Abstract
Gestational diabetes mellitus (GDM) increases the future risk of developing type 2 diabetes mellitus (T2DM). There is now a growing evidence that breastfeeding has short- and long-term health benefits for mothers with GDM. Mothers with GDM who breastfeed have improved lipid and glucose metabolic profiles for the first 3 months after birth. However, women with GDM are less likely to breastfeed and, if they do, breastfeeding is usually continued for a shorter duration compared with women without GDM. One long-term prospective study followed women with GDM from delivery for up to 19 years postpartum, and found that breastfeeding for ≥3 months reduced the risk of T2DM and delayed the development of T2DM by a further 10 years compared with breastfeeding for <3 months. However, the physiological mechanisms underlying the protective effects of breastfeeding are still unknown, even though it is important to gain a full understanding of the pathways involved in these effects. Therefore, the purpose of this review is to provide a comprehensive analysis of the recent developments in the field of GDM and breastfeeding. We reviewed data from animal experiments and human studies. We also provide insight into the molecular pathways and describe promising topics for future research.
Keywords: Breastfeeding, Gestational diabetes mellitus, Glucose homeostasis, Type 2 diabetes mellitus
1. Introduction
The International Diabetes Federation has estimated that 382 million adults worldwide have diabetes mellitus. The incidence of diabetes is escalating to epidemic proportions and by 2035, the figure is expected to reach 592 million [1].
Reflecting the upward trend of diabetes, the incidence of gestational diabetes mellitus (GDM) is also spiraling upwards, now affecting up to 5% of all pregnancies worldwide [2]. GDM is defined by glucose intolerance of variable severity that begins in or is first diagnosed during pregnancy [3]. Although most women with GDM commonly revert to normal glucose tolerance after pregnancy, they have an increased risk of developing type 2 diabetes mellitus (T2DM) later in life. In fact, a meta-analysis of 20 retrospective and prospective cohort studies showed that the risk of developing T2DM later in life was 7 times higher in women with GDM than in women without GDM [4].
Appropriate management programs and prevention strategies are strongly recommended for this high-risk group of women with GDM. Breastfeeding has well-known health benefits for the infant; however, its benefits on maternal health were often overlooked or even unknown. Since the 1980s, there has been renewed focus on the health promoting effects of breastfeeding in women, including protection against diabetes [5], osteoporosis [6], and cancer [7].
Here, we critically review the scientific evidence from human and animal studies describing that breastfeeding confers short- and long-term benefits on maternal glucose tolerance and the risk of progression to T2DM in women with GDM. We also describe the effects of breastfeeding on the metabolic profile of women with GDM.
2. Breastfeeding habits of women with GDM
Breastfeeding is recommended and encouraged for mothers. Despite its well-recognized benefits on the health outcomes of the mother and child, it has been suggested that the rate of breastfeeding is lower in women with GDM. Unfortunately, the literature on this topic is scarce [8,9].
In a German prospective post-partum study performed between 1989 and 1999 [8], breastfeeding habits were recorded in 257 mothers with GDM, and were compared with the breastfeeding habits of 527 healthy mothers enrolled in the prospective BABYDIAB study between 1989 and 2000. The proportion of mothers with GDM who breastfed their children was significantly lower (75% vs. 86%) and it was continued for a shorter duration (median duration of breastfeeding: 9 vs. 17 weeks) compared with healthy mothers. These values were even lower in women with GDM who required insulin therapy during pregnancy or if they were obese [8].
A retrospective study investigated breastfeeding rates in hospitals and on discharge in women with preexisting diabetes (insulin-treated diabetes and non-insulin treated diabetes before gestation), women with GDM, or women without diabetes [9]. The study showed that women with GDM (n=1291) were less likely to breastfeed in hospital or on discharge compared with healthy women (n>23,000). Notably, the breastfeeding rate was lowest in women with insulin-treated diabetes [9].
Several reasons have been proposed to explain the lower rates of breastfeeding in women with diabetes. First, the initiation of breastfeeding may be more difficult for women with insulin-dependent diabetes [10] and overweight/obese women [11] because maternal diabetes and obesity can delay lactogenesis. Matias et al. [12] reported that one-third of women with GDM experienced delayed onset of lactogenesis, and that maternal obesity, insulin treatment, and suboptimal in-hospital breastfeeding were the key risk factors for early breastfeeding failure. Rasmussen et al. [11] proposed that being overweight or obese may affect the hormonal response to suckling in the first week postpartum by impairing the prolactin response, for example, which may contribute to the delayed onset of lactation. Because women with GDM often have a higher body mass index (BMI) than women without GDM [13,14], this could partly explain the lower breastfeeding rates in women with GDM. Second, the higher rates of pregnancy and neonatal complications in women with GDM (e.g. prematurity, cesarean delivery, infant macrosomia, and hypoglycemia) can pose significant challenges to breastfeeding success, as reviewed by Taylor et al. [15]. The rate of early breastfeeding (any initiation or at hospital discharge) was reported to be lower after cesarean section than after vaginal delivery (pooled odds ratio 0.57, 95% confidence interval 0.50–0.64) [16]. Considering that the frequency of cesarean deliveries is much higher in women with GDM than in women without GDM [17], this might contribute to the lower breastfeeding rates in women with GDM. It is also possible that medical management of newborns that involves prolonged separation of the mother and child after birth and the provision of supplemental milk feedings may also interfere with maternal milk production, as reviewed by Taylor et al. [15].
3. Long-term effects of breastfeeding on T2DM development and glucose metabolism in women with a history of GDM
Very few studies have examined the relationship between breastfeeding duration and the incidence of T2DM in women with GDM. To our knowledge, only four studies have investigated whether the favorable effects of breastfeeding persist after weaning and protect women with GDM against T2DM in later life [18–21] (Table 1). Only one study assessed the development of T2DM in women with GDM for up to 20 years after delivery [21]. Limitations of the studies include a lack of standardized oral glucose tolerance tests (OGTT) at specific times after pregnancy, lack of detailed measures on breastfeeding (i.e. duration and intensity), self-report of GDM or T2DM diagnosis, recall bias regarding lactation duration, lack of control for confounders (e.g. physical activity and diet, differences in postpartum BMI, medication), retrospective study design, and insufficient duration of follow-up.
Table 1.
Overview of long-terms studies of women with GDM that examined the association between breastfeeding and subsequent risk of T2DM or metabolic disorders.
| Author, year | Study design, year | Study population, location | n | Breastfeeding assessment | Method of T2DM diagnosis | Follow-up time postpartum | Main findings |
|---|---|---|---|---|---|---|---|
| Kjos et al. (1995) [22] | Retrospective analysis 1997–1994 | Latino women, Los Angeles, USA | 671 | At 4–16 wk postpartum; questionnaire (yes vs. no) | 75-g 2-h OGTT | Variable (within 7.5 yr) | Breastfeeding at 4–16 wk postpartum was not associated with progression to T2DM within 7.5 yr |
| Buchanan et al. (1999) [19] | Longitudinal study 1993–1997 | Latino women, Los Angeles, USA | 91 | At 11–26 months postpartum (yes vs. no) | 75-g 2-h OGTT and IVGTT | 11–26 months (15-month intervals after pregnancy) | No significant differences in T2DM diagnosis or OGTT results according to yes/no breastfeeding status (25% vs. 15.4%) |
| Stuebe et al. (2005) [20] | Retrospective cohort 1989–2003 | Subgroup of the Nurses Health Study II, 14 states, USA | Not explicitly stated; estimated to be >3000 based on the number of person years | Total breastfeeding duration for each pregnancy; questionnaires distributed in 1993, 1997, and 2003 | Self-report of T2DM; confirmed using medical charts | Variable (up to 14 years postpartum) | No effect of breastfeeding on risk of T2DM (HR 0.96; 95% CI 0.84–1.09) after adjusting for lifestyle factors, sociodemographic factors, family history of diabetes, and BMI |
| Ziegler et al. (2012) [21] | Prospective cohort 1989–1999 | Caucasian women, Munich, Germany | 264 | Duration ≤3 vs. >3 months; prospectively recorded by questionnaires at 9 months and 2 years after birth | 75-g 2-h OGTT during each follow-up visit | For up to 19 yr postpartum (follow-up at 2 and 9 mo, and 2, 5, 8, 11, 15, and 19 years postpartum) | Breastfeeding for >3 months decreased the risk of T2DM by >40% (HR 0.54, 95% CI 0.34–0.85) after adjusting for islet autoantibodies, family history of diabetes, maternal BMI, age, and smoking |
| Chouinard-Castonguay et al. (2013) [23] | Cross-sectional study. Data recorded in 2009–2011 for women with a GDM-complicated pregnancy between 2003 and 2010 | Women from the Quebec region, Canada | 144 | Total breastfeeding duration for each pregnancy; retrospective self-report | 75-g 2-h OGTT | Variable (1–7 yr postpartum; mean 4±1.9 yr) | Breastfeeding increased insulin sensitivity (HOMA-IS), decreased fasting insulin, 2-h insulin, and AUC insulin Breastfeeding for >10 months improved glucose tolerance, and increased insulin sensitivity and insulin secretion/insulin sensitivity |
AUC: area under the concentration curve; CI: confidence interval; GDM: gestational diabetes mellitus; HOMA-IS: homeostatic model assessment of insulin secretion; HR: hazard ratio; IVGTT: intravenous glucose tolerance test; OGTT: oral glucose tolerance test; mo: months; T2DM: type 2 diabetes mellitus; wk: weeks; yr: years.
In a retrospective study, Kjos et al. reported that breastfeeding at 4–16 weeks postpartum (recorded as yes/no) was not associated with the progression to T2DM within a follow-up of 7.5 years after delivery in 671 Latino women with a history of GDM and variable postpartum screening by 2-h plasma glucose levels in an OGTT [18]. In a longitudinal analysis of 91 women included in that study, Buchanan et al., found no difference in the prevalence of diabetes, which was diagnosed based on an OGTT, at 11–26 months postpartum according to breastfeeding status (measured as yes/no) [19]. Maternal BMI was similar between the two groups. However, the duration of follow-up was limited to 26 months and may be too short to detect a difference in the progression to diabetes between the two groups of women because <30% of women develop T2DM within 2 years of the diagnosis of GDM (Figure 1) [21].
Figure 1.
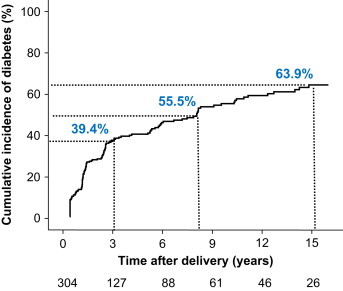
Cumulative life-table risk of postpartum diabetes in 304 women with GDM who were followed prospectively for up to 19 years postpartum. The numbers below the graph indicate the number of women at each follow-up. Published previously in Ziegler et al. Long term protective effect of lactation on the development of type 2 diabetes mellitus in women with recent gestational diabetes mellitus.
Diabetes 2012, 61(12):3167–3171. Copyright 2012 by the American Diabetes Association.
In a subgroup analysis of the Nurses Health study II [20], a retrospective observational cohort study, breastfeeding did not affect the risk of diabetes in women with GDM, which included >3000 women (the exact number was not reported) who were followed up for up to 14 years by questionnaires. By contrast, the duration of breastfeeding was inversely associated with the risk of T2DM in young and middle-aged women who were never diagnosed with GDM (>50,000 women). The authors reported that for every year a woman had breastfed, her risk of developing T2DM was reduced by 15% percent. This protective effect appeared to begin after 6 months of breastfeeding and was independent of maternal BMI and lifestyle behaviors (i.e. amount of exercise, diet, and smoking). However, breastfeeding had a clinically insignificant effect on the risk of T2DM among high-risk women with GDM, with a risk reduction of just 4%. This lack of effect in women with GDM may be due to the retrospective design of the study and that the diagnosis of T2DM/GDM was self-reported.
In 2012, we reported the results of the German GDM study, the first prospective study to show that breastfeeding may be beneficial for the long-term protection against T2DM [21]. The study prospectively followed 304 women with GDM from delivery for up to 19 years postpartum for the development of diabetes. As a part of this study, we investigated whether breastfeeding influenced the short- or long-term postpartum diabetes outcomes [21]. Postpartum diabetes developed in 147 women and was dependent on the treatment received during pregnancy (insulin vs. diet), BMI, and the presence or absence of islet autoantibodies. Unsurprisingly, breastfeeding offered no protection from diabetes in women who were antibody-positive at the time of GDM diagnosis, almost certainly because of the ongoing autoimmune destruction of pancreatic β cells. However, in women with GDM without islet autoantibodies, breastfeeding reduced the long-term risk of diabetes by >40%, and delayed the onset of T2DM by a further 10 years, independent of maternal BMI and insulin use during pregnancy. The risk reduction was greatest for women who continued lactation for >3 months (Figure 2). Strengths of the prospective study include the long duration of follow-up (up to 19 years after delivery) and regular screening for T2DM by 2-h OGTTs. Some limitations of the study include limited access to information regarding diet and physical activity postpartum, which possibly contributed to the risk reduction associated with breastfeeding. However, this is the only prospective study to show a sustained, protective effect of lactation against diabetes that was maintained for up to 15 years postpartum in women with GDM. Therefore, the results of the study provide compelling evidence that breastfeeding has substantial long-lasting health benefits for mothers with GDM.
Figure 2.
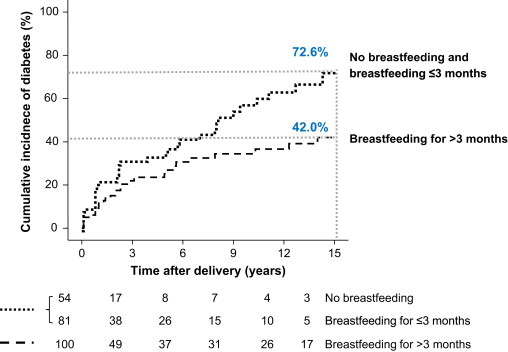
Cumulative life-table risk of postpartum diabetes in islet autoantibody negative women with gestational diabetes who breastfed for >3 months (dashed line) compared with those who did not breastfeed or breastfed for ≤3 months (dotted line). The numbers below the graph indicate the number of subjects at each follow-up. Published previously in Ziegler et al. Long term protective effect of lactation on the development of type 2 diabetes mellitus in women with recent gestational diabetes mellitus.
Diabetes 2012, 61(12):3167–3171. Copyright 2012 by the American Diabetes Association.
If breastfeeding is not possible after birth for medical, personal, or social reasons (e.g. stillbirth, death of a newborn, adoption of the newborn, and maternal/newborn medical conditions) it becomes essential to suppress lactation with medications. So far, there is very little information describing the impact of breastfeeding suppression on subsequent T2DM development. To the best of our knowledge, the only study to investigate this issue was the Nurses Health Study II [20]. The authors found that suppression of breastfeeding with medications (by bromocriptine-related or estrogen preparations) increased the risk of T2DM by 46% compared with women who never breastfed but did not use such medications. Although the study was retrospective and had a considerable variation in follow-up time of up to 14 years, these findings suggest that disrupting the hormonal changes involved in breastfeeding may have significant metabolic consequences [20].
In tentative support of this concept, Kjos et al. [22] found that, in a retrospective study, women with GDM who had chosen to use progestin hormonal contraceptives during breastfeeding nearly tripled their risk of developing T2DM at 7.5 years postpartum compared with the other women included in that study. The annual incidence rate of T2DM in women who used non-hormonal forms of contraception (n=443) was 8.7% versus 10.4% in women who used an estrogen and progestin combination oral contraceptive (n=383). Meanwhile, the incidence of T2DM was 26.5% in women who used oral progestin contraceptives (n=78).
Recently, Chouinard-Castonguay et al. [23] investigated the postpartum insulin and glucose responses in 144 breastfeeding women with a history of GDM between 2003 and 2010 living in the greater Quebec region, Canada. The women performed 75-g OGTTs at a mean of 4 years after the last GDM-complicated pregnancy. At the clinical visit, the breastfeeding duration for each pregnancy was retrospectively self-reported in months, and the total duration of breastfeeding (not necessarily exclusive) for all of the participants׳ infants (with or without GDM) was calculated. At 4 years postpartum, women who reported lactating for ≥10 months (mean 13 months) had a better insulin sensitivity-secretion index, higher homeostatic model assessment of insulin sensitivity and Matsuda indices, lower fasting and 2-h plasma glucose and serum insulin concentrations, lower area under the curve (AUC) for insulin, and less frequently had impaired glucose intolerance, compared with women who lactated for <10 months. There were no significant differences in postpartum BMI between the two comparison groups. Breastfeeding duration also emerged as an independent predictor of fasting insulin concentrations and insulin sensitivity indices. Of note, the analysis was adjusted for sociodemographic and lifestyle factors, insulin therapy during gestation, and time since last GDM complicated pregnancy. Overall, the results suggest that a longer duration of breastfeeding is associated with improved insulin and glucose responses in women with GDM, and that these associations are independent of lifestyle factors [23]. However, because of the cross-sectional nature of the study, no causal interferences can be drawn and long-term prospective studies are needed to clarify the precise interrelationship.
In summary, evidence from prospective studies that breastfeeding may be beneficial to women׳s health, including the protection against T2DM development in later life, is accumulating but still limited (Table 1). Further large, well-designed, prospective studies with long-term follow-up are needed to conclusively determine whether breastfeeding can reduce the incidence of T2DM occurring many years after a GDM-complicated pregnancy. Nevertheless, there is increasing awareness that confounding factors associated with breastfeeding may determine the duration of breastfeeding and the risk of T2DM. These factors, including adequate control of lifestyle behaviors and the metabolic state during pregnancy, must be taken into account in future breastfeeding studies.
4. Short-term effects of breastfeeding on glucose metabolism in women with GDM
Several human studies have examined whether breastfeeding exerts short-term effects on maternal glucose homeostasis, for example within the first 3–12 months postpartum. Most of these studies had a cross-sectional design [24–27], although some were prospective [28,29].
In an early cross-sectional study that involved subsequent follow-up assessments, Kjos et al. [24] determined glucose tolerance using 2-h OGTTs in 809 primarily Latino women with GDM. Of these women, 404 chose to breast-feed while 405 did not lactate after delivery. At the first follow-up visit at 4–12 weeks postpartum, glucose and lipid metabolism were improved in the lactating group in terms of significantly lower total AUC for glucose, lower mean fasting serum glucose, lower mean 2-h glucose, and higher mean serum high-density lipoprotein-cholesterol after adjusting for maternal age, BMI, and the use of insulin during pregnancy [24].
A smaller clinical study by McManus et al. [26] assessed postpartum glucose tolerance using the frequently sampled intravenous glucose tolerance test (FSIGT) in 26 Caucasian women with GDM. Fourteen mothers were breastfeeding after hospital discharge, while the other 12 women did not breastfeed. The two groups were matched for age, weight, weight loss by 3 months postpartum, and their exercise habits. At 3 months postpartum, insulin sensitivity, glucose responses, or adiposity (subcutaneous and visceral fact measured by computed tomography) were not significantly different between the two groups. However, the disposition index (DI), which was calculated as insulin sensitivity×acute insulin response to glucose, was significantly higher in breastfeeding women than in non-breastfeeding women. The disposition index represents the ability of pancreatic β cells to compensate for insulin resistance. Accordingly, the results of this study support the concept breastfeeding in mothers with GDM is associated with improved pancreatic β cell function, at least in the short term [26].
In the Atlantic Diabetes in Pregnancy study, O׳Reilly et al. compared the results of a 75-g OGTT performed at 12 weeks postpartum between 300 women with GDM and 220 women with normal glucose tolerance during pregnancy [27,30]. The prevalence of persistent hyperglycemia, defined as impaired fasting glucose, impaired glucose tolerance, or T2DM, was significantly decreased by 10% in women who breastfed compared with women who bottle-fed their infant. Based on these results, the authors concluded that breastfeeding may confer beneficial metabolic effects after a GDM-complicated pregnancy [27,30].
Diniz and Costa [25] conducted a cross-sectional study of 67 Brazilian women with a history of GDM, and found that breastfeeding was inversely associated with the insulin response (AUC for insulin) and the peak insulin concentration during the OGTT at 12–18 months postpartum. Accordingly, their study showed, for the first time, a protective effect of breastfeeding that was maintained beyond 1 year postpartum. However, the results of that study should be extrapolated with caution because of its methodological weaknesses.
An important limitation of the studies by Kjos et al. [24] and Diniz and Costa [25] is the fact that 50–60% of women with GDM were breastfeeding at the time of the postpartum OGTT. Consequently, it is unclear whether the findings reflect a sustained effect of breastfeeding or the metabolic state during lactation.
In this context, the results of the Study of Women, Infant Feeding, and Type 2 Diabetes (SWIFT) [31] seem particularly interesting. SWIFT was a large, prospective, observational cohort study that enrolled and followed women with GDM. As a part of that study, Gunderson et al. prospectively examined the metabolic effects of breastfeeding during an OGTT [28]. Of 835 lactating women with GDM, 205 breastfed their infants for a mean duration of 15 min during the 2-h OGTT, was generally performed at 6–9 weeks postpartum. Breastfeeding was associated with a small but significant reduction in the 2-h glucose concentration (mean reduction 5%) and the 2-h insulin concentration, as well as higher insulin sensitivity indices at 0 and 120 min. However, there were no differences in the fasting plasma glucose or fasting serum insulin concentrations. These findings indicate that breastfeeding has immediate effects in terms of reducing glucose and insulin concentrations within 2 h after a glucose challenge.
SWIFT was primarily designed to assess whether prolonged, intensive breastfeeding reduced the 2-year incidence of T2DM among women with GDM relative to formula feeding. In a recent analysis of 522 mothers at 6–9 weeks postpartum, Gunderson et al. [29] found that women who were exclusively breastfeeding or mostly breastfeeding at this time had lower fasting plasma glucose and lower fasting and 2-h serum insulin concentrations, as well as improved insulin sensitivity indices compared with women who were exclusively or mostly formula feeding. These results suggest a dose–response relationship between increasing intensity of breastfeeding and improvements in fasting plasma glucose, fasting and 2-h insulin and insulin sensitivity at 6–9 weeks postpartum. The mean fasting plasma glucose concentration was 4–5 mg/dL lower in exclusively breastfeeding women than in women who were exclusively formula feeding. The prevalence rates of T2DM and prediabetes at this time were also significantly lower in women who were breastfeeding at 6–9 weeks postpartum, with rates of 3.3% and 24.6%, respectively, in women with GDM who exclusively breastfed (n=211) compared with 3.7% and 41.5%, respectively, in women who exclusively or mostly formula fed (n=135). When stratified by maternal obesity, the relationship between glucose intolerance categories and infant feeding groups remained in obese women (n=241). An important strength of SWIFT is that it was the first large-scale, prospective, community-based study involving >1000 diverse women with GDM. It included detailed assessments of breastfeeding duration and glucose tolerance, and accounted for numerous potential confounders, including physical activity, dietary intake, sleep, maternal BMI, sociodemographic, time since birth, fasting parameters, and breastfeeding behavior before the test. Further results of this study are eagerly awaited, particularly the incidence rates of T2DM at 2 years postpartum.
In summary, the findings from human studies seem to support the concept that breastfeeding has favorable immediate and short-term effects on glucose homeostasis. However, very few studies have examined whether breastfeeding has long-term benefits on glucose homeostasis several years after a GDM-complicated birth [23]. Therefore, it is vital that studies evaluate glucose tolerance or β cell function several years after stopping breastfeeding after a GDM-complicated birth.
5. Mechanisms underlying the possible protective effects of breastfeeding against progression to T2DM
Epidemiologic studies suggest that breastfeeding reduces the risk of progression to T2DM after a GDM-complicated pregnancy. However, the pathways underlying these protective effects of breastfeeding are still poorly understood, and most relevant studies in this context have focused on animal models (Figure 3).
Figure 3.
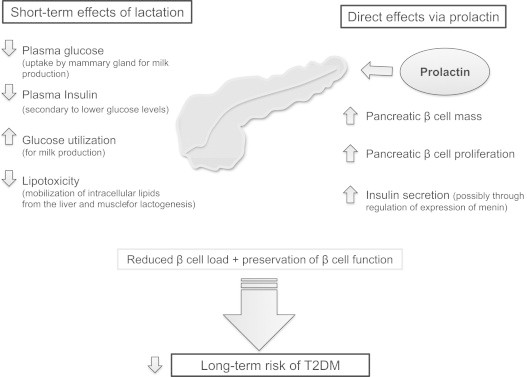
Potential mechanisms involved in the short-term effects of breastfeeding on glucose metabolism and its long-term effect on the development of T2DM based on evidence from animal models. T2DM: type 2 diabetes mellitus.
In rodent studies, lactation decreases blood glucose and plasma insulin concentrations in the postpartum period [32,33], with reductions of 20% and 35%, respectively, in lactating rats at 12 days postpartum compared with non-lactating rats [32]. Lactation was also reported to improve insulin resistance [33]. The mammary gland expresses insulin receptors and is extremely sensitive to insulin during lactation [34]. Therefore, Jones et al. [33] suggested that the lower plasma insulin concentrations during lactation are primarily due to increased insulin sensitivity as a result of increased glucose disposal by the mammary gland.
Results of an early human study by Butte et al. [35] indicated that circulating glucose could be preferentially diverted to the mammary gland via an non-insulin-independent pathway to satisfy the demands of lactogenesis. This diversion may reduce the glucose load on pancreatic β cells and preserve long-term insulin production.
Breastfeeding-related hormones, such as prolactin, may also play an important role in the regulation of insulin secretion and glucose homeostasis. For example, prolactin stimulates insulin secretion from rat pancreatic islets or isolated β cells [36–38]. One mechanism underlying this effect might involve downregulation of menin, as observed in pregnant animals. Menin is a protein expressed from the Men1 gene which regulates islet cell proliferation by histone-methylation. The overexpression of the protein had anti-proliferative effects on β cells, leading to impaired glucose tolerance in the pregnant animals. Of importance, Men1 expression is regulated by prolactin [39]. Further evidence for a stimulatory effect of prolactin came from mice with targeted deletion of the prolactin receptor. These mice had reductions in pancreatic islet density and β cell mass, together with blunted insulin secretory responses to glucose compared with wild-type littermates [40]. Physiologically elevated prolactin levels induce normal adaptive processes, including expansion of the β cell mass and improvements in hepatic insulin sensitivity, that ultimately increase glucose-stimulated insulin secretion in diabetic rats [41]. Animal and human studies have clearly shown that the β cell mass influences the insulin secretory capacity and the risk of developing T2DM [42,43]
Women with GDM have a chronic defect in pancreatic β cell function [44–46] and that their β cell function declines within 1 year postpartum [47]. Therefore, the amelioration of insulin resistance, which is common in women with a history of GDM, may be a critical target for the preservation of β cell function and the prevention of T2DM [48]. In this context, a comparison of the effects of breastfeeding and treatment with an insulin-sensitizing drug, such as thiazolidinediones (peroxisome proliferator-activated receptor-γ [PPARγ] agonists) may be of interest. In the Troglitazone in Prevention of Diabetes study [48], 266 women with GDM were randomized 1:1 to either pharmacological treatment with troglitazone for 3 months or placebo within 4 years after delivery. The risk of progressing to T2DM was 55% lower in the troglitazone group at a median follow-up of 42 months. This protection from T2DM was closely associated with the initial reductions in endogenous insulin requirements and with the preservation of pancreatic β cell function. In other words, a reduction in the secretory demands placed on β cells otherwise induced by chronic insulin resistance greatly reduced the risk of progression to diabetes. Unloading of β cells may also be relevant to breastfeeding, even though breastfeeding may improve insulin sensitivity via mechanisms distinct from those of PPARγ agonists [49].
Prolactin and PPARγ agonists directly or indirectly modulate the transcription factors STAT5 and PPARγ, and the expression of lipoprotein lipase (LPL). These co-factors are expressed in breast, adipose tissue, and skeletal muscle, and regulate each other׳s expression through negative feedback. PPARγ agonists redistribute intracellular lipid from insulin-responsive tissues (e.g. liver and muscle) to insulin-sensitive adipocytes. It was suggested, that lactation improves postpartum insulin sensitivity by mobilizing lipids derived from liver and muscle (non-adipose tissues) into breast milk rather than redirecting lipids into adipocytes that are already enlarged. In the postpartum period, non-lactating, insulin-resistant women may be more likely to store lipids in non-adipose tissues, further disrupting the balance between insulin secretion and insulin sensitivity [49].
Of interest, repeated pregnancies without lactation seem to disrupt glucose homeostasis. For example, Zhong et al. [50] reported that, after three cycles of pregnancy without lactation, there was a marked deterioration in glucose regulation in rats, in terms of fasting hyperglycemia and insulin resistance, together with a higher rate of spontaneous abortion during the third pregnancy, compared with rats that had lactated after each gestation. Bromocriptine use to suppress lactation in postpartum women may disrupt mechanisms of appetite regulation [20], because bromocriptine administration to lactating rats not only decreased milk production, but also impaired patterns of dietary intake and leptin release, compared with weaning rats whose pubs were removed from the nest [51].
Little is known about the role of the circulating metabolite profile after a GDM-complicated pregnancy on the long-term risk of developing T2DM. It was recently reported that serotonin acts downstream of lactogen signaling to stimulate β cell proliferation in pregnant mice [52]. Blocking 5-hydroxytryptamine receptor-2b signaling suppressed β cell expansion and caused glucose intolerance. However, the role of serotonin in regulating β cell mass after birth in women with GDM, particularly during the breastfeeding period, is poorly understood and animal studies are needed to examine how serotonin and/or associated metabolites may influence maternal progression to T2DM in the postpartum period.
6. Social impact of breastfeeding
Many mothers are aware of the benefits of breastfeeding for their babies, but the benefits on the mother׳s health are often overlooked. It is now becoming clear that breastfeeding not only provides mothers with GDM with immediate, short-term benefits after birth (e.g. improvements in glucose tolerance), but also with long-lasting health benefits that include prevention of metabolic syndrome [53], T2DM [21,29], and preservation of β cell function [23].
The decision of a mother to start and continue breastfeeding is usually based on the benefits for the baby. Considering the emerging data regarding the benefits of breastfeeding in women with GDM, it is essential that healthcare providers educate women with a history of GDM on the potential benefits for their own future health, help support mothers to continue breastfeeding if they experience perinatal problems. Women with GDM breastfeed less frequently and for shorter durations than healthy women [8,54]. These differences are especially apparent in obese women and in those with severe GDM. The onset of breastfeeding is also frequently delayed in women with GDM [12,55]. The higher rates of obstetric and neonatal complications might explain the lower breastfeeding rate and high frequency of unsuccessful breastfeeding in this population [15]. Counseling on the benefits of breastfeeding for the mother and child should be provided by certified nurse midwives before delivery. However, it was reported that the discussions of breastfeeding at the first prenatal visit to an obstetric clinic were infrequent (in just 29% of women), brief (mean duration 39 s), and initiated by clinicians in an ambivalent manner [56]. Healthcare professionals should have an adequate knowledge of the process of breastfeeding and be able to apply a problem-solving approach to address the difficulties that women with GDM may encounter to provide appropriate evidence-based care. On the basis of these findings, we suggest that breastfeeding education programs delivered to healthcare professionals should be optimized to encourage knowledge transfer to women, particularly for high-risk women, such as those with GDM.
Recommendations for the length of breastfeeding do not differ among women with diabetes, women with GDM, and women without diabetic complications during pregnancy. The World Health Organization recommends exclusive breastfeeding until 6 months of age, and that breastfeeding should be continued with appropriate complementary foods for 2 years or longer [57]. Breastfeeding for ≥3 months should be encouraged in women with GDM because it reduces the risk of or delays the onset of T2DM in women with GDM [21].
7. Breastfeeding as low-cost intervention for preventing T2DM
The personal costs of T2DM and its complications (e.g. heart disease, stroke, and death) are enormous, not to mention the financial burden placed on the healthcare system. Recently, the estimated life-time direct medical costs of treating T2DM and its complications in women at ages 25–44 years and 45–54 years was estimated to be $130.800 and $111.400, respectively [58]. Although women have fewer T2DM derived complications, women have greater medical costs than men, primarily because they on average life longer than men. The cost of breastfeeding education programs are likely to be minimal relative to the cost of treating a patient with T2DM, but future studies on the cost-effectiveness of breastfeeding in terms of avoidance of T2DM need to be conducted. The onset of T2DM is preventable or can be delayed in many patients by adequate lifestyle changes, including increased physical activity, healthy eating, and weight loss [59]. In the light of emerging data, breastfeeding may also represent a lifestyle factor for preventing T2DM. In high-risk groups, such as women with GDM, and in women with a low socioeconomic status, breastfeeding may offer a safe and feasible low-cost intervention to prevent T2DM development. Support should be provided to instruct and encourage breastfeeding, especially for women with GDM.
8. Conclusion and further perspectives
In conclusion, the results of observational studies and a small number of prospective studies suggest that breastfeeding is associated with improvements in glucose and lipid metabolism together with reduced risk of T2DM in women with GDM. However, because women who breastfeed are more likely to engage with other healthy behaviors and are more likely to be highly educated or have a lower BMI, the results of observational and retrospective studies must be interpreted with caution. It is essential to prospectively collect data, including the duration and intensity of breastfeeding, changes in lifestyle behaviors, body weight, and biochemical factors during the reproductive years, and to control for metabolic disorders before pregnancy, maternal BMI, time postpartum, and breastfeeding at follow-up OGTT to avoid residual confounding and reverse causation. The limited data available to date highlight the need for prospective studies with a long duration of follow-up that ideally involve assessments of biomarkers to substantiate the aggregating epidemiological evidence that breastfeeding reduces the risk of T2DM in later life. Future studies should also elucidate the molecular and physiological mechanisms involved in the protective effects of breastfeeding on the development of T2DM many years after a GDM-complicated pathway. In particular, we recommend metabolomics studies, as well as studies examining the underlying signaling pathways to identify new physiological and pharmacological targets for preventing T2DM.
Authors׳ contributions
DM wrote the manuscript; AGZ gave input into the scope of the paper. All authors critically revised the manuscript.
Conflict of interest
The authors have no conflicts of interest relevant to this article.
Acknowledgments
This study was supported in part by grants from the German Federal Ministry of Education and Research (BMBF) to the German Center for Diabetes Research (DZD e.V.) and the German Competence Net for Diabetes Mellitus.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Contributor Information
Daniela Much, Email: daniela.much@helmholtz-muenchen.de.
Andreas Beyerlein, Email: andreas.beyerlein@helmholtz-muenchen.de.
Michaela Roßbauer, Email: michaela.rossbauer@helmholtz-muenchen.de.
Sandra Hummel, Email: sandra.hummel@lrz.uni-muenchen.de.
Anette-G. Ziegler, Email: anette-g.ziegler@helmholtz-muenchen.de.
References
- 1.International Diabetes Federation, 2013. IDF diabetes atlas. 6th ed. Brussels, Belgium: International Diabetes Federation. 〈http://www.idf.org/diabetesatlas〉.
- 2.2008. The global challenge of diabetes. Lancet 371:1723. [DOI] [PubMed]
- 3.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Report of the Expert Committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 4.Bellamy L., Casas J.P., Hingorani A.D., Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373:1773–1779. doi: 10.1016/S0140-6736(09)60731-5. [DOI] [PubMed] [Google Scholar]
- 5.1984. Breast-feeding and diabetes. Lancet 2:1283. [PubMed]
- 6.Melton L.J., 3rd, Bryant S.C., Wahner H.W., O׳Fallon W.M., Malkasian G.D., Judd H.L. Influence of breastfeeding and other reproductive factors on bone mass later in life. Osteoporosis International. 1993;3:76–83. doi: 10.1007/BF01623377. [DOI] [PubMed] [Google Scholar]
- 7.McTiernan A., Thomas D.B. Evidence for a protective effect of lactation on risk of breast cancer in young women. Results from a case-control study. American Journal of Epidemiology. 1986;124:353–358. doi: 10.1093/oxfordjournals.aje.a114405. [DOI] [PubMed] [Google Scholar]
- 8.Hummel S. Stillverhalten bei Frauen mit Gestationsdiabetes. Deutsche Medizinische Wochenschrift. 2008;133:180. doi: 10.1055/s-2008-1017493. [DOI] [PubMed] [Google Scholar]
- 9.Finkelstein S.A., Keely E., Feig D.S., Tu X., Yasseen A.S., 3rd, Walker M. Breastfeeding in women with diabetes: lower rates despite greater rewards. A population-based study. Diabetic Medicine. 2013;30:1094–1101. doi: 10.1111/dme.12238. [DOI] [PubMed] [Google Scholar]
- 10.Neubauer S.H., Ferris A.M., Chase C.G., Fanelli J., Thompson C.A., Lammi-Keefe C.J. Delayed lactogenesis in women with insulin-dependent diabetes mellitus. American Journal of Clinical Nutrition. 1993;58:54–60. doi: 10.1093/ajcn/58.1.54. [DOI] [PubMed] [Google Scholar]
- 11.Rasmussen K.M., Kjolhede C.L. Prepregnant overweight and obesity diminish the prolactin response to suckling in the first week postpartum. Pediatrics. 2004;113:e465–e471. doi: 10.1542/peds.113.5.e465. [DOI] [PubMed] [Google Scholar]
- 12.Matias S.L., Dewey K.G., Quesenberry C.P., Jr., Gunderson E.P. Maternal prepregnancy obesity and insulin treatment during pregnancy are independently associated with delayed lactogenesis in women with recent gestational diabetes mellitus. American Journal of Clinical Nutrition. 2013;99(1):115–121. doi: 10.3945/ajcn.113.073049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim S.Y., England L., Wilson H.G., Bish C., Satten G.A., Dietz P. Percentage of gestational diabetes mellitus attributable to overweight and obesity. American Journal of Public Health. 2010;100:1047–1052. doi: 10.2105/AJPH.2009.172890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torloni M.R., Betran A.P., Horta B.L., Nakamura M.U., Atallah A.N., Moron A.F. Prepregnancy BMI and the risk of gestational diabetes: a systematic review of the literature with meta-analysis. Obesity Reviews. 2009;10:194–203. doi: 10.1111/j.1467-789X.2008.00541.x. [DOI] [PubMed] [Google Scholar]
- 15.Taylor J.S., Kacmar J.E., Nothnagle M., Lawrence R.A. A systematic review of the literature associating breastfeeding with type 2 diabetes and gestational diabetes. Journal of the American College of Nutrition. 2005;24:320–326. doi: 10.1080/07315724.2005.10719480. [DOI] [PubMed] [Google Scholar]
- 16.Prior E., Santhakumaran S., Gale C., Philipps L.H., Modi N., Hyde M.J. Breastfeeding after cesarean delivery: a systematic review and meta-analysis of world literature. American Journal of Clinical Nutrition. 2012;95:1113–1135. doi: 10.3945/ajcn.111.030254. [DOI] [PubMed] [Google Scholar]
- 17.Naylor C.D., Sermer M., Chen E., Sykora K. Cesarean delivery in relation to birth weight and gestational glucose tolerance: pathophysiology or practice style? Toronto Trihospital Gestational Diabetes Investigators. Journal of the American Medical Association. 1996;275:1165–1170. [PubMed] [Google Scholar]
- 18.Kjos S.L., Peters R.K., Xiang A., Henry O.A., Montoro M., Buchanan T.A. Predicting future diabetes in Latino women with gestational diabetes. Utility of early postpartum glucose tolerance testing. Diabetes. 1995;44:586–591. doi: 10.2337/diab.44.5.586. [DOI] [PubMed] [Google Scholar]
- 19.Buchanan T.A., Xiang A.H., Kjos S.L., Trigo E., Lee W.P., Peters R.K. Antepartum predictors of the development of type 2 diabetes in Latino women 11–26 months after pregnancies complicated by gestational diabetes. Diabetes. 1999;48:2430–2436. doi: 10.2337/diabetes.48.12.2430. [DOI] [PubMed] [Google Scholar]
- 20.Stuebe A.M., Rich-Edwards J.W., Willett W.C., Manson J.E., Michels K.B. Duration of lactation and incidence of type 2 diabetes. Journal of the American Medical Association. 2005;294:2601–2610. doi: 10.1001/jama.294.20.2601. [DOI] [PubMed] [Google Scholar]
- 21.Ziegler A.G., Wallner M., Kaiser I., Rossbauer M., Harsunen M.H., Lachmann L. Long-term protective effect of lactation on the development of type 2 diabetes in women with recent gestational diabetes mellitus. Diabetes. 2012;61:3167–3171. doi: 10.2337/db12-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kjos S.L., Peters R.K., Xiang A., Thomas D., Schaefer U., Buchanan T.A. Contraception and the risk of type 2 diabetes mellitus in Latina women with prior gestational diabetes mellitus. Journal of the American Medical Association. 1998;280:533–538. doi: 10.1001/jama.280.6.533. [DOI] [PubMed] [Google Scholar]
- 23.Chouinard-Castonguay S., Weisnagel J.S., Tchernof A., Robitaille J. Relationship between lactation duration and insulin and glucose response among women with prior gestational diabetes. European Journal of Endocrinology. 2013;168:515–523. doi: 10.1530/EJE-12-0939. [DOI] [PubMed] [Google Scholar]
- 24.Kjos S.L., Henry O., Lee R.M., Buchanan T.A., Mishell D.R., Jr. The effect of lactation on glucose and lipid metabolism in women with recent gestational diabetes. Obstetrics and Gynecology. 1993;82:451–455. [PubMed] [Google Scholar]
- 25.Diniz J.M., Da Costa T.H. Independent of body adiposity, breast-feeding has a protective effect on glucose metabolism in young adult women. British Journal of Nutrition. 2004;92:905–912. doi: 10.1079/bjn20041288. [DOI] [PubMed] [Google Scholar]
- 26.McManus R.M., Cunningham I., Watson A., Harker L., Finegood D.T. Beta-cell function and visceral fat in lactating women with a history of gestational diabetes. Metabolism: Clinical and Experimental. 2001;50:715–719. doi: 10.1053/meta.2001.23304. [DOI] [PubMed] [Google Scholar]
- 27.O׳Reilly M., Avalos G., Dennedy M.C., O׳Sullivan E.P., Dunne F.P. Breast-feeding is associated with reduced postpartum maternal glucose intolerance after gestational diabetes. Irish Medical Journal. 2012;105:31–36. [PubMed] [Google Scholar]
- 28.Gunderson E.P., Crites Y., Chiang V., Walton D., Azevedo R.A., Fox G. Influence of breastfeeding during the postpartum oral glucose tolerance test on plasma glucose and insulin. Obstetrics and Gynecology. 2012;120:136–143. doi: 10.1097/AOG.0b013e31825b993d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gunderson E.P., Hedderson M.M., Chiang V., Crites Y., Walton D., Azevedo R.A. Lactation intensity and postpartum maternal glucose tolerance and insulin resistance in women with recent GDM: the SWIFT cohort. Diabetes Care. 2012;35:50–56. doi: 10.2337/dc11-1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O׳Reilly M.W., Avalos G., Dennedy M.C., O׳Sullivan E.P., Dunne F. Vol. 165. 2011. Atlantic DIP: high prevalence of abnormal glucose tolerance post partum is reduced by breast-feeding in women with prior gestational diabetes mellitus; pp. 953–959. (European Journal of Endocrinology). [DOI] [PubMed] [Google Scholar]
- 31.Gunderson E.P., Matias S.L., Hurston S.R., Dewey K.G., Ferrara A., Quesenberry C.P., Jr. Study of women, infant feeding, and type 2 diabetes mellitus after GDM pregnancy (SWIFT), a prospective cohort study: methodology and design. BMC Public Health. 2011;11:952. doi: 10.1186/1471-2458-11-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burnol A.F., Leturque A., Ferre P., Kande J., Girard J. Increased insulin sensitivity and responsiveness during lactation in rats. American Journal of Physiology. 1986;251:E537–E541. doi: 10.1152/ajpendo.1986.251.5.E537. [DOI] [PubMed] [Google Scholar]
- 33.Jones R.G., Ilic V., Williamson D.H. Physiological significance of altered insulin metabolism in the conscious rat during lactation. Biochemical Journal. 1984;220:455–460. doi: 10.1042/bj2200455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burnol A.F., Loizeau M., Girard J. Insulin receptor activity and insulin sensitivity in mammary gland of lactating rats. American Journal of Physiology. 1990;259:E828–E834. doi: 10.1152/ajpendo.1990.259.6.E828. [DOI] [PubMed] [Google Scholar]
- 35.Butte N.F., Hopkinson J.M., Mehta N., Moon J.K., Smith E.O. Adjustments in energy expenditure and substrate utilization during late pregnancy and lactation. American Journal of Clinical Nutrition. 1999;69:299–307. doi: 10.1093/ajcn/69.2.299. [DOI] [PubMed] [Google Scholar]
- 36.Nielsen J.H. Effects of growth hormone, prolactin, and placental lactogen on insulin content and release, and deoxyribonucleic acid synthesis in cultured pancreatic islets. Endocrinology. 1982;110:600–606. doi: 10.1210/endo-110-2-600. [DOI] [PubMed] [Google Scholar]
- 37.Brelje T.C., Parsons J.A., Sorenson R.L. Regulation of islet beta-cell proliferation by prolactin in rat islets. Diabetes. 1994;43:263–273. doi: 10.2337/diab.43.2.263. [DOI] [PubMed] [Google Scholar]
- 38.Crepaldi S.C., Carneiro E.M., Boschero A.C. Long-term effect of prolactin treatment on glucose-induced insulin secretion in cultured neonatal rat islets. Hormone and Metabolic Research. 1997;29:220–224. doi: 10.1055/s-2007-979025. [DOI] [PubMed] [Google Scholar]
- 39.Karnik S.K., Chen H., McLean G.W., Heit J.J., Gu X., Zhang A.Y. Menin controls growth of pancreatic beta-cells in pregnant mice and promotes gestational diabetes mellitus. Science (New York, NY) 2007;318:806–809. doi: 10.1126/science.1146812. [DOI] [PubMed] [Google Scholar]
- 40.Freemark M., Avril I., Fleenor D., Driscoll P., Petro A., Opara E. Targeted deletion of the PRL receptor: effects on islet development, insulin production, and glucose tolerance. Endocrinology. 2002;143:1378–1385. doi: 10.1210/endo.143.4.8722. [DOI] [PubMed] [Google Scholar]
- 41.Park S., Kang S., Lee H.W., Ko B.S. Central prolactin modulates insulin sensitivity and insulin secretion in diabetic rats. Neuroendocrinology. 2012;95:332–343. doi: 10.1159/000336501. [DOI] [PubMed] [Google Scholar]
- 42.Leahy J.L., Bonner-Weir S., Weir G.C. Abnormal glucose regulation of insulin secretion in models of reduced B-cell mass. Diabetes. 1984;33:667–673. doi: 10.2337/diab.33.7.667. [DOI] [PubMed] [Google Scholar]
- 43.Butler A.E., Janson J., Bonner-Weir S., Ritzel R., Rizza R.A., Butler P.C. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 44.Ryan E.A., Imes S., Liu D., McManus R., Finegood D.T., Polonsky K.S. Defects in insulin secretion and action in women with a history of gestational diabetes. Diabetes. 1995;44:506–512. doi: 10.2337/diab.44.5.506. [DOI] [PubMed] [Google Scholar]
- 45.Kautzky-Willer A., Prager R., Waldhausl W., Pacini G., Thomaseth K., Wagner O.F. Pronounced insulin resistance and inadequate beta-cell secretion characterize lean gestational diabetes during and after pregnancy. Diabetes Care. 1997;20:1717–1723. doi: 10.2337/diacare.20.11.1717. [DOI] [PubMed] [Google Scholar]
- 46.Buchanan T.A. Pancreatic B-cell defects in gestational diabetes: implications for the pathogenesis and prevention of type 2 diabetes. Journal of Clinical Endocrinology and Metabolism. 2001;86:989–993. doi: 10.1210/jcem.86.3.7339. [DOI] [PubMed] [Google Scholar]
- 47.Retnakaran R., Qi Y., Sermer M., Connelly P.W., Hanley A.J., Zinman B. Beta-cell function declines within the first year postpartum in women with recent glucose intolerance in pregnancy. Diabetes Care. 2010;33:1798–1804. doi: 10.2337/dc10-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buchanan T.A., Xiang A.H., Peters R.K., Kjos S.L., Marroquin A., Goico J. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk hispanic women. Diabetes. 2002;51:2796–2803. doi: 10.2337/diabetes.51.9.2796. [DOI] [PubMed] [Google Scholar]
- 49.Ramos-Roman M.A. Prolactin and lactation as modifiers of diabetes risk in gestational diabetes. Hormone and Metabolic Research. 2011;43:593–600. doi: 10.1055/s-0031-1284353. [DOI] [PubMed] [Google Scholar]
- 50.Zhong S., Almario R., Dubrinsky M., Rose K., Lin P.K., Grunberger G. Repeated pregnancy without lactation: effects on maternal glycemic control, pregnancy outcome, carcass composition, and fat distribution in rats. Metabolism: Clinical and Experimental. 1990;39:1127–1132. doi: 10.1016/0026-0495(90)90083-o. [DOI] [PubMed] [Google Scholar]
- 51.Denis R., Williams G., Vernon R. Regulation of serum leptin and its role in the hyperphagia of lactation in the rat. Journal of Endocrinology. 2003;176:193–203. doi: 10.1677/joe.0.1760193. [DOI] [PubMed] [Google Scholar]
- 52.Kim H., Toyofuku Y., Lynn F.C., Chak E., Uchida T., Mizukami H. Serotonin regulates pancreatic beta cell mass during pregnancy. Nature Medicine. 2010;16:804–808. doi: 10.1038/nm.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gunderson E.P., Jacobs D.R., Jr., Chiang V., Lewis C.E., Feng J., Quesenberry C.P., Jr. Duration of lactation and incidence of the metabolic syndrome in women of reproductive age according to gestational diabetes mellitus status: a 20-year prospective study in CARDIA (Coronary Artery Risk Development in Young Adults) Diabetes. 2010;59:495–504. doi: 10.2337/db09-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cordero L., Treuer S.H., Landon M.B., Gabbe S.G. Management of infants of diabetic mothers. Archives of Pediatrics & Adolescent Medicine. 1998;152:249–254. doi: 10.1001/archpedi.152.3.249. [DOI] [PubMed] [Google Scholar]
- 55.Nommsen-Rivers L.A., Chantry C.J., Dewey K.G. Early breastfeeding outcomes in gestational diabetic primiparas delivering term infants. Journal of the Federation of American Societies for Experimental Biology. 2010;24:91–94. [Google Scholar]
- 56.Demirci J.R., Bogen D.L., Holland C., Tarr J.A., Rubio D., Li J. Characteristics of breastfeeding discussions at the initial prenatal visit. Obstetrics and Gynecology. 2013;122(6):1263–1270. doi: 10.1097/01.AOG.0000435453.93732.a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.World Health Organization and UNICEF, 2003. Global strategy for infant and young child feeding. Available at: 〈http://www.who.int/maternal_child_adolescent/documents/9241562218/en/index.html〉.
- 58.Zhuo X., Zhang P., Hoerger T.J. Lifetime direct medical costs of treating type 2 diabetes and diabetic complications. American Journal of Preventive Medicine. 2013;45:253–261. doi: 10.1016/j.amepre.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 59.Canadian Diabetes Association, 2012. The prevalence and costs of diabetes. Available at: 〈http://www.diabetes.ca/diabetes-and-you/what/prevalence/〉.


