Abstract
In a collaboration involving 11 groups with research interests in cerebral amyloid angiopathy (CAA), we used a two-stage process to develop and in turn validate a new consensus protocol and scoring scheme for the assessment of CAA and associated vasculopathic abnormalities in post-mortem brain tissue. Stage one used an iterative Delphi-style survey to develop the consensus protocol. The resultant scoring scheme was tested on a series of digital images and paraffin sections that were circulated blind to a number of scorers. The scoring scheme and choice of staining methods were refined by open-forum discussion. The agreed protocol scored parenchymal and meningeal CAA on a 0-3 scale, capillary CAA as present/absent and vasculopathy on 0-2 scale, in the 4 cortical lobes that were scored separately. A further assessment involving three centres was then undertaken. Neuropathologists in three centres (Bristol, Oxford and Sheffield) independently scored sections from 75 cases (25 from each centre) and high inter-rater reliability was demonstrated. Stage two used the results of the three-centre assessment to validate the protocol by investigating previously described associations between APOE genotype (previously determined), and both CAA and vasculopathy. Association of capillary CAA with or without arteriolar CAA with APOE ε4 was confirmed. However APOE ε2 was also found to be a strong risk factor for the development of CAA, not only in AD but also in elderly non-demented controls. Further validation of this protocol and scoring scheme is encouraged, to aid its wider adoption to facilitate collaborative and replication studies of CAA.
Keywords: Angiopathy, amyloid, dementia, Delphi, validation, APOE, CAA, consensus, parenchymal, meningeal
Introduction
Cerebral amyloid angiopathy (CAA) is a disease in which amyloid is deposited within the walls of meningeal and parenchymal blood vessels – predominantly arteriolar and scanty venous deposits but occasionally involving capillaries. Most cases are sporadic and result from the accumulation of the Alzheimer’s disease (AD)-associated amyloid-β peptide (Aβ), although rare autosomal dominant mutations of a number of genes produce vascular accumulation of amyloid composed of other proteins such as cystatin C, ABri and ADan. Sporadic CAA affects approximately 30% of the neurologically-normal elderly [1] and 90-100% of those with AD [2]. It is most often asymptomatic but can cause or contribute to dementia [3], cerebral haemorrhage [4,5] and ischaemic damage [2,6-8].
The aetiology of sporadic CAA is unclear. However extensive work carried out by Weller [9] and others over the last decade suggests that sporadic CAA is a protein-elimination-failure arteriopathy [10-12], whereby impairment of perivascular ‘lymphatic’ drainage, endothelial transport and degradation of Aβ by cerebrovascular smooth muscle cells and perhaps perivascular macrophages participate (to varying degrees) in the perivascular build up of Aβ and development of CAA. Contributing factors are likely to include the effects of age on the thickness, composition and compliance of the perivascular basement membranes of cerebral arterioles along which interstitial fluid drains from the brain. Weller et al. proposed that this drainage is driven by the systolic pulsation of the blood vessels [13]. Genetic factors are probably also important, particularly the APOE genotype, which was reported to influence the development of CAA by altering the relative proportions of Aβ40:Aβ42 [14] and is also associated with the level and activity of the Aβ-degrading enzyme neprilysin in cerebrovascular smooth muscle cells [11,15]. Capillary CAA on the other hand has been suggested to be primarily caused by reduced transendothelial clearance of Aβ-APOE4 protein complexes [16,17].
Several early studies found a relationship between APOE genotype and the severity of CAA in patients with AD, patients with one or two copies of the APOE ε4 allele having more severe CAA than those without an ε4 allele [4,18,19], although others found that there was no relationship [20,21]. A recent systematic review and meta-analysis of all published studies on APOE and CAA supported the association for the presence of a dose dependent association between APOE ε4 and sporadic CAA [22,23]. Thal et al. [16,17] has also reported that sporadic CAA could be subdivided into two distinct entities: (A) type 1 CAA, characterised by the presence of capillary Aβ with or without Aβ deposition in arterial vessels; (B) type 2 CAA in which Aβ deposition is restricted to arterial vessels.
These authors found that regardless of the presence or absence of AD, only CAA type 1 was strongly associated with APOE ε4 [16,17], whereas type 2 was associated with APOE ε2. APOE genotype was also reported to be associated with vasculopathic abnormalities and cerebral haemorrhage in CAA. Nicoll et al. found that APOE ε2 allele was significantly over-represented in CAA-associated cerebral haemorrhage [5]. Greenberg et al. concluded that APOE ε4 increased CAA severity by enhancing Aβ deposition within cerebral blood vessels whilst APOE ε2 promoted the vasculopathic changes which led to vessel rupture [24].
Rationale
Sporadic CAA is a major focus of research by many groups in several countries. CAA has considerable co-morbidity with AD, the most common form of dementia and one of the largest health care challenges of modern times. It is therefore somewhat surprising that there is no generally agreed protocol for the assessment of CAA in post-mortem brain tissue [25-27]. In contrast there are widely used protocols for the diagnosis and staging of AD pathology [28] and these have greatly facilitated AD research. The need to develop a broadly-accepted protocol for the assessment of CAA, to facilitate research collaboration and improve reproducibility of findings between different groups, was strongly endorsed at the 2nd International Conference on CAA in Newcastle, UK in 2002. The present paper describes the use of the Delphi method co-ordinated by the Dementia Research Group in the University of Bristol to develop, appraise, validate and apply such a protocol in collaboration with other research groups in the United Kingdom, mainland Europe, North America and Japan. The process is outlined in Figure 1.
Figure 1.
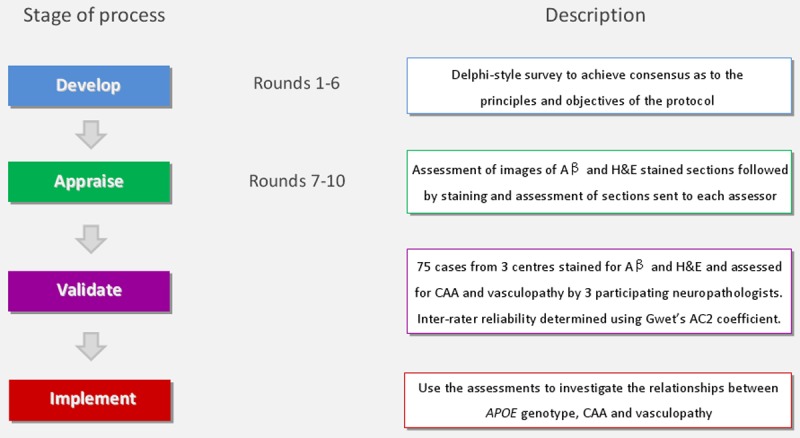
Flow diagram of the CAA consensus protocol process. A protocol was developed by CAA researchers using a modified Delphi method before being implemented by participants using images and sections of Aβ immunolabelled and H&E stained brain sections. The protocol was then validated by the assessment of 75 cases by 3 neuropathologists and inter-rater reliability determined. The results from the validation process were then used to investigate the relationship between APOE genotype, CAA severity and vasculopathy.
Methods
Stage 1 – development of protocol
A literature review was first carried out to identify research groups that had published on CAA in post-mortem human brain tissue. Sixteen research groups were contacted by email and asked if they were they willing to participate in the development of a consensus protocol for assessment of CAA. Representatives from 12 of the 16 groups consented. A Delphi-style survey [29,30] was initiated using an online survey tool (Bristol Online Surveys (http://www.survey.bris.ac.uk/)) hosted by the University of Bristol. A uniform resource locator (URL) web-link specific to each round of the survey was provided by email to the participants and all questions were completed online completely anonymised. The protocol development phase took 6 rounds to complete.
In round 1, participants were asked to list the principles and objectives that they believed should form the structure of the protocol. In round 2, participants were asked to score each principle or objective and for its perceived importance (Tables 1 and 2). In round 3 the cumulative scores were circulated to all participants, who were asked again, in the light of these scores, for their views, particularly in relation to potentially contradictory principles or objectives (e.g. that the assessment protocol should (i) avoid prior assumptions about the relationship of CAA to infarcts or haemorrhage, but (ii) integrate clinical and radiological information in the assessment). This yielded agreement which implied that the highest scores should determine the principles and objectives to be adopted for the assessment protocol. In round 4 participants were asked to consider more detailed aspects of an assessment protocol: to indicate what antibodies or stains they would use, at what magnification they would make the assessments, and to submit a sample protocol taking account of the principles and objectives previously agreed. In round 5 all sample protocols were fed back to the participants, who were asked to score each one according to how well it was thought to fulfil the agreed principles and objectives. The protocols were then ranked according to cumulative score, the common elements were extracted from the protocols with the highest scores and a hybrid protocol was formulated (Table 3). In round 6 the hybrid protocol was sent to all participants, who were asked to comment on its suitability. All participants agreed that the suggested protocol was suitable for the assessment of CAA.
Table 1.
Principles suggested and scored by participants
| Principle | Cumulative score |
|---|---|
| Enable valid comparisons between cohorts | 33 |
| Subject to scientific validation | 33 |
| Achieve high intra- and inter-rater reliability | 32 |
| User-friendly and easy to apply | 30 |
| Include several relevant regions of brain | 29 |
| Should not require specialised equipment | 29 |
| Record association with other pathological findings | 25 |
| Avoid prior assumptions about biological relevance of CAA (i.e. avoid assessing severity according to the absence of presence of findings such as infarcts or haemorrhage) | 25 |
| Assessment of CAA based on labelling with antibodies | 24 |
| Highly sensitive to detection of mild CAA | 22 |
| Relate score to clinical as well as pathological features | 19 |
| Based on same blocks as used for CERAD assessment | 18 |
| Demonstrate amyloid nature of vascular deposits, in addition to Aβ on immunohistochemistry | 15 |
Table 2.
Objectives suggested and scored by participants
| Objective | Cumulative score |
|---|---|
| Separate assessment of leptomeningeal and parenchymal CAA | 28 |
| Clear indication in assessment summary of regions affected | 28 |
| Record ‘secondary’ pathologies such as fibrinoid necrosis | 26 |
| Obtain an overall score of CAA severity | 26 |
| Categorise according to type of affected vessel in each region (i.e. arterioles and capillaries) | 26 |
| Allow for grading of CAA in biopsy as well as autopsy material | 22 |
| Allow for integration of clinical and radiological information in the assessment | 18 |
| Score severity according to maximum severity observed in any region | 17 |
| Take APOE genotype into account | 13 |
Table 3.
Hybrid protocol for scoring CAA and CAA-associated vasculopathy
| Score | Parenchymal CAA | Meningeal CAA | Capillary CAA | Vasculopathy |
|---|---|---|---|---|
| 0 | Absent | Absent | Absent | Absent |
| 1 | Scant Aβ deposition | Scant Aβ deposition | Present | Occasional vessel |
| 2 | Some circumferential Aβ | Some circumferential Aβ | Many vessels | |
| 3 | Widespread circumferential Aβ | Widespread circumferential Aβ |
Stage 2 – appraisal
To assess the ease of use of the protocol and the reproducibility of the assessments, sections from 3 cases of CAA were (i) stained with Harris’s haematoxylin and eosin (H&E); (ii) labelled with an agreed antibody to Aβ (Signet 4G8, distributed by Covance, UK; 1:16,000 overnight) by standard avidin-biotin-peroxidase immunohistochemistry after pre-treatment with 100% formic acid for 20 minutes, and (iii) sections were also stained with Martius scarlet blue (MSB) and phosphotungstic acid/haematoxylin (PTAH) when fibrinoid necrosis was suspected. Many photomicroscope images of the stained sections were circulated to the participants for scoring of the severity of CAA and for comment on the presence or absence or vasculopathy and capillary CAA, according to the agreed protocol. Scores were collated and fed back to the participants. The results showed that inter-rater reliability was good when scoring meningeal, parenchymal and capillary CAA but there was variability in the scoring of vasculopathy. Participants were asked to comment on how the identification of vasculopathy could be improved and the responses were circulated.
Unstained formalin-fixed paraffin-embedded sections of frontal and temporal cortex from 3 AD cases with differing CAA severity were then sent to all participants for staining (Aβ and H&E) and scoring. Once again the consistency of assessment of parenchymal and meningeal CAA was good. However there was variation in the detection of capillary CAA and vasculopathic abnormalities.
The 3rd International Conference on CAA in Reykjavik, Iceland in 2007 provided an opportunity for the study to be discussed in an open meeting chaired by KC, who presented the results of the appraisal rounds. Most of the researchers who were involved in the study, as well as some who were not, discussed ways to improve the recognition of capillary CAA and to score and record vasculopathic abnormalities.
In the final round of the protocol appraisal, opinions were solicited on the stains to be used to detect capillary CAA and fibrinoid necrosis. Agreement was reached that the pan-Aβ antibody was adequate to detect capillary CAA. Participants felt that fibrinoid necrosis was too infrequent to warrant the routine use of MSB or PTAH to detect it but that either of these stains should be used when fibrinoid necrosis was suspected on examination of H&E-stained sections.
Stage 3 – validation
Following the development of the protocol, a Network Cooperation grant was obtained from the Alzheimer’s Research Trust (now Alzheimer’s Research UK) to carry out a validation study of the consensus protocol. Three centres with access to post-mortem brain tissue (Bristol, Oxford and Sheffield (CFAS cohort)) agreed to take part in the validation study. The intention was to examine 20 AD (CERAD definite, Braak tangle stage IV-VI) and 5 control cases (neurologically normal, CERAD no AD, Braak 0-II) with available APOE genotypes, were selected from each centre. Details regarding the composition of the cohorts received from each centre, including mean age, age range and standard deviation are provided in Table 4. Paraffin sections (7 μm) were cut from the frontal, temporal, parietal and occipital lobes blocks for all cases, and were sent to Bristol for staining and immunolabelling. Sections from Bristol were cut from large brain blocks and mounted on glass slides measuring 3 x 1.5 inches. Sections from Oxford and CFAS cohorts were cut from smaller blocks and mounted on 3 x 1 inch glass slides; whilst the blocks were smaller than those from Bristol; the sections nonetheless largely filled the area of the slide adjacent to the label.
Table 4.
Mean age, SD and minimum and maximum ages of AD and control cases in the three participating cohorts
| Cohort | N | Mean | SD | Min | Max |
|---|---|---|---|---|---|
| Oxford - AD | 20 | 80.9 | 6.8 | 66 | 91 |
| Oxford - control | 5 | 83.2 | 7.3 | 77 | 92 |
| Oxford - total | 25 | 81.4 | 6.8 | 66 | 92 |
| CFAS - AD | 11 | 89.5 | 6.4 | 79 | 102 |
| CFAS - control | 4 | 83.0 | 6.3 | 76 | 91 |
| CFAS - total | 15 | 87.7 | 6.8 | 76 | 102 |
| Bristol - AD | 20 | 78.2 | 10.2 | 60 | 95 |
| Bristol - control | 5 | 87.6 | 5.6 | 80 | 94 |
| Bristol - total | 25 | 80.0 | 10.1 | 60 | 95 |
| Total - AD | 51 | 81.7 | 9.1 | 60 | 102 |
| Total - control | 14 | 84.7 | 6.32 | 76 | 94 |
| Total | 65 | 82.3 | 8.7 | 60 | 102 |
For CAA staining, approximately 300 sections (one each from the frontal, temporal, parietal and occipital lobes for each of the cases studied) were immunolabelled with 4G8 antibody to Aβ as described above. For assessment of vasculopathy, sections from all of the blocks were also stained with H&E. All of the stained sections from each centre were anonymised in terms of diagnosis and circulated to the participating neuropathologists (ME, PI, SL) for assessment of CAA and vasculopathy according to the agreed protocol that is summarised in Table 3. Under x10 objective the whole section was scanned and allocated a score for CAA severity that best fitted the description in Table 3. More detailed descriptions of the type of vasculopathy (thrombosis, micro-haemorrhage, concentric splitting of vessel wall, or fibrinoid necrosis) were recorded for future reference. Figures 2, 3 and 4 provide illustrative examples of arteriolar CAA of varying severity, capillary CAA and examples of vasculopathy. Scores were sent to Bristol for collation and then externally to FM and TM for statistical analysis independent of the project co-ordinators. To determine the prevalence of parenchymal, meningeal and capillary CAA and vasculopathy the median score for each of the four brain regions was assessed for each case. The median score of the 3 assessors for each case was also calculated for all CAA types and vasculopathy.
Figure 2.
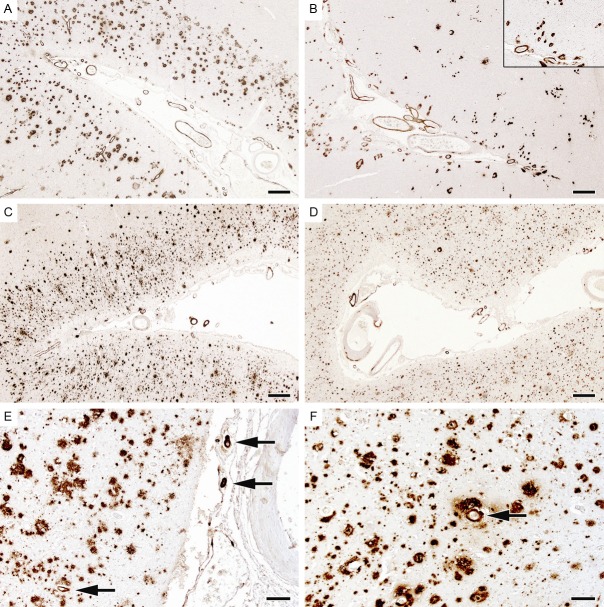
CAA severity scores – representative examples. The sections have been immunolabelled for Aβ. A and B. Illustrate an arteriolar CAA severity score of 3 in the leptomeninges and cerebral cortex, with Aβ in most arterioles and circumferential deposition in many. C and D. Illustrate a CAA severity score of 2 in both meninges and parenchyma. Several arterioles show Aβ deposition, in some cases circumferential. E and F. Illustrate sections in which the arteriolar CAA score was 1. Most fields lacked any vascular Aβ; the arrows indicate the only Aβ-positive vessels within the sections. The scale bars in A-D represent 400 μm, those in E and F 100 μm.
Figure 3.

Aβ immunolabelling of capillary CAA in the occipital cortex. A. Florid capillary CAA. B. Capillary CAA with concomitant severe arteriolar CAA. Sections with any capillary CAA score 1, those with no capillary CAA score zero. The scale bars represent 50 μm in A, 100 μm in B.
Figure 4.
Examples of CAA-associated vasculopathy. A. Fibrinoid necrosis, confirmed here by staining with phosphotungstic acid/haematoxylin. B. Thrombosis of a vessel shown in an adjacent section to contain Aβ. C. Micro-haemorrhage adjacent to a small amyloid-laden arteriole. D. Concentric splitting of the wall of amyloid-laden vessels (‘double-barrelling’). Sections are scored as zero if there is no vasculopathy, 1 if only occasional vessels are affected and 2 if many vessels are affected. The scale bars represent 100 μm in A and B, and 50 μm in C and D.
Statistical analysis
Inter-rater reliability
The extent of agreement between the three raters in relation to: severity of parenchymal, meningeal and capillary CAA as well as vasculopathy, in the four different brain areas, was assessed by calculating Gwet’s AC2 coefficients. The choice of this method was based on the fact that this is a paradox-resistant alternative to Kappa’s coefficient when the overall percentage agreement is high [31]. The coefficient calculations were performed using Agreestat 2011.2 programme (Advanced Analytics, Gaithersburg, MD, USA). An AC2 coefficient of zero suggested that any agreement was due to chance. The extent of agreement was assessed using the benchmark proposed by Landis and Koch [32], a coefficient >0.6 indicating substantial agreement and a value >0.8 near-perfect agreement. A p value <0.05 suggested that agreement was not due to chance.
Relationships between APOE, CAA and vasculopathy
The effect of the number of APOE ε2 and ε4 alleles on the occurrence of AD was assessed by Fisher’s exact test. The effects of APOE on CAA and vasculopathy were investigated by a weighted cross-tabulation for multiple readers, design-based F test, and then further quantified by weighted logistic, ordered logistic regression adjusted for cohort source and case-control status. The presence of APOE alleles (ε2 and ε4) was adjusted (for each other) within the analysis. Results presented are the odds of the APOE ε2 or ε4 allele increasing the likelihood of CAA and vasculopathy. A 95% confidence interval was calculated for the odds ratio (OR). A p value <0.05 was regarded as statistically significant.
Results
Agreement of protocol
The aim of this study was to develop a protocol for the assessment of CAA severity in post-mortem tissue by collating opinions from experts in the CAA field. This was achieved using a Delphi-style questionnaire methodology. The most prevalently suggested objectives by participants were integrated to form a protocol that was then put forward for validation. The key objectives were to score parenchymal and meningeal CAA separately; to record individual regional scores; to identify capillary CAA and to record secondary pathologies such as fibrinoid necrosis, all in a number of regions of cortex to include the occipital lobe (Table 2).
These objectives were incorporated into a protocol (Table 3) that involved scoring parenchymal and meningeal CAA individually on a 0-3 scale, capillary CAA as present/absent and vasculopathy on a 0-2 scale in designated Brodmann areas from the frontal, temporal, parietal and occipital lobes of a cohort of brains from three collaborating centres (Table 4).
Outcome of validation exercise
Table 5 shows Gwet’s AC2 coefficients for parenchymal, meningeal and capillary CAA and vasculopathy for each individual brain region assessed and all brain regions combined. In general all levels of severity of CAA, presence or absence of capillary CAA and presence or absence of vasculopathy were seen to have AC2 coefficients greater than 0.8, implying near-perfect agreement. The only exceptions were for parenchymal CAA in the temporal lobe (AC2=0.776) and parenchymal (0.785) and capillary CAA (0.638) in the occipital lobes, where agreement was nonetheless substantial. Importantly all variables had p values <0.001 indicating that agreement was not due to chance.
Table 5.
Inter-rater reliability on the neuropathological burden according to brain regions
| Brain Areas | Neuropathological burden | Gwet’s AC2 coefficient | Standard Error | 95% CI (AC2) | p-value*** |
|---|---|---|---|---|---|
| All | pCAA | 0.857 | 0.050 | 0.758 to 0.957 | <0.001 |
| mCAA | 0.886 | 0.042 | 0.801 to 0.971 | <0.001 | |
| capCAA | 0.875 | 0.047 | 0.782 to 0.969 | <0.001 | |
| vasculopathy | 0.969 | 0.019 | 0.93 to 1.000 | <0.001 | |
| Frontal | pCAA | 0.823 | 0.040 | 0.743 to 0.902 | <0.001 |
| mCAA | 0.853 | 0.041 | 0.771 to 0.935 | <0.001 | |
| capCAA | 0.939 | 0.032 | 0.876 to 1.000 | <0.001 | |
| vasculopathy | 0.959 | 0.035 | 0.889 to 1.000 | <0.001 | |
| Temporal | pCAA | 0.776 | 0.071 | 0.635 to 0.917 | <0.001 |
| mCAA | 0.887 | 0.056 | 0.774 to 0.999 | <0.001 | |
| capCAA | 0.810 | 0.077 | 0.656 to 0.964 | <0.001 | |
| vasculopathy | 0.931 | 0.034 | 0.862 to 0.999 | <0.001 | |
| Parietal | pCAA | 0.865 | 0.032 | 0.802 to 0.929 | <0.001 |
| mCAA | 0.882 | 0.040 | 0.803 to 0.961 | <0.001 | |
| capCAA | 0.902 | 0.035 | 0.832 to 0.972 | <0.001 | |
| vasculopathy | 0.993 | 0.006 | 0.982 to 1.000 | <0.001 | |
| Occipital | pCAA | 0.785 | 0.058 | 0.670 to 0.901 | <0.001 |
| mCAA | 0.849 | 0.068 | 0.714 to 0.984 | <0.001 | |
| capCAA | 0.638 | 0.086 | 0.467 to 0.808 | <0.001 | |
| vasculopathy | 0.972 | 0.020 | 0.932 to 1.000 | <0.001 |
Significance at
p<0.001.
pCAA: parenchymal CAA, mCAA: meningeal CAA, capCAA: capillary CAA.
Having achieved near perfect agreement in most assessments of parenchymal, meningeal and capillary CAA and vasculopathy, we determined whether relationships previously reported between CAA, vasculopathy and APOE genotype applied to the data obtained by the three independent raters in our validation study. The analysis was performed on a final cohort of 51 AD and 14 control cases for which APOE genotype data was available.
APOE as a risk factor for AD
Before assessing relationships with CAA and vasculopathy we firstly looked to confirm the established relationship between the APOE ε4 allele and the occurrence of AD [33] (Table 6). The APOE ε4 allele frequency was higher in AD cases and the number of ε4 homozygotes was also greater than in controls. The cumulative frequency of the APOE ε2 allele was clearly elevated in controls compared to AD cases (Fisher’s exact test, p=0.030). When comparing the cumulative frequency between cohorts, there were no significant differences in the distribution of the number of the APOE ε2 alleles among controls (p=0.667) or among AD (p=0.521), no difference was noticed also in the distribution of the number of the APOE ε4 alleles (controls, p=0.800; AD, p=0.856) according to Fisher’s exact test.
Table 6.
APOE allele frequencies in AD and control cases
| Diagnosis | Centre | APOE alleles | Number of ε4 alleles | Number of ε2 alleles | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| ε2 | ε3 | ε4 | 0 | 1 | 2 | 0 | 1 | 2 | ||
| AD | Bristol | 0.025 | 0.625 | 0.350 | 0.500 | 0.300 | 0.200 | 0.950 | 0.050 | 0.000 |
| n=51 | Oxford | 0.075 | 0.500 | 0.425 | 0.400 | 0.350 | 0.250 | 0.850 | 0.150 | 0.000 |
| CFAS | 0.000 | 0.727 | 0.273 | 0.545 | 0.364 | 0.091 | 1.000 | 0.000 | 0.000 | |
| Cumulative | 0.039 | 0.598 | 0.363 | 0.471 | 0.338 | 0.153 | 0.922 | 0.078 | 0.000 | |
| Control | Bristol | 0.200 | 0.700 | 0.100 | 0.800 | 0.200 | 0.000 | 0.800 | 0.000 | 0.200 |
| n=14 | Oxford | 0.100 | 0.700 | 0.200 | 0.600 | 0.400 | 0.000 | 0.800 | 0.200 | 0.000 |
| CFAS | 0.375 | 0.375 | 0.250 | 0.500 | 0.500 | 0.000 | 0.500 | 0.250 | 0.250 | |
| Cumulative | 0.214 | 0.607 | 0.179 | 0.643 | 0.357 | 0.000 | 0.714 | 0.142 | 0.142 | |
| p (Fisher’s exact test) | 0.007 | 1.000 | 0.072 | 0.192 | 0.032 | |||||
CAA prevalence
Non-demented elderly controls
Across all four regions investigated (frontal, temporal, parietal and occipital lobes), CAA was evident in 78.5-85.7% of cases: 50.0-64.3% of all cases had parenchymal CAA, 78.5-85.7% had meningeal CAA and 7.1-14.3% had capillary CAA.
CAA was most prevalent in the occipital lobe 85.7% followed by the temporal (78.6%), parietal (71.4%) and frontal (64-71.4%) lobes. Capillary CAA was considerably more abundant in the occipital lobe (21.4%) than the frontal (7.1%), parietal (7%) and temporal (0-7.1%) lobes.
AD cases
CAA was found in 82.4-94.1% of the cases assessed: 66.680.4% of all cases had parenchymal CAA, 80.4-92.2% meningeal CAA and 7.8-23.5% capillary CAA.
The most affected region was the occipital lobe, in which 92.2-100.0% of cases had some evidence of arteriolar CAA. The parietal lobe was least affected (76.5-80.4%). Capillary CAA was most commonly observed in the occipital lobe (35.3-47.1%), followed by the temporal (7.8-19.6%), parietal (7.8-17.7% and frontal (5.9-11.8%) lobes.
APOE as a risk factor for CAA
We investigated the association between APOE genotype and the presence and distribution of CAA (Tables 7, 8, 9 and 10). When AD and non-demented elderly controls were combined (Table 7), the association between CAA and APOE ε4 was strong in the frontal lobe, where ε4 was associated with meningeal CAA, and in the occipital lobe, where ε4 was associated with parenchymal, meningeal and capillary CAA and vasculopathy. However possession of APOE ε2 was associated with a considerably increased likelihood of CAA in AD and controls combined (Table 8), especially so for parenchymal and meningeal CAA and capillary CAA in the parietal lobe and 4 regions combined.
Table 7.
Relationship between APOE ε4, CAA and vasculopathy in control and AD brains
| Unadj p | OR | 95% CI (OR) | p | ||
|---|---|---|---|---|---|
| All | pCAA | 0.030 | 1.79 | (1.00-3.21) | 0.050 |
| mCAA | <0.001 | 1.69 | (0.92-3.10) | 0.089 | |
| capCAA | 0.176 | 1.55 | (0.68-3.50) | 0.293 | |
| vasculopathy | 0.967 | 0.66 | (0.28-1.54) | 0.337 | |
| Frontal | pCAA | 0.204 | 1.73 | (0.99-3.02) | 0.056 |
| mCAA | <0.001 | 2.29 | (1.28-4.12) | 0.006** | |
| capCAA | 0.936 | 0.94 | (0.32-2.75) | 0.916 | |
| vasculopathy | 0.688 | 0.59 | (0.21-1.64) | 0.310 | |
| Temporal | pCAA | 0.130 | 1.27 | (0.71-2.30) | 0.421 |
| mCAA | 0.045 | 1.37 | (0.78-2.40) | 0.271 | |
| capCAA | 0.606 | 1.33 | (0.53-3.35) | 0.547 | |
| vasculopathy | 0.159 | 0.41 | (0.14-1.22) | 0.109 | |
| Parietal | pCAA | 0.182 | 1.27 | (0.71-2.28) | 0.418 |
| mCAA | 0.000 | 1.32 | (0.73-2.37) | 0.356 | |
| capCAA | 0.708 | 0.87 | (0.34-2.25) | 0.779 | |
| vasculopathy | 0.589 | 0.75 | (0.27-2.05) | 0.572 | |
| Occipital | pCAA | 0.001 | 2.79 | (1.54-5.04) | 0.001*** |
| mCAA | 0.012 | 1.87 | (1.01-3.49) | 0.048* | |
| capCAA | <0.001 | 3.60 | (1.88-6.90) | <0.001*** | |
| vasculopathy | 0.024 | 2.61 | (1.19-5.71) | 0.017* |
Significance at
p<0.05;
significance at
p<0.01;
significance at
p<0.001.
Table 8.
Relationship between APOE ε2, CAA and vasculopathy in control and AD brains
| Unadj p | OR | 95% CI (OR) | p | ||
|---|---|---|---|---|---|
| All | pCAA | 0.001 | 4.38 | (2.45-7.80) | <0.001*** |
| mCAA | 0.001 | 10.93 | (4.33-27.57) | <0.001*** | |
| capCAA | 0.160 | 2.91 | (1.10-7.66) | 0.031* | |
| vasculopathy | 0.255 | 0.52 | (0.13-2.09) | 0.357 | |
| Frontal | pCAA | 0.019 | 3.93 | (2.11-7.30) | <0.001*** |
| mCAA | 0.010 | 5.74 | (2.89-11.38) | <0.001*** | |
| capCAA | 0.288 | 1.69 | (0.55-5.24) | 0.361 | |
| vasculopathy | 0.359 | 0.37 | (0.05-3.02) | 0.352 | |
| Temporal | pCAA | <0.001 | 5.11 | (2.64-9.89) | <0.001*** |
| mCAA | 0.001 | 9.88 | (3.56-27.43) | <0.001*** | |
| capCAA | 0.504 | 2.71 | (0.77-9.60) | 0.121 | |
| vasculopathy | 0.556 | 2.20 | (0.56-8.68) | 0.257 | |
| Parietal | pCAA | 0.002 | 3.28 | (1.77-6.08) | <0.001*** |
| mCAA | <0.001 | 6.56 | (2.32-18.59) | <0.001*** | |
| capCAA | 0.004 | 5.69 | (1.70-18.99) | 0.005** | |
| vasculopathy | 0.649 | 0.40 | (0.05-3.09) | 0.376 | |
| Occipital | pCAA | 0.001 | 3.15 | (1.49-6.68) | 0.003** |
| mCAA | 0.007 | 7.16 | (2.31-22.13) | <0.001*** | |
| capCAA | 0.381 | 1.23 | (0.41-3.70) | 0.714 | |
| vasculopathy | 0.152 | 1.48 | (0.27-8.24) | 0.654 |
Significance at
p<0.05;
significance at
p<0.01;
significance at
p<0.001.
Table 9.
Relationship between APOE ε2 and ε4 and CAA in control brains
| APOE ε2 | APOE ε4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| OR | 95% CI (OR) | p | OR | 95% CI (OR) | p | |||||
| All | pCAA | 17.09 | (3.25-89.90) | 0.001*** | 1.64 | (0.48-5.64) | 0.425 | |||
| mCAA | 38.73 | (8.67-173.12) | <0.001*** | 0.53 | (0.11-2.64) | 0.432 | ||||
| Frontal | pCAA | 6.47 | (1.13-37.07) | 0.037* | 2.99 | (0.91-9.86) | 0.071 | |||
| mCAA | 7.18 | (1.20-42.90) | 0.032* | 2.21 | (0.59-8.21) | 0.229 | ||||
| Temporal | pCAA | 11.15 | (2.51-49.61) | 0.002** | 0.49 | (0.14-1.76) | 0.266 | |||
| mCAA | 23.05 | (5.30-100.18) | <0.001*** | 0.38 | (0.10-1.39) | 0.138 | ||||
| Parietal | pCAA | 6.31 | (1.43-27.87) | 0.016* | 0.85 | (0.23-3.10) | 0.798 | |||
| mCAA | 19.21 | (4.61-80.14) | <0.001*** | 0.66 | (0.17-2.60) | 0.549 | ||||
| Occipital | pCAA | 11.09 | (2.04-60.29) | 0.006** | 4.56 | (1.05-19.79) | 0.043* | |||
| mCAA | 8.87 | (2.11-37.25) | 0.004** | 1.03 | (0.20-5.34) | 0.969 | ||||
There were not enough cases with capillary CAA or vasculopathy for statistical analysis. Significance at
p<0.05;
significance at
p<0.01;
significance at
p<0.001.
Table 10.
Relationship between APOE ε2 and ε4 and CAA in AD brains
| APOE ε2 | APOE ε4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| OR | 95% CI (OR) | p | OR | 95% CI (OR) | p | |||||
| All | pCAA | 2.60 | (1.35-5.03) | 0.005** | 1.71 | (0.90-3.27) | 0.102 | |||
| mCAA | 8.03 | (2.77-23.27) | <0.001** | 2.14 | (1.09-4.21) | 0.028* | ||||
| Frontal | pCAA | 2.56 | (1.22-5.36) | 0.013* | 1.44 | (0.78-2.67) | 0.243 | |||
| mCAA | 6.03 | (2.14-16.96) | 0.001*** | 2.37 | (1.21-4.65) | 0.012* | ||||
| Temporal | pCAA | 3.80 | (1.59-9.08) | 0.003** | 1.43 | (0.73-2.81) | 0.295 | |||
| mCAA | 6.67 | (1.75-25.39) | 0.006** | 1.78 | (0.95-3.33) | 0.069 | ||||
| Parietal | pCAA | 2.50 | (1.25-5.00) | 0.010* | 1.38 | (0.72-2.62) | 0.328 | |||
| mCAA | 4.74 | (1.42-15.78) | 0.012* | 1.65 | (0.85-3.18) | 0.135 | ||||
| Occipital | pCAA | 2.18 | (0.74-6.47) | 0.159 | 2.55 | (1.35-4.83) | 0.004** | |||
| mCAA | 6.27 | (1.13-34.66) | 0.036* | 2.18 | (1.11-4.27) | 0.024* | ||||
There were not enough cases with capillary CAA or vasculopathy for statistical analysis. Significance at
p<0.05;
significance at
p<0.01;
significance at
p<0.001.
When we looked at the non-demented elderly controls separately (Table 9), APOE ε2 was strongly associated with CAA in both the parenchyma and meninges in all four regions separately and combined. Conversely, APOE ε4 was significantly associated only with parenchymal CAA in the occipital lobe. Only one control case had capillary CAA and very few had any vasculopathy.
In AD the association of the APOE ε2 allele with CAA was strong in both the parenchyma and meninges in all four regions both separately and combined (Table 10) except for the occipital parenchymal CAA. In AD the association between meningeal CAA and APOE ε4 allele was strong in the four areas combined, frontal and occipital lobes, and parenchymal CAA in the occipital lobe were associated with APOE ε4.
Relationship between APOE genotype and CAA type
Tables 11A, 11B summarise the relationship between APOE genotype and the different types of CAA in our series. All individuals with the APOE ε2/2 genotype had CAA-Type 2 (i.e. Aβ deposition restricted to arterial vessels). In the uncontrolled weighted analysis, the odds ratio for CAA-Type 1 (i.e. characterised by the presence of capillary Aβ with or without Aβ deposition in arterial vessels) versus non-CAA-Type 1 for the presence of APOE ε4/4 genotype was 10.6 (95% CI 3.8; 29.3). When we controlled the analyses for cohort and diagnosis,this relationship was still very strong (OR 8.0 95% CI 2.8; 23.3).
Table 11A.
Weighted percentage of individuals with each APOE genotype among each CAA type (row percentages)
| 22 | 23 | 24 | 33 | 34 | 44 | |
|---|---|---|---|---|---|---|
| Type 0 | 0.0 | 0.0 | 0.0 | 58.1 | 41.9 | 0.0 |
| Type 1 | 0.0 | 9.0 | 3.8 | 25.6 | 29.5 | 32.0 |
| Type 2 | 7.0 | 5.8 | 3.5 | 50.0 | 27.9 | 5.8 |
Table 11B.
Weighted percentage of individuals with each CAA type among each genotype (column percentages)
| 22 | 23 | 24 | 33 | 34 | 44 | |
|---|---|---|---|---|---|---|
| Type 0 | 0.0 | 0.0 | 0.0 | 22.2 | 21.7 | 0.0 |
| Type 1 | 0.0 | 58.3 | 50.0 | 24.7 | 38.3 | 83.3 |
| Type 2 | 100 | 41.7 | 50.0 | 53.1 | 40.0 | 16.7 |
Discussion
Developed criteria
The aim of this study was to develop novel criteria for scoring the severity of CAA and diagnosing concomitant vasculopathy, in a standardised, reproducible and biologically meaningful way. The reliability of the criteria was verified by three experienced neuropathologists (ME, PI and SL), whose assessment of CAA and vasculopathy in a cohort of AD cases and non-demented elderly controls from 3 centres was analysed using Gwet’s AC2 statistic, a new statistical method designed to allow the calculation of agreement in situations where there is high agreement of a rare event such as that of the presence of capillary CAA or vasculopathy [31]. When we applied the arbitrary boundaries of Landis and Koch [32], our coefficients were found, in the main, to be outstanding (near-perfect) for arteriolar and capillary CAA and vasculopathy in the four brain regions assessed. This was particularly encouraging for parenchymal and meningeal forms of CAA that show very variable severity and might therefore have been expected to generate low AC2 coefficients. The lowest coefficient in our study was for capillary CAA in the occipital lobe. Although the agreement was still considered substantial, the lower AC2 coefficient probably reflects the greater difficulty in identifying capillary CAA, particularly in the context of abundant plaque pathology. The recognition of capillary CAA as a discrete pathological process is relatively recent and the greater experience of the neuropathologists in identifying arteriolar CAA may also have contributed to the slightly different levels of consistency in identifying the two processes.
The AC2 coefficient was near-perfect for vasculopathy. Whilst this is an encouraging finding, in our AD cohort the occurrence of vasculopathy was relatively rare, more so than that of capillary CAA. In part the high coefficient reflects the agreement amongst the assessors that there was no vasculopathy in the majority of cases. The robustness of identifying vasculopathy should perhaps be evaluated in a series of cases with CAA-associated haemorrhage.
Application of criteria
Prevalence
CAA was previously reported to be present in between 90-100% of AD cases and in approximately 30% of non-demented controls [1,26,34-37]. In this study we found CAA to be present in 80-92% of AD cases and 79-86% of controls. The seemingly inflated occurrence of CAA in control brains is likely to be due to a number of methodological issues. The first of these was the inclusion of the occipital lobe, which is the most commonly affected part of the brain but which was not assessed in most previous studies [38]. Another contributor may be the sampling method for CAA, which in the current protocol involves scanning the whole section rather than assessing a specific number of randomly selected fields only. Lastly, the use of the 4G8 antibody may be relevant as it seems to be more effective than some other Aβ antibodies at detecting CAA. Clearly it would be of benefit to apply this protocol to a larger series of post-mortem brain tissue samples from non-demented elderly to determine whether our findings can be replicated.
APOE, CAA and vasculopathy
The present study suggests that APOE ε2 may have a more important role in the development of arteriolar CAA than has been previously appreciated. This is particularly so in the absence of AD - in control brains possession of APOE ε2 was a strong risk factor for the development of arteriolar CAA in all brain regions assessed. Possession of APOE ε4 seemed to raise the risk of CAA mainly in the occipital lobe and predominantly in patients with AD who had both arteriolar and capillary accumulation of Aβ.
Conclusions
We have developed an assessment protocol for CAA that can be reliably used to assess the presence and/or severity of parenchymal, meningeal and capillary CAA, and CAA-associated vasculopathy, in post-mortem brain tissue. The novel identification of significant association between APOE ε2 and CAA in control brains highlights the value of the assessment protocol and its utility in promoting collaboration and pooling of data between centres.
Acknowledgements
We should like to thank Shane McParland and Nicky Price for their technical assistance, Drs Matthew Frosch and Catharine Joachim for their contributions throughout the rounds of the protocol development and also Professors James Nicoll and Dietmar Thal for their advice in the later stages of the protocol development. This study was supported by grants from ARUK (Alzheimer’s Research UK) and BRACE (Bristol Research into Alzheimer’s and Care of the Elderly).
Disclosure of conflict of interest
No conflicts of interest to be declared.
References
- 1.Love S, Nicoll JA, Hughes A, Wilcock GK. APOE and cerebral amyloid angiopathy in the elderly. Neuroreport. 2003;14:1535–1536. doi: 10.1097/00001756-200308060-00027. [DOI] [PubMed] [Google Scholar]
- 2.Love S, Miners S, Palmer J, Chalmers K, Kehoe P. Insights into the pathogenesis and pathogenicity of cerebral amyloid angiopathy. Front Biosci (Landmark Ed) 2009;14:4778–4792. doi: 10.2741/3567. [DOI] [PubMed] [Google Scholar]
- 3.Keage HA, Carare RO, Friedland RP, Ince PG, Love S, Nicoll JA, Wharton SB, Weller RO, Brayne C. Population studies of sporadic cerebral amyloid angiopathy and dementia: a systematic review. BMC Neurol. 2009;9:3. doi: 10.1186/1471-2377-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenberg SM, Rebeck GW, Vonsattel JP, Gomez-Isla T, Hyman BT. Apolipoprotein E epsilon 4 and cerebral hemorrhage associated with amyloid angiopathy. Ann Neurol. 1995;38:254–259. doi: 10.1002/ana.410380219. [DOI] [PubMed] [Google Scholar]
- 5.Nicoll JA, Burnett C, Love S, Graham DI, Dewar D, Ironside JW, Stewart J, Vinters HV. High frequency of apolipoprotein E epsilon 2 allele in hemorrhage due to cerebral amyloid angiopathy. Ann Neurol. 1997;41:716–721. doi: 10.1002/ana.410410607. [DOI] [PubMed] [Google Scholar]
- 6.Cadavid D, Mena H, Koeller K, Frommelt RA. Cerebral beta amyloid angiopathy is a risk factor for cerebral ischemic infarction. A case control study in human brain biopsies. J Neuropathol Exp Neurol. 2000;59:768–773. doi: 10.1093/jnen/59.9.768. [DOI] [PubMed] [Google Scholar]
- 7.Olichney JM, Hansen LA, Hofstetter CR, Lee JH, Katzman R, Thal LJ. Association between severe cerebral amyloid angiopathy and cerebrovascular lesions in Alzheimer disease is not a spurious one attributable to apolipoprotein E4. Arch Neurol. 2000;57:869–874. doi: 10.1001/archneur.57.6.869. [DOI] [PubMed] [Google Scholar]
- 8.Vinters HV. Cerebral amyloid angiopathy. A critical review. Stroke. 1987;18:311–324. doi: 10.1161/01.str.18.2.311. [DOI] [PubMed] [Google Scholar]
- 9.Weller RO, Preston SD, Subash M, Carare RO. Cerebral amyloid angiopathy in the aetiology and immunotherapy of Alzheimer disease. Alzheimers Res Ther. 2009;1:6. doi: 10.1186/alzrt6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herzig MC, Van Nostrand WE, Jucker M. Mechanism of cerebral beta-amyloid angiopathy: murine and cellular models. Brain Pathol. 2006;16:40–54. doi: 10.1111/j.1750-3639.2006.tb00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miners JS, Kehoe P, Love S. Neprilysin protects against cerebral amyloid angiopathy and Abeta-induced degeneration of cerebrovascular smooth muscle cells. Brain Pathol. 2011;21:594–605. doi: 10.1111/j.1750-3639.2011.00486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pahnke J, Wolkenhauer O, Krohn M, Walker LC. Clinico-pathologic function of cerebral ABC transporters - implications for the pathogenesis of Alzheimer’s disease. Curr Alzheimer Res. 2008;5:396–405. doi: 10.2174/156720508785132262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weller RO, Subash M, Preston SD, Mazanti I, Carare RO. Perivascular drainage of amyloid-beta peptides from the brain and its failure in cerebral amyloid angiopathy and Alzheimer’s disease. Brain Pathol. 2008;18:253–266. doi: 10.1111/j.1750-3639.2008.00133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fryer JD, Simmons K, Parsadanian M, Bales KR, Paul SM, Sullivan PM, Holtzman DM. Human apolipoprotein E4 alters the amyloid-beta 40:42 ratio and promotes the formation of cerebral amyloid angiopathy in an amyloid precursor protein transgenic model. J Neurosci. 2005;25:2803–2810. doi: 10.1523/JNEUROSCI.5170-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miners JS, Van Helmond Z, Chalmers K, Wilcock G, Love S, Kehoe PG. Decreased expression and activity of neprilysin in Alzheimer disease are associated with cerebral amyloid angiopathy. J Neuropathol Exp Neurol. 2006;65:1012–1021. doi: 10.1097/01.jnen.0000240463.87886.9a. [DOI] [PubMed] [Google Scholar]
- 16.Thal DR, Ghebremedhin E, Rub U, Yamaguchi H, Del Tredici K, Braak H. Two types of sporadic cerebral amyloid angiopathy. J Neuropathol Exp Neurol. 2002;61:282–293. doi: 10.1093/jnen/61.3.282. [DOI] [PubMed] [Google Scholar]
- 17.Thal DR, Papassotiropoulos A, Saido TC, Griffin WS, Mrak RE, Kolsch H, Del Tredici K, Attems J, Ghebremedhin E. Capillary cerebral amyloid angiopathy identifies a distinct APOE epsilon4-associated subtype of sporadic Alzheimer’s disease. Acta Neuropathol. 2010;120:169–183. doi: 10.1007/s00401-010-0707-9. [DOI] [PubMed] [Google Scholar]
- 18.Premkumar DR, Cohen DL, Hedera P, Friedland RP, Kalaria RN. Apolipoprotein E-epsilon4 alleles in cerebral amyloid angiopathy and cerebrovascular pathology associated with Alzheimer’s disease. Am J Pathol. 1996;148:2083–2095. [PMC free article] [PubMed] [Google Scholar]
- 19.Schmechel DE, Saunders AM, Strittmatter WJ, Crain BJ, Hulette CM, Joo SH, Pericak-Vance MA, Goldgaber D, Roses AD. Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90:9649–9653. doi: 10.1073/pnas.90.20.9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heinonen O, Lehtovirta M, Soininen H, Helisalmi S, Mannermaa A, Sorvari H, Kosunen O, Paljarvi L, Ryynanen M, Riekkinen PJ Sr. Alzheimer pathology of patients carrying apolipoprotein E epsilon 4 allele. Neurobiol Aging. 1995;16:505–513. doi: 10.1016/0197-4580(95)00076-q. [DOI] [PubMed] [Google Scholar]
- 21.Olichney JM, Hansen LA, Galasko D, Saitoh T, Hofstetter CR, Katzman R, Thal LJ. The apolipoprotein E epsilon 4 allele is associated with increased neuritic plaques and cerebral amyloid angiopathy in Alzheimer’s disease and Lewy body variant. Neurology. 1996;47:190–196. doi: 10.1212/wnl.47.1.190. [DOI] [PubMed] [Google Scholar]
- 22.Rannikmae K, Kalaria RN, Greenberg SM, Chui HC, Schmitt FA, Samarasekera N, Al-Shahi Salman R, Sudlow CL. APOE associations with severe CAA-associated vasculopathic changes: collaborative meta-analysis. J Neurol Neurosurg Psychiatry. 2014;85:300–5. doi: 10.1136/jnnp-2013-306485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rannikmae K, Samarasekera N, Martinez-Gonzalez NA, Al-Shahi Salman R, Sudlow CL. Genetics of cerebral amyloid angiopathy: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2013;84:901–908. doi: 10.1136/jnnp-2012-303898. [DOI] [PubMed] [Google Scholar]
- 24.Greenberg S, Vonsattel J, Segal A, Chiu R, Clatworthy A, Liao A, Hyman B, Rebeck G. Association of apolipoprotein E epsilon2 and vasculopathy in cerebral amyloid angiopathy. Neurology. 1998;50:961–965. doi: 10.1212/wnl.50.4.961. [DOI] [PubMed] [Google Scholar]
- 25.Olichney JM, Hansen LA, Hofstetter CR, Grundman M, Katzman R, Thal LJ. Cerebral infarction in Alzheimer’s disease is associated with severe amyloid angiopathy and hypertension. Arch Neurol. 1995;52:702–708. doi: 10.1001/archneur.1995.00540310076019. [DOI] [PubMed] [Google Scholar]
- 26.Vinters HV, Gilbert JJ. Cerebral amyloid angiopathy: incidence and complications in the aging brain. II. The distribution of amyloid vascular changes. Stroke. 1983;14:924–928. doi: 10.1161/01.str.14.6.924. [DOI] [PubMed] [Google Scholar]
- 27.Vonsattel JP, Myers RH, Hedley-Whyte ET, Ropper AH, Bird ED, Richardson EP Jr. Cerebral amyloid angiopathy without and with cerebral hemorrhages: a comparative histological study. Ann Neurol. 1991;30:637–649. doi: 10.1002/ana.410300503. [DOI] [PubMed] [Google Scholar]
- 28.Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 29.Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, Hall K, Hasegawa K, Hendrie H, Huang Y, Jorm A, Mathers C, Menezes PR, Rimmer E, Scazufca M Alzheimer’s Disease International. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366:2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Linstone HA, Turoff M. Advanced Book Program. Addison-Wesley Pub. Co.; 1975. The Delphi method: techniques and applications. [Google Scholar]
- 31.Gwet KL. Computing inter-rater reliability and its variance in the presence of high agreement. Br J Math Stat Psychol. 2008;61:29–48. doi: 10.1348/000711006X126600. [DOI] [PubMed] [Google Scholar]
- 32.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 33.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 34.Esiri MM, Wilcock GK. Cerebral amyloid angiopathy in dementia and old age. J Neurol Neurosurg Psychiatry. 1986;49:1221–1226. doi: 10.1136/jnnp.49.11.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masuda J, Tanaka K, Ueda K, Omae T. Autopsy study of incidence and distribution of cerebral amyloid angiopathy in Hisayama, Japan. Stroke. 1988;19:205–210. doi: 10.1161/01.str.19.2.205. [DOI] [PubMed] [Google Scholar]
- 36.Tomonaga M. Cerebral amyloid angiopathy in the elderly. J Am Geriatr Soc. 1981;29:151–157. doi: 10.1111/j.1532-5415.1981.tb01757.x. [DOI] [PubMed] [Google Scholar]
- 37.Yamada M, Tsukagoshi H, Otomo E, Hayakawa M. Cerebral amyloid angiopathy in the aged. J Neurol. 1987;234:371–376. doi: 10.1007/BF00314080. [DOI] [PubMed] [Google Scholar]
- 38.Attems J, Jellinger KA, Lintner F. Alzheimer’s disease pathology influences severity and topographical distribution of cerebral amyloid angiopathy. Acta Neuropathol. 2005;110:222–231. doi: 10.1007/s00401-005-1064-y. [DOI] [PubMed] [Google Scholar]