFS of patients with stable disease was 19.2 weeks and 6.7 weeks for all patients (95% CI: 6.43-7.14). PFS after 1 year was 10.3% with median OS of 18.1 weeks.[29]
Yttrium clivatuzumab tetraxetan (hPAM4-Cide®)
A monoclonal antibody, clivatuzumab, combined with yttrium (a radioisotope) where tetraxetan acts as a chelator (YCT). YCT is directed to mucin antigen found in 85% pancreatic adenocarcinoma but absent in normal pancreatic tissue.[30] This unique combo was studied along with gemcitabine, a radio sensitizer, in advanced pancreatic cancer based on preclinical data. Previously untreated 38 patients (n = 33, stage IV; n = 6, stage III) where 19 patients received escalating dose of YCT in repeat cycles. Grade III/IV toxicities were observed in all patients after second cycle. The median OS was 7.7 mo for all 38 patients, including 11.8 mo for those who received repeated cycles (46% [6 of 13 patients] ≥1 year), with improved efficacy at higher doses.[31]
Conatumumab (AMG 655)
Conatumumab, an agonist monoclonal antibody directed towards the death receptor 5, causing the activation of capcase and subsequent apoptosis. Patients with mutant KRAS metastatic colorectal cancer refractory to fluoropyrimidine-and oxaliplatin-based chemotherapy were randomized to FOLFIRI plus conatumumab, ganitumab, or placebo. Median PFS in conatumumab, ganitumab, and placebo group were 6.5 mo (HR: 0.69; P = 0.147), 4.5 mo (HR: 1.01; P = 0.998) and 4.6 mo, respectively. median OS were 12.3 mo (HR: 0.89; P = 0.650), 12.4 mo (HR: 1.27; P = 0.357) and 12.0 mo, respectively.[32] In metastatic pancreatic adenocarcinoma, addition of conatumumab to gemcitabine showed trend towards improved 6 mo survival rate compared to gemcitabine alone (59% vs. 50%).[33]
Ganitumab (AMG 479)
Ganitumab, a monoclonal antibody directed against insulin-like growth factor-1. In a multicenter, phase II, open-label trial, ganitumab was studied in metastatic progressive carcinoid or pancreatic neuroendocrine tumor (PNET) but failed to show any significant tumor response.[34] However, in metastatic pancreatic adenocarcinoma, addition of ganitumab to gemcitabine showed trend toward improved 6 mo survival rate compared to gemcitabine alone (57% vs. 50%).[33] As mentioned earlier, ganitumab + FOLFIRI in metastatic colorectal cancer failed to show any significant tumor response.
TYROSINE KINASE INHIBITORS
Erlotinib (Tarceva®)
Erlotinib is a reversible human HER1 and EGFR tyrosine kinase inhibitor. Erlotinib is approved in combination with gemcitabine for unresectable locally advanced or metastatic pancreatic adenocarcinoma based on a double-blind, placebo controlled, phase III trial.[35] Though modest, median OS was significantly greater in erlotinib + gemcitabine group compared with gemcitabine alone group (OS 6.24 mo. vs. 5.91 mo, HR: 0.82, 95% CI: 0.69-0.99, P = 0.038). One year survival (23% vs. 17%; P = 0.023) and PFS (3.75 mo vs. 3.55 mo, HR: 0.77, 95% CI: 0.64-0.92, P = 0.004) were also greater in erlotinib arm. Though well-tolerated, erlotinib arm had higher incidence of rash, diarrhea, infection and stomatitis. Erlotinib in combination with radiotherapy for locally advanced unresectable esophageal cancer was studied in 16 patients where median OS of 7.3 mo (95% CI: 3.8-22.3), estimated PFS of 4.5 mo (95% CI: 2.4-7.3) and estimated 1-year survival of 29% (95% CI: 11-51) were observed.[36] The addition of bevacizumab and erlotinib to neoadjuvant concurrent chemoradiation therapy for localized esophageal/gastroesophagel cancer did not demonstrate survival benefit but targeted agent specific toxicities were evident.[37] Erlotinib monotherapy when studied as a second line agent in a phase II trial in metastatic esophageal cancer, showed some benefit in squamous cell tumors but not in adenocarcinoma (3.3 mo vs. 1.6 mo, P = 0.026).[38] A phase II trial (SWOG 0127) evaluated erlotinib as a monotherapy in unresectable/metastatic gastroesophagel junction and gastric adenocarcinoma and found its activity in gastroesophageal junction tumors but not in gastric adenocarcinoma.[39] A phase III, randomized, double blind, placebo controlled trial compared sorafenib + erlotinib (n = 362) and sorafenib + placebo (n = 358) in advanced HCC. There was no significant advantage of adding erlotinib to sorafenib (median OS 9.5 mo vs. 8.5 mo, HR: 0.929, 95% CI: 0.781-1.106, P = 0.204 and time to progression [TTP] was 3.2 mo vs. 4.0 mo, HR: 1.135, 95% CI: 0.944-1.366, P = 0.91).[40] The combination of erlotinib + bevacizumab in unresectable/metastatic HCC did not show any improvement in PFS compared to sorafenib alone.[41] Erlotinib has also been studied in a phase III trial in biliary tract cancer where it did not show any benefit when added to gemcitabine and platinum based chemotherapy.[42] The addition of erlotinib to bevacizumab as maintenance treatment after first-line chemotherapy in metastatic colorectal cancer did not improve PFS significantly.[43] In a phase II trial, combination of FOLFOX, bevacizumab and erlotinib could not be tolerated as a first line agent in previously untreated metastatic colorectal cancer due to toxicity profile resulting in high withdrawal rates.[44]
Sorafenib (Nexavar®)
Sorafenib, a small molecule multi kinase inhibitor, is approved for unresectable primary HCC. In a phase III, double-blind, placebo controlled trial, 297 and 302 evaluable patients received sorafenib and placebo respectively. Median OS in sorafenib group was 10.7 mo versus 7.9 mo in placebo group (HR: 0.69; 95% CI: 0.55-0.87, P < 0.001). Though no difference in symptomatic progression between two groups, median time to radiologic progression was 5.5 mo in sorafenib group versus 2.8 mo in placebo group (P < 0.001).[45] In a phase II trial of advanced or metastatic gastric and gastroesophageal junction adenocarcinoma, sorafenib with docetaxel + cisplatin showed 41% partial response (95% CI: 28-54). Median PFS was 5.8 mo (90% CI: 5.4-7.4) and median OS was 13.6 mo (90% CI: 8.6-16.1).[46] Addition of sorafenib to gemcitabine for advanced pancreatic cancer has not shown any added benefit in a phase III trial.[47] Sorafenib sensitizes the colon cancer cells to radiation induced cytotoxicity in xenograft models.[48,49,50] and helps to overcome irinotecan resistance by inhibiting drug efflux pump.[51]
Imatinib (Gleevec®)
Imatinib, a multi kinase inhibitor, is approved for unresectable/metastatic gastrointestinal stromal tumor (GIST). In two phase III trials, comparing 400 mg daily dose (conventional dose) versus 400 mg twice a day dose (higher dose), objective response rate (45% in both groups) and median OS (55 mo [CI: 47-62] in conventional dose vs. 51 mo [CI: 46-60] in higher dose, P = 0.83) were similar in both groups. However, the results of PFS were conflicting in both trials. Dose reduction and treatment interruption were more frequent with twice a day regimen.[52,53] When different c-KIT (stem cell factor receptor) mutations and treatment response were analyzed in these patients, exon-9 c-KIT mutation was the strongest adverse prognostic factor for response to imatinib and higher dose regimen resulted in significant superior median PFS (P = 0.0013).[54] Possibly, indefinite treatment in imatinib responders may be required in GIST as its discontinuation after successful use for 3 years resulted in rapid progression in a phase III, open label trial (2-year PFS was 80% [95% CI: 58-91] in continuation arm vs. 16% [95% CI: 5-33] in the interruption group, P < 0·0001).[55]
Sunitinib (Sutent®)
Sunitinib, a multikinase inhibitor, is approved for GIST after imatinib failure. In a randomized, double blind, placebo controlled, phase III study, 228 patients received 6 week cycles of sunitinib (50 mg daily for 4 weeks followed by 2 weeks break) and 114 patients received placebo.[56] Blinding was terminated after the interim analysis showed significantly prolonged survival in sunitinib arm and respectively 63% in sunitinib and 87% in placebo arm, received open label sunitinib afterward. Median follow-up of 41.7 mo (95% CI: 40.3-43.8), median OS for sunitinib and placebo arms were respectively 72.7 weeks (95% CI: 61.3-83.0) and 64.9 weeks (95% CI: 45.7-96.0) when calculated for the entire study period (blind and open label phase). Sunitinib nearly doubled median OS and halved the hazard of death compare to placebo (HR: 0.505; 95% CI: 0.262-1.134, P = 0.306). Disease progression was 3 fold greater in placebo arm (HR: 0.339; 95% CI: 0.244-0.472, P ≤ 0.001). The median TTP among all patients in the final intent to treat population was 26.6 weeks (95% CI: 16.0-32.1) in sunitinib and 6.4 weeks (95% CI: 4.4-10.0) in placebo arm, respectively. In a maintenance therapy in metastatic pancreatic adenocarcinoma, PFS at 6 mo was 3.6% (95% CI: 0-10.6) and 22.2% (95% CI: 6.2-38.2; P < 0.01); 2 year OS was 7.1% (95% CI: 0-16.8) and 22.9% (95% CI: 5.8-40.0%; P = 0.11) and stable disease was 21.4% and 51.9% (P = 0.02) in placebo and sunitinib groups, respectively.[57] Addition of sunitinib to FOLFIRI or as a single agent in metastatic colorectal cancer refractory to standard chemotherapy did not offer any advantage.[58,59] A phase III, open-label trial (SUN 1170) comparing sunitinib with sorafenib in patients with advanced HCC was discontinued due to increased sunitinib related serious adverse events and the improbability of achieving noninferior or superior efficacy.
Regorafenib (Stivarga®)
Regorafenib is a small molecule multikinase inhibitor. It is approved for patients with metastatic colorectal cancer who have been previously treated with fluoropyrimidine-, oxaliplatin-and irinotecan-based chemotherapy, an anti-VEGF therapy and if KRAS wild type, an anti-EGFR therapy based on the results of a placebo controlled, randomized, phase III trial (CORRECT trial).[60] In this study, median OS was 6·4 mo in regorafenib group (n = 500) versus 5·0 mo in placebo group (n = 253) (HR: 0·77, 95% CI: 0·64-0·94, one-sided P = 0·0052). Regorafenib is also approved for patients with locally advanced, unresectable or metastatic GIST who have been previously treated with imatinib and sunitinib. Efficacy was confirmed in a phase III trial in which 199 patients were randomly assigned to best supportive care with either regorafenib (160 mg daily for 3 of every 4 weeks) or placebo. Median PFS was 4·8 mo (range 1·4-9·2) for regorafenib and 0·9 mo (range 0·9-1·8) for placebo (HR: 0·27, 95% CI: 0·19-0·39, P < 0·0001).[61] In a phase II trial of regorafenib in HCC after failure of first line sorafenib, 36 patients were included. Median TTP and OS were 4.3 mo and 13.8 mo respectively, but 89% patients had treatment interruption due to adverse drug reaction.[62]
Gefitinib (Iressa®)
Gefitinib, an EGFR tyrosine kinase inhibitor, when studied in phase II trials in metastatic or recurrent esophageal/gastroesophageal junction adenocarcinoma and squamous cell cancers, has been well tolerated. Gefitinib increases the radiosensitivity of gastric cancer cell lines.[63] In pancreatic cell lines, gefitinib has shown to reverse multidrug resistance via RAF1/ERK signaling pathway.[64] Moreover, gefitinib has shown antiproliferative effect on pancreatic cancer cell lines as well as on cholangiocarcinoma cells and gallbladder carcinoma when combined with gemcitabine. In a phase II trial of gefitinib combined with gemcitabine, 6 mo PFS was 30%. Median PFS and OS were 4.1 and 7.3 mo, respectively.[65] In human and murine HCC cells, gefitinib induced cell cycle arrest, apoptosis and cell growth inhibition.[66,67] In phase II trials, FOLFOX4 + gefitinib showed median OS of 12 mo and median event free survival of 5.4 mo in previously treated metastatic colorectal cancer,[68] as well as median OS of 20.5 mo and median TTP of 9.3 mo in previously untreated metastatic cases.[69] These results discourage the addition of gefitinib to FOLFOX-4 in metastatic colorectal cancer. In chemotherapy naïve metastatic colorectal cancer, addition of gefitinib to capecitabine + oxaliplatin showed 80% disease control rate, median TTP was 7.3 mo (95% CI: 4.76-9.2) and median OS was 21.9 mo (95% CI: 15.1-not reached).[70] EGFR phosphorylation status in colon cancer is predictive of response to gefitinib, even synergistic when used with platinum based chemotherapy.[71] Expression of p21 gene in combination with p53 gene mutation is a predictor of resistance to combination of chemotherapy + gefitinib.[72] The combination of gefitinib + cetuximab has synergistic effect on cell proliferation and apoptosis when studied in colon cancer cell lines.[73]
Cediranib (AZD 2171)
Cediranib, a vascular EGFR tyrosine kinase inhibitor, inhibits tumor cell migration and invasion, with no effects on cell proliferation when studied in colorectal, pancreatic and HCC cell lines.[74] Cediranib, when studied in advanced HCC, median OS of 5.8 mo (95% CI: 3.4-7.3) and median TTP of 2.8 mo (95% CI: 2.3-4.4) were observed. Grade ≥ 3 adverse events were observed in 93%.[75] In a phase III, randomized, double blind trial (HORIZON II), previously untreated metastatic colorectal cancer patients received either cediranib (n = 502) or placebo (n = 358) in addition to FOLFOX or capecitabine + oxaliplatin. The addition of cediranib to FOLFOX or capecitabine + oxaliplatin resulted in median PFS prolongation from 8.3 mo in placebo arm to 8.6 mo in cediranib arm (HR: 0.84, 95% CI: 0.73-0.98, P = 0.0121) but had no impact on OS (median OS, 19.7 mo for cediranib vs. 18.9 mo for placebo, HR: 0.94; 95% CI: 0.79-1.12, P = 0.5707). There were no significant differences in objective response rate, duration of response, or liver resection rate.[76] In a phase II, randomized trial of cediranib in metastatic colorectal cancer, 20 mg daily dose reached primary objective, median PFS increased from 8.3 mo to 10.2 mo (HR: 0.70, 95% CI: 0.44-1.11, P = 0.167).[77] Cediranib was comparable to bevacizumab when combined with modified FOLFOX6 regimen (HORIZON III trial).[78]
Lapatinib (Tykerb®)
Lapatinib, a dual tyrosine kinase inhibitor associated with HER2/neu and EGFR receptor. The combination of lapatinib with cisplatin or 5-FU or trastuzumab synergistically inhibits cell proliferation and exhibits an enhanced pro-apoptotic effect on esophageal cancer cells. Lapatinib has not proved to be beneficial in HCC, pancreatic, biliary or refractory colorectal cancers when studied in small preclinical or phase II studies.
Vandetanib (Caprelsa®)
Vandetanib inhibits tyrosine kinase activity of EGFR, vascular EGFR families, RET, BRK and TIE2. In a preclinical trial, vandetanib synergistically enhanced the sunitinib-associated inhibition of gastric cancer cell growth.[79] Preclinical trial has shown beneficial effect of vandetanib in liver and early intestinal cancer in mice. In several phase I trials, vandetanib is being studied with different chemotherapy combination in advanced colorectal cancer.
Linifanib (ABT 869)
Linifanib is a selective inhibitor of vascular EGFR and platelet-derived growth factor receptor (GFR) tyrosine kinases. In a phase II trial of unresectable or metastatic HCC, linifanib showed median time to disease progression of 3.7 mo and median OS of 9.7 mo and thus raised hopes for another effective targeted agent in HCC beyond sorafenib.[80]
Vatalanib (PTK787)
Vatalanib is an oral antiangiogenic agent that acts as a vascular EGFR inhibitor. In a phase III, randomized, placebo controlled trial of previously untreated metastatic colorectal cancer, patients were randomly assigned to FOLFOX4 + vatalanib (evaluable n = 581) or FOLFOX4 + placebo (evaluable n = 575) arm.[81] Median PFS in vatalanib arm was 7.7 mo versus 7.6 mo in placebo arm (HR: 0.88, 95% CI: 0.74-1.03, P = 0.118); median OS in vatalanib and placebo arms were respectively 21.4 mo and 20.5 mo (HR: 1.08, 95% CI: 0.94-1.24, P = 0.260). In post hoc analysis of PFS in patients (n = 158/arm) with high serum lactate dehydrogenase, a potential marker of hypoxia, PFS was longer with vatalanib versus placebo (7.7 vs. 5.8 mo, respectively; HR: 0.67, 95% CI: 0.49-0.91, P = 0.009). Similar results were observed in another phase III, randomized trial when FOLFOX4 + vatalanib or placebo combination was studied in previously treated metastatic colorectal cancer.[82] Subsequently, vascular density analysis was performed in biopsy specimens of 141 colorectal cancer patients (placebo arm [n = 70] and vatalanib [n = 71]) in above phase III trials. The vascular density correlated with response to therapy, PFS and OS. The response rate increased from 15% (3/20) to 50% (11/22) in tumors with high vascular density, when vatalanib was added to chemotherapy (P = 0.02).[83]
Brivanib (BMS 582664)
Brivanib is an inhibitor of vascular EGFR and fibroblast GFR. The results of large phase III trials are disappointing despite encouraging phase II trials, where brivanib was used as a first and second line agent in advanced HCC. In a phase III, multicenter, double blind, placebo controlled, randomized trial of advanced HCC (BRISK-PS), patients failed/intolerant to sorafenib, were randomly assigned to receive brivanib and best supportive care (n = 263) versus placebo and best supportive care (n = 132).[84] Median OS was 9.4 mo for brivanib and 8.2 mo for placebo (HR: 0.89, 95.8% CI: 0.69-1.15, P = 0.3307) and median TTP was 4.2 mo for brivanib and 2.7 mo for placebo (HR: 0.56, 95% CI: 0.42-0.76, P < 0.001). Moreover, brivanib related side-effect caused discontinuation in 23%. In another, phase III, randomized, double blind trial, brivanib (n = 577) and sorafenib (n = 578) were compared head to head in untreated advanced HCC (BRISK-FL). The primary end point of OS non-inferiority for brivanib versus sorafenib in the per-protocol population (n = 1150) did not meet (HR: 1.06, 95.8% CI: 0.93-1.22). Median OS was 9.9 mo for sorafenib and 9.5 mo for brivanib. TTP, objective response rate and disease control rate were similar between two arms.[85] The combination of cetuximab and brivanib has been studied in metastatic, chemotherapy refractory, wild type-KRAS colorectal cancer in a phase III, randomized, placebo controlled trial (AGITG CO.20). Median OS in the intent-to-treat population was 8.8 mo in brivanib arm (n = 376) and 8.1 mo in placebo arm (n = 374) (HR: 0.88; 95% CI: 0.74-1.03, P = 0.12). Median PFS was 5.0 mo in brivanib and 3.4 mo in placebo arm (HR: 0.72, 95% CI: 0.62-0.84; P < 0.001). Any grade ≥ 3 adverse events were 78% in brivanib and 53% in placebo arms.[86]
Nilotinib (Tasigna®)
Nilotinib, a second generation tyrosine kinase inhibitor, prevents autophosphorylation of c-KIT and platelet GFR. In a phase III, open label trial, nilotinib was studied in advanced GIST resistant to imatinib and sunitinib.[87] Patients (n = 248) were randomized to nilotinib or best supportive care with imatinib/sunitinib/placbo. Median PFS was similar in both arms (nilotinib 109 days vs. best supportive care 111 days; P = 0.56) based on central radiology review. However, intent-to-treat analysis favored nilotinib over best supportive care (119 days vs. 70 days, P = 0.0007). A nonsignificant but positive trend was noted for OS in nilotinib group. Post hoc subset analyses in patients with progression and only one prior regimen each of imatinib and sunitinib revealed a significant difference in median OS of > 4 months in favor of nilotinib (405 vs. 280 days; P = 0.02).
NON TYROSINE KINASE TARGET INHIBITORS
Everolimus (Afinitor®)
Everolimus is a mammalian target of rapamycin inhibitor. Everolimus causes dose dependent decrease in cell proliferation, cell cycle arrest in G1/S phase and damages cell shape.[88] Everolimus with capecitabine showed favorable toxicity profile and modest benefit (median PFS 1.8 months [95% CI: 0.8-2.8] in previously treated metastatic gastric cancer).[89] Everolimus monotherapy in previously chemotherapy treated advanced gastric cancer was studied in a phase II trial where no complete or partial response were observed. However, a decrease in tumor size from baseline was observed in 45%, disease control rate was 56.0% (95% CI: 41.3-70.0), median PFS was 2.7 mo (95% CI: 1.6-3.0) and median OS was 10.1 mo (95% CI: 6.5-12.1).[90] Everolimus augments the effects of sorafenib synergistically in orthotopic HCC model.[91] In a phase II trial of everolimus in advanced HCC with 0-2 previous regimens, median PFS and OS were 3.8 mo (95% CI: 2.1-4.6) and 8.4 mo (95% CI: 3.9-21.1), respectively. The estimated PFS rate at 24 weeks was 28.6% (95% CI: 7.9-49.3).[92] In a phase III, randomized trial, low-grade or intermediate-grade PNETs with radiologic progression within the previous 12 mo to receive everolimus (n = 207), or placebo (n = 203). Median PFS was 11.0 mo in everolimus versus 4.6 mo in placebo (HR: 0.35, 95% CI: 0.27-0.45, P < 0.001), representing a 65% reduction in the estimated risk of progression or death.[93] Everolimus and octreotide are effective independently in PNETs. However, this combination was recently studied in a phase II trial in treatment naïve 50 patients with well differentiated metastatic neuroendocrine tumors. Disease control rate was 92% and median TTP was 16.3 mo (95% CI: 10.7-20.1).[94] Everolimus is ineffective in metastatic colorectal cancer resistant to multiple chemotherapy agents.[95]
Selumetinib (AZD 6244)
Selumetinib inhibits mitogen-activated protein kinase (MEK or MAPK/ERK kinase) 1 and 2. Single agent (selumetinib or capecitabine) trial in colorectal cancer patients who previously failed one or two chemotherapeutic agent showed similar median PFS (81 days in selumetinib vs. 88 days in capecitabine arm).[96] In a phase II trial, erlotinib + selumetinib combination was studied in pancreatic adenocarcinoma (n = 46) treated with one prior chemotherapy. Estimated median PFS and OS by Kaplan-Meier were 2.6 and 7.5 mo, respectively and disease control rate was 51% in 41 evaluable patients.[97] Combination of selumetinib and vorinostat synergistically inhibits cell proliferation in two colorectal cancer cell lines with KRAS mutation.[98] Another phase II trial compared selumetinib or capecitabine as a single agent in advanced or metastatic pancreatic cancer patients (n = 70) who failed gemcitabine. Median OS was 5.4 mo in selumetinib arm and 5.0 mo in capecitabine arm (HR: 1.03, two-sided 80% CI: 0.68-1.57, P = 0.92).[99] In a phase II study of selumetinib in metastatic biliary cancer, median PFS and OS were 3.7 (95% CI: 3.5-4.9) and 9.8 (95% CI: 5.97-not available) mo, respectively.[100]
Trametinib (Mekinist®)
Trametinib is a reversible MEK 1 and 2 inhibitor. In a pre-clinical study of pancreatic cancer cell lines treated with trametinib, cancer cell proliferation was inhibited and addition of EGFR/HER2 inhibitor, lapatinib enhanced the inhibition elicited by trametinib in three of eight cell lines.[101] Trametinib when combined with 5-FU markedly decreased colony numbers of colon cancer cell lines.[102]
CLINICALLY USED BIOMARKERS FOR TARGETED THERAPY SELECTION IN GI CANCERS
In GI cancers, tumor receptor tyrosine kinase-targeted therapies (i.e., trastuzumab, imatinib) and antibodies (cetuximab, panitumumab) demonstrate a robust clinical response in patients that exhibit overexpression of their intended targets or certain genetic alterations. Trastuzumab is used only in gastric or gastroesophageal junction cancers that overexpress HER2. Imatinib is used for GIST expressing c-KIT and further studies of KIT mutation and platelet derived growth factor mutations have also been shown to correlate with response/resistance to imatinib. Although initially approved for all metastatic colon cancers, the label was changed to indicate cetuximab should only be used for the treatment of KRAS mutation-negative (wild-type), EGFR-expressing metastatic colorectal cancer (in combination with FOLFIRI as first-line treatment, in combination with irinotecan [in patients refractory to irinotecan-based chemotherapy], or as a single agent in patients who have failed oxaliplatin and irinotecan based chemotherapy or who are intolerant to irinotecan). Similarly, monotherapy with panitumumab in treatment of EGFR-expressing refractory metastatic colorectal cancer with disease progression on or following fluoropyrimidine-, oxaliplatin- and irinotecan-based regimens. Currently, no biomarkers for antiangiogenic therapies have been shown to correlate with survival.
Pharmacokinetic/pharmacodynamic markers: Erlotinib is cleared rapidly by smokers with 25-40% lower exposure and hence cautiously increasing the dose in active smokers is recommended in the package insert. Exposure to imatinib was assessed in a study of 73 patients who were randomly assigned to 400 or 600 mg of imatinib daily for advanced GIST, there was a 10-fold variance in trough levels with either dose (from 414 to 4182 ng/mL).[103] Clinical outcomes were correlated with trough levels at steady state. Trough values below 1100 ng/mL correlated strongly with a significantly shorter time to tumor progression and a lower rate of clinical benefit as compared to higher trough levels and authors have suggested lower exposure may contribute to drug resistance.
CONCLUSION
The “one size fits all’ approach for cancer therapy has long gone. With integration of optimum technology and better understanding of how cancers evolve at the molecular level, newer potential therapeutic targets are discovered faster than ever before. This understanding has helped us to embrace personalized approaches to treat cancer. Since the approval of bevacizumab in 2004 for colon cancer, there are now nine approved targeted drugs for treatment of GI cancers (five for colon, two for PNET, one for gastric/gastroesophageal, one for HCC, one for pancreas) and small incremental benefits have been seen in survival in each of these cancers. The era of personalized medicine is here, identifying validated assays and targets and doing studies in selected populations where the effect size is large as the population is preselected with patients who are most likely to benefit, has set a new standard for developing novel targeted agents in GI cancers. As their survival is generally poor and cost of drugs and toxicity is high, reducing sample size of trials and focusing on clinically meaningful rather than merely statistically significant benefit has become our driving principle for the future.
The results of ongoing and planned trials in colon cancer seek to expand the current approved indications for these agents, since the successful approval of bevacizumab as second line after failure of bevacizumab containing first line regimen. Notable phase 3 trials that are ongoing or planned include using ziv-aflibercept in the first line setting and adjuvant trials post metastatectomy that include regorafenib. Other studies plan to evaluate integration of targeted therapies with liver directed therapies, e.g. FOXFIRE global study evaluating incorporation of sirspheres with FOLFOX bevacizumab versus FOLFOX bevacizumab alone in advanced colon cancer and sorafenib with or without chemoembolization. The RADIANT-4 trial that seeks to re-examine the role of everolimus in carcinoids has completed accrual and is expected to be analyzed at the end of 2014. Given the success of trastuzumab in gastric cancer, the analysis of the LOGIC trial that has completed accrual and examines the role of adding lapatinib to standard chemotherapy in HER-2 positive gastric cancer is eagerly awaited. With the exception of erlotinib, all tyrosine kinase inhibitors approved for treatment of GI malignancies have been approved as single agents. More trials evaluating the benefits of targeted therapy in the adjuvant setting for GI cancers are needed, with better risk assessment to aid decision making as is used now for imatinib in the adjuvant setting for GIST tumors. The STORM study evaluating the value of adjuvant sorafenib 400 mg BID in delaying/preventing recurrence after radio frequency ablation or surgery for potentially curable HCC has completed accrual and results are awaited. Several ongoing trials of chemotherapy plus a tyrosine kinase inhibitor are ongoing or planned and despite many notable failures of this approach especially in pancreatic cancer, many investigators remain optimistic that the preclinical success with this approach may translate into clinical benefit. Biomarker driven approaches are ideal, but limited availability of tissue for such studies and lack of validated assays has been a major challenge. As a class of agents combined VEGF and FGFR inhibitors appear promising, but as yet have not received approval for any malignancy. In addition, many trials of vaccines and immune modulatory agents are planned or ongoing and integration with chemo and targeted therapies is also of great interest.
AUTHOR'S PROFILE
Dr. Ravi Chhatrala: Virginia Commonwealth University, Richmond, VA 23219, USA.
Dr. Yasmin Thanavala: Roswell Park Cancer Institute Buffalo, NY 14263, USA.
Dr. Renuka Iyer: Roswell Park Cancer Institute Buffalo, NY 14263, USA.
Footnotes
Source and Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1. [Last assessed on 2014 Jan 6]. Available from: http://www.cancer.gov/cancertopics/factsheet/Therapy/targeted .
- 2.Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–17. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 3.De Vita F, Orditura M, Innocente R, Pinto C, Chiarion Sileni V, Martinelli E, et al. Induction primary CT with Folfox-4 and cetuximab followed by RT and cetuximab in locally advanced esophageal cancer (LAEC): Analysis of preliminary data from B152 Trial. J Clin Oncol. 2008;26 Abstract: 15524. [Google Scholar]
- 4.Kanzler S, Trarbach T, Seufferlein T, Kubicka S, Lordick F, Geissler M, Daum S, et al. Cetuximab with irinotecan/folinic acid/5-FU as first-line treatment in advanced gastric cancer: A nonrandomized multicenter AIO phase II study. J Thorac Oncol. 2009;27:4534. [Google Scholar]
- 5.Philip PA, Benedetti J, Corless CL, Wong R, O’Reilly EM, Flynn PJ, et al. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205. J Clin Oncol. 2010;28:3605–10. doi: 10.1200/JCO.2009.25.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asnacios A, Fartoux L, Romano O, Tesmoingt C, Louafi SS, Mansoubakht T, et al. Gemcitabine plus oxaliplatin (GEMOX) combined with cetuximab in patients with progressive advanced stage hepatocellular carcinoma: Results of a multicenter phase 2 study. Cancer. 2008;112:2733–9. doi: 10.1002/cncr.23489. [DOI] [PubMed] [Google Scholar]
- 7.Malka D, Trarbach T, Fartoux L, Mendiboure J, de la Fouchardière C, Viret F, et al. A multicenter, randomized phase II trial of gemcitabine and oxaliplatin (GEMOX) alone or in combination with biweekly cetuximab in the first-line treatment of advanced biliary cancer: Interim analysis of the BINGO trial. J Clin Oncol. 2009;27 Abstract: 4520. [Google Scholar]
- 8.Van Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658–64. doi: 10.1200/JCO.2006.08.1620. [DOI] [PubMed] [Google Scholar]
- 9.Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–34. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 10.Poulin-Costello M, Azoulay L, Van Cutsem E, Peeters M, Siena S, Wolf M. An analysis of the treatment effect of panitumumab on overall survival from a phase 3, randomized, controlled, multicenter trial (20020408) in patients with chemotherapy refractory metastatic colorectal cancer. Target Oncol. 2013;8:127–36. doi: 10.1007/s11523-013-0271-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: The PRIME study. J Clin Oncol. 2010;28:4697–705. doi: 10.1200/JCO.2009.27.4860. [DOI] [PubMed] [Google Scholar]
- 12.Seymour MT, Brown SR, Middleton G, Maughan T, Richman S, Gwyther S, et al. Panitumumab and irinotecan versus irinotecan alone for patients with KRAS wild-type, fluorouracil-resistant advanced colorectal cancer (PICCOLO): A prospectively stratified randomised trial. Lancet Oncol. 2013;14:749–59. doi: 10.1016/S1470-2045(13)70163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peeters M, Price TJ, Cervantes A, Sobrero AF, Ducreux M, Hotko Y, et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol. 2010;28:4706–13. doi: 10.1200/JCO.2009.27.6055. [DOI] [PubMed] [Google Scholar]
- 14.Hecht JR, Mitchell E, Chidiac T, Scroggin C, Hagenstad C, Spigel D, et al. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol. 2009;27:672–80. doi: 10.1200/JCO.2008.19.8135. [DOI] [PubMed] [Google Scholar]
- 15.Yakes FM, Chinratanalab W, Ritter CA, King W, Seelig S, Arteaga CL. Herceptin-induced inhibition of phosphatidylinositol-3 kinase and Akt Is required for antibody-mediated effects on p27, cyclin D1, and antitumor action. Cancer Res. 2002;62:4132–41. [PubMed] [Google Scholar]
- 16.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–97. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 17.Harder J, Ihorst G, Heinemann V, Hofheinz R, Moehler M, Buechler P, et al. Multicentre phase II trial of trastuzumab and capecitabine in patients with HER2 overexpressing metastatic pancreatic cancer. Br J Cancer. 2012;106:1033–8. doi: 10.1038/bjc.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 19.Petrelli F, Borgonovo K, Cabiddu M, Ghilardi M, Lonati V, Maspero F, et al. FOLFIRI-bevacizumab as first-line chemotherapy in 3500 patients with advanced colorectal cancer: A pooled analysis of 29 published trials. Clin Colorectal Cancer. 2013;12:145–51. doi: 10.1016/j.clcc.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Allegra CJ, Yothers G, O’Connell MJ, Sharif S, Petrelli NJ, Colangelo LH, et al. Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: Results of NSABP protocol C-08. J Clin Oncol. 2011;29:11–6. doi: 10.1200/JCO.2010.30.0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmiegel W, Reinacher-Schick A, Arnold D, Kubicka S, Freier W, Dietrich G, et al. Capecitabine/irinotecan or capecitabine/oxaliplatin in combination with bevacizumab is effective and safe as first-line therapy for metastatic colorectal cancer: A randomized phase II study of the AIO colorectal study group. Ann Oncol. 2013;24:1580–7. doi: 10.1093/annonc/mdt028. [DOI] [PubMed] [Google Scholar]
- 22.Uronis HE, Bendell JC, Altomare I, Blobe GC, Hsu SD, Morse MA, et al. A phase II study of capecitabine, oxaliplatin, and bevacizumab in the treatment of metastatic esophagogastric adenocarcinomas. Oncologist. 2013;18:271–2. doi: 10.1634/theoncologist.2012-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah MA, Ramanathan RK, Ilson DH, Levnor A, D’Adamo D, O’Reilly E, et al. Multicenter phase II study of irinotecan, cisplatin, and bevacizumab in patients with metastatic gastric or gastroesophageal junction adenocarcinoma. J Clin Oncol. 2006;24:5201–6. doi: 10.1200/JCO.2006.08.0887. [DOI] [PubMed] [Google Scholar]
- 24.Fang P, Hu JH, Cheng ZG, Liu ZF, Wang JL, Jiao SC. Efficacy and safety of bevacizumab for the treatment of advanced hepatocellular carcinoma: A systematic review of phase II trials. PLoS One. 2012;7:e49717. doi: 10.1371/journal.pone.0049717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hapani S, Chu D, Wu S. Risk of gastrointestinal perforation in patients with cancer treated with bevacizumab: A meta-analysis. Lancet Oncol. 2009;10:559–68. doi: 10.1016/S1470-2045(09)70112-3. [DOI] [PubMed] [Google Scholar]
- 26.Van Cutsem E, Tabernero J, Lakomy R, Prenen H, Prausová J, Macarulla T, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012;30:3499–506. doi: 10.1200/JCO.2012.42.8201. [DOI] [PubMed] [Google Scholar]
- 27.Rougier P, Riess H, Manges R, Karasek P, Humblet Y, Barone C, et al. Randomised, placebo-controlled, double-blind, parallel-group phase III study evaluating aflibercept in patients receiving first-line treatment with gemcitabine for metastatic pancreatic cancer. Eur J Cancer. 2013;49:2633–42. doi: 10.1016/j.ejca.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Ma NY, Cai XW, Fu XL, Li Y, Zhou XY, Wu XH, et al. Safety and efficacy of nimotuzumab in combination with radiotherapy for patients with squamous cell carcinoma of the esophagus. Int J Clin Oncol. 2013 doi: 10.1007/s10147-013-0564-3. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 29.Strumberg D, Schultheis B, Scheulen ME, Hilger RA, Krauss J, Marschner N, et al. Phase II study of nimotuzumab, a humanized monoclonal anti-epidermal growth factor receptor (EGFR) antibody, in patients with locally advanced or metastatic pancreatic cancer. Invest New Drugs. 2012;30:1138–43. doi: 10.1007/s10637-010-9619-8. [DOI] [PubMed] [Google Scholar]
- 30.Gold DV, Goggins M, Modrak DE, Newsome G, Liu M, Shi C, et al. Detection of early-stage pancreatic adenocarcinoma. Cancer Epidemiol Biomarkers Prev. 2010;19:2786–94. doi: 10.1158/1055-9965.EPI-10-0667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ocean AJ, Pennington KL, Guarino MJ, Sheikh A, Bekaii-Saab T, Serafini AN, et al. Fractionated radioimmunotherapy with (90) Y-clivatuzumab tetraxetan and low-dose gemcitabine is active in advanced pancreatic cancer: A phase 1 trial. Cancer. 2012;118:5497–506. doi: 10.1002/cncr.27592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohn AL, Tabernero J, Maurel J, Nowara E, Sastre J, Chuah BY, et al. A randomized, placebo-controlled phase 2 study of ganitumab or conatumumab in combination with FOLFIRI for second-line treatment of mutant KRAS metastatic colorectal cancer. Ann Oncol. 2013;24:1777–85. doi: 10.1093/annonc/mdt057. [DOI] [PubMed] [Google Scholar]
- 33.Kindler HL, Richards DA, Garbo LE, Garon EB, Stephenson JJ, Jr, Rocha-Lima CM, et al. A randomized, placebo-controlled phase 2 study of ganitumab (AMG 479) or conatumumab (AMG 655) in combination with gemcitabine in patients with metastatic pancreatic cancer. Ann Oncol. 2012;23:2834–42. doi: 10.1093/annonc/mds142. [DOI] [PubMed] [Google Scholar]
- 34.Strosberg JR, Chan JA, Ryan DP, Meyerhardt JA, Fuchs CS, Abrams T, et al. A multi-institutional, phase II open-label study of ganitumab (AMG 479) in advanced carcinoid and pancreatic neuroendocrine tumors. Endocr Relat Cancer. 2013;20:383–90. doi: 10.1530/ERC-12-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–6. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 36.Iyer R, Chhatrala R, Shefter T, Yang G, Malhotra U, Tan W, et al. Erlotinib and radiation therapy for elderly patients with esophageal cancer-clinical and correlative results from a prospective multicenter phase 2 trial. Oncology. 2013;85:53–8. doi: 10.1159/000351617. [DOI] [PubMed] [Google Scholar]
- 37.Bendell JC, Meluch A, Peyton J, Rubin M, Waterhouse D, Webb C, et al. A phase II trial of preoperative concurrent chemotherapy/radiation therapy plus bevacizumab/erlotinib in the treatment of localized esophageal cancer. Clin Adv Hematol Oncol. 2012;10:430–7. [PubMed] [Google Scholar]
- 38.Ilson DH, Kelsen D, Shah M, Schwartz G, Levine DA, Boyd J, et al. A phase 2 trial of erlotinib in patients with previously treated squamous cell and adenocarcinoma of the esophagus. Cancer. 2011;117:1409–14. doi: 10.1002/cncr.25602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dragovich T, McCoy S, Fenoglio-Preiser CM, Wang J, Benedetti JK, Baker AF, et al. Phase II trial of erlotinib in gastroesophageal junction and gastric adenocarcinomas: SWOG 0127. J Clin Oncol. 2006;24:4922–7. doi: 10.1200/JCO.2006.07.1316. [DOI] [PubMed] [Google Scholar]
- 40.Mok TS, Paz-Ares L, Wu YL, Novello S, Juhasz E, Aren O, et al. Breast cancer, early stage. Ann Oncol. 2012;23(Suppl 9):ixe1–ixe30. [Google Scholar]
- 41.Govindarajan R, Siegel E, Makhoul I, Williamson S. Bevacizumab and erlotinib in previously untreated inoperable and metastatic hepatocellular carcinoma. Am J Clin Oncol. 2013;36:254–7. doi: 10.1097/COC.0b013e318248d83f. [DOI] [PubMed] [Google Scholar]
- 42.Lee J, Park SH, Chang HM, Kim JS, Choi HJ, Lee MA, et al. Gemcitabine and oxaliplatin with or without erlotinib in advanced biliary-tract cancer: A multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2012;13:181–8. doi: 10.1016/S1470-2045(11)70301-1. [DOI] [PubMed] [Google Scholar]
- 43.Johnsson A, Hagman H, Frödin JE, Berglund A, Keldsen N, Fernebro E, et al. A randomized phase III trial on maintenance treatment with bevacizumab alone or in combination with erlotinib after chemotherapy and bevacizumab in metastatic colorectal cancer: The Nordic ACT Trial. Ann Oncol. 2013;24:2335–41. doi: 10.1093/annonc/mdt236. [DOI] [PubMed] [Google Scholar]
- 44.Meyerhardt JA, Stuart K, Fuchs CS, Zhu AX, Earle CC, Bhargava P, et al. Phase II study of FOLFOX, bevacizumab and erlotinib as first-line therapy for patients with metastatic colorectal cancer. Ann Oncol. 2007;18:1185–9. doi: 10.1093/annonc/mdm124. [DOI] [PubMed] [Google Scholar]
- 45.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 46.Sun W, Powell M, O’Dwyer PJ, Catalano P, Ansari RH, Benson AB., 3rd Phase II study of sorafenib in combination with docetaxel and cisplatin in the treatment of metastatic or advanced gastric and gastroesophageal junction adenocarcinoma: ECOG 5203. J Clin Oncol. 2010;28:2947–51. doi: 10.1200/JCO.2009.27.7988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gonçalves A, Gilabert M, François E, Dahan L, Perrier H, Lamy R, et al. BAYPAN study: A double-blind phase III randomized trial comparing gemcitabine plus sorafenib and gemcitabine plus placebo in patients with advanced pancreatic cancer. Ann Oncol. 2012;23:2799–805. doi: 10.1093/annonc/mds135. [DOI] [PubMed] [Google Scholar]
- 48.Kim YB, Jeung HC, Jeong I, Lee K, Rha SY, Chung HC, et al. Mechanism of enhancement of radiation-induced cytotoxicity by sorafenib in colorectal cancer. J Radiat Res. 2013;54:52–60. doi: 10.1093/jrr/rrs074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jeong YK, Kim MS, Lee JY, Kim EH, Kim W, Ha H, et al. Sorafenib acts synergistically in combination with radiotherapy without causing intestinal damage in colorectal cancer. Tumori. 2013;99:176–82. doi: 10.1177/030089161309900210. [DOI] [PubMed] [Google Scholar]
- 50.Kuo YC, Lin WC, Chiang IT, Chang YF, Chen CW, Su SH, et al. Sorafenib sensitizes human colorectal carcinoma to radiation via suppression of NF-κB expression in vitro and in vivo. Biomed Pharmacother. 2012;66:12–20. doi: 10.1016/j.biopha.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 51.Mazard T, Causse A, Simony J, Leconet W, Vezzio-Vie N, Torro A, et al. Sorafenib overcomes irinotecan resistance in colorectal cancer by inhibiting the ABCG2 drug-efflux pump. Mol Cancer Ther. 2013;12:2121–34. doi: 10.1158/1535-7163.MCT-12-0966. [DOI] [PubMed] [Google Scholar]
- 52.Blanke CD, Rankin C, Demetri GD, Ryan CW, von Mehren M, Benjamin RS, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008;26:626–32. doi: 10.1200/JCO.2007.13.4452. [DOI] [PubMed] [Google Scholar]
- 53.Verweij J, Casali PG, Zalcberg J, LeCesne A, Reichardt P, Blay JY, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: Randomised trial. Lancet. 2004;364:1127–34. doi: 10.1016/S0140-6736(04)17098-0. [DOI] [PubMed] [Google Scholar]
- 54.Debiec-Rychter M, Sciot R, Le Cesne A, Schlemmer M, Hohenberger P, van Oosterom AT, et al. KIT mutations and dose selection for imatinib in patients with advanced gastrointestinal stromal tumours. Eur J Cancer. 2006;42:1093–103. doi: 10.1016/j.ejca.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 55.Le Cesne A, Ray-Coquard I, Bui BN, Adenis A, Rios M, Bertucci F, et al. Discontinuation of imatinib in patients with advanced gastrointestinal stromal tumours after 3 years of treatment: An open-label multicentre randomised phase 3 trial. Lancet Oncol. 2010;11:942–9. doi: 10.1016/S1470-2045(10)70222-9. [DOI] [PubMed] [Google Scholar]
- 56.Demetri GD, Garrett CR, Schöffski P, Shah MH, Verweij J, Leyvraz S, et al. Complete longitudinal analyses of the randomized, placebo-controlled, phase III trial of sunitinib in patients with gastrointestinal stromal tumor following imatinib failure. Clin Cancer Res. 2012;18:3170–9. doi: 10.1158/1078-0432.CCR-11-3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reni M, Cereda S, Milella M, Novarino A, Passardi A, Mambrini A, et al. Maintenance sunitinib or observation in metastatic pancreatic adenocarcinoma: A phase II randomised trial. Eur J Cancer. 2013;49:3609–15. doi: 10.1016/j.ejca.2013.06.041. [DOI] [PubMed] [Google Scholar]
- 58.Carrato A, Swieboda-Sadlej A, Staszewska-Skurczynska M, Lim R, Roman L, Shparyk Y, et al. Fluorouracil, leucovorin, and irinotecan plus either sunitinib or placebo in metastatic colorectal cancer: A randomized, phase III trial. J Clin Oncol. 2013;31:1341–7. doi: 10.1200/JCO.2012.45.1930. [DOI] [PubMed] [Google Scholar]
- 59.Saltz LB, Rosen LS, Marshall JL, Belt RJ, Hurwitz HI, Eckhardt SG, et al. Phase II trial of sunitinib in patients with metastatic colorectal cancer after failure of standard therapy. J Clin Oncol. 2007;25:4793–9. doi: 10.1200/JCO.2007.12.8637. [DOI] [PubMed] [Google Scholar]
- 60.Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–12. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 61.Demetri GD, Reichardt P, Kang YK, Blay JY, Rutkowski P, Gelderblom H, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:295–302. doi: 10.1016/S0140-6736(12)61857-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bruix J, Tak WY, Gasbarrini A, Santoro A, Colombo M, Lim HY, et al. Regorafenib as second-line therapy for intermediate or advanced hepatocellular carcinoma: Multicentre, open-label, phase II safety study. Eur J Cancer. 2013;49:3412–9. doi: 10.1016/j.ejca.2013.05.028. [DOI] [PubMed] [Google Scholar]
- 63.Cao WG, Ma T, Li JF, Li H, Ji YB, Chen XH, et al. Effect of gefitinib on radiosensitivity of gastric cancer cell lines. Ai Zheng. 2007;26:1330–5. [PubMed] [Google Scholar]
- 64.Xiao Z, Ding N, Xiao G, Wang S, Wu Y, Tang L. Reversal of multidrug resistance by gefitinib via RAF1/ERK pathway in pancreatic cancer cell line. Anat Rec (Hoboken) 2012;295:2122–8. doi: 10.1002/ar.22552. [DOI] [PubMed] [Google Scholar]
- 65.Fountzilas G, Bobos M, Kalogera-Fountzila A, Xiros N, Murray S, Linardou H, et al. Gemcitabine combined with gefitinib in patients with inoperable or metastatic pancreatic cancer: A phase II Study of the Hellenic Cooperative Oncology Group with biomarker evaluation. Cancer Invest. 2008;26:784–93. doi: 10.1080/07357900801918611. [DOI] [PubMed] [Google Scholar]
- 66.Höpfner M, Sutter AP, Huether A, Schuppan D, Zeitz M, Scherübl H. Targeting the epidermal growth factor receptor by gefitinib for treatment of hepatocellular carcinoma. J Hepatol. 2004;41:1008–16. doi: 10.1016/j.jhep.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 67.Zhu BD, Yuan SJ, Zhao QC, Li X, Li Y, Lu QY. Antitumor effect of Gefitinib, an epidermal growth factor receptor tyrosine kinase inhibitor, combined with cytotoxic agent on murine hepatocellular carcinoma. World J Gastroenterol. 2005;11:1382–6. doi: 10.3748/wjg.v11.i9.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kuo T, Cho CD, Halsey J, Wakelee HA, Advani RH, Ford JM, et al. Phase II study of gefitinib, fluorouracil, leucovorin, and oxaliplatin therapy in previously treated patients with metastatic colorectal cancer. J Clin Oncol. 2005;23:5613–9. doi: 10.1200/JCO.2005.08.359. [DOI] [PubMed] [Google Scholar]
- 69.Fisher GA, Kuo T, Ramsey M, Schwartz E, Rouse RV, Cho CD, et al. A phase II study of gefitinib, 5-fluorouracil, leucovorin, and oxaliplatin in previously untreated patients with metastatic colorectal cancer. Clin Cancer Res. 2008;14:7074–9. doi: 10.1158/1078-0432.CCR-08-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gelibter AJ, Gamucci T, Pollera CF, Di Costanzo F, Nuzzo C, Gabriele A, et al. A phase II trial of gefitinib in combination with capecitabine and oxaliplatin as first-line chemotherapy in patients with advanced colorectal cancer. Curr Med Res Opin. 2007;23:2117–23. doi: 10.1185/030079907X226113. [DOI] [PubMed] [Google Scholar]
- 71.Van Schaeybroeck S, Karaiskou-McCaul A, Kelly D, Longley D, Galligan L, Van Cutsem E, et al. Epidermal growth factor receptor activity determines response of colorectal cancer cells to gefitinib alone and in combination with chemotherapy. Clin Cancer Res. 2005;11:7480–9. doi: 10.1158/1078-0432.CCR-05-0328. [DOI] [PubMed] [Google Scholar]
- 72.Ogino S, Meyerhardt JA, Cantor M, Brahmandam M, Clark JW, Namgyal C, et al. Molecular alterations in tumors and response to combination chemotherapy with gefitinib for advanced colorectal cancer. Clin Cancer Res. 2005;11:6650–6. doi: 10.1158/1078-0432.CCR-05-0738. [DOI] [PubMed] [Google Scholar]
- 73.Yuan HH, Han Y, Bian WX, Liu L, Bai YX. The effect of monoclonal antibody cetuximab (C225) in combination with tyrosine kinase inhibitor gefitinib (ZD1839) on colon cancer cell lines. Pathology. 2012;44:547–51. doi: 10.1097/PAT.0b013e32835817a2. [DOI] [PubMed] [Google Scholar]
- 74.Morelli MP, Brown AM, Pitts TM, Tentler JJ, Ciardiello F, Ryan A, et al. Targeting vascular endothelial growth factor receptor-1 and -3 with cediranib (AZD2171): Effects on migration and invasion of gastrointestinal cancer cell lines. Mol Cancer Ther. 2009;8:2546–58. doi: 10.1158/1535-7163.MCT-09-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alberts SR, Fitch TR, Kim GP, Morlan BW, Dakhil SR, Gross HM, et al. Cediranib (AZD2171) in patients with advanced hepatocellular carcinoma: A phase II North Central Cancer Treatment Group Clinical Trial. Am J Clin Oncol. 2012;35:329–33. doi: 10.1097/COC.0b013e3182118cdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hoff PM, Hochhaus A, Pestalozzi BC, Tebbutt NC, Li J, Kim TW, et al. Cediranib plus FOLFOX/CAPOX versus placebo plus FOLFOX/CAPOX in patients with previously untreated metastatic colorectal cancer: A randomized, double-blind, phase III study (HORIZON II) J Clin Oncol. 2012;30:3596–603. doi: 10.1200/JCO.2012.42.6031. [DOI] [PubMed] [Google Scholar]
- 77.Kato T, Muro K, Yamaguchi K, Bando H, Hazama S, Amagai K, et al. Cediranib in combination with mFOLFOX6 in Japanese patients with metastatic colorectal cancer: Results from the randomised phase II part of a phase I/II study. Ann Oncol. 2012;23:933–41. doi: 10.1093/annonc/mdr359. [DOI] [PubMed] [Google Scholar]
- 78.Schmoll HJ, Cunningham D, Sobrero A, Karapetis CS, Rougier P, Koski SL, et al. Cediranib with mFOLFOX6 versus bevacizumab with mFOLFOX6 as first-line treatment for patients with advanced colorectal cancer: A double-blind, randomized phase III study (HORIZON III) J Clin Oncol. 2012;30:3588–95. doi: 10.1200/JCO.2012.42.5355. [DOI] [PubMed] [Google Scholar]
- 79.Lyros O, Mueller A, Heidel F, Schimanski CC, Gockel I, Galle PR, et al. Analysis of anti-proliferative and chemosensitizing effects of sunitinib on human esophagogastric cancer cells: Synergistic interaction with vandetanib via inhibition of multi-receptor tyrosine kinase pathways. Int J Cancer. 2010;127:1197–208. doi: 10.1002/ijc.25137. [DOI] [PubMed] [Google Scholar]
- 80.Toh HC, Chen PJ, Carr BI, Knox JJ, Gill S, Ansell P, et al. Phase 2 trial of linifanib (ABT-869) in patients with unresectable or metastatic hepatocellular carcinoma. Cancer. 2013;119:380–7. doi: 10.1002/cncr.27758. [DOI] [PubMed] [Google Scholar]
- 81.Hecht JR, Trarbach T, Hainsworth JD, Major P, Jäger E, Wolff RA, et al. Randomized, placebo-controlled, phase III study of first-line oxaliplatin-based chemotherapy plus PTK787/ZK 222584, an oral vascular endothelial growth factor receptor inhibitor, in patients with metastatic colorectal adenocarcinoma. J Clin Oncol. 2011;29:1997–2003. doi: 10.1200/JCO.2010.29.4496. [DOI] [PubMed] [Google Scholar]
- 82.Van Cutsem E, Bajetta E, Valle J, Köhne CH, Hecht JR, Moore M, et al. Randomized, placebo-controlled, phase III study of oxaliplatin, fluorouracil, and leucovorin with or without PTK787/ZK 222584 in patients with previously treated metastatic colorectal adenocarcinoma. J Clin Oncol. 2011;29:2004–10. doi: 10.1200/JCO.2010.29.5436. [DOI] [PubMed] [Google Scholar]
- 83.Giatromanolaki A, Koukourakis MI, Sivridis E, Gatter KC, Trarbach T, Folprecht G, et al. Vascular density analysis in colorectal cancer patients treated with vatalanib (PTK787/ZK222584) in the randomised CONFIRM trials. Br J Cancer. 2012;107:1044–50. doi: 10.1038/bjc.2012.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Llovet JM, Decaens T, Raoul JL, Boucher E, Kudo M, Chang C, et al. Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: Results from the randomized phase III BRISK-PS study. J Clin Oncol. 2013;31:3509–16. doi: 10.1200/JCO.2012.47.3009. [DOI] [PubMed] [Google Scholar]
- 85.Johnson PJ, Qin S, Park JW, Poon RT, Raoul JL, Philip PA, et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: Results from the randomized phase III BRISK-FL study. J Clin Oncol. 2013;31:3517–24. doi: 10.1200/JCO.2012.48.4410. [DOI] [PubMed] [Google Scholar]
- 86.Siu LL, Shapiro JD, Jonker DJ, Karapetis CS, Zalcberg JR, Simes J, et al. Phase III randomized, placebo-controlled study of cetuximab plus brivanib alaninate versus cetuximab plus placebo in patients with metastatic, chemotherapy-refractory, wild-type K-RAS colorectal carcinoma: The NCIC Clinical Trials Group and AGITG CO. 20 Trial. J Clin Oncol. 2013;31:2477–84. doi: 10.1200/JCO.2012.46.0543. [DOI] [PubMed] [Google Scholar]
- 87.Reichardt P, Blay JY, Gelderblom H, Schlemmer M, Demetri GD, Bui-Nguyen B, et al. Phase III study of nilotinib versus best supportive care with or without a TKI in patients with gastrointestinal stromal tumors resistant to or intolerant of imatinib and sunitinib. Ann Oncol. 2012;23:1680–7. doi: 10.1093/annonc/mdr598. [DOI] [PubMed] [Google Scholar]
- 88.Wang ZG, Fukazawa T, Nishikawa T, Watanabe N, Sakurama K, Motoki T, et al. RAD001 offers a therapeutic intervention through inhibition of mTOR as a potential strategy for esophageal cancer. Oncol Rep. 2010;23:1167–72. [PubMed] [Google Scholar]
- 89.Lim T, Lee J, Lee DJ, Lee HY, Han B, Baek KK, et al. Phase I trial of capecitabine plus everolimus (RAD001) in patients with previously treated metastatic gastric cancer. Cancer Chemother Pharmacol. 2011;68:255–62. doi: 10.1007/s00280-011-1653-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Doi T, Muro K, Boku N, Yamada Y, Nishina T, Takiuchi H, et al. Multicenter phase II study of everolimus in patients with previously treated metastatic gastric cancer. J Clin Oncol. 2010;28:1904–10. doi: 10.1200/JCO.2009.26.2923. [DOI] [PubMed] [Google Scholar]
- 91.Piguet AC, Saar B, Hlushchuk R, St-Pierre MV, McSheehy PM, Radojevic V, et al. Everolimus augments the effects of sorafenib in a syngeneic orthotopic model of hepatocellular carcinoma. Mol Cancer Ther. 2011;10:1007–17. doi: 10.1158/1535-7163.MCT-10-0666. [DOI] [PubMed] [Google Scholar]
- 92.Zhu AX, Abrams TA, Miksad R, Blaszkowsky LS, Meyerhardt JA, Zheng H, et al. Phase 1/2 study of everolimus in advanced hepatocellular carcinoma. Cancer. 2011;117:5094–102. doi: 10.1002/cncr.26165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–23. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bajetta E, Catena L, Fazio N, Pusceddu S, Biondani P, Blanco G, et al. Everolimus in combination with octreotide LAR as the first-line treatment for advanced neuroendocrine tumors: A phase II trial of the I.T.M.O (Italian Trials in Medical Oncology) group. ASCO Meeting Abstracts. 2013;31(Suppl 15):4136. [Google Scholar]
- 95.Ng K, Tabernero J, Hwang J, Bajetta E, Sharma S, Del Prete SA, et al. Phase II study of everolimus in patients with metastatic colorectal adenocarcinoma previously treated with bevacizumab-, fluoropyrimidine-, oxaliplatin-, and irinotecan-based regimens. Clin Cancer Res. 2013;19:3987–95. doi: 10.1158/1078-0432.CCR-13-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bennouna J, Lang I, Valladares-Ayerbes M, Boer K, Adenis A, Escudero P, et al. A Phase II, open-label, randomised study to assess the efficacy and safety of the MEK1/2 inhibitor AZD6244 (ARRY-142886) versus capecitabine monotherapy in patients with colorectal cancer who have failed one or two prior chemotherapeutic regimens. Invest New Drugs. 2011;29:1021–8. doi: 10.1007/s10637-010-9392-8. [DOI] [PubMed] [Google Scholar]
- 97.Ko AH, Tempero MA, Bekaii-Saab TB, Kuhn P, Courtin R, Ziyeh S, et al. Dual MEK/EGFR inhibition for advanced, chemotherapy-refractory pancreatic cancer: A multicenter phase II trial of selumetinib (AZD6244; ARRY-142886) plus erlotinib. ASCO Meeting Abstracts. 2013;31(Suppl 15):4014. [Google Scholar]
- 98.Morelli MP, Tentler JJ, Kulikowski GN, Tan AC, Bradshaw-Pierce EL, Pitts TM, et al. Preclinical activity of the rational combination of selumetinib (AZD6244) in combination with vorinostat in KRAS-mutant colorectal cancer models. Clin Cancer Res. 2012;18:1051–62. doi: 10.1158/1078-0432.CCR-11-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bodoky G, Timcheva C, Spigel DR, La Stella PJ, Ciuleanu TE, Pover G, et al. A phase II open-label randomized study to assess the efficacy and safety of selumetinib (AZD6244 [ARRY-142886]) versus capecitabine in patients with advanced or metastatic pancreatic cancer who have failed first-line gemcitabine therapy. Invest New Drugs. 2012;30:1216–23. doi: 10.1007/s10637-011-9687-4. [DOI] [PubMed] [Google Scholar]
- 100.Bekaii-Saab T, Phelps MA, Li X, Saji M, Goff L, Kauh JS, et al. Multi-institutional phase II study of selumetinib in patients with metastatic biliary cancers. J Clin Oncol. 2011;29:2357–63. doi: 10.1200/JCO.2010.33.9473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Walters DM, Lindberg JM, Adair SJ, Newhook TE, Cowan CR, Stokes JB, et al. Inhibition of the growth of patient-derived pancreatic cancer xenografts with the MEK inhibitor trametinib is augmented by combined treatment with the epidermal growth factor receptor/HER2 inhibitor lapatinib. Neoplasia. 2013;15:143–55. doi: 10.1593/neo.121712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Watanabe M, Sowa Y, Yogosawa M, Sakai T. Novel MEK inhibitor trametinib and other retinoblastoma gene (RB)-reactivating agents enhance efficacy of 5-fluorouracil on human colon cancer cells. Cancer Sci. 2013;104:687–93. doi: 10.1111/cas.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Demetri GD, Wang Y, Wehrle E, Racine A, Nikolova Z, Blanke CD, et al. Imatinib plasma levels are correlated with clinical benefit in patients with unresectable/metastatic gastrointestinal stromal tumors. J Clin Oncol. 2009;27:3141–7. doi: 10.1200/JCO.2008.20.4818. [DOI] [PubMed] [Google Scholar]


