Summary
Skeletal muscle stem cells, or “satellite cells” (SCs), are required for the regeneration of damaged muscle tissue. Although SCs self-renew during regeneration, the mechanisms that govern SC re-entry into quiescence remain elusive. We show that FOXO3, a member of the forkhead family of transcription factors, is expressed in quiescent SCs (QSCs). Conditional deletion of Foxo3 in QSCs impairs self-renewal and increases the propensity of SCs to adopt a differentiated fate. Transcriptional analysis of SCs lacking FOXO3 revealed a downregulation of Notch signaling, a key regulator of SC quiescence. Conversely, overexpression of Notch intracellular domain (NICD) rescued the self-renewal deficit of FOXO3-deficient SCs. We show that FOXO3 regulates NOTCH1 and NOTCH3 receptor expression and that decreasing expression of NOTCH1 and NOTCH3 receptors phenocopies the effect of FOXO3 deficiency in SCs. We demonstrate that FOXO3, perhaps by activating Notch signaling, promotes the quiescent state during SC self-renewal in adult muscle regeneration.
Highlights
-
•
FOXO3 is expressed in quiescent adult SCs
-
•
FOXO3 is required for self-renewal of SCs
-
•
FOXO3-deficient SCs display an increased propensity to differentiate
-
•
FOXO3 promotes Notch signaling, a key regulator of quiescence in adult SCs
Little is known about the molecular mechanisms that govern muscle stem cell self-renewal. Rando and colleagues show that FOXO3, a forkhead family transcription factor, activates Notch signaling, a key regulator of the quiescent state in muscle stem cells, and thereby promotes self-renewal during muscle regeneration.
Introduction
A fundamental characteristic of adult stem cells is the ability to serve as source of cells both to give rise to differentiated cells and to replenish the stem cell pool. In skeletal muscle, myogenic stem cells, or “satellite cells” (SCs), exist in a quiescent state, a state of reversible mitotic arrest and reduced metabolic activity that is characteristic of many stem cell populations (Cheung and Rando, 2013). In response to muscle fiber injury, SCs activate, proliferate, and either differentiate into multinucleated myofibers or self-renew (Yoshida et al., 1998, Schmalbruch and Lewis, 2000, Heslop et al., 2001). The process of self-renewal requires that a subset of activated SCs (ASCs) returns to quiescence and involves a complex orchestration of cell-cycle and metabolic transitions (Groszer et al., 2001, He et al., 2009). In adult skeletal muscle, the molecular mechanisms that regulate the self-renewal of SCs have only just begun to be explored (Abou-Khalil et al., 2009, Shea et al., 2010, Le Grand et al., 2012).
The FOXO family of forkhead transcription factors functions downstream of the PI3K/AKT pathway and regulates a wide variety of physiological processes including cell proliferation, differentiation, survival, and metabolism (Greer and Brunet, 2005). Mammals have four members of the FOXO gene family: FOXO1, FOXO3, FOXO4, and FOXO6 (Jacobs et al., 2003, van der Heide et al., 2005, Lam et al., 2006). FOXO family members bind similar DNA sequences (Furuyama et al., 2000) and may therefore display some redundancies in function (Paik et al., 2007, Tothova et al., 2007). However, the presence of specific phenotypes that result from null mutations in Foxo1, Foxo3, and Foxo4 in mice indicates that each Foxo gene has unique physiological roles and is functionally divergent (Castrillon et al., 2003, Hosaka et al., 2004, Lin et al., 2004, Jonsson et al., 2005).
Members of the FOXO family of transcription factors regulate stem cell and progenitor pools in many adult tissues. For example, ablation of Foxo3 alone or in combination with Foxo1 and Foxo4 results in increased cell cycling and reduction of the hematopoietic stem cell pool (Miyamoto et al., 2007, Tothova et al., 2007). Hematopoietic stem cells from FOXO3-deficient mice also have increased levels of reactive oxygen species resulting in apoptosis and a limitation in repopulating ability in vivo (Tothova et al., 2007). In neural tissue, a loss of Foxo3 alone or in combination with Foxo1 and Foxo4 results in a depletion of adult neural stem cells, which is due, at least in part, to the premature proliferative amplification and differentiation of these cells (Renault et al., 2009, Paik et al., 2007). Furthermore, FOXO3 loss decreases the ability of neural stem cells to self-renew in vitro (Renault et al., 2009, Paik et al., 2007). Although the FOXO factors were shown to be expressed in different cell types in muscle and FOXO3 germline knockout mice were reported to display a delay in muscle regeneration (Bosnakovski et al., 2008, Hu et al., 2008), none of the members of the FOXO family have been shown to regulate SC function.
In the present study, we investigated the function of FOXO3 in adult SCs using an inducible genetic system to ablate the Foxo3 gene specifically in SCs. We found that FOXO3 is required for the self-renewal of SCs during muscle regeneration. FOXO3-deficient SCs proliferate more rapidly than control SCs and differentiate more readily, thereby resulting in reduced self-renewal of the SC pool. In addition, we observed that FOXO3 regulates the Notch signaling pathway, an essential regulator of quiescence in adult SCs, such that a deletion of Foxo3 results in reduced levels of Notch signaling in SCs. We propose that the FOXO3-Notch axis is important for SC self-renewal by promoting the reacquisition of quiescence during the self-renewal process.
Results
Foxo3 mRNA and FOXO3 Protein Are Expressed in Quiescent SCs
By immunohistochemical analysis using a FOXO3-specific antibody (Figures S1A and S1B available online), we found that FOXO3 is expressed in quiescent SCs (QSCs), identified as PAX7+ve cells, in adult mouse muscle (Figure 1A). To compare Foxo3 mRNA and FOXO3 protein levels between QSCs and ASCs, we isolated SCs by fluorescence activated cell sorting (FACS) from uninjured hindlimb muscles and from muscles 2.5 days after injury, respectively (Cheung et al., 2012, Bjornson et al., 2012). Quantitative RT-PCR (qRT-PCR) analysis revealed that Foxo3 mRNA was expressed at higher levels in QSCs compared to ASCs (Figure 1B). Western blot analysis also showed a clear reduction in FOXO3 protein in ASCs compared to QSCs (Figures 1C and 1D).
Figure 1.
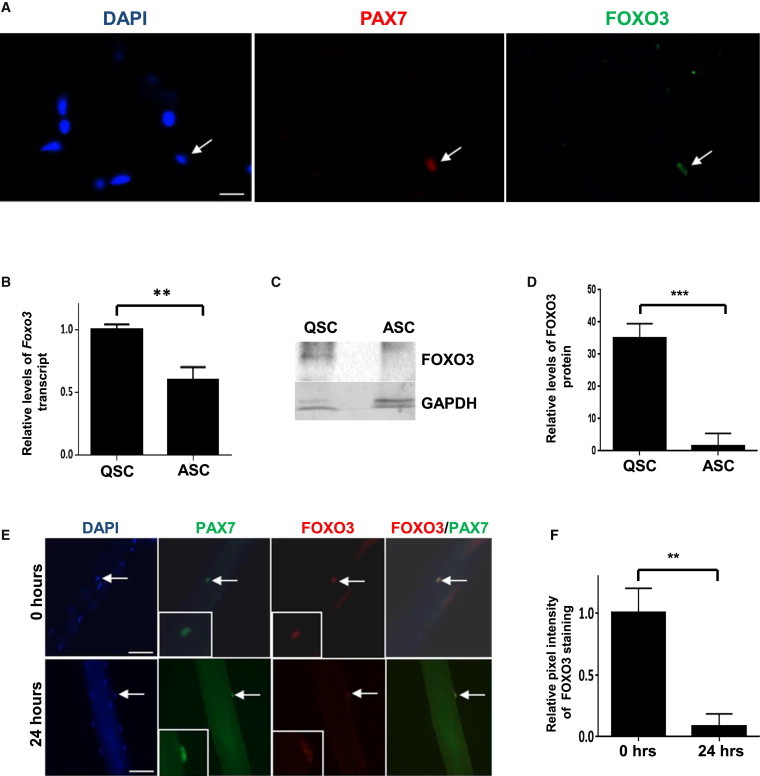
FOXO3 Is Expressed in QSCs and Is Downregulated upon Activation
(A) Cryosections of uninjured tibialis anterior (TA) muscle contain PAX7+ve SCs that stain positive for FOXO3 (arrow).
(B) FACS-purified QSCs and ASCs from injured muscle isolated 2.5 days after injury were assessed for levels of Foxo3 transcript by qRT-PCR. Foxo3 transcript levels in ASCs are normalized to those in quiescence. QSC and ASC samples represent triplicate experiments of pooled RNA from two mice for each experiment (∗∗p < 0.01).
(C) A representative western blot analysis of lysates from FACS-purified QSCs and ASCs plated for 1 day in culture. Blots were probed with an antibody to FOXO3. FOXO3 protein levels are higher in QSCs compared to ASCs.
(D) The graph shows quantitative analyses of replicate blots of relative intensities of FOXO3 bands normalized to intensities of GAPDH for the corresponding sample. QSC and ASC samples represent triplicate experiments of pooled lysates from SCs sorted from two mice for each experiment (n = 3).
(E) Single myofibers with associated SCs were fixed either immediately after isolation (0 hr) or after 24 hr in culture and then immunostained for PAX7 and FOXO3. FOXO3 expression is reduced in SCs after 24 hr in culture compared to SCs at 0 hr. The insets in the lower left of the PAX7 and FOXO3 panels show high-magnification images of the SCs (indicated by the arrows) in the images. The FOXO3/PAX7 panels show merged images of PAX7 and FOXO3 staining, which show colocalization of the two proteins in the nucleus at 0 hr but that, at 24 hr, FOXO3 staining is much reduced.
(F) The graph shows the mean relative pixel intensities for nuclear FOXO3 staining at 0 hr and 24 hr that was quantified and expressed as a ratio after normalization with the pixel intensities for the corresponding PAX7 staining. There is a significant reduction in FOXO3 staining after 24 hr in culture (n = 10 myofibers per sample).
Scale bars represent 25 μm in (A) and 100 μm in (E).
To assess FOXO3 subcellular localization in QSCs and ASCs, we isolated single muscle fibers and fixed them immediately after isolation or cultured them for 1 day prior to fixation and staining with a FOXO3 antibody. In QSCs, FOXO3 localization was restricted to the nucleus where it colocalized with PAX7 expression (Figure 1E), whereas the intensity of nuclear staining of FOXO3 was markedly reduced in ASCs (Figure 1F). Thus, FOXO3 is likely to be more active in QSCs than in ASCs.
FOXO3 Is Required for the Self-Renewal of SCs
To investigate the functional consequences of disrupting FOXO3 expression on SC function, we generated mice in which Foxo3 was specifically deleted in SCs. To do this, we generated Pax7CreER/+; Foxo3fl/fl mice (which we refer to as Foxo3cKO mice) in which a tamoxifen-inducible Cre recombinase (CreER) is expressed from the 3′ untranslated region of the Pax7 locus (Nishijo et al., 2009), and the second exon of the Foxo3 allele is flanked by loxP sites (Castrillon et al., 2003). The activation of CreER by administration of tamoxifen to Foxo3cKO mice results in the deletion of the Foxo3 gene specifically in QSCs. Quantitative RT-PCR analysis confirmed that, upon tamoxifen injection in adult mice, Foxo3 transcript levels in QSCs were reduced to levels that correlated with the efficiency of recombination (∼65% as estimated by the population of SCs expressing eYFP in FACS analyses) (Figure S2A). One month after tamoxifen administration to Foxo3cKO mice, the gross histology of muscles appeared normal, and there were no statistically significant differences in SC numbers between Foxo3cKO and control muscles (Figures S2B and S2C). To test directly for a functional requirement of FOXO3 in SCs in the context of regeneration, tibialis anterior (TA) muscles of tamoxifen-treated Foxo3cKO and control mice were injured by BaCl2 injection (Caldwell et al., 1990). One week after injury, histological analysis of Foxo3cKO muscles revealed no gross differences in fiber morphology or fiber sizes compared to control muscles (Figures S2D and S2E). However, an analysis of the regenerating muscle, which is characterized by the presence of centrally nucleated fibers (CNFs), revealed a 60% decline in the number of SCs associated with CNFs in Foxo3cKO muscles compared to control muscles (Figure 2A). This suggests that FOXO3 is required for efficient SC self-renewal.
Figure 2.
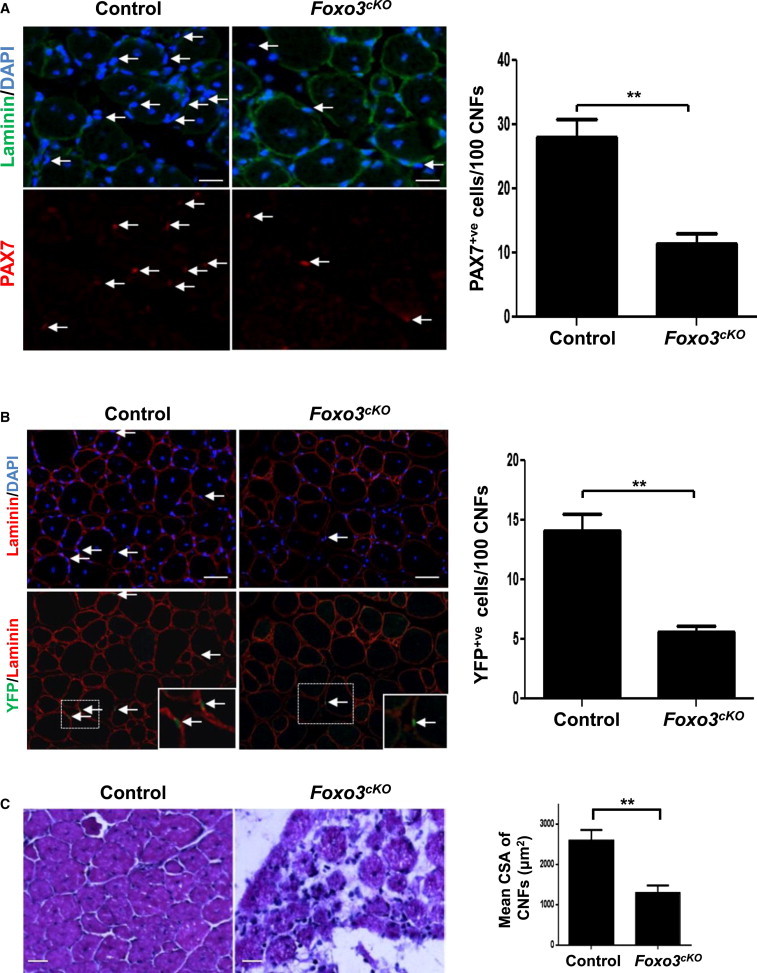
FOXO3 Is Required for SCs to Self-Renew during Muscle Regeneration
(A) Four weeks after the initiation of tamoxifen treatment, Foxo3cKO and control mice were injured, and muscles were harvested 7 days later. In the panels, the arrows point to PAX7+ve SCs associated with CNFs in control and Foxo3cKO muscles. The graph shows the average number of PAX7+ve SCs per 100 CNFs from multiple sections in control and Foxo3cKO muscles (n = 4 mice per genotype) (∗∗p < 0.01).
(B) One month after tamoxifen treatment, Foxo3cKO-YFP and control-YFP mice were injured, and muscles were harvested 1 month later. Cryosections were stained for Laminin and YFP. In the panels, the arrows point to YFP+ve SCs associated with CNFs in control and Foxo3cKO muscles. The insets show high-magnification images of the areas in dotted rectangles. The graph shows the average number of YFP+ve SCs per 100 CNFs from multiple sections in control and Foxo3cKO muscles (n = 4 mice per genotype) (∗∗p < 0.05).
(C) Muscles of tamoxifen-treated Foxo3cKO and control mice were injured twice, with a spacing of 2 weeks between successive injuries. Cryosections stained with hematoxylin and eosin reveal impaired regeneration in the Foxo3cKO muscles. The graph shows the mean cross-sectional area of CNFs from multiple sections in control and Foxo3cKO muscles (n = 3 mice per genotype) (∗∗p < 0.05).
To investigate if quiescent SC numbers are restored in the Foxo3cKO mice over a longer period, we used Foxo3cKO mice in which eYFP is conditionally expressed from the ROSA locus (Foxo3cKO-YFP mice) to mark FOXO3-deficient SCs after tamoxifen administration. FOXO3 protein could not be detected in YFP+ve SCs in these mice (Figure S2F). Mice were injured 1 month after tamoxifen treatment, and muscles were harvested 1 month later. Foxo3cKO-YFP mice displayed a significant decrease in the number of self-renewed SCs compared to control mice, as determined by the number of YFP+ve SCs associated with CNFs (Figure 2B). We confirmed that these YFP+ve SCs indeed represented a self-renewing population by the presence of PAX7 and absence of MYOD expression (Figure S2G). The functional consequence of the self-renewal deficit was demonstrated by the markedly impaired regeneration of muscle in Foxo3cKO mice following sequential rounds of muscle injury (Figure 2C). These experiments confirm the importance of FOXO3 in SC return to quiescence during self-renewal.
The induction of quiescence in proliferating SC progeny is essential for the self-renewal process and can be studied in vitro using the “reserve cell” model in which proliferating cells are switched to mitogen-poor medium, resulting in the terminal differentiation of most cells and a reversible cell-cycle withdrawal into a quiescent state of a subpopulation of cells (Yoshida et al., 1998). Because FOXO3 appeared to be critical for SC self-renewal in vivo, we sought to determine whether increasing or decreasing FOXO3 activity would alter the entry of proliferating SCs into a quiescent state in vitro using this reserve cell model. On the basis of immunocytochemistry, we characterized myogenic cells in low serum cultures as quiescent reserve cells (PAX7+ve) or differentiated cells (Myogenin+ve or MyHC+ve). In order to test further the hypothesis that FOXO3 promotes SC quiescence, we overexpressed a constitutively active form of FOXO3 (GFP-FOXO3-TM) (Brunet et al., 1999) in proliferating SC progeny isolated from mice lacking Foxo3 (Foxo3−/−). Strikingly, we observed that all FOXO3-TM-expressing (i.e., GFP+ve) cells were PAX7+ve and Myogenin−ve/MyHC−ve after 3 days in low serum medium (Figure S3A). In the absence of FOXO3, the ability of proliferating SC progeny to return to quiescence was almost completely abolished (Figure S3B). Furthermore, when grown in high serum medium, FOXO3-TM-expressing cells failed to proliferate compared to control transfected cells, consistent with the ability of FOXO3 activity to promote cellular quiescence (Figure S3C).
Conversely, we wanted to investigate the effects of the absence of FOXO3 on the ability of quiescent SCs to break quiescence and enter the cell cycle. Therefore, we isolated FOXO3-deficient SCs associated with single fibers from Foxo3−/− and control mice, and we pulse-labeled fiber cultures with 5-ethynyl-2′-deoxyuridine (EdU). Although the absence of FOXO3 was, itself, insufficient to induce SCs to break quiescence in vivo, the additional stimulus imposed by ex vivo explantation led to a much greater activation of FOXO3-deficient SCs compared to controls. By 36 hr, there were twice as many EdU-incorporating SC progeny in cultures from Foxo3−/− mice as in cultures from control mice (Figure 3A). Additionally, proliferating SC progeny isolated from Foxo3−/− muscles displayed a significantly greater percentage of cells in S phase compared to wild-type SC progeny (Figure 3B). These data support the role of FOXO3 in maintaining SC quiescence and suppressing cell-cycle entry.
Figure 3.
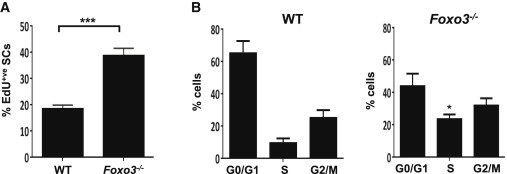
FOXO3 Suppresses Cell-Cycle Entry of SCs
(A) SCs from Foxo3−/− and wild-type mice were tested for entry into the cell cycle by the incorporation of EdU at different times. The graph shows the percentage of SCs from Foxo3−/− and wild-type (WT) mice that incorporated EdU in the first 36 hr in culture. A greater number of Foxo3−/− SCs incorporated EdU than wild-type SCs (∗∗∗p < 0.005).
(B) FACS analysis was performed on proliferating SC progeny isolated from Foxo3−/− and wild-type mice. The distribution of SC progeny in G0/G1, S, and G2/M phases of the cell cycle are plotted as a percentage of the total number of cells. The percentage of Foxo3−/− myogenic progenitors in S phase was twice that of wild-type cells (n = 3 mice per genotype).
FOXO3-Deficient SCs Display Increased Differentiation
To test for the possibility that the apparent inhibition of self-renewal in FOXO3-deficient SCs was due to an alteration in potential cell-fate choices, we used single myofiber preparations isolated from extensor digitorum longus (EDL) muscles of control and Foxo3cKO mice 1 month after tamoxifen administration. Immediately after isolation and prior to activation, >95% of SCs associated with myofibers exhibited features of QSCs (i.e., PAX7+ve and MYOD−ve) in both control and Foxo3cKO fibers. The remaining cells exhibited features of ASCs (i.e., PAX7+ve and MYOD+ve). During the subsequent 48 and 72 hr of culturing, SC progeny were characterized as activated (PAX7+ve, MYOD+ve), self-renewing (PAX7+ve, MYOD−ve), or differentiating (PAX7−ve, MYOD+ve) (Zammit et al., 2004). After 48 and 72 hr in culture, no self-renewing SCs could be detected in the FOXO3-deficient cultures, whereas a small but clear subset of control SCs was undergoing self-renewal at these times (Figures 4A–4C). Strikingly, the population of SC progeny undergoing differentiation was far greater in the Foxo3cKO population compared to control cells (Figures 4A, 4B, and 4D). Therefore, we conclude that FOXO3 increases the propensity of SCs to self-renew and decreases their propensity to differentiate.
Figure 4.
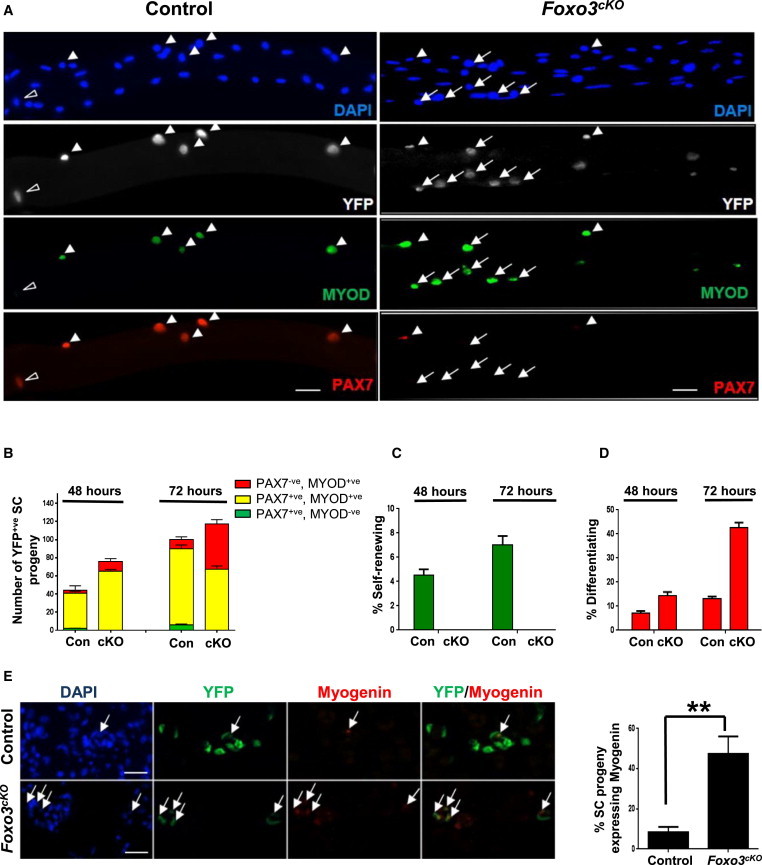
FOXO3-Deficient SCs Exhibit Enhanced Propensity to Differentiate
(A) One week after tamoxifen treatment, single myofibers were isolated from control (Con) and Foxo3cKO (cKO) EDL muscles and cultured for 72 hr. YFP+ve SCs associated with individual myofibers were coimmunostained for PAX7 and MYOD to distinguish self-renewing (PAX7+ve, MYOD−ve; open arrowheads), activated (PAX7+ve, MYOD+ve; closed arrowheads), and differentiation-committed (PAX7−ve, MYOD+ve; arrows) SCs. Nuclei were counterstained with DAPI.
(B) The distribution of the three populations of SCs illustrated in (A) was quantified after 48 or 72 hr in culture. Data from multiple myofibers (n = 50 fibers per sample) were pooled to give a population mean (±SEM) for cells in each category.
(C) SCs from control and Foxo3cKO fibers undergoing self-renewal after 48 and 72 hr in culture are plotted as a percentage of the total number of YFP+ve SCs counted in each category. Compared to control SCs, Foxo3cKO SCs failed to self-renew.
(D) SCs from control and Foxo3cKO fibers that were committed to differentiation after 48 and 72 hr are plotted as a percentage of the total number of YFP+ve SCs counted in each category. Foxo3cKO SCs displayed an increased propensity to differentiate over time in culture compared to control SCs.
(E) One month after tamoxifen treatment, muscles of Foxo3cKO and control mice were injured and harvested 3 days later. Cryosections were immunostained for Myogenin and GFP. The arrows point to the YFP+ve,Myogenin+ve cells. The graph on the right shows that Foxo3cKO SCs have a much greater propensity to differentiate compared to control SCs (n = 3 mice per genotype) (∗∗p < 0.01).
Scale bars represent 100 μm in (A) and 25 μm in (E).
To confirm that a reduction in the self-renewal potential of FOXO3-deficient SCs was associated with an increased propensity of the progeny to undergo differentiation, the muscles of Foxo3cKO or control mice were injured 1 month after tamoxifen treatment, harvested 3 days later, and stained for YFP and Myogenin. We observed that a significantly greater fraction of SC progeny from Foxo3cKO muscles expressed Myogenin compared to those from control mice (Figure 4E). Likewise, when YFP+ve cells were obtained from such muscles and plated in differentiation-promoting conditions, a much greater percentage of FOXO3-deficient cells underwent myogenic differentiations (Figure S4). These results suggest that FOXO3 does in fact suppress the propensity of SC progeny to undergo terminal differentiation, thus allowing a greater percentage of cells to self-renew and re-enter the quiescent state.
FOXO3 Promotes the Notch Signaling Pathway
Because a major characteristic of the stem cell self-renewal process is the reacquisition of the quiescent state, we tested the hypothesis that FOXO3 might control SC self-renewal by modulating signaling pathways that promote quiescence. To this end, we isolated QSCs from hindlimb muscles from control and Foxo3−/− mice and assessed the expression of genes in the Notch pathway, which has been clearly associated with the promotion or maintenance of SC quiescence (Bjornson et al., 2012, Mourikis et al., 2012, Wen et al., 2012). Intriguingly, we observed that Foxo3−/− SCs displayed a significant downregulation of several Notch target genes (Hes1, Hes2, Hes6, HeyL) compared to wild-type SCs (Figure 5A). Of the Notch receptors that have been reported to be highly expressed in adult SCs (Notch1, Notch2, and Notch3) (Mourikis et al., 2012), both Notch1 and Notch3 were downregulated in Foxo3−/− QSCs, as was Notch4 (Figure 5B). To determine the status of Notch signaling in Foxo3cKO SCs, we assessed the levels of active Notch intracellular domain (NICD) in sorted SCs from control and Foxo3cKO mice. Western blot analysis revealed a reduction of NICD levels in Foxo3cKO SCs compared to control SCs (Figure 5C), consistent with a decrease in the quiescence-promoting activity of Notch signaling in Foxo3cKO SCs.
Figure 5.
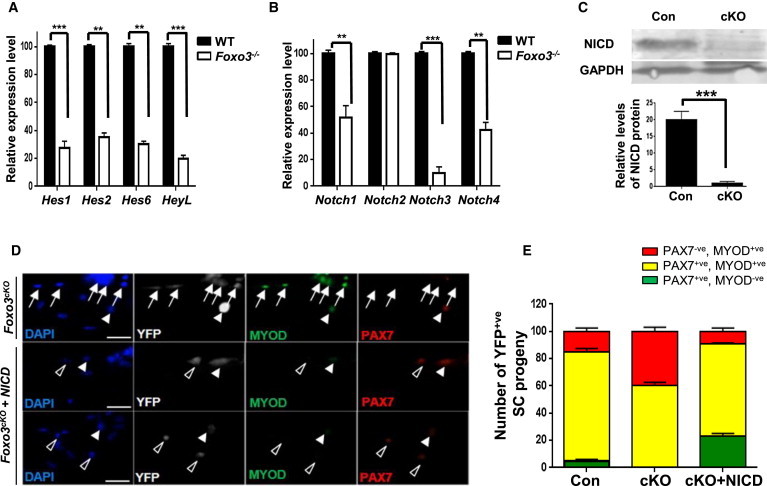
FOXO3 Promotes Activation of Notch Signaling, an Essential Pathway for the Maintenance of SC Quiescence
(A and B) FACS-purified QSCs were isolated from Foxo3−/− and wild-type muscles and analyzed for levels of expression of Notch target genes and Notch receptors by qRT-PCR. RNA expression levels in Foxo3−/− SCs are normalized to those in wild-type SCs. Samples represent triplicate experiments of pooled RNA from two mice for each experiment (∗∗∗p < 0.005; ∗∗p < 0.01).
(C) A western blot analysis with an anti-NICD antibody was performed with lysates from FACS-sorted SCs isolated from Foxo3cKO and control mice. Control represents wild-type SCs isolated from littermate offspring that were not treated with tamoxifen. The graph below shows that NICD levels are reduced in Foxo3cKO SCs. GAPDH serves as a loading control. Con and cKO samples represent triplicate experiments of pooled lysates from SCs sorted from two mice for each experiment (∗∗∗p < 0.005).
(D) Single myofibers isolated from EDL muscles from tamoxifen-treated Foxo3cKO mice were infected with retroviral vectors expressing NICD or control virus for 72 hr. Myofibers from EDL muscles from control mice were also isolated. YFP+ve SCs were coimmunostained with PAX7 and MYOD to determine self-renewing (PAX7+ve, MYOD−ve; open arrowheads), proliferating (PAX7+ve, MYOD+ve; closed arrowheads), and differentiating (PAX7−ve, MYOD+ve; arrows) populations. Nuclei were counterstained with DAPI (scale bar represents 100 μm).
(E) The distribution of the three populations of SCs illustrated in (D) was quantified after 72 hr in culture. NICD expression in cKO SCs results in an increase in self-renewing SCs. Data from multiple myofibers were pooled to give a population mean (±SEM) for cells in each category (n = 80 fibers per sample).
To test if, conversely, FOXO3 can induce Notch signaling, we overexpressed wild-type FOXO3 in proliferating SC progeny. qRT-PCR experiments revealed that FOXO3 overexpression resulted in an upregulation of a subset of Notch targets (Hes1, Hes2, and HeyL) and receptors (Notch1, Notch2, and Notch3) in SC progeny (Figures S5A and S5B). To confirm further that increasing FOXO3 activity can enhance Notch activity, SC progeny were transfected with GFP-FOXO3-TM and a Notch reporter construct (pHes1-Luc). We observed an increase in luciferase activity in cells expressing FOXO-TM compared to cells expressing a control vector (Figure S5C). To determine whether increasing Notch activity would enhance self-renewal in FOXO3-deficient SCs, we used a retroviral vector to express NICD in single fiber-associated SCs isolated from Foxo3cKO mice (Figures 5D, 5E, and S5D). After 72 hr in culture, we observed an increase in self-renewing SCs upon NICD expression in FOXO3-deficient cultures compared to control-infected cultures, demonstrating that an increase in Notch activity can rescue the self-renewal deficit of FOXO3-deficient SCs (Figures 5D and 5E).
We next addressed whether FOXO3 could directly regulate Notch receptor expression as a mechanism by which FOXO3 might modulate Notch signaling. By bioinformatic analysis, we identified putative FOXO-responsive-elements (FREs) in the promoters of the Notch1 and Notch3 genes (Figure S5E). To determine if Notch1and Notch3 are direct targets of FOXO3, we performed chromatin immunoprecipitation (ChIP) of endogenous FOXO3 in proliferating SC progeny. To induce translocation of endogenous FOXO3 to the nucleus, we treated the cells with LY294002 (LY), a specific PI3kinase inhibitor, which has been used successfully for FOXO3 ChIP (Brunet et al., 1999, Renault et al., 2009, Webb et al., 2013). The induction of FOXO3 translocation and binding was confirmed by the demonstration of FOXO3 binding to the FRE of a well-known FOXO target, p27KIP1 (Renault et al., 2009) (Figure 6A). Under these conditions, we observed binding of FOXO3 to each of the FREs in the Notch1 and Notch3 promoters, suggesting that FOXO3 may regulate the expression of Notch receptors in SC progeny (Figure 6A).
Figure 6.
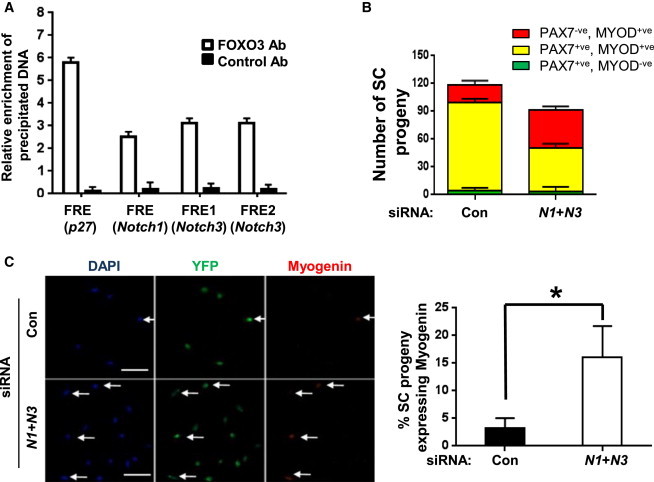
FOXO3 Regulates NOTCH1 and NOTCH3 Receptor Expression, Thereby Promoting Notch Signaling
(A) SC progeny were incubated in medium containing 20 μm LY for 2 hr to activate endogenous FOXO3. Cells were lysed and processed for ChIP using FOXO3 antibody and the isotype control antibody, followed by qRT-PCR. FOXO3 binding to p27KIP1, a known target gene of FOXO3, was used to confirm activation of FOXO3. FOXO3 binding to the putative FREs located at 1.2 kb upstream of the transcriptional start site of the of the Notch1 gene and 1.5 and 2.9 kb upstream of the transcriptional start site of the Notch3 gene were significantly higher compared to immunoprecipitates with the control antibody. Data are normalized for background levels and input chromatin for each sample and are calculated as a percentage of the input. The graph shows relative levels of enrichment of FOXO3 binding in input samples precipitated with FOXO3 antibody and control antibody and represents the quantitation of two independent ChIP experiments.
(B) Single myofibers with associated SCs were treated with 50 nM of siRNA against Notch1 (N1) and 20 nM of siRNA against Notch3 (N3) or a cyclophilin siRNA control for 24 hr. After 72 hr, fibers were fixed and immunostained for PAX7 and MYOD. Cultures treated with siRNAs against Notch receptors displayed an increased percentage of differentiating cells. Data from multiple myofibers were pooled to give a population mean (±SEM) for cells in each category (n = 50 fibers per sample).
(C) FACS-sorted SCs were plated in the presence of N1and N3 siRNAs or control siRNAs for 24 hr. After 48 hr, cells were fixed and stained for GFP, Myogenin, and DAPI. Representative fields are shown on the left and quantitative analysis on the right. Arrows point to YFP+ve SC progeny that are Myogenin+ve. The graph represents values obtained from separate FACS sorts from three mice (n = 3). Scale bar represents 100 μm.
Next, we tested whether knocking down Notch1 and Notch3 receptor expression using specific small interfering RNAs (siRNAs) would phenocopy the effect of absence of Foxo3 in SCs. We observed an increase in differentiating cells upon treatment of SCs with siRNAs to Notch1 and Notch3 (Figures 6B and S5F), similar to the effect of Foxo3 deficiency in SCs (Figures 4A, 4B, and 4D). To examine the effect of knocking down Notch1 and Notch3, we sorted SCs and plated them in the presence of siRNAs against Notch1 and Notch3 and observed an increase in the propensity of these cells, like FOXO3-deficient SC progeny, to undergo differentiation compared to control cells (Figure 6C). Taken together, our results show that a FOXO3-Notch axis suppresses the ability of SC progeny to differentiate and promotes their propensity to undergo self-renewal, thus playing a critical role in the retention of the quiescent SC pool during the process of muscle regeneration.
Discussion
Our results demonstrate a functional requirement for FOXO3 in the induction of SC quiescence and the process of SC self-renewal during adult muscle regeneration, such that a loss of FOXO3 results in a reduction of the SC pool after injury. We show that FOXO3 promotes self-renewal by a mechanism that involves the prevention of premature terminal differentiation in SCs. Importantly, our data implicate the modulation of Notch signaling, a key regulator of quiescence in SCs, as a target of FOXO3 activity and as a potential mediator of the promotion of self-renewal by FOXO3.
Our experiments show that after a single round of injury, Foxo3cKO muscles regenerated as well as control muscles. An earlier study reported impaired regeneration in Foxo3−/− muscles upon cardiotoxin-induced injury (Hu et al., 2008). This study used mice in which Foxo3 is deleted in every tissue, whereas our study used mice in which Foxo3 is ablated specifically in SCs. Because FOXO3 expression is not restricted to SCs and is expressed in other cell types such as endothelial cells and vascular smooth muscle cells (Potente et al., 2005, Lee et al., 2007), it is possible that SCs in Foxo3−/− mice are subject to influences from FOXO3-deficient cells in the niche, leading to impaired muscle regeneration.
In the absence of growth factors or in the presence of stress stimuli, FOXO members reside in the nucleus and are active as transcription factors (Tothova et al., 2007). In response to growth factor stimulation, AKT phosphorylates FOXOs at three conserved serine and threonine residues (Thr32, Ser253, and Ser315 in FOXO3), permitting the binding of 14-3-3 chaperone proteins that facilitate the translocation of FOXOs from the nucleus to the cytoplasm (Brunet et al., 1999). Cytoplasmic FOXOs are then targeted for ubiquitination and proteosomal degradation (Huang and Tindall, 2011). Using RT-PCR analysis and western blot analysis, we detected higher levels of FOXO3 expression in QSCs compared to ASCs, leading us to hypothesize that FOXO3 activity is important in the quiescent state in SCs (Figures 1B–1F). In regenerating muscle, the milieu is rich with growth factors (Jennische and Hansson, 1987), a condition that is likely to contribute to the inhibition of FOXO3 function. After a peak, growth factor levels decline during the regenerative process (Allen and Boxhorn, 1989). This would lead to an increase in FOXO3 activity during the phase of SC self-renewal in which a subset of SC progeny return to the quiescent state.
Our experiments demonstrate that the modulation of Notch signaling by FOXO3 is a critical component in the molecular mechanisms that FOXO3 utilizes to regulate self-renewal of the muscle stem cell compartment. A recent study reported the repression of p38 MAPK in one of the daughter cells following SC division as a mechanism in daughter cells that undergo self-renewal (Troy et al., 2012). In the hematopoietic system as well, modulation of the p38 MAPK pathway has been proposed to mediate the function of FOXO3 to maintain the pool of hematopoietic stem cells (HSCs) (Miyamoto et al., 2007). In a study aimed to determine the crosstalk between Notch signaling and the MAPK pathways, it was found that active Notch signaling suppresses p38 MAPK activity by the induction of a MAPK phosphatase, MKP-1, in C2C12 cells (Kondoh et al., 2007). It is possible that active Notch signaling suppresses p38 MAPK in SCs as well, thus representing a nodal point of interaction between these pathways and enabling self-renewal in these cells.
A previous report showed that Notch signaling is important in the process of asymmetric SC divisions such that pharmacological inhibition of Notch activity results in a reduction of self-renewing cells (Kuang et al., 2007). Indeed, inhibition of Notch signaling in SCs by the deletion of the transcriptional coactivator of the Notch pathway, RBP-Jκ, results in a gradual depletion of the SC pool due to the spontaneous activation and differentiation of SCs and their failure to self-renew (Bjornson et al., 2012, Mourikis et al., 2012). Interestingly, reduced levels of Notch signaling in FOXO3-deficient SCs did not deplete the SC pool in resting muscle as seen in SCs deficient for RBP-Jκ. This suggests that there could be a functional redundancy in the maintenance of the quiescent state in SCs by other FOXO homologs, FOXO1 and FOXO4, as well as by regulators unrelated to the FOXO family. Alternatively, reduced levels of Notch signaling in FOXO3-deficient SCs may not quantitatively equate to levels resulting from deleting RBP-Jκ. This could be indicative of dosage in Notch signaling affecting different outcomes in regulating the quiescent state. Along these lines, studies on the function of NOTCH2 and DLL4 signaling in the vasculature have shown that the functional deletion of one allele results in phenotypic abnormalities that are less severe than those that occur when both the alleles are deleted (McCright et al., 2001, Duarte et al., 2004).
In summary, our findings demonstrate a functional requirement for FOXO3 as a regulator of Notch signaling in the self-renewal of SCs during muscle regeneration. These findings add to the growing list of cellular mechanisms that have been studied in the context of the proliferation and differentiation of stem cells and their progeny but are also important to the maintenance and attainment of quiescence (Cheung and Rando, 2013). The regulation of cellular quiescence is clearly a central aspect of SC self-renewal, a process that appears to be dysregulated in both aging and disease and leads ultimately to an impairment of stem cell function (Conboy et al., 2005, Brack et al., 2007).
Experimental Procedures
Animals
Foxo−/− and Foxo3fl/fl mice were generated in the lab of Dr. Ronald DePinho (Dana Farber Cancer Institute, Boston). Wild-type FVB/N mice were purchased from Charles River Laboratories. Foxo3fl/fl mice were crossed with Pax7CreERtm (referred in the text to as Pax7CreER/+) mice (Nishijo et al., 2009) to generate Pax7CreER/+; Foxofl/+ mice. F1 mice were crossed with Foxo3fl/+ to generate Pax7CreER/+; Foxo3fl/fl (experimental) and Pax7CreER/+; Foxo3+/+ (control) mice. For lineage tracing, tamoxifen-regulated enhanced yellow fluorescence protein (YFP) was introduced into the control and experimental genetic backgrounds using ROSA26tm1 (EYFP)/Cas mice (Srinivas et al., 2001) (referred to as ROSAeYFP/+ in the text) to generate Pax7CreER/+; Foxo3fl/fl; ROSAeYFP/+ (experimental) and Pax7CreER/+; Foxo3+/+; ROSAeYFP/+ (control) mice. All mice used in this study were between 4 and 6 months of age. Animal strain maintenance, surgical procedures, drug treatments, and husbandry were carried out at the Veterinary Medical Unit at the Veterans Affairs Health Care System in Palo Alto, and all procedures were approved by the Institutional Animal Care and Use Committee.
Muscle Injury
Mice were anesthetized with isofluorane and TA muscles were injured by the injection of 30 μl of 1.2% BaCl2 solution (w/v in ddH2O, Sigma-Aldrich).
Single Fiber Preparations
To isolate single myofibers, EDL muscles were digested with 0.2% type 11 Collagenase (Worthington Biochemical) in Ham’s F10 medium for 75 min at 37°C with shaking. Muscles were dissociated by gentle triturating and were washed several times to eliminate cellular debris and contaminating cells. EDL myofibers were then fixed immediately or after various times using 2% paraformaldehyde (PFA, Sigma-Aldrich) in PBS. For suspension culture, myofibers were incubated in Ham’s F10 medium containing 10% horse serum (v/v) (Invitrogen) at 37°C in 5% CO2.
Immunofluorescence and Histology
Details regarding antibodies and antigen retrieval are given in Supplemental Experimental Procedures. Fluorescence was visualized and photographed using a Zeiss Axiovert Microscope (Carl Zeiss), and images were processed using Volocity software (PerkinElmer).
Quantitative RT-PCR
Quantitative RT-PCR (RT-PCR) was performed using the LightCycler 480 Real-Time PCR System with the LightCycler 480 SYBR Green 1 Mastermix (Roche). Each sample was amplified in triplicate using primers specific to the Notch signaling pathway (Bjornson et al., 2012). Primer sets used were Foxo3 (5′-TCACCCATGCAGACTATCCA-3′, 5′-GTCTGGTTGCCGTAGTGTGA-3′) and glyceraldehade 3-phosphate dehydrogenase (Gapdh) (5′-TGCGACTTCAACAG CAACTC-3′, 5′-ATGTAGGCCATGAGGTCCAC-3′). Expression levels were normalized to Gapdh.
Statistical Analyses
A minimum of three replicates in terms of individual animals was used per experiment, and data are represented as mean ± SEM. For isolation of protein and RNA from SCs, a minimum of three animals was used and FACS-sorted cells were pooled for subsequent analysis. Two-tailed Student’s t tests were used to test for statistically significant differences between groups using GraphPad Prism software. Differences were considered significant at the p < 0.05 level.
Acknowledgments
We thank members of the Rando Lab and the Brunet lab for critical discussions and reading of the manuscript. In particular, we thank Dr. Valérie Renault from the Brunet lab and Drs. Stefano Biressi and Stéphane Boutet from the Rando lab for advice on experiments. Pax7CreER mice were kindly provided by Dr. Charles Keller (OHSU, Portland, OR). We also thank Dr. Ronald DePinho (MD Anderson Cancer Center, Houston) for the Foxo−/− and Foxo3fl/fl mice. This work was supported by the Glenn Foundation for Medical Research and an Ellison Medical Foundation/AFAR postdoctoral fellowship to A.E.W., a grant from the NIH (P01 AG036695) to A.B., and grants from the NIH (P01 AG036695, R37 AG023806, and R01 AR062185) and the Department of Veteran Affairs to T.A.R.
Published: March 20, 2014
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/3.0/).
Supplemental Information includes Supplemental Experimental Procedures and five figures and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2014.02.002.
Supplemental Information
References
- Abou-Khalil R., Le Grand F., Pallafacchina G., Valable S., Authier F.J., Rudnicki M.A., Gherardi R.K., Germain S., Chretien F., Sotiropoulos A. Autocrine and paracrine angiopoietin 1/Tie-2 signaling promotes muscle satellite cell self-renewal. Cell Stem Cell. 2009;5:298–309. doi: 10.1016/j.stem.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen R.E., Boxhorn L.K. Regulation of skeletal muscle satellite cell proliferation and differentiation by transforming growth factor-beta, insulin-like growth factor I, and fibroblast growth factor. J. Cell. Physiol. 1989;138:311–315. doi: 10.1002/jcp.1041380213. [DOI] [PubMed] [Google Scholar]
- Bjornson C.R., Cheung T.H., Liu L., Tripathi P.V., Steeper K.M., Rando T.A. Notch signaling is necessary to maintain quiescence in adult muscle stem cells. Stem Cells. 2012;30:232–242. doi: 10.1002/stem.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosnakovski D., Xu Z., Li W., Thet S., Cleaver O., Perlingeiro R.C., Kyba M. Prospective isolation of skeletal muscle stem cells with a Pax7 reporter. Stem Cells. 2008;26:3194–3204. doi: 10.1634/stemcells.2007-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brack A.S., Conboy M.J., Roy S., Lee M., Kuo C.J., Keller C., Rando T.A. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–810. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- Brunet A., Bonni A., Zigmond M.J., Lin M.Z., Juo P., Hu L.S., Anderson M.J., Arden K.C., Blenis J., Greenberg M.E. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Caldwell C.J., Mattey D.L., Weller R.O. Role of the basement membrane in the regeneration of skeletal muscle. Neuropathol. Appl. Neurobiol. 1990;16:225–238. doi: 10.1111/j.1365-2990.1990.tb01159.x. [DOI] [PubMed] [Google Scholar]
- Castrillon D.H., Miao L., Kollipara R., Horner J.W., DePinho R.A. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 2003;301:215–218. doi: 10.1126/science.1086336. [DOI] [PubMed] [Google Scholar]
- Cheung T.H., Rando T.A. Molecular regulation of stem cell quiescence. Nat. Rev. Mol. Cell Biol. 2013;14:329–340. doi: 10.1038/nrm3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung T.H., Quach N.L., Charville G.W., Liu L., Park L., Edalati A., Yoo B., Hoang P., Rando T.A. Maintenance of muscle stem-cell quiescence by microRNA-489. Nature. 2012;482:524–528. doi: 10.1038/nature10834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy I.M., Conboy M.J., Wagers A.J., Girma E.R., Weissman I.L., Rando T.A. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- Duarte A., Hirashima M., Benedito R., Trindade A., Diniz P., Bekman E., Costa L., Henrique D., Rossant J. Dosage-sensitive requirement for mouse Dll4 in artery development. Genes Dev. 2004;18:2474–2478. doi: 10.1101/gad.1239004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuyama T., Nakazawa T., Nakano I., Mori N. Identification of the differential distribution patterns of mRNAs and consensus binding sequences for mouse DAF-16 homologues. Biochem. J. 2000;349:629–634. doi: 10.1042/0264-6021:3490629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer E.L., Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24:7410–7425. doi: 10.1038/sj.onc.1209086. [DOI] [PubMed] [Google Scholar]
- Groszer M., Erickson R., Scripture-Adams D.D., Lesche R., Trumpp A., Zack J.A., Kornblum H.I., Liu X., Wu H. Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. Science. 2001;294:2186–2189. doi: 10.1126/science.1065518. [DOI] [PubMed] [Google Scholar]
- He S., Nakada D., Morrison S.J. Mechanisms of stem cell self-renewal. Annu. Rev. Cell Dev. Biol. 2009;25:377–406. doi: 10.1146/annurev.cellbio.042308.113248. [DOI] [PubMed] [Google Scholar]
- Heslop L., Beauchamp J.R., Tajbakhsh S., Buckingham M.E., Partridge T.A., Zammit P.S. Transplanted primary neonatal myoblasts can give rise to functional satellite cells as identified using the Myf5nlacZl+ mouse. Gene Ther. 2001;8:778–783. doi: 10.1038/sj.gt.3301463. [DOI] [PubMed] [Google Scholar]
- Hosaka T., Biggs W.H., 3rd, Tieu D., Boyer A.D., Varki N.M., Cavenee W.K., Arden K.C. Disruption of forkhead transcription factor (FOXO) family members in mice reveals their functional diversification. Proc. Natl. Acad. Sci. USA. 2004;101:2975–2980. doi: 10.1073/pnas.0400093101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P., Geles K.G., Paik J.H., DePinho R.A., Tjian R. Codependent activators direct myoblast-specific MyoD transcription. Dev. Cell. 2008;15:534–546. doi: 10.1016/j.devcel.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H., Tindall D.J. Regulation of FOXO protein stability via ubiquitination and proteasome degradation. Biochim. Biophys. Acta. 2011;1813:1961–1964. doi: 10.1016/j.bbamcr.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs F.M., van der Heide L.P., Wijchers P.J., Burbach J.P., Hoekman M.F., Smidt M.P. FoxO6, a novel member of the FoxO class of transcription factors with distinct shuttling dynamics. J. Biol. Chem. 2003;278:35959–35967. doi: 10.1074/jbc.M302804200. [DOI] [PubMed] [Google Scholar]
- Jennische E., Hansson H.A. Regenerating skeletal muscle cells express insulin-like growth factor I. Acta Physiol. Scand. 1987;130:327–332. doi: 10.1111/j.1748-1716.1987.tb08144.x. [DOI] [PubMed] [Google Scholar]
- Jonsson H., Allen P., Peng S.L. Inflammatory arthritis requires Foxo3a to prevent Fas ligand-induced neutrophil apoptosis. Nat. Med. 2005;11:666–671. doi: 10.1038/nm1248. [DOI] [PubMed] [Google Scholar]
- Kondoh K., Sunadome K., Nishida E. Notch signaling suppresses p38 MAPK activity via induction of MKP-1 in myogenesis. J. Biol. Chem. 2007;282:3058–3065. doi: 10.1074/jbc.M607630200. [DOI] [PubMed] [Google Scholar]
- Kuang S., Kuroda K., Le Grand F., Rudnicki M.A. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129:999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam E.W., Francis R.E., Petkovic M. FOXO transcription factors: key regulators of cell fate. Biochem. Soc. Trans. 2006;34:722–726. doi: 10.1042/BST0340722. [DOI] [PubMed] [Google Scholar]
- Le Grand F., Grifone R., Mourikis P., Houbron C., Gigaud C., Pujol J., Maillet M., Pagès G., Rudnicki M., Tajbakhsh S., Maire P. Six1 regulates stem cell repair potential and self-renewal during skeletal muscle regeneration. J. Cell Biol. 2012;198:815–832. doi: 10.1083/jcb.201201050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.Y., Chung J.W., Youn S.W., Kim J.Y., Park K.W., Koo B.K., Oh B.H., Park Y.B., Chaqour B., Walsh K., Kim H.S. Forkhead transcription factor FOXO3a is a negative regulator of angiogenic immediate early gene CYR61, leading to inhibition of vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ. Res. 2007;100:372–380. doi: 10.1161/01.RES.0000257945.97958.77. [DOI] [PubMed] [Google Scholar]
- Lin L., Hron J.D., Peng S.L. Regulation of NF-kappaB, Th activation, and autoinflammation by the forkhead transcription factor Foxo3a. Immunity. 2004;21:203–213. doi: 10.1016/j.immuni.2004.06.016. [DOI] [PubMed] [Google Scholar]
- McCright B., Gao X., Shen L., Lozier J., Lan Y., Maguire M., Herzlinger D., Weinmaster G., Jiang R., Gridley T. Defects in development of the kidney, heart and eye vasculature in mice homozygous for a hypomorphic Notch2 mutation. Development. 2001;128:491–502. doi: 10.1242/dev.128.4.491. [DOI] [PubMed] [Google Scholar]
- Miyamoto K., Araki K.Y., Naka K., Arai F., Takubo K., Yamazaki S., Matsuoka S., Miyamoto T., Ito K., Ohmura M. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell. 2007;1:101–112. doi: 10.1016/j.stem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Mourikis P., Sambasivan R., Castel D., Rocheteau P., Bizzarro V., Tajbakhsh S. A critical requirement for notch signaling in maintenance of the quiescent skeletal muscle stem cell state. Stem Cells. 2012;30:243–252. doi: 10.1002/stem.775. [DOI] [PubMed] [Google Scholar]
- Nishijo K., Hosoyama T., Bjornson C.R., Schaffer B.S., Prajapati S.I., Bahadur A.N., Hansen M.S., Blandford M.C., McCleish A.T., Rubin B.P. Biomarker system for studying muscle, stem cells, and cancer in vivo. FASEB J. 2009;23:2681–2690. doi: 10.1096/fj.08-128116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik J.H., Kollipara R., Chu G., Ji H., Xiao Y., Ding Z., Miao L., Tothova Z., Horner J.W., Carrasco D.R. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell. 2007;128:309–323. doi: 10.1016/j.cell.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potente M., Urbich C., Sasaki K., Hofmann W.K., Heeschen C., Aicher A., Kollipara R., DePinho R.A., Zeiher A.M., Dimmeler S. Involvement of Foxo transcription factors in angiogenesis and postnatal neovascularization. J. Clin. Invest. 2005;115:2382–2392. doi: 10.1172/JCI23126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renault V.M., Rafalski V.A., Morgan A.A., Salih D.A., Brett J.O., Webb A.E., Villeda S.A., Thekkat P.U., Guillerey C., Denko N.C. FoxO3 regulates neural stem cell homeostasis. Cell Stem Cell. 2009;5:527–539. doi: 10.1016/j.stem.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmalbruch H., Lewis D.M. Dynamics of nuclei of muscle fibers and connective tissue cells in normal and denervated rat muscles. Muscle Nerve. 2000;23:617–626. doi: 10.1002/(sici)1097-4598(200004)23:4<617::aid-mus22>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Shea K.L., Xiang W., LaPorta V.S., Licht J.D., Keller C., Basson M.A., Brack A.S. Sprouty1 regulates reversible quiescence of a self-renewing adult muscle stem cell pool during regeneration. Cell Stem Cell. 2010;6:117–129. doi: 10.1016/j.stem.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas S., Watanabe T., Lin C.S., William C.M., Tanabe Y., Jessell T.M., Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tothova Z., Kollipara R., Huntly B.J., Lee B.H., Castrillon D.H., Cullen D.E., McDowell E.P., Lazo-Kallanian S., Williams I.R., Sears C. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Troy A., Cadwallader A.B., Fedorov Y., Tyner K., Tanaka K.K., Olwin B.B. Coordination of satellite cell activation and self-renewal by Par-complex-dependent asymmetric activation of p38α/β MAPK. Cell Stem Cell. 2012;11:541–553. doi: 10.1016/j.stem.2012.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heide L.P., Jacobs F.M., Burbach J.P., Hoekman M.F., Smidt M.P. FoxO6 transcriptional activity is regulated by Thr26 and Ser184, independent of nucleo-cytoplasmic shuttling. Biochem. J. 2005;391:623–629. doi: 10.1042/BJ20050525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb A.E., Pollina E.A., Vierbuchen T., Urbán N., Ucar D., Leeman D.S., Martynoga B., Sewak M., Rando T.A., Guillemot F. FOXO3 shares common targets with ASCL1 genome-wide and inhibits ASCL1-dependent neurogenesis. Cell Rep. 2013;4:477–491. doi: 10.1016/j.celrep.2013.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Y., Bi P., Liu W., Asakura A., Keller C., Kuang S. Constitutive Notch activation upregulates Pax7 and promotes the self-renewal of skeletal muscle satellite cells. Mol. Cell. Biol. 2012;32:2300–2311. doi: 10.1128/MCB.06753-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida N., Yoshida S., Koishi K., Masuda K., Nabeshima Y. Cell heterogeneity upon myogenic differentiation: down-regulation of MyoD and Myf-5 generates ‘reserve cells’. J. Cell Sci. 1998;111:769–779. doi: 10.1242/jcs.111.6.769. [DOI] [PubMed] [Google Scholar]
- Zammit P.S., Golding J.P., Nagata Y., Hudon V., Partridge T.A., Beauchamp J.R. Muscle satellite cells adopt divergent fates: a mechanism for self-renewal? J. Cell Biol. 2004;166:347–357. doi: 10.1083/jcb.200312007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


