Abstract
Since the discovery of insulin in 1921, protein therapeutics have become vital tools in the treatment of diabetes mellitus. This heritage has been extended with the comparatively recent introduction of recombinant and re-engineered insulins, in addition to the advent of GLP1 agonists. FGF21 represents an example of a novel experimental protein therapy which is able to induce favorable metabolic effects in various species ranging from rodents to man.
The aim of this review is to communicate the story of the FGF21 drug discovery path from identification in a functional in vitro screen, to the eventual evaluation of its utility in patients. Given that the development of FGF21 advanced hand-in-hand with rapidly evolving scientific research around this target, we have also attempted to describe our view of recent developments regarding the mechanistic understanding of FGF21 biology.
Keywords: FGF21, LY2405319, Metabolism, Drug discovery
1. Discovery of FGF21 as an in vitro activity hit in a phenotypic screen
Fibroblast Growth Factor 21 (FGF21) belongs to the FGF superfamily. Since the identification of the first FGF (FGF1 or αFGF) in 1976, this family has grown steadily and now comprises 22 members in man. Given that FGFs were classified together purely on a structural basis [1] the diversity in their biological functions and modes of action is not surprising. To date, FGFs have been shown to regulate a myriad of fundamental processes such as cell differentiation, proliferation, morphogenesis and metabolism while aberrant FGF signaling has been linked to pathological conditions of cancer and metabolic disease [1–6].
The mouse and human FGF21 genes were originally cloned by Dr. Nobuyuki Itoh's group via a PCR approach utilizing FGF-derived degenerative primers, and the highest expression of FGF21 transcript was found in mouse liver and thymus [7]. This finding remained the sole biology-related hint prior to 2005, when the first report identifying FGF21 as a novel metabolic regulator was communicated [8]. Subsequent studies demonstrated that FGF21 represented a secreted factor which was controlled by important metabolic pathways such as PPARγ [9] and PPARα [10,11].
The initial breakthrough in uncovering the function of FGF21 came as a result of a phenotypic screen carried out at Lilly Research Laboratories in early 2000. In particular, a cassette consisting of several novel secreted proteins of unknown function (PUF) was tested in a glucose uptake assay in mouse 3T3-L1 adipocytes. Since the main goal of this effort was to identify molecules able to induce a quick “insulin-like” response, the screening was performed in a short term cell treatment paradigm with insulin alone or in combination with the PUFs. One of the PUFs examined was human recombinant FGF21 which was able to augment insulin activity in the glucose uptake assay. However, while the effect was both statistically significant and reproducible it was relatively modest in nature (Figure 1A). Follow up studies were focused on improving the FGF21 response window via tweaking the assay conditions and yielded positive but unexpected results, suggestive of a unique mechanism of action. Firstly, when the time of cell stimulation was extended from 1 h to 6 h, FGF21 exhibited a biologically meaningful signal in glucose uptake as compared to the magnitude of response with insulin. Secondly, FGF21 appeared to induce glucose uptake in adipocytes without requiring the presence of exogenous insulin (Figure 1B). Thus, even though the primary aim of the screen at Lilly was to identify molecules that acted in a rapid hormonal fashion – as insulin mimetics or sensitizers, our experimentation yielded an activity hit in FGF21 that was neither [8].
Figure 1.
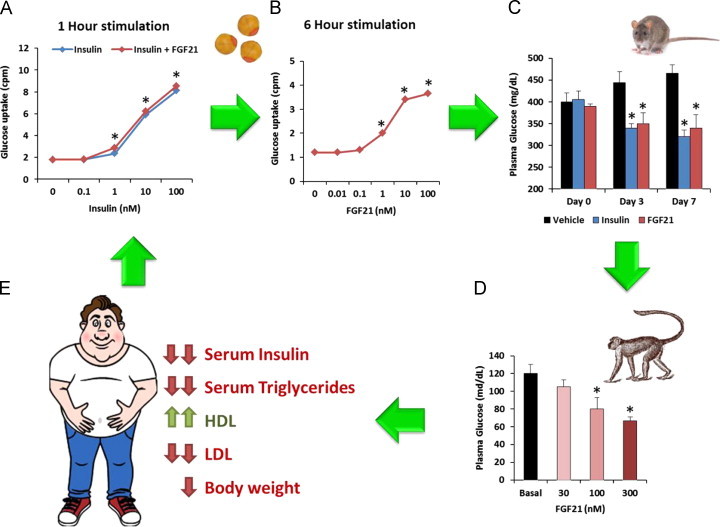
Schematic representation of the identification and evaluation of FGF21 therapeutic utility through the drug discovery process. (A) The functional screening assay involved the assessment of FGF21's effects on glucose uptake in combination with insulin utilizing in an adipocyte model. (B) Following the initial activity hit the glucose uptake screening system was refined to more accurately capture the magnitude of FGF21 action in a longer term treatment paradigm. (C) Subsequent work focused on demonstrating translatability of this in vitro effect into metabolic efficacy in rodent models of insulin resistance and hyperglycemia such as ob/ob mice, where FGF21 demonstrated glucose lowering comparable to that observed with insulin (A, B and C are adapted from [8]). (D) The next study confirmed that FGF21 is bioactive in diabetic non-human primates, a model of metabolic disease which closely resembles the human condition (adapted from [40]). (E) Finally, LY2405319, an FGF21 analog, was shown to be efficacious in humans, suggesting that FGF21-based therapies may indeed represent a novel therapeutic option [49].
These early in vitro results led to the idea that FGF21 is able to act in an insulin-independent manner. At the time there existed significant evidence to this end: FGF21 functioned via activation of FGF receptors, FGF21 and insulin action on glucose utilization in mouse 3T3-L1 and human primary adipocytes were additive, and the FGF21 glucose uptake effect required transcriptional activation and up-regulation of the glucose transporter GLUT1, as opposed to insulin acting in a traditional hormonal manner utilizing GLUT4 translocation. While this hypothesis was valid in a relatively simple cell based system such as 3T3-L1 adipocytes, it was successfully challenged later on in a more complex environment – at the whole body level in mice – where insulin and FGF21 pathways appear to be interdependent [12–15].
2. Evidence of FGF21 in vivo bioactivity
Even though identifying target cells and a relevant readout of bioactivity was important, it represented only the initial step in uncovering the true nature of FGF21 biology. Given these exciting initial findings the goal of subsequent studies was to establish translatability of the FGF21 effect in vitro into meaningful glucose lowering in diabetic animals, and whether FGF21 was devoid of side effects such as hypoglycemia and weight gain, issues commonly associated with traditional insulin therapy [16]. Needless to say there was also heightened awareness of growth factor like mitogenic potential with FGF21, which also required careful assessment. Thus, the need to decisively establish FGF21 as a viable and safe drug target forced the FGF21 research team right at the beginning of the discovery process to consider hard line go/no go experimentation, in animals rather than exploring the specifics of FGF21 action in adipocytes.
Such accelerated drug evaluation path for FGF21 shortly revealed the impressive metabolic benefits of this protein in vivo. FGF21 administration to ob/ob and db/db mice – standard animal models of hyperglycemia and insulin resistance – led to a profound plasma glucose lowering comparable to that of insulin [8] (Figure 1C). Importantly, while insulin induced occasional hypoglycemia in normal animals, no signs of plasma glucose below the basal level were observed with FGF21 in diabetic or lean mice, in the fed or fasted state, even at suprapharmacologic doses. Opposite to insulin, the glucose lowering effect of FGF21 was also sustained in nature lasting up to 24 h post-injection. Furthermore, animals did not gain weight upon chronic FGF21 treatment. The phenotype of mice with stable overexpression of human FGF21 at a level of approximately 100 ng/ml in plasma (500 times higher than basal [8,17,18]) was also consistent with FGF21 pharmacology, as transgenic animals were able to maintain tight glycemic control. Curiously, when compared to wild type littermates, chow- or high fat diet-fed FGF21 overexpressing mice consumed almost twice as much food and yet were lean and protected from the development of diet-induced obesity, a facet of FGF21 biology which was further defined in subsequent studies [14,19]. Importantly, no overt signs of proliferation were detected in tissues of rodents either injected with FGF21 protein or in FGF21 transgenic mice. Quite to the contrary, FGF21 transgenic animals appeared to be partially protected from chemically-induced malignancies [20] and demonstrated significantly delayed age-related mortality [21].
The first pharmacological report on FGF21 [8] also provided hints as to both mechanisms and relevant biomarkers underlying FGF21 action, indicative of the complexity and richness of FGF21 biology and serving to pave the way for future research. While FGF21 appeared to function via a classical FGFR-mediated mechanism, namely phosphorylation of FRS2 and subsequent activation of the MAPK signaling cascade, a mystery remained as to how FGF21 was able to activate FGFR since it was not able to bind the receptor directly. While it was proposed that FGF21 may require an adipocyte specific co-factor [8], it took several years and various approaches to finally identify the transmembrane protein βKlotho (KLB) as the necessary co-receptor able to permit FGF21 mediated activation of FGFRs in vitro [22–25] and promote FGF21 action in vivo [26–28].
The rapid reduction of plasma insulin in preclinical species [8] later led to the hypothesis that insulin sensitization represented the main mechanism underlying FGF21's glycemic action [12–15]. Subsequently, FGF21 was demonstrated to attenuate glucagon secretion ex vivo [8], raising the possibility of interplay between the FGF21 and glucagon pathways. Conversely, it was also shown that glucagon elevates plasma FGF21 levels in human subjects [29,30]. Further supporting a link between FGF21 and glucagon pathways, Habegger et al. demonstrated that a glucagon receptor agonist significantly increased FGF21 expression in isolated hepatocytes from wild-type, but not glucagon receptor knock out animals [29]. Critically, this report also shows that FGF21 null mice do not exhibit the normal profile of beneficial metabolic effects induced upon glucagon receptor engagement, including absence of improved glycemia, enhanced energy expenditure and corrected lipid homeostasis [29,31]. Leptin levels were also massively reduced in FGF21 transgenic mice, indicative of recovery in systemic leptin resistance and suggestive of possible leptin-FGF21 cross-talk specifically with regard to the anti-obesity effects of FGF21 [14,19]. While the potent lowering of plasma triglycerides [8] was a pleasant bonus to the improvements in glycemia and insulin sensitivity, it came as a relative surprise as such an in vivo effect could not be predicted for an activity hit originating in an in vitro glucose uptake assay. Finally, the initial histological observation of brown fat enrichment in FGF21 transgenic animals [8] later led to the idea of FGF21 being a “browning” agent [8,32–34], a concept which has recently been put forward to explain FGF21-driven increases in energy expenditure in animals [35].
3. FGF21 target validation in non-human primates
Given at times the poor translatability of pharmacologic findings in rodents to higher species [36–38], FGF21 was then fast-tracked to another quick win/kill experiment. This study was carried out in collaboration with Dr. Barbara Hansen with the intent to verify FGF21's metabolic activity in diabetic rhesus monkeys. Since Type 2 diabetes in Dr. Hansen's colony of non-human primates is developed in a “natural” way – via unrestricted food supply and limitations in physical activity throughout monkeys' lifespan in the maintenance facility, these animals present with similar disease characteristics to those observed in man. Due to the latter, the outcomes of pharmacological interventions in this in vivo model are highly translatable to the human condition [39].
The study design involved treating a total of 6 monkeys with daily injections of FGF21 in a dose-escalating manner for 6 weeks. The protein was dosed at 30, 100, and 300 μg/kg for 2 weeks in each dosing phase [40], causing a multitude of striking metabolic outcomes.
FGF21 administration led to a dramatic decline in fasting plasma glucose (Figure 1D) [39], insulin, glucagon, and triglycerides, as had been observed earlier in rodents. The glucose effect was durable, lasted 24 h post injection, and was corroborated by lowering of plasma fructosamine, a more integrated measure of glycemic control. Importantly, as in rodents, no incidence of hypoglycemia was noted at any FGF21 dose level in the monkeys. Reductions in plasma insulin and glucagon were observed within 7 days of FGF21 administration and preceded the glucose lowering effect, which became apparent only after 3 weeks of dosing. Given that onset of glucose lowering was significantly faster in a follow up diabetic monkey study when much higher doses of an FGF21 analog, LY2405319, were used [41], the timing of FGF21 administration and the dose required to induce a meaningful glucose lowering effect appear to be interdependent.
This study also highlighted the wealth of FGF21 pharmacology toward various lipid endpoints that could not be evaluated in rodents [40]. In particular, at the end of FGF21 administration plasma triglycerides, total cholesterol, and LDLc were lowered by 70%, 35% and 28%, respectively, while HDLc was elevated by 80% with beneficial changes in lipoprotein particle size/numbers and cardiovascular risk markers also observed. Plasma lipids trended upward and reached pre-treatment levels at the end of the washout phase suggesting that continued pharmacological pressure on the FGF21 signaling pathway is required to maintain efficacy. The magnitude of the effects on triglycerides, total cholesterol, and LDL-c levels were comparable to what has been clinically documented for lipid-lowering therapies such as statins [42]; however, the 80% increase in “good” HDL-c achieved over such a short (6-week) period of protein administration is remarkable. The substantial and beneficial impact of FGF21 on multiple aspects of lipid homeostasis in diabetic monkeys was suggestive that FGF21 could potentially be considered as a standalone therapy to treat cardiovascular dysfunctions and familial lipid disorders.
Finally, the reduction in body weight we observed was indicative of the substantial potential of FGF21 to treat obesity. Given that only a weak FGF21 weight lowering effect was seen in leptin signaling-deficient animals such as ob/ob or db/db mice [14,17,43,44], this finding provided an additional to [8] clue to the mechanistic role of leptin in FGF21's anti-obesity action [44,45]. Overall, the pharmacological package obtained in diabetic monkeys firmly established FGF21 as a viable “glucose plus” drug candidate to treat metabolic disease.
4. FGF21 optimization at Lilly
While the benefits of FGF21 therapy in animals were unmistakable it quickly became apparent that the native FGF21 protein had a low probability for successful development as a molecule suitable for clinical use. Indeed, wild type protein retained various biophysical deficiencies. In addition, the exponential rise in diabetes prevalence worldwide [46] dictated a need to identify a cost-efficient and scalable expression/purification process. Thus, the strategic intent of FGF21 engineering at Lilly was principally focused on biopharmaceutical optimization – designing the molecule with enhanced stability and to ensure it was amenable to wide range of dosing demands in a broad patient population, while maintaining a favorable bioactivity profile of the native protein. Improving the latter was impractical during this first optimization attempt as it was carried out at a time when understanding of FGF21 biology was still in its infancy. This made the effort rather risky but worth exploring given the promise of FGF21's preclinical pharmacology.
Detailed biophysical characterization revealed two major issues with the wild type FGF21 molecule. First, the protein was prone to rapid aggregation in preservative containing solutions. Second, FGF21 appeared to be highly conformationally unstable, undergoing at least three thermal-unfolding states in differential scanning calorimetry analysis. A disulfide stabilization engineering strategy based on FGF21 structure modeling yielded a biopharmaceutically improved variant with an engineered disulfide bond at Leu118Cys–Ala134Cys in the C-terminal domain of FGF21.
Another developmental difficulty was selecting a large scale expression and purification process for FGF21. Initial attempts to express FGF21 in Pichia pastoris yielded low productivity along with issues related to the heterogeneity of the protein. While the introduction of a second disulfide bond in FGF21 resulted in major improvements in productivity, the integrity of the FGF21 variant produced in yeast remained inadequate due to high levels of N-terminal proteolysis and O-linked glycosylation.
As Ser167 was identified as the major site for O-linked glycosylation, it was subjected to site-specific mutagenesis, and the preferred variant, Ser167Ala, was chosen. To address N-terminal proteolysis, FGF21 was further modified to remove the first four amino acids, HPIP, given that ΔHPIP FGF21 is fully biologically active [22,47]. Thus, the optimized FGF21 variant for Pichia pastoris expression, FGF21 Leu118Cys–Ala134Cys, Ser167Ala, ΔHPIP, or LY2405319 (LY), was essentially devoid of the major biopharmaceutical deficiencies associated with native FGF21 [48].
LY2405319 was next profiled for bioactivity in vitro and in animal models of diabetes that have been previously utilized to characterize the effects of FGF21 [48]. In brief, in vitro LY was not mitogenic, its activity was comparable to wild type FGF21 and strictly KLB-dependent; in rodents LY showed nearly identical to native protein pharmacologic activity. Finally, LY was tested in diabetic monkeys at Dr. Hansen's facility. Apart from a significantly enhanced and accelerated onset of pharmacologic response likely due to the suprapharmacologic doses of LY at 3, 9 and 50 mg/kg as compared considerably lower doses in our earlier experiment [40], this engineered FGF21 variant showed robust potency toward all known metabolic components of FGF21 action [41].
Thus, the drug discovery efforts at Lilly yielded LY2405319, an investigational FGF21 variant suitable for early-phase clinical development to study the effects of pharmacologic administration of an FGF21 based therapeutic in human patients [41,49].
5. Outcomes of the LY2405319 clinical trials
LY2405319 (LY) was first characterized for safety and pharmacokinetics in a single ascending dose study with LY levels ranging from 0.6 to 30 mg and then in a subsequent 7-day experiment with doses ranging from 1 to 20 mg daily in healthy volunteers [49]. These early investigations provided safety, tolerability, pharmacokinetic, and some pharmacodynamic data to support a 28-day proof-of-concept trial in obese subjects with T2DM at 3, 10 and 20 mg daily doses of LY that were generally well tolerated. Skin rash and hypersensitivity were observed in two 20 mg subjects with injection-site-related adverse events most frequently reported across all doses.
Statistically and clinically meaningful effects on all four lipid parameters (total cholesterol, LDL, HDL, and TGs; Figure 1E) and body weight in comparison to baseline were observed during the trial, consistent with the results of FGF21 or LY administration to obese rhesus monkeys with dyslipidemia [40,41]. The impact of LY treatment on fasting triglycerides was rapid, occurring as early as 2 days of dosing, and for all lipids appeared to reach a maximum effect at 1–3 weeks with both 10 mg and top end 20 mg LY doses providing maximal efficacy. These effects were accompanied by the expected lowering of ApoCIII and ApoB in plasma [49].
Body weight was also reduced over the 28-day treatment period with 10 mg being the maximum effective dose. Weight loss in humans coincided with increases in plasma β-hydroxybutyrate suggestive of an LY-mediated increase in fatty acid oxidation similar to the findings in rodents [14,50–52].
A prominent reduction in mean fasting insulin levels was observed. This replicates the effects of FGF21 in animals and is indicative of FGF21 mechanism of glycemic improvement being driven by insulin sensitization at the whole body level. Importantly, total plasma adiponectin levels were also robustly increased in a dose-dependent fashion providing another evidence of translatability of FGF21 pharmacology across species [41,49,53]. High molecular-weight adiponectin, a correlate of human efficacy in the case of TZD treatment [54], was also substantially elevated by LY [49].
However, in spite of the positive outcomes with regard to corollary measures, the LY effect on glucose was not as robust as anticipated based on prior experiments in diabetic rodents and monkeys [8,40,41]. Only a dose-dependent trend in the lowering of fasting glucose was observed, even though plasma LY levels in the study were within the range of efficacious exposures of FGF21 in animal models [49].
Thus, even though the overall results of the study were indicative that FGF21 is bioactive in humans and suggested that FGF21-based therapies may be effective for the treatment of select metabolic disorders, the question whether targeting the FGF21 pathway will one day yield a drug to treat hyperglycemia remained unanswered.
6. Alternative optimization attempts
While the focus of engineering effort at Lilly was on improving FGF21 biopharmaceutical properties, others explored additional optimization possibilities. The relatively short half-life of FGF21 [40,55,56] forced the need for once daily injections in man [49] therefore limiting patient convenience. Thus, to improve time-action of FGF21-based molecules alternative re-engineering strategies utilizing conventional size-extension approaches were undertaken. Notably, the report of Mu et al. was the first to describe the introduction of a non-natural amino acid, p-acetylphenylalanine, into the FGF21 sequence for the site-specific attachment of polyethylene glycol (PEG), a process which yielded PEGylated analogs with improved duration of in vivo action [57]. Several other PEGylated FGF21 molecules were also described [58,59], all exhibiting improved pharmacokinetics while maintaining pharmacological profiles in animals similar to the native protein.
In parallel, many other long acting FGF21 variants were identified. Wang et al. described a mouse/human FGF21 conjugate fused to SUMO protein [60], Huang et al. 2013 reported FGF21-CovX-body chimera [61], and Veniant et al. communicated the generation of an FGF21 therapeutic by fusing an immunoglobulin Fc to an FGF21 variant [62]. The importance of the latter report and the following work [63] is further stressed by identification of a specific C-terminal cleavage in human FGF21 in animals' and human plasma that renders the protein inactive [22,47].
Since early/mid 2000 the breadth of understanding of FGF21 biology grew considerably, specifically in regard to the preferred FGF21 receptor complex, FGFR1c and KLB, both – in vitro and in vivo [23–25,27,35]. This knowledge enabled exploration of innovative “FGF21 receptor-driven” engineering strategies in the search for FGF21 mimetics. Wu et al. put forward the initial proof-of-concept report to demonstrate that an agonistic FGFR1 antibody, R1Mab, could mirror several metabolic effects of FGF21 in animals [64]. Nevertheless, given that this antibody is not selective to the adipose-specific FGFR1c isoform and its action is not limited to KLB-expressing tissues as in the case for FGF21, this molecule suffered from several negative off target effects. More recently, Foltz et al. reported the generation of a KLB/FGFR1c-specific antibody, mimAb1 [65]. As this antibody did not cross-react with mouse FGFR1 and KLB, the researchers in a bold move skipped a customary step of target validation in rodent models. Instead, they proceeded with their molecule straight into obese monkeys to demonstrate that mimAb1 functions as an FGF21 mimetic [66]. Finally, Smith et al. used phage display techniques to generate AvimerTm polypeptides that can simultaneously bind with high affinity FGFR1c and KLB, thus maintaining selectivity for the FGF21 receptor complex [66]. Since the Avimer proteins showed “FGF21-like” pharmacology in obese monkeys, these molecules can be considered the first artificial bi-specific activators of the FGF21 pathway [67].
7. Uncovering FGF21 biology
The early reports on FGF21 fostered substantial interest in further study of the protein both in industry and academia. This groundswell of excitement translated into a large number of follow up publications which keep growing exponentially year-on-year thus contributing to significantly expanded knowledge around the biology of FGF21 both in the preclinical setting and in man. Even though many of these studies focused on discrete and basic aspects of mechanism of action, they are also vital to the overall success of the FGF21 drug discovery.
Key to understanding the mechanisms behind FGF21 pharmacology was appreciation of the role of the endogenous FGF21 system that began with delineating tissues which either expressed or were capable of responding to this protein. As stated earlier FGF21 was identified in the liver [7], however, further expression was subsequently reported in white adipose tissue [68], brown adipose tissue [69], muscle [13] and pancreas [70]. In 2010 Dushay et al. published the first report on FGF21 expression in human liver and its elevation in subjects with non-alcoholic fatty liver disease (NAFLD) [71]. Interestingly, in the same study no expression of FGF21 in human adipose tissue was detected, suggestive of potential differences in tissue expression pattern between humans and rodents [27,71]. An earlier report demonstrated the presence of FGF21 mRNA in human skeletal muscle, however, this expression was not robust and was not modulated by diabetic status [72]. In human pancreatic sections FGF21 protein and mRNA is present (Kharitonenkov A & Adams AC, unpublished observations); nonetheless, the relative contribution of this tissue to circulating levels in man has yet to be rigorously evaluated.
Basal circulating FGF21 concentrations in healthy adult humans have been estimated to fall within a range of 0.05–2 ng/ml dependent on the assay methodology employed. In essence ELISA assays seem to approximate circulating FGF21 levels in the low hundred picogram range, whereas RIA based methods produce an estimate almost 10× higher in the 1–2 ng/ml range. Thus one must be cautious when comparing data between studies in which assay methods differ [11,51,73]. In rodents FGF21 is known to be regulated by nutrient deprivation, however, in humans the effect of prolonged fasting on FGF21 concentrations is not robust and remains to be elucidated further [11,74]. There have also been limited studies indicating that FGF21 is regulated in a circadian manner under various physiological conditions therefore this possible confounding factor must be taken into account when estimating FGF21 levels [74]. Furthermore, there is an extremely wide range of FGF21 concentrations observed in human serum [11,75] raising doubts as to the relevance of circulating FGF21 to its biological actions [74,76–78]. Of note, a recent report suggests that FGF21 in human plasma is partially truncated [63] a change which renders the protein inactive in the in vitro setting [24].
In tandem with efforts to uncover the physiology of FGF21 in humans, preclinical studies were advancing in rodents. As is common to most efforts to quickly grasp of the function of novel proteins, mice with whole body ablation of FGF21 (FGF21KO) were obtained. The Lilly generated FGF21KO were found to display obesity and glucose intolerance [52,79], a phenotype which was consistent with that of an independently generated strain [80]. Interestingly, a third FGF21KO line was shown to be lipodystrophic [81]. The phenotype of both the Kyoto University generated and Lilly FGF21KO mice is consistent with that of FGF21-overexpressing animals (FGF21Tg) [8]. FGF21Tg mice exhibit lower body weight and are lean when compared to WT mice [8] with significantly improved glycemia in the face of vanishingly low insulin levels. In addition to the lean phenotype and resistance to diet induced obesity observed in our own strain of FGF21Tg mice, an independent line of FGF21 overexpressing animals also displays significantly augmented lifespan [21].
With the phenotypes of genetic models largely supporting the previous literature regarding the effects of exogenous FGF21 administration the next logical step was to determine tissue (s) in which FGF21 can act. Acute FGF21 signaling, determined by increased immediate early gene signaling is observed in liver, adipose tissue and pancreas [27,35,50,70]. Physiologically, this activation is coupled to a reduction in serum glucose, insulin, triglycerides, and free fatty acids, as well as an increase in adiponectin secretion [14,19,26,27,44].
Various techniques were successfully used to demonstrate that in cells FGF21 requires the co-factor KLB for its action [22–25,47,82]. Nevertheless, the role of KLB in the propagation of the FGF21 signal in vivo was initially challenged by the finding that FGF21 signaling was fully preserved in mice with total body KLB ablation [83]. Therefore, to critically evaluate this hypothesis, independent lines of KLB null mice were generated. However, in these KLBKO animals the distinctive induction of immediate early gene expression was noticeably absent [26,27]. As a result of the absence of the initial receptor engagement, all FGF21 driven metabolic effects were lost in KLBKO animals, demonstrating the ultimate requirement for KLB in the mediation of FGF21 in vivo pharmacology [26,27].
FGF21 is active on 3T3-L1 adipocytes that express KLB and FGFR1 (FGFR2 is also present but at a significantly lower level). Both FGF receptors in adipocytes are tyrosine phosphorylated/activated when cells are treated with FGF21 [8], supporting the idea that several FGFRs in a context of KLB expression are sufficient to facilitate FGF21 in vitro signaling [23,24,43]. Extrapolating this data to the in vivo condition it was also plausible to propose that tissues co-expressing this FGFR/KLB complex such as the liver, pancreas and adipose are either responsible for specific aspects of FGF21 pharmacology or function in concert/sequence to support FGF21 in vivo action [84]. To assess those possibilities, and given the fact that fat cells are enriched with FGFR1 and KLB, FGF21 was first tested in mice with adipose specific deletion of FGFR1 (FR1KO) [35]. Unexpectedly, the majority of the metabolic effects of FGF21 were lost in the FR1KO animals in both acute and chronic dosing conditions, and only discreet facets of FGF21 biology such as lowering of free fatty acids remained [35]. This striking result indicated that for whole body effects in mice FGF21 primarily requires targeting of FGFR1 in adipose tissue and came in a surprising contrast to the pale cast of thought above. Importantly, animals with neuronal FGFR1 loss were still fully responsive to exogenous FGF21 suggestive of peripheral FGF21 having no direct action on neurons [35] even though some recent reports suggested otherwise [85,86].
Supporting the adipose tissue-driven hypothesis of FGF21 mode of in vivo action is a dramatically diminished response to FGF21 treatment in lipodystrophic mice [44] and animals with fat-specific KLB ablation [26]. Additionally, the plethora of FGF21 pharmacologic effects are retained in animals with the use of size-extended FGF21 variants that cannot penetrate into brain [58,59,67] suggestive of a straightforward tailoring strategy for FGF21 based therapies if these adipose centric mouse data bear relevance in man.
Adiponectin levels are also significantly increased in FGF21Tg mice [8], reduced in FGF21KO animals and treatment with recombinant FGF21 increases the circulating levels of all three forms of adiponectin [53]. Noteworthy, the latter was completely lost in the FR1KO mice [35] leading to investigations on the role of adiponectin downstream of FGF21. Since acute FGF21 signaling is not compromised in adiponectin null (Adn−/−) mice, the FGF21 receptor machinery appear to be intact in these animals. Nevetheless, Adn−/− mice were refractory to the glycemic effects of FGF21 [53,87] while full FGF21 efficacy remained intact with regard to lipid lowering. Thus, individual endpoints of FGF21 pharmacology appear to be mediated by distinct downstream factors, such as adiponectin or leptin. Further work is required to elucidate other downstream effectors of FGF21 action, a strategy which holds the possibility of finding novel drug targets within the realm of FGF21 biology.
8. Caveat emptor?
Despite a large body of evidence regarding the positive metabolic actions of FGF21, safety concerns associated with targeting the FGF21 pathway have also emerged. Indeed, while above we attempted to show how basic research can uncover data to aid and guide drug discovery, the same process is also expected to reveal negative issues with any given target. Once identified these issues need to be fully explored as patient safety is of the utmost importance.
FGF21Tg animals are shorter than WT mice [8,88]; indeed the increased longevity and reduced body length in these animals is reminiscent of that observed in the snell dwarf mouse [89]. The FGF21Tg mice were shown to be prone to the early onset of torpor [90], a state of energy conservation marked by reduced body temperature and physical activity. While in a follow up study [91] FGF21 did not appear to be sufficient to induce hibernation in rodents and no reports of FGF21 effects on body temperature have been reported in higher mammals this finding remains a potential concern. Recent studies have also suggested that FGF21 can delay growth plate development [92–94], and may have deleterious effects on bone [95], and reproduction [96]. Wei et al. utilized both genetic and pharmacologic FGF21 gain of function paradigms to demonstrate a striking decrease in bone mass in mice. Furthermore, FGF21KO mice exhibited a phenotype of increased bone mass [95] all together leading to the hypothesis that FGF21 is a critical regulator of bone turnover. Nevertheless, and given that FGF21Tg mice present with extended lifespan during which they remain apparently healthy [21], the question remains if this observed reduction in bone mass has any negative consequences. Furthermore, a significant positive association between plasma FGF21 levels and BMD was recently reported in healthy women suggestive that the association between bone loss and FGF21 in rodents may not be directly translated to humans [97]. With regard to reproductive effects, Owen et al. demonstrated that FGF21 promotes infertility in female mice via direct action on the suprachiasmatic nucleus (SCN) of the hypothalamus to suppress the vasopressin–kisspeptin signaling cascade and to inhibit luteinizing hormone release. In addition, mice lacking KLB specifically in the SCN are refractory to this FGF21 mediated female fertility effect, further suggestive of its central origin and the potential involvement of FGF21 in a liver-to-brain axis that modulates female reproduction in response to nutritional challenge [96]. If this observation in rodents holds in man, size-extended FGF21 variants that cannot enter brain will present with safety advantages in the clinic.
9. Concluding remarks
The history of the discovery and subsequent maturation of FGF21 can teach several lessons about the wonders one may encounter during the drug discovery process. While nature itself is unpredictable, the search to find novel drug candidates is even more complex since in essence the latter are required to beneficially modulate a pathological environment which is on the whole still poorly understood. As an example, FGF21 was discovered via an in vitro assay as a potential insulin mimetic, emerged as a viable “glucose plus” agent in animals, and ended up providing several metabolic benefits yet failed to deliver sustained glucose lowering in man.
In the post whole genome sequencing era, tried and tested strategies of exploiting “low hanging fruit” and “avoiding risks” are long gone. Consistent investment and diligent focus is needed to evaluate and enable the best novel targets. As FGF21 demonstrates, phenotypic screens can deliver new drug candidates, but the need for a concise, reproducible and translatable in vitro assay is paramount. Knowledge of robust and easily measurable in vivo biomarkers of action in addition to the presence of robust functional endpoints is also vital in determining translation from the preclinical setting into man. One key lesson in the development of FGF21 is that risk taking and wiliness to commit to early go/no go experimentation are paramount to increasing the pace of the drug development process.
Finally, successful drug discovery can be framed around novel first-in-class targets if the process in parallel is accompanied by evolving basic scientific research. Appreciation that the vast majority of these initially obscure molecules will not pass reproducibility tests [98] is not a negative factor but instead should serve as motivation to quickly explore the next potential target. Collaborations between industrial teams and academic scientists even though thwarted at times by bureaucratic barriers are crucially important in conceiving novel hypotheses and partitioning research expenses. The essential character of things can be unearthed via working in concert and transparency on the part of both. At the very end, timely publications are essential in creating interest that feeds back to accelerate innovation and lead the development of novel targets.
Given all the challenges the pharmaceutical industry is currently experiencing [37], the odds of delivering revolutionary therapies remain slim. Yet FGF21 serves as a prime example that novel and potentially better medicines are within the reach. After 10+ years of intense investigations FGF21 still holds promise to eventually become a powerful drug in the treatment of a wide array of chronic human diseases.
Conflict of interest
Alexei Kharitonenkov and Andrew C. Adams are full time employees of Eli Lilly and Company. No other conflict of interest exist.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Itoh N. Hormone-like (endocrine) Fgfs: their evolutionary history and roles in development, metabolism, and disease. Cell and Tissue Research. 2010;342(1):1–11. doi: 10.1007/s00441-010-1024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelleher F.C. Fibroblast growth factor receptors, developmental corruption and malignant disease. Carcinogenesis. 2013;34(10):2198–2205. doi: 10.1093/carcin/bgt254. [DOI] [PubMed] [Google Scholar]
- 3.Evans S.J. Dysregulation of the fibroblast growth factor system in major depression. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(43):15506–15511. doi: 10.1073/pnas.0406788101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hutley L. Fibroblast growth factor 1: a key regulator of human adipogenesis. Diabetes. 2004;53(12):3097–3106. doi: 10.2337/diabetes.53.12.3097. [DOI] [PubMed] [Google Scholar]
- 5.Yamagata H. Promoter polymorphism in fibroblast growth factor 1 gene increases risk of definite Alzheimer's disease. Biochemical and Biophysical Research Communications. 2004;321(2):320–323. doi: 10.1016/j.bbrc.2004.06.142. [DOI] [PubMed] [Google Scholar]
- 6.van der Walt J.M. Fibroblast growth factor 20 polymorphisms and haplotypes strongly influence risk of Parkinson disease. American Journal of Human Genetics. 2004;74(6):1121–1127. doi: 10.1086/421052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishimura T. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochimica et Biophysica Acta. 2000;1492(1):203–206. doi: 10.1016/s0167-4781(00)00067-1. [DOI] [PubMed] [Google Scholar]
- 8.Kharitonenkov A. FGF-21 as a novel metabolic regulator. Journal of Clinical Investigation. 2005;115(6):1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muise E.S. Adipose fibroblast growth factor 21 is up-regulated by peroxisome proliferator-activated receptor gamma and altered metabolic states. Molecular Pharmacology. 2008;74(2):403–412. doi: 10.1124/mol.108.044826. [DOI] [PubMed] [Google Scholar]
- 10.Mraz M. Serum concentrations and tissue expression of a novel endocrine regulator fibroblast growth factor-21 in patients with type 2 diabetes and obesity. Clinical Endocrinology (Oxford) 2009;71(3):369–375. doi: 10.1111/j.1365-2265.2008.03502.x. [DOI] [PubMed] [Google Scholar]
- 11.Galman C. The circulating metabolic regulator FGF21 is induced by prolonged fasting and PPARalpha activation in man. Cell Metabolism. 2008;8(2):169–174. doi: 10.1016/j.cmet.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 12.Berglund E.D. Fibroblast growth factor 21 controls glycemia via regulation of hepatic glucose flux and insulin sensitivity. Endocrinology. 2009;150(9):4084–4093. doi: 10.1210/en.2009-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Izumiya Y. FGF21 is an Akt-regulated myokine. FEBS Letters. 2008;582(27):3805–3810. doi: 10.1016/j.febslet.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coskun T. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology. 2008;149(12):6018–6027. doi: 10.1210/en.2008-0816. [DOI] [PubMed] [Google Scholar]
- 15.Arner P. FGF21 attenuates lipolysis in human adipocytes – a possible link to improved insulin sensitivity. FEBS Letters. 2008;582(12):1725–1730. doi: 10.1016/j.febslet.2008.04.038. [DOI] [PubMed] [Google Scholar]
- 16.Hermansen K., Davies M. Does insulin detemir have a role in reducing risk of insulin-associated weight gain? Diabetes, Obesity and Metabolism. 2007;9(3):209–217. doi: 10.1111/j.1463-1326.2006.00665.x. [DOI] [PubMed] [Google Scholar]
- 17.Fisher F.M. Obesity is a fibroblast growth factor 21 (FGF21)-resistant state. Diabetes. 2010;59(11):2781–2789. doi: 10.2337/db10-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berglund E.D. Glucagon and lipid interactions in the regulation of hepatic AMPK signaling and expression of PPARalpha and FGF21 transcripts in vivo. American Journal of Physiology – Endocrinology and Metabolism. 2010;299(4):E607–E614. doi: 10.1152/ajpendo.00263.2010. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Hale C. Lack of overt FGF21 resistance in two mouse models of obesity and insulin resistance. Endocrinology. 2012;153(1):69–80. doi: 10.1210/en.2010-1262. [DOI] [PubMed] [Google Scholar]
- 20.Huang X. Forced expression of hepatocyte-specific fibroblast growth factor 21 delays initiation of chemically induced hepatocarcinogenesis. Molecular Carcinogenesis. 2006;45(12):934–942. doi: 10.1002/mc.20241. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y. The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. Elife. 2012;1:e00065. doi: 10.7554/eLife.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Micanovic R. Different roles of N- and C- termini in the functional activity of FGF21. Journal of Cellular Physiology. 2009;219(2):227–234. doi: 10.1002/jcp.21675. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki M. betaKlotho is required for fibroblast growth factor (FGF) 21 signaling through FGF receptor (FGFR) 1c and FGFR3c. Molecular Endocrinology. 2008;22(4):1006–1014. doi: 10.1210/me.2007-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kharitonenkov A. FGF-21/FGF-21 receptor interaction and activation is determined by betaKlotho. Journal of Cellular Physiology. 2008;215(1):1–7. doi: 10.1002/jcp.21357. [DOI] [PubMed] [Google Scholar]
- 25.Ogawa Y. BetaKlotho is required for metabolic activity of fibroblast growth factor 21. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(18):7432–7437. doi: 10.1073/pnas.0701600104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ding X. betaKlotho is required for fibroblast growth factor 21 effects on growth and metabolism. Cell Metabolism. 2012;16(3):387–393. doi: 10.1016/j.cmet.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adams A.C. FGF21 requires betaklotho to act in vivo. PLoS One. 2012;7(11):e49977. doi: 10.1371/journal.pone.0049977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang C. Differential specificity of endocrine FGF19 and FGF21 to FGFR1 and FGFR4 in complex with KLB. PLoS One. 2012;7(3):e33870. doi: 10.1371/journal.pone.0033870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Habegger K.M. Fibroblast growth factor 21 mediates specific glucagon actions. Diabetes. 2013;62(5):1453–1463. doi: 10.2337/db12-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arafat A.M. Glucagon increases circulating fibroblast growth factor 21 independently of endogenous insulin levels: a novel mechanism of glucagon-stimulated lipolysis? Diabetologia. 2013;56(3):588–597. doi: 10.1007/s00125-012-2803-y. [DOI] [PubMed] [Google Scholar]
- 31.Berglund E.D., Unger R.H. Role of fibroblast growth factor 21 in biology of glucagon. Diabetes. 2013;62(5):1376. doi: 10.2337/db12-1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim K.H. Autophagy deficiency leads to protection from obesity and insulin resistance by inducing Fgf21 as a mitokine. Nature Medicine. 2013;19(1):83–92. doi: 10.1038/nm.3014. [DOI] [PubMed] [Google Scholar]
- 33.Lee P. Mild cold exposure modulates fibroblast growth factor 21 (FGF21) diurnal rhythm in humans: relationship between FGF21 levels, lipolysis, and cold-induced thermogenesis. Journal of Clinical Endocrinology and Metabolism. 2013;98(1):E98–E102. doi: 10.1210/jc.2012-3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fisher F.M. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes and Development. 2012;26(3):271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adams A.C. The breadth of FGF21's metabolic actions are governed by FGFR1 in adipose tissue. Molecular Metabolism. 2012;2(1):31–37. doi: 10.1016/j.molmet.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moller D.E. Metabolic disease drug discovery – hitting the target is easier said than done. Cell Metabolism. 2012;15(1):19–24. doi: 10.1016/j.cmet.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 37.Munos B.H., Chin W.W. How to revive breakthrough innovation in the pharmaceutical industry. Science Translational Medicine. 2011;3(89):89cm16. doi: 10.1126/scitranslmed.3002273. [DOI] [PubMed] [Google Scholar]
- 38.Ford J. Unreliable research: trouble at the lab. Economist. 2013;409(8858):26–31. [Google Scholar]
- 39.Hansen B.C. Investigation and treatment of type 2 diabetes in nonhuman primates. Methods in Molecular Biology. 2012;933:177–185. doi: 10.1007/978-1-62703-068-7_11. [DOI] [PubMed] [Google Scholar]
- 40.Kharitonenkov A. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology. 2007;148(2):774–781. doi: 10.1210/en.2006-1168. [DOI] [PubMed] [Google Scholar]
- 41.Adams A.C. LY2405319, an engineered FGF21 variant, improves the metabolic status of diabetic monkeys. PLoS One. 2013;8(6):e65763. doi: 10.1371/journal.pone.0065763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schaefer E.J., Asztalos B.F. The effects of statins on high-density lipoproteins. Current Atherosclerosis Reports. 2006;8(1):41–49. doi: 10.1007/s11883-006-0063-3. [DOI] [PubMed] [Google Scholar]
- 43.Adams A.C. Fundamentals of FGF19 and FGF21 action in vitro and in vivo. PLoS One. 2012;7(5):e38438. doi: 10.1371/journal.pone.0038438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Veniant M.M. FGF21 promotes metabolic homeostasis via white adipose and leptin in mice. PLoS One. 2012;7(7):e40164. doi: 10.1371/journal.pone.0040164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muller T.D. Restoration of leptin responsiveness in diet-induced obese mice using an optimized leptin analog in combination with exendin-4 or FGF21. Journal of Peptide Science. 2012;18(6):383–393. doi: 10.1002/psc.2408. [DOI] [PubMed] [Google Scholar]
- 46.Maruthur N.M. The growing prevalence of type 2 diabetes: increased incidence or improved survival? Current Diabetes Reports. 2013;13(6):786–794. doi: 10.1007/s11892-013-0426-4. [DOI] [PubMed] [Google Scholar]
- 47.Yie J. FGF21 N- and C-termini play different roles in receptor interaction and activation. FEBS Letters. 2009;583(1):19–24. doi: 10.1016/j.febslet.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 48.Kharitonenkov A. Rational design of a fibroblast growth factor 21-based clinical candidate, LY2405319. PLoS One. 2013;8(3):e58575. doi: 10.1371/journal.pone.0058575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gaich G. The effects of LY2405319, an FGF21 analog, in obese human subjects with Type 2 diabetes. Cell Metabolism. 2013;18(3):333–340. doi: 10.1016/j.cmet.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 50.Fisher F.M. Integrated regulation of hepatic metabolism by fibroblast growth factor 21 (FGF21) in vivo. Endocrinology. 2011;152(8):2996–3004. doi: 10.1210/en.2011-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mai K. Physiological modulation of circulating FGF21: relevance of free fatty acids and insulin. American Journal of Physiology – Endocrinology and Metabolism. 2010;299(1):E126–E130. doi: 10.1152/ajpendo.00020.2010. [DOI] [PubMed] [Google Scholar]
- 52.Badman M.K. Fibroblast growth factor 21-deficient mice demonstrate impaired adaptation to ketosis. Endocrinology. 2009;150(11):4931–4940. doi: 10.1210/en.2009-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Holland W.L. An FGF21-adiponectin-ceramide axis controls energy expenditure and insulin action in mice. Cell Metabolism. 2013;17(5):790–797. doi: 10.1016/j.cmet.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Turer A.T., Scherer P.E. Adiponectin: mechanistic insights and clinical implications. Diabetologia. 2012;55(9):2319–2326. doi: 10.1007/s00125-012-2598-x. [DOI] [PubMed] [Google Scholar]
- 55.Hecht R. Rationale-based engineering of a potent long-acting FGF21 analog for the treatment of type 2 diabetes. PLoS One. 2012;7(11):e49345. doi: 10.1371/journal.pone.0049345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu J. Acute glucose-lowering and insulin-sensitizing action of FGF21 in insulin-resistant mouse models – association with liver and adipose tissue effects. American Journal of Physiology – Endocrinology and Metabolism. 2009;297(5):E1105–E1114. doi: 10.1152/ajpendo.00348.2009. [DOI] [PubMed] [Google Scholar]
- 57.Mu J. FGF21 analogs of sustained action enabled by orthogonal biosynthesis demonstrate enhanced antidiabetic pharmacology in rodents. Diabetes. 2012;61(2):505–512. doi: 10.2337/db11-0838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu J. Polyethylene glycol modified FGF21 engineered to maximize potency and minimize vacuole formation. Bioconjugate Chemistry. 2013;24(6):915–925. doi: 10.1021/bc300603k. [DOI] [PubMed] [Google Scholar]
- 59.Camacho R.C. Pegylated Fgf21 rapidly normalizes insulin-stimulated glucose utilization in diet-induced insulin resistant mice. European Journal of Pharmacology. 2013;715(1–3):41–45. doi: 10.1016/j.ejphar.2013.06.023. [DOI] [PubMed] [Google Scholar]
- 60.Wang H. High-level expression and purification of soluble recombinant FGF21 protein by SUMO fusion in Escherichia coli. BMC Biotechnology. 2010;10:14. doi: 10.1186/1472-6750-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang J. Development of a novel long-acting antidiabetic FGF21 mimetic by targeted conjugation to a scaffold antibody. Journal of Pharmacology and Experimental Therapeutics. 2013;346(2):270–280. doi: 10.1124/jpet.113.204420. [DOI] [PubMed] [Google Scholar]
- 62.Veniant M.M. Long-acting FGF21 has enhanced efficacy in diet-induced obese mice and in obese rhesus monkeys. Endocrinology. 2012;153(9):4192–4203. doi: 10.1210/en.2012-1211. [DOI] [PubMed] [Google Scholar]
- 63.Hager T. Differential enzyme-linked immunosorbent assay and ligand-binding mass spectrometry for analysis of biotransformation of protein therapeutics: application to various FGF21 modalities. Analytical Chemistry. 2013;85(5):2731–2738. doi: 10.1021/ac303203y. [DOI] [PubMed] [Google Scholar]
- 64.Wu A.L. Amelioration of type 2 diabetes by antibody-mediated activation of fibroblast growth factor receptor 1. Science Translational Medicine. 2011;3(113):113ra126. doi: 10.1126/scitranslmed.3002669. [DOI] [PubMed] [Google Scholar]
- 65.Foltz I.N. Treating diabetes and obesity with an FGF21-mimetic antibody activating the betaKlotho/FGFR1c receptor complex. Science Translational Medicine. 2012;4(162):162ra153. doi: 10.1126/scitranslmed.3004690. [DOI] [PubMed] [Google Scholar]
- 66.Smith R. FGF21 can be mimicked in vitro and in vivo by a novel anti-FGFR1c/beta-Klotho bispecific protein. PLoS One. 2013;8(4):e61432. doi: 10.1371/journal.pone.0061432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smith R. A novel approach to improve the function of FGF21. BioDrugs. 2013;27(2):159–166. doi: 10.1007/s40259-013-0013-x. [DOI] [PubMed] [Google Scholar]
- 68.Wang H., Qiang L., Farmer S.R. Identification of a domain within peroxisome proliferator-activated receptor gamma regulating expression of a group of genes containing fibroblast growth factor 21 that are selectively repressed by SIRT1 in adipocytes. Molecular and Cellular Biology. 2008;28(1):188–200. doi: 10.1128/MCB.00992-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hondares E. Hepatic FGF21 expression is induced at birth via PPARalpha in response to milk intake and contributes to thermogenic activation of neonatal brown fat. Cell Metabolism. 2010;11(3):206–212. doi: 10.1016/j.cmet.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Johnson C.L. Fibroblast growth factor 21 reduces the severity of cerulein-induced pancreatitis in mice. Gastroenterology. 2009;137(5):1795–1804. doi: 10.1053/j.gastro.2009.07.064. [DOI] [PubMed] [Google Scholar]
- 71.Dushay J. Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology. 2010;139(2):456–463. doi: 10.1053/j.gastro.2010.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hojman P. Fibroblast growth factor-21 is induced in human skeletal muscles by hyperinsulinemia. Diabetes. 2009;58(12):2797–2801. doi: 10.2337/db09-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen C. High plasma level of fibroblast growth factor 21 is an Independent predictor of type 2 diabetes: a 5.4-year population-based prospective study in Chinese subjects. Diabetes Care. 2011;34(9):2113–2115. doi: 10.2337/dc11-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Andersen B., Beck-Nielsen H., Hojlund K. Plasma FGF21 displays a circadian rhythm during a 72-h fast in healthy female volunteers. Clinical Endocrinology (Oxford) 2011;75(4):514–519. doi: 10.1111/j.1365-2265.2011.04084.x. [DOI] [PubMed] [Google Scholar]
- 75.Angelin B., Larsson T.E., Rudling M. Circulating fibroblast growth factors as metabolic regulators – a critical appraisal. Cell Metabolism. 2012;16(6):693–705. doi: 10.1016/j.cmet.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 76.Gariani K. Increased FGF21 plasma levels in humans with sepsis and SIRS. Endocrine Connections. 2013;2(3):146–153. doi: 10.1530/EC-13-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xiao Y. Distinct changes in serum fibroblast growth factor 21 levels in different subtypes of diabetes. Journal of Clinical Endocrinology and Metabolism. 2012;97(1):E54–E58. doi: 10.1210/jc.2011-1930. [DOI] [PubMed] [Google Scholar]
- 78.Christodoulides C. Circulating fibroblast growth factor 21 is induced by peroxisome proliferator-activated receptor agonists but not ketosis in man. Journal of Clinical Endocrinology and Metabolism. 2009;94(9):3594–3601. doi: 10.1210/jc.2009-0111. [DOI] [PubMed] [Google Scholar]
- 79.Adams A.C. Fibroblast growth factor 21 is not required for the antidiabetic actions of the thiazoladinediones. Molecular Metabolism. 2013;2(3):205–214. doi: 10.1016/j.molmet.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hotta Y. Fibroblast growth factor 21 regulates lipolysis in white adipose tissue but is not required for ketogenesis and triglyceride clearance in liver. Endocrinology. 2009;150(10):4625–4633. doi: 10.1210/en.2009-0119. [DOI] [PubMed] [Google Scholar]
- 81.Dutchak P.A. Fibroblast growth factor-21 regulates PPARgamma activity and the antidiabetic actions of thiazolidinediones. Cell. 2012;148(3):556–567. doi: 10.1016/j.cell.2011.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kurosu H. Tissue-specific expression of betaKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. Journal of Biological Chemistry. 2007;282(37):26687–26695. doi: 10.1074/jbc.M704165200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tomiyama K. Relevant use of Klotho in FGF19 subfamily signaling system in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(4):1666–1671. doi: 10.1073/pnas.0913986107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kharitonenkov A., Larsen P. FGF21 reloaded: challenges of a rapidly growing field. Trends in Endocrinology & Metabolism. 2011;22(3):81–86. doi: 10.1016/j.tem.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 85.Sarruf D.A. Fibroblast growth factor 21 action in the brain increases energy expenditure and insulin sensitivity in obese rats. Diabetes. 2010;59(7):1817–1824. doi: 10.2337/db09-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bookout A.L. FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nature Medicine. 2013;19(9):1147–1152. doi: 10.1038/nm.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lin Z. Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metabolism. 2013;17(5):779–789. doi: 10.1016/j.cmet.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 88.Inagaki T. Inhibition of growth hormone signaling by the fasting-induced hormone FGF21. Cell Metabolism. 2008;8(1):77–83. doi: 10.1016/j.cmet.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li Y., Knapp J.R., Kopchick J.J. Enlargement of interscapular brown adipose tissue in growth hormone antagonist transgenic and in growth hormone receptor gene-disrupted dwarf mice. Experimental Biology and Medicine (Maywood) 2003;228(2):207–215. doi: 10.1177/153537020322800212. [DOI] [PubMed] [Google Scholar]
- 90.Inagaki T. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metabolism. 2007;5(6):415–425. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 91.Nelson B.T. Metabolic hormone FGF21 is induced in ground squirrels during hibernation but its overexpression is not sufficient to cause torpor. PLoS One. 2013;8(1):e53574. doi: 10.1371/journal.pone.0053574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wu S. Increased expression of fibroblast growth factor 21 (FGF21) during chronic undernutrition causes growth hormone insensitivity in chondrocytes by inducing Leptin Receptor Overlapping Transcript (LEPROT) and Leptin Receptor Overlapping Transcript-like 1 (LEPROTL1) Expression. Journal of Biological Chemistry. 2013;288(38):27375–27383. doi: 10.1074/jbc.M113.462218. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 93.Wu S. Fibroblast growth factor 21 (FGF21) inhibits chondrocyte function and growth hormone action directly at the growth plate. Journal of Biological Chemistry. 2012;287(31):26060–26067. doi: 10.1074/jbc.M112.343707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kubicky R.A. Role of fibroblast growth factor 21 (FGF21) in undernutrition-related attenuation of growth in mice. Endocrinology. 2012;153(5):2287–2295. doi: 10.1210/en.2011-1909. [DOI] [PubMed] [Google Scholar]
- 95.Wei W. Fibroblast growth factor 21 promotes bone loss by potentiating the effects of peroxisome proliferator-activated receptor gamma. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(8):3143–3148. doi: 10.1073/pnas.1200797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Owen B.M. FGF21 contributes to neuroendocrine control of female reproduction. Nature Medicine. 2013;19(9):1153–1156. doi: 10.1038/nm.3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee P. Fibroblast growth factor 21 (FGF21) and bone: is there a relationship in humans? Osteoporosis International. 2013;24(12):3053–3057. doi: 10.1007/s00198-013-2464-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mullard A. Reliability of ‘new drug target’ claims called into question. Nature Reviews Drug Discovery. 2011;10(9):643–644. doi: 10.1038/nrd3545. [DOI] [PubMed] [Google Scholar]


