Abstract
Background
Gut microbiota is regarded as one of the major factors involved in the control of body weight. The antiobesity effects of ginseng and its main constituents have been demonstrated, but the effects on gut microbiota are still unknown.
Methods
To investigate the effect of ginseng on gut microbiota, 10 obese middle-aged Korean women took Panax ginseng extracts for 8 wk and assessment of body composition parameters, metabolic biomarkers, and gut microbiota composition was performed using 16S rRNA gene-based pyrosequencing at baseline and at 8 wk. Significant changes were observed in body weight and body mass index; however, slight changes were observed in gut microbiota. We divided the participants into two groups, the effective and the ineffective weight loss groups, depending on weight loss effect, in order to determine whether the antiobesity effect was influenced by the composition of gut microbiota, and the composition of gut microbiota was compared between the two groups.
Results
Prior to ginseng intake, significant differences of gut microbiota were observed between both at phyla and genera and the gut microbiota of the effective and ineffective weight loss groups was segregated on a principal coordinate analysis plot.
Conclusion
Results of this study indicate that ginseng exerted a weight loss effect and slight effects on gut microbiota in all participants. In addition, its antiobesity effects differed depending on the composition of gut microbiota prior to ginseng intake.
Keywords: gut microbiota, obesity, Panax ginseng, pyrosequencing
1. Introduction
Ginseng (the root of Panax ginseng Meyer, Araliaceae) has been used in herbal medicine as a general tonic for the promotion of health in Asian countries, including Korea, China, and Japan for 1,000 years [1]. The pharmacological properties of ginseng are attributed to ginsenosides, also referred to as steroid saponins, which are found in extracts of ginseng [2]. The pharmacological effects of ginseng extracts and ginsenosides have been reported to show various biological activities in inflammation, immunology, and cancer [3–5]. The effects of ginseng on obesity and metabolic disease, such as hypertension, diabetes, and hyperlipidemia, have also been reported [6–10].
Obesity is a serious health problem that has become prevalent in developed countries in recent years and is a risk factor for metabolic disease [11]. Recent studies have demonstrated a link between diet-induced obesity and changes in the gut microbial ecology, resulting in an increased capacity of the distal gut microbiota to promote host adiposity [12,13]. Animal and human studies have revealed a remarkable microbial influence on host metabolism, energy utilization and storage, and metabolic disease [14–16]. Gut microbiota is regarded as one of the major etiological factors involved in the control of body weight, so that drugs or components that help to maintain balance in the composition of the gut microbiota can increase the antiobesity effect [17,18].
Although the antiobesity effects of ginseng have been reported, whether or not it has an effect on the gut microbiota is still unknown. Other studies on ginseng-related gut microbiota have reported that metabolic activity of ginsenoside Rb1 to compound K (a metabolite of ginseng saponin) is variable between individuals, depending on the composition of gut microbiota in particular [19,20].
Therefore, this study was conducted to assess the effects of ginseng on obesity and gut microbiota using pyrosequencing based on the 16S rRNA gene. In addition, the difference of its antiobesity effects depending on gut microbiota composition was also investigated.
2. Materials and methods
2.1. Study participants and design
This study was approved by the Institutional Review Board of Dongguk University Ilsan Hospital (Gyeonggi-do, Korea; approval no. 2012-SR-25). Participants were recruited by advertisements in the local newspaper or by posters in the hospital. For qualification, participants should be obese [body mass index (BMI) ≥25 kg/m2] and female aged 40–60 yr. They must have been weight-stable within ±10% during the past 6 mo, and free from antibiotics, probiotics, or any drugs that could impact their weight for the past 3 mo. Participants with weight-influencing diseases, including hyper/hypothyroidism, heart diseases, psychogenic diseases, or other chronic systemic diseases were excluded. Smokers or pregnant women confirmed by a positive hCG screening test were also excluded. Nineteen participants were recruited, and 10 of them completed the study. The participants were asked not to change their exercise or diet habits during the 8-wk clinical trial. During the study, participants who failed to take <80% of the required dose of medicine, retracted their consents due to inconvenience (personal choices), or refused to have communication with members of the research staff were dropped from the study.
2.2. Herbal preparation
Panax ginseng extracts were manufactured and provided by Korea Medicine Biofermentation Co., Ltd (Andong, Korea). Quantities of the freeze-dried granulated extracts weighing 4 g each were packed in paper medicine pockets. The medicines were distributed to the participants per 2 wk at every visit. The participants were asked to take one packet two times/d. The herbal medicines were distributed to the participants by the administrative pharmacist in the dispensary of the hospital.
2.3. Ginsenosides analysis
Ginsenosides were determined using high performance liquid chromatography (HP 1050, AGILENT, Santa Clara, CA, USA), the analytical column was an Ultrasphere ODS (C18, 5 μm, 4.6 mm × 250 mm, Shiseido, Tokyo, Japan). The two mobile phases were water (Samchun Chemical, Gyeonggi, Korea) and acetonitrile (Samchun Chemical, Gyeonggi, Korea). The flow rate was set at 0.4 mL/min and wavelength was 203 nm. Seventeen saponins (Rb2, Rb3, Rc, Rd, Re, Rf, Rg1, Rg2S, Rg2R, Rg3S, Rg3R, Rh1, Rh2S, Rh2R, C-K, F1, F2; Sigma–Aldrich, St Louis, MO, USA) were used as standards for this purpose. Rg1, Rf, and Rc were the main contents, the retention time of Rg1 was 16.12 min, that of Rf was 19.18 min, and that of Rc was 19.53 min. The concentration of Rg1 was 3.73%, that of Rf was 3.57%, and that of Rc was 1.87% (Fig. 1).
Fig. 1.
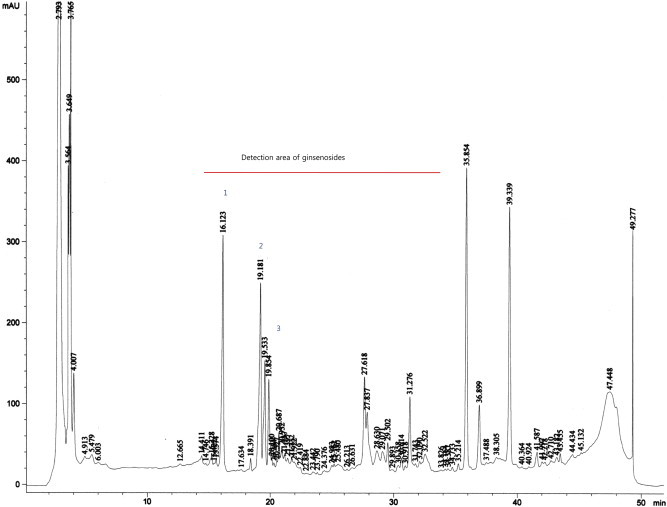
High performance liquid chromatography analysis of Panax ginseng extracts. Peaks: (1) Rg1; (2) Rf; and (3) Rc.
2.4. Assessment
Progress of analytical measurements in the study is shown in Table 1.The participants were asked to visit every 2nd wk. Blood pressure, body weight, waist circumference, and body composition were measured at every visit, and blood analysis and stool analysis were checked on the 1st visit day (wk 0) and last day (wk 8).
Table 1.
Progress of Analysis in the Course of the Study
| Blood analysis | Stool analysis | BP | BW | WC | Body composition analysis | |
|---|---|---|---|---|---|---|
| Visit 1 (wk 0) | O | O | O | O | O | O |
| Visit 2 (wk 2) | O | O | O | O | ||
| Visit 3 (wk 4) | O | O | O | O | ||
| Visit 4 (wk 6) | O | O | O | O | ||
| Visit 5 (wk 8) | O | O | O | O | O | O |
BP, blood pressure; BW, body weight; WC, waist circumference.
2.5. Physical examination and anthropometry
Blood pressure and heart rate were measured using an automatic digital sphygmomanometer. Wearing a hospital gown, body weight and height were measured to the nearest 0.1 kg and 0.5 cm, respectively. Waist circumference was measured three times according to the World Health Organization method [21] by the same observer.
2.6. Body composition analysis
Body composition was measured at every visit using the bioelectrical impedance analysis method (InBody 3.0; Biospace, Seoul, Korea). This device measures impedance through eight tactile electrodes placed on palms, thumbs, heels, and soles. Each participant stood upright, stepping onto the foot electrodes and loosely gripping the pipe-shaped hand electrodes with arms held vertically. Lean body mass, body mass index, and percent fat were measured and recorded.
2.7. Blood chemistry
Blood tests including fasting glucose, high-density lipoprotein-cholesterol, triglyceride, and total cholesterol were performed prior to the start of the experiment and 8 wk later. At baseline, participants with high fasting blood glucose (>140 mg/dL) or possible liver problems (aspartate aminotransferase or alanine aminotransferase >100 IU/L) were excluded.
2.8. Pyrosequencing
The participants were asked to bring their stool samples on the 1st visit day (wk 0) and last day (wk 8) in the stool-sampling container. The fresh human stools were collected and immediately stored at –70°C. Genomic DNA were extracted from fecal samples of participants using a Fast DNA SPIN extraction kit (MP Biomedicals, Santa Ana, CA, USA), and fragments of the 16S rRNA gene (V1–V3) were amplified from the extracted DNA. The amplifications were performed according to previous reports using a barcoded fusion primer [22,23] using a C1000 Touch thermal cycler (Bio-Rad, Hercules, CA, USA). The amplified products were visualized on 2% agarose gel electrophoresis using the Gel Doc system (Bio-Rad). Amplicons were purified using the QIA quick PCR purification kit (Qiagen, Valencia, CA, USA) and quantified using the PicoGreen dsDNA Assay kit (Invitrogen, Carlsbad, CA, USA). Equimolar concentrations of each amplicon from different samples were pooled and purified using the AMPure bead kit (Agencourt Bioscience, Beverly, MA, USA) and then amplified on sequencing beads by emulsion PCR. Sequencing reactions were performed using a Roche/454 GS Junior system (454 Life Sciences, Branford, CT, USA) following the manufacturer's instructions.
2.9. Sequence data analysis
Obtained sequences were sorted according to their unique barcode in the demultiplexing step, and low quality reads (average quality score <25 or read length <300 bp) were removed for further analysis. Primer sequences were trimmed by pairwise sequence alignment and the hmm-search program of the HMMER 3.0 package [24]. To modify sequencing errors, representative sequences in clusters of trimmed sequences were chosen for taxonomy identification. Each read was characterized by their taxonomic positions according to the highest pairwise similarity among the top five BLASTN hits against the EzTaxon-e database [25]. Chimera sequences were removed by UCHIME [26]. Various read numbers in samples were normalized by random subsampling, and the diversity indices were calculated using the mothur program [27]. Pyrosequencing reads obtained from this study are available in the European Molecular Biology Laboratory Sequence Read Archive database under study number PRJEB4531 [28].
2.10. Statistical analysis
Results are presented as mean ± standard deviation. Comparison of prior to and after treatment was performed using paired t test and Wilcoxon signed-rank test and the two groups divided according to weight loss effect were compared using the Mann–Whitney U test. Values of p < 0.05 were considered statistically significant. All analyses were performed using SPSS version 15.0 for Windows (SPSS Inc., Chicago, IL, USA).
3. Results and discussion
3.1. Anthropometric and biochemical changes
Differences of gut microbial communities are related to gender and age [29,30], therefore we limited our inclusion criteria to a specific gender and the participants were middle-aged (40–59 yr) women. A total of 10 participants completed the trial; their general characteristics are shown in Table 2. Age was 50.40 ± 4.95 yr and body weight and BMI were 71.39 ± 4.95 kg and 28.35 ± 2.00 kg/m2, respectively.
Table 2.
Changes of Anthropometric and Biochemical Measurement
| Parameters | Prior to | After | p |
|---|---|---|---|
| Age | 50.40 ± 4.95 | ||
| BW (kg) | 71.39 ± 4.95 | 70.33 ± 5.38 | 0.042* |
| BMI (kg/m2) | 28.35 ± 2.00 | 27.87 ± 1.97 | 0.031* |
| WC (cm) | 94.08 ± 7.12 | 92.50 ± 7.08 | 0.058 |
| Body fat percentage (%) | 37.89 ± 4.27 | 37.24 ± 5.21 | 0.544 |
| TC (mg/dL) | 209.90 ± 27.23 | 211.00 ± 27.30 | 0.821 |
| TG (mg/dL) | 136.50 ± 67.48 | 139.20 ± 53.07 | 0.841 |
| HDL (mg/dL) | 56.80 ± 14.54 | 56.20 ± 13.81 | 0.853 |
| Glucose (mg/dL) | 108.10 ± 13.30 | 113.60 ± 13.15 | 0.100 |
Data are presented as mean ± SD.
* p < 0.05.
BMI, body mass index; BW, body weight; HDL, high-density lipoprotein-cholesterol; TC, total cholesterol; TG, triglyceride; WC, waist circumference.
After ginseng intake, significant decreases were observed in weight and BMI, with difference of –1.06 ± 1.41 kg and −0.48 ± 0.59 kg/m2, respectively. However, no significant decrease was observed in waist circumference, body fat percentage, high-density lipoprotein-cholesterol, triglyceride, total cholesterol, and glucose. In contrast to this result, the effects of ginseng, ginsenosides, or compound K on antiobesity have been reported as lowering cholesterol and controlling blood glucose via inhibition of lipid accumulation in adipocyte and increase of phosphorylation of insulin receptor substrate-1, Akt, membranous glucose transporter 4 in muscle [7–9]. However, there was no significant effect on obesity related parameters in this study. No effects of ginseng on these parameters were reported in randomized controlled studies for healthy obese participants during 12 wk, [31,32]. A longer duration of taking ginseng might be needed to confirm significant effects for human beings. Different bacterial ginsenoside-hydrolyzing effects between humans and experimental mice [33] and individual difference of metabolic ability to ginseng could be a reason for this result.
3.2. Analysis of gut microbiota
We performed pyrosequencing for analysis of the gut microbiota of prior to and after in ginseng treated participants. Bacterial richness and diversity obtained from pyrosequencing after normalization of reads number are shown in Table 3. A total of 73,611 sequences were obtained and analyzed, and the normalized read number of each sample for comparison of diversity indices was 2,000. Good's coverage of samples was over 80%, except for the after treatment sample of Participant 5. Increased Shannon diversity indices were detected in the after treatment sample compared to the prior to treatment sample for Participants 1, 2, 5, 6, and 7, whereas deceased indices were detected for samples of Participants 4, 8, 9, and 10.
Table 3.
Summary of Estimated Diversities Obtained from Pyrosequences
| Sample | Analyzed reads | Normalized reads | Observed OTUs | Estimated OTUs (Chao1) | Shannon diversity | Good's coverage | |
|---|---|---|---|---|---|---|---|
| Prior to | 1 | 1,866 | 1,866 | 336 | 1,728.13 | 3.67 | 0.86 |
| 2 | 2,923 | 2,000 | 340 | 3,087.25 | 3.20 | 0.85 | |
| 3 | 2,753 | 2,000 | 243 | 1,743.23 | 2.96 | 0.90 | |
| 4 | 5,354 | 2,000 | 246 | 1,103.37 | 3.68 | 0.91 | |
| 5 | 4,863 | 2,000 | 350 | 1,988.45 | 3.89 | 0.87 | |
| 6 | 2,485 | 2,000 | 227 | 595.88 | 3.63 | 0.93 | |
| 7 | 3,320 | 2,000 | 179 | 485.71 | 3.01 | 0.94 | |
| 8 | 3,334 | 2,000 | 213 | 717.79 | 3.38 | 0.93 | |
| 9 | 4,503 | 2,000 | 427 | 3,647.56 | 4.32 | 0.83 | |
| 10 | 5,070 | 2,000 | 218 | 784.25 | 3.37 | 0.92 | |
| After | 1 | 2,056 | 2,000 | 372 | 1,440.03 | 4.14 | 0.87 |
| 2 | 2,262 | 2,000 | 343 | 2,893.88 | 3.38 | 0.85 | |
| 3 | 2,953 | 2,000 | 244 | 1,544.20 | 2.96 | 0.90 | |
| 4 | 4,922 | 2,000 | 297 | 1,394.29 | 3.49 | 0.89 | |
| 5 | 5,137 | 2,000 | 522 | 3,800.79 | 4.65 | 0.79 | |
| 6 | 4,345 | 2,000 | 415 | 1,555.36 | 4.21 | 0.85 | |
| 7 | 3,209 | 2,000 | 229 | 913.00 | 3.65 | 0.92 | |
| 8 | 2,417 | 2,000 | 96 | 255.00 | 2.59 | 0.97 | |
| 9 | 3,510 | 2,000 | 159 | 366.83 | 3.29 | 0.96 | |
| 10 | 6,329 | 2,000 | 163 | 649.77 | 2.47 | 0.94 |
OTU, operational taxonomic unit.
Predominant phyla in average community compositions were Firmicutes, Actinobacteria, and Bacteroidetes, and no significant change in phylum level was observed between prior to and after. Selected genera having over 1% proportion of median value were compared. The main dominants were changed after ginseng intake; those prior to intake were genera of Blautia, Bifidobacterium, and Anaerostipes whereas those after intake were Bifidobacterium, Blautia, and Faecalibacterium, in order of abundance (Fig. 2). Significant change was observed only in the relative abundance of Anaerostipes; prior to was 6.70 ± 3.35%, and after was 3.11 ± 3.24% (data not shown).
Fig. 2.
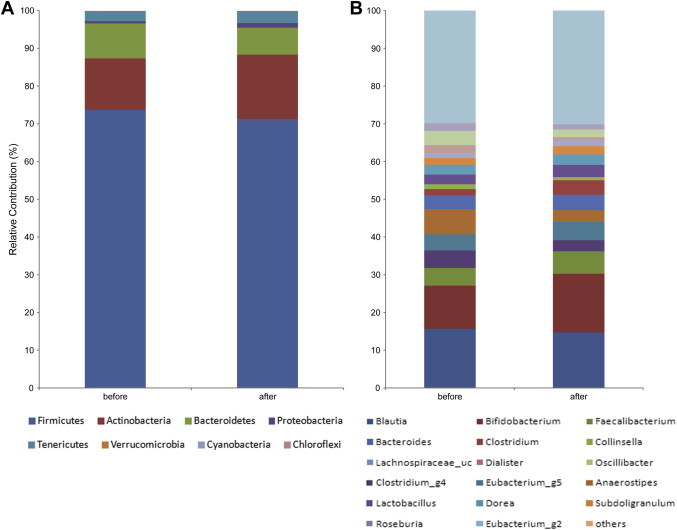
The phylum and genus composition of fecal bacteria were compared between prior to and after treatment in a total of 10 participants. The relative abundance of dominant phyla identified from pyrosequencing data is shown. (A) Phylum composition of fecal bacteria. (B) Genus composition.
3.3. Comparison of gut microbiota between the effective and ineffective weight loss groups
To express the pharmacological actions of ginseng saponins, it is presumed that ginsenosides, the main constituent of ginseng, must be metabolized by human intestinal microbes after being taken orally [34]. The ginsenoside Rb1 in orally administered ginseng is metabolized to compound K by gut microbiota prior to its absorption into the blood. Beta-glucosidase, produced by intestinal microbiota, plays an important role in the pharmacological actions of ginsenoside and the components of ginseng; it is the representative ginsenoside-transforming enzyme. This enzyme activity of gut microbiota varies significantly between individuals, so that the metabolizing activities of ginsenoside Rb1inindividuals are significantly different [19]. People with different levels of ginsenoside Rb1 degradation to compound K had different gut microbiota [20].
To investigate whether the antiobesity effect of ginseng might be influenced by the composition of gut microbiota, we analyzed bacterial communities of all participants at the baseline using principal coordinate analysis (PCoA). In the PCoA plot, gut microbiota of each member was clustered according to the degree of weight loss (Fig. 3). The groups were designated as: the effective weight loss group (EWG; Participants 1, 2, 5, and 6; weight change, −2.4 ± 0.38 kg); and ineffective weight loss group (IWG; Participants 3, 4, and 10; weight change, 0.63 ± 0.64 kg). Although there was no significant difference in general characteristics such as age and obesity related parameters (Table 4), different gut microbiota was observed between groups. The rarefaction curves showed the difference of gut microbiota between the two groups (Fig. 4). The richness of bacterial communities obtained from EWG was relatively higher than that of IWG. Phyla of Firmicutes, Actinobacteria, Tenericutes, and Bacteroidetes were predominant in EWG samples of prior to ginseng intakes, whereas Firmicutes, Actinobacteria, and Proteobacteria were dominant in IWG samples (Table 5, Fig. 5A). Relative abundances of Actinobacteria and Proteobacteria in EWG were lower than those in IWG, whereas phyla of Tenericutes, Bacteroidetes, and Firmicutes were more abundant in the EWG than IWG. Furthermore the relative abundances of Firmicutes, Actinobacteria and Proteobacteria were significantly different between both groups. These results partly correspond with the earlier one. Samples with fecal activity potently metabolizing ginsenoside Rb1 to compound K had lower levels of Proteobacteria and higher levels of Tenericutes and Bacteriodetes than in samples with fecal activity non-metabolizing ginsenoside Rb1 to compound K [20]. For detailed microbial composition, we analyzed the composition of genera, it had also noteworthy differences between groups (Table 5, Fig. 5B). The three predominant genera in EWG were Blautia, Anaerostipes, and Oscillibacter, whereas those in IWG were Bifidobacterium, Blautia, and Clostridium_g4. The relative abundances of Anaerostipes and Eubacterium_g5 were increased in EWG, whereas that of Lactobacillus was increased in IWG. Furthermore, the relative abundance of Bifidobacterium, Escherichia, and Clostridium_g23 in EWG were significantly lower than those in IWG. However, the genera that had significant differences between the groups (Clostridiales_uc_g, Oscillibacter, Ruminococcus, Holdemania, and Sutterella) were not consistent with a previous study [20]. Individual variations of gut microbiota [35] can generate these different results, so it is not easy to compare directly between the two limited sample sized studies.
Fig. 3.
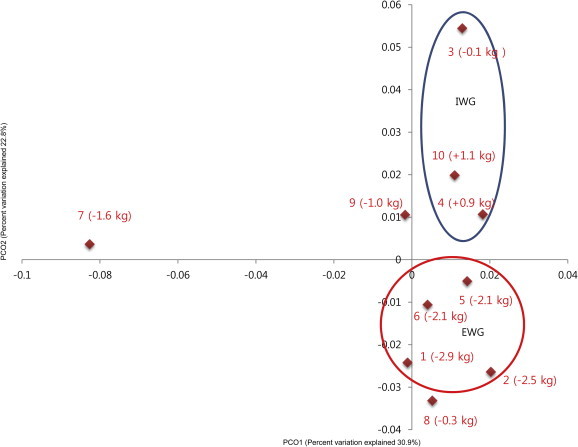
Principal coordinate analysis (PCoA) plot in a total of 10 participants. The plot shows the clustering pattern depending degree of weight loss. The participants' number and body weight change are shown. EWG, effective weight loss group; IWG, ineffective weight loss group.
Table 4.
Comparison of General Characteristics Between the Effective Weight Loss Group (EWG) and Ineffective Weight Loss Group (IWG) at Baseline
| Parameters | EWG | IWG | p |
|---|---|---|---|
| Age (y) | 53.25 ± 5.06 | 49.25 ± 4.65 | 0.182 |
| BW (kg) | 71.65 ± 4.55 | 74.57 ± 3.49 | 0.200 |
| BMI (kg/m2) | 28.83 ± 2.83 | 28.57 ± 0.95 | 0.887 |
| TC (mg/dL) | 207.75 ± 18.66 | 209.67 ± 33.84 | 0.926 |
| TG (mg/dL) | 111.75 ± 55.99 | 187.00 ± 67.51 | 0.199 |
| HDL (mg/dL) | 53.25 ± 5.31 | 62.33 ± 26.27 | 0.612 |
| Glucose (mg/dL) | 100.25 ± 16.62 | 108.00 ± 6.92 | 0.446 |
Data are presented as mean ± SD.
BMI, body mass index; BW, body weight; HDL, high-density lipoprotein -cholesterol; TC, total cholesterol; TG, triglyceride.
Fig. 4.
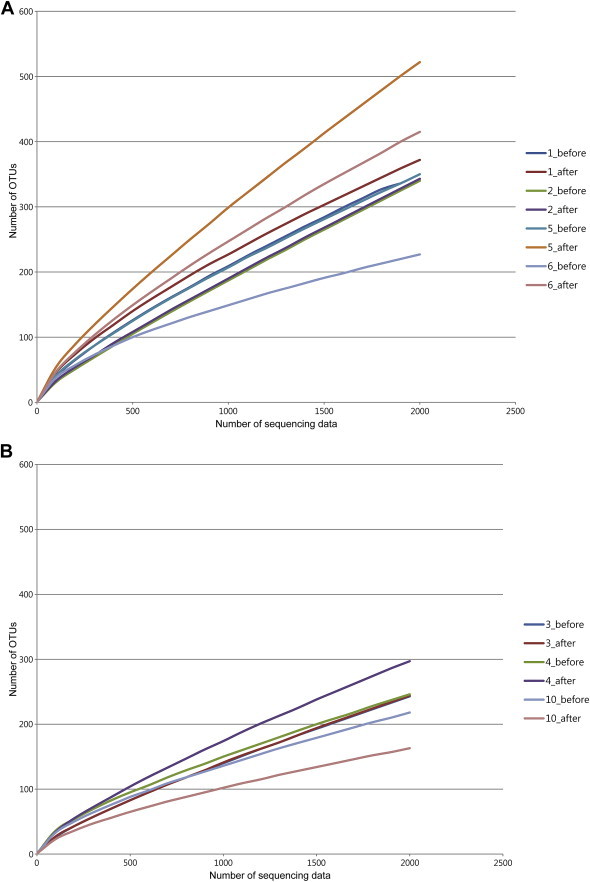
Rarefaction curves of (A) effective weight loss group (EWG) and (B) ineffective weight loss group (IWG) samples were compared. The rarefaction curves were obtained after obtaining a normalized read size in each sample.
Table 5.
Comparison of Gut Microbiota Between the EWG and IWG at Baseline
| Gut microbiota (%) | EWG (%) | IWG (%) | p | |
|---|---|---|---|---|
| Phylum | Firmicutes | 84.25 ± 10.33 | 68.44 ± 5.20 | 0.034* |
| Actinobacteria | 7.29 ± 7.21 | 28.46 ± 6.81 | 0.034* | |
| Bacteroidetes | 3.42 ± 2.74 | 1.48 ± 1.71 | 0.480 | |
| Proteobacteria | 0.07 ± 0.06 | 1.54 ± 1.73 | 0.034* | |
| Tenericutes | 4.86 ± 4.90 | 0.01 ± 0.01 | 0.142 | |
| Verrucomicrobia | 0.13 ± 0.27 | 0.07 ± 0.08 | 0.172 | |
| Genus | Blautia | 23.78 ± 23.80 | 14.06 ± 8.61 | 0.724 |
| Bifidobacterium | 5.75 ± 5.40 | 25.43 ± 9.74 | 0.034* | |
| Faecalibacterium | 3.49 ± 3.82 | 7.13 ± 3.42 | 0.289 | |
| Clostridium_g4 | 3.97 ± 3.26 | 3.86 ± 5.84 | 1.000 | |
| Eubacterium_g5 | 12.99 ± 7.60 | 4.20 ± 3.96 | 0.480 | |
| Anaerostipes | 12.99 ± 7.60 | 1.87 ± 1,48 | 0.157 | |
| Bacteroides | 1.87 ± 1.48 | 1.00 ± 1.48 | 0.289 | |
| Clostridium | 1.09 ± 1.03 | 1.80 ± 2.73 | 0.724 | |
| Collinsella | 1.01 ± 1.80 | 2.38 ± 4.13 | 1.000 | |
| Lactobacillus | 0.51 ± 0.63 | 7.08 ± 12.73 | 0.724 | |
| Dorea | 4.08 ± 4.73 | 1.74 ± 1.26 | 0.480 | |
| Subdoligranulum | 1.49 ± 1.50 | 1.40 ± 1.42 | 0.858 | |
| Lachnospiraceae_uc | 1.57 ± 1.01 | 1.29 ± 0.54 | 0.480 | |
| Dialister | 8.32 ± 15.90 | 0.10 ± 0.09 | 0.714 | |
| Oscillibacter | 2.53 ± 2.34 | 1.17 ± 1.14 | 0.212 | |
| Roseburia | 2.53 ± 2.34 | 1.17 ± 1.14 | 0.289 | |
| Escherichia | 0.00 ± 0.00 | 0.89 ± 0.57 | 0.019* | |
| Clostridium_g23 | 0.00 ± 0.00 | 0.17 ± 0.21 | 0.019* |
Data are presented as mean ± SD.
∗ p < 0.05.
Fig. 5.
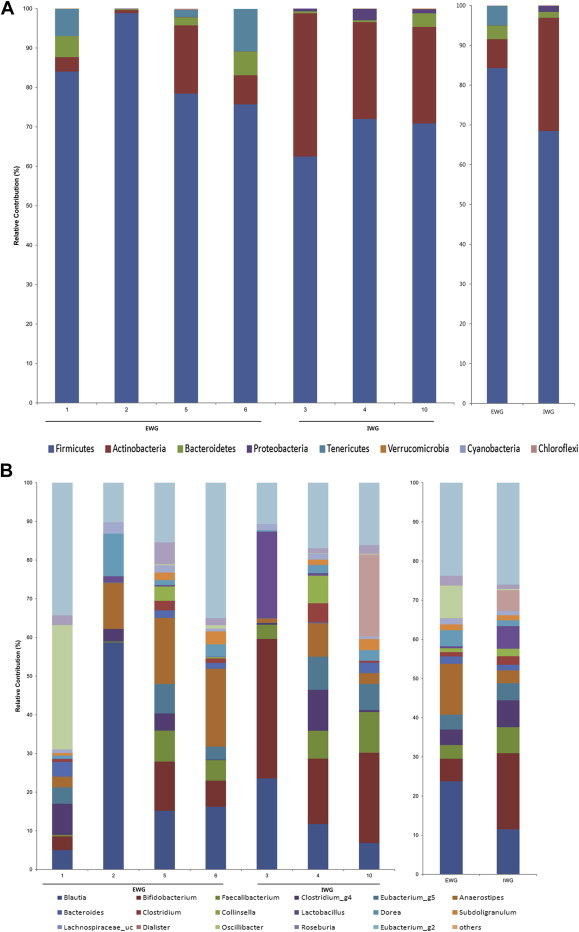
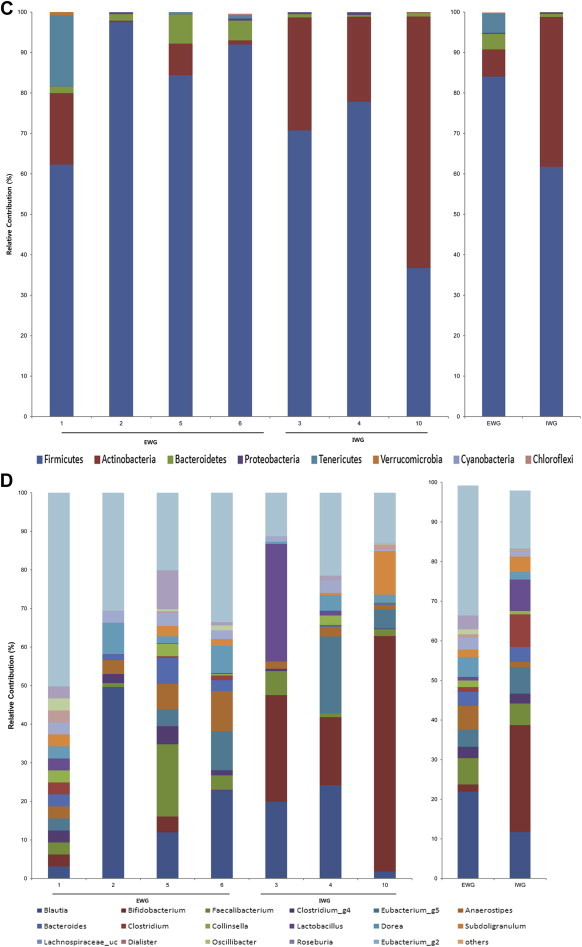
The phylum and genus compositions of fecal bacteria were compared between prior to and after treatment in the effective weight loss group (EWG) and ineffective weight loss group (IWG). The relative abundance of dominant phyla identified from pyrosequencing data is shown (individual samples are on the left panels and pooled samples are on the right panels). (A) Phylum composition of fecal bacteria prior to ginseng intake. (B) Genus prior to intake. (C) Phylum after intake. (D) Genus after intake.
The antiobesity effect of ginseng could work differently depending on gut microbiota composition as explained above. We also wanted to know whether ginseng could make changes of gut microbial composition. Therefore, we investigated changes of microbial composition after ginseng intake. Each group showed changes in microbial composition; the three main dominant genera of EWG were changed to Blautia, Faecalibacterium, and Anaerostipes, and those of IWG were changed to Bifidobacterium, Blautia, and Clostridium at the genus level (Fig. 5C and D). However, neither group showed statistically significant changes at the phylum or genus level (data not shown). In addition, we compared the changes of gut microbiota after ginseng treatment between the two groups; only the relative composition of Proteobacteria had significant change (Table 6).
Table 6.
Changes of Gut Microbiota Between Effective Weight Loss Group (EWG) and Ineffective Weight Loss Group (IWG) Samples After Ginseng Intake
| Gut microbiota (%) | EWG (%) | IWG (%) | p | |
|---|---|---|---|---|
| Phylum | Firmicutes | −0.20 ± 16.06 | −6.69 ± 23.82 | 0.724 |
| Actinobacteria | −0.57 ± 10.43 | 8.59 ± 25.34 | 0.724 | |
| Bacteroidetes | 0.41 ± 3.77 | −0.77 ± 1.57 | 0.724 | |
| Proteobacteria | 0.21 ± 0.25 | −1.21 ± 1.01 | 0.034* | |
| Tenericutes | −0.12 ± 8.42 | 0.01 ± 0.01 | 0.714 | |
| Verrucomicrobia | 0.19 ± 0.36 | −0.03 ± 0.28 | 0.067 | |
| Genus | Blautia | −1.88 ± 6.48 | 1.25 ± 9.70 | 1.000 |
| Bifidobacterium | −0.36 ± 10.11 | 10.00 ± 24.39 | 0.289 | |
| Faecalibacterium | 2.52 ± 5.55 | −4.14 ± 5.94 | 0.157 | |
| Clostridium_g4 | −0.02 ± 0.93 | −1.10 ± 2.69 | 0.480 | |
| Eubacterium_g5 | 1.03 ± 4.11 | 3.16 ± 7.19 | 0.724 | |
| Anaerostipes | −7.03 ± 4.95 | 2.36 ± 3.46 | 0.157 | |
| Bacteroides | 1.18 ± 3.02 | −0.64 ± 1.36 | 0.157 | |
| Clostridium | −0.43 ± 1.20 | −1.75 ± 2.64 | 0.289 | |
| Collinsella | −0.12 ± 0.26 | −1.55 ± 2.75 | 0.593 | |
| Lactobacillus | −0.32 ± 0.71 | 2.88 ± 4.45 | 0.289 | |
| Dorea | 0.38 ± 2.76 | 0.47 ± 1.34 | 0.480 | |
| Subdoligranulum | −0.23 ± 1.00 | 2.57 ± 5.14 | 0.858 | |
| Lachnospiraceae_uc | 0.80 ± 0.94 | 0.34 ± 1.09 | 0.289 | |
| Dialister | 0.10 ± 0.17 | −6.64 ± 11.54 | 0.372 | |
| Oscillibacter | −6.21 ± 12.86 | −0.05 ± 0.47 | 0.372 | |
| Roseburia | 0.29 ± 2.88 | −0.07 ± 1.25 | 0.480 |
Data are presented as mean ± SD.
* p < 0.05.
The comparisons of bacterial communities between prior to and after ginseng intake in both groups were analyzed by PCoA plot (Fig. 6). Prior to ginseng intake, bacterial communities were segregated depending on weight loss effect, but there was no remarkable change of bacterial communities in both groups after ginseng intake. This indicates that the influence of ginseng intakes on bacterial community was not considerable, however the compositions of gut bacteria could determine whether weight loss is effective or not.
Fig. 6.
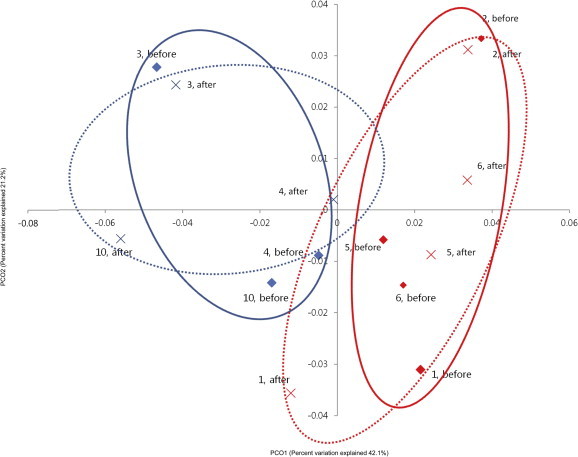
Principal coordinate analysis plot. The plot shows the clustering pattern between the effective weight loss group (EWG) and ineffective weight loss group (IWG) based on weighted pairwise Fast UniFrac analysis.  , the community of EWG in the prior to ginseng intake;
, the community of EWG in the prior to ginseng intake;  , that of EWG in the after ginseng intake,
, that of EWG in the after ginseng intake,  , that of IWG in the prior to ginseng intake;
, that of IWG in the prior to ginseng intake;  , that of IWG in the after ginseng intake. Unbroken circles indicate grouping of the communities in the prior to ginseng intake and broken circles indicate that in the after ginseng intake.
, that of IWG in the after ginseng intake. Unbroken circles indicate grouping of the communities in the prior to ginseng intake and broken circles indicate that in the after ginseng intake.
Ginseng exerted a weight loss effect and slight effects on gut microbiota in all participants. It is an important result that its antiobesity effects differed depending on the composition of gut microbiota prior to ginseng intake. The biotransformation activity from ginsenoside-Rb1 to compound K was significantly different among individuals [36], and intestinal bacterial metabolism of ginseng is dependent on the composition of gut microbiota [19,20]. Therefore, a single ginsenoside or a ginseng extract may lead to different effects among participants [33]. However, we did not analyze the biotransformation activity ginsenoside to compound K, for example, so supplemental studies are necessary to confirm the metabolism of ginseng by gut microbiota for antiobesity.
There were other limitations in this study including: no controlled study, a limited number of participants, and a limited study period. Therefore, the present study can be considered explorative research, which can motivate a full-scaled one. However, it was the first trial to assess the effects of ginseng on obesity and gut microbiota as well different weight loss effects depending on the composition of gut microbiota.
Conflicts of interest
All contributing authors declare no conflicts of interest.
Acknowledgments
This research was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (No. 2006-2005173).
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- 1.Li C.P., Li R.C. An introductory note to ginseng. Am J Chin Med. 1973;1:249–261. doi: 10.1142/s0192415x73000279. [DOI] [PubMed] [Google Scholar]
- 2.Kim D.H. Chemical diversity of Panax ginseng, Panax quinquifolium and Panax notoginseng. J Ginseng Res. 2012;6:1–15. doi: 10.5142/jgr.2012.36.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joh E.H., Lee I.A., Jung I.H., Kim D.H. Ginsenoside Rb1 and its metabolite compound K inhibit IRAK-1 activation—the key step of inflammation. Biochem Pharmacol. 2011;2:278–286. doi: 10.1016/j.bcp.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Choo M.K., Sakurai H., Kim D.H., Saiki I. A ginseng saponin metabolite suppresses tumor necrosis factor-alpha-promoted metastasis by suppressing nuclear factor-kappaB signaling in murine colon cancer cells. Oncol Rep. 2008;9:595–600. [PubMed] [Google Scholar]
- 5.Kang S., Min H. Ginseng, the ‘immunity boost’: the effects of Panax ginseng on immune system. J Ginseng Res. 2012;36:354–368. doi: 10.5142/jgr.2012.36.4.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gu J., Li W., Xiao D., Wei S., Cui W., Chen W., Hu Y., Bi X., Kim Y., Li J. Compound K, a final intestinal metabolite of ginsenosides, enhances insulin secretion in MIN6 pancreatic β-cells by upregulation of GLUT2. Fitoterapia. 2013;7:84–88. doi: 10.1016/j.fitote.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 7.Oh J., Lee H., Park D., Ahn J., Shin S.S., Yoon M. Ginseng and its active components ginsenosides inhibit adipogenesis in 3T3-L1 cells by regulating MMP-2 and MMP-9. Evid Based Complement Alternat Med. 2012;2012:265023. doi: 10.1155/2012/265023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee H.J., Lee Y.H., Park S.K., Kang E.S., Kim H.J., Lee Y.C., Choi C.S., Park S.E., Ahn C.W., Cha B.S. Korean red ginseng (Panax ginseng) improves insulin sensitivity and attenuates the development of diabetes in Otsuka Long-Evans Tokushima fatty rats. Metabolism. 2009;58:1170–1177. doi: 10.1016/j.metabol.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 9.Lee S.H., Lee H.J., Lee Y.H., Lee B.W., Cha B.S., Kang E.S., Ahn C.W., Park J.S., Kim H.J., Lee E.Y. Korean red ginseng (Panax ginseng) improves insulin sensitivity in high fat fed Sprague–Dawley rats. Phytother Res. 2012;26:142–147. doi: 10.1002/ptr.3610. [DOI] [PubMed] [Google Scholar]
- 10.Hong S.Y., Kim J.Y., Ahn H.Y., Shin J.H., Kwon O. Panax ginseng extract rich in ginsenoside protopanaxatriol attenuates blood pressure elevation in spontaneously hypertensive rats by affecting the Akt-dependent phosphorylation of endothelial nitric oxide synthase. J Agric Food Chem. 2012;60:3086–3091. doi: 10.1021/jf204447y. [DOI] [PubMed] [Google Scholar]
- 11.James P.T., Rigby N., Leach R., International Obesity Task Force The obesity epidemic, metabolic syndrome and future prevention strategies. Eur J Cardiovasc Prev Rehabil. 2004;11:3–8. doi: 10.1097/01.hjr.0000114707.27531.48. [DOI] [PubMed] [Google Scholar]
- 12.Bäckhed F., Crawford P.A. Coordinated regulation of the metabolome and lipidome at the host-microbial interface. Biochim Biophys Acta. 2010;1801:240–245. doi: 10.1016/j.bbalip.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turnbaugh P.J., Bäckhed F., Fulton L., Gordon J.I. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3:213–223. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cani P.D., Delzenne N.M. The role of the gut microbiota in energy metabolism and metabolic disease. Curr Pharm Des. 2009;15:1546–1558. doi: 10.2174/138161209788168164. [DOI] [PubMed] [Google Scholar]
- 15.Larsen N., Vogensen F.K., van den Berg F.W., Nielsen D.S., Andreasen A.S., Pedersen B.K., Al-Soud W.A., Sørensen S.J., Hansen L.H., Jakobsen M. Gut microbiota in human adults with type 2 diabetes differs from nondiabetic adults. PLoS One. 2010;5:e9085. doi: 10.1371/journal.pone.0009085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Angelakis E., Armougom F., Million M., Raoult D. The relationship between gut microbiota and weight gain in humans. Future Microbiol. 2012;7:91–109. doi: 10.2217/fmb.11.142. [DOI] [PubMed] [Google Scholar]
- 17.Harris K., Kassis A., Major G., Chou C.J. Is the gut microbiota a new factor contributing to obesity and its metabolic disorders? J Obes. 2012;2012:879151. doi: 10.1155/2012/879151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Million M., Raoult D. The role of the manipulation of the gut microbiota in obesity. Curr Infect Dis Rep. 2013;15:25–30. doi: 10.1007/s11908-012-0301-5. [DOI] [PubMed] [Google Scholar]
- 19.Choi J.R., Hong S.W., Kim Y., Jang S.E., Kim N.J., Han M.J., Kim D.H. Metabolic activities of ginseng and its constituents, ginsenoside Rb1 and Rg1, by human intestinal microflora. J Ginseng Res. 2011;35:301–307. doi: 10.5142/jgr.2011.35.3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim K.A., Jung I.H., Park S.H., Ahn Y.T., Huh C.S., Kim D.H. Comparative analysis of the gut microbiota in people with different levels of ginsenoside Rb1 degradation to compound K. PLoS One. 2013;8:e62409. doi: 10.1371/journal.pone.0062409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization . WHO; Copenhagen: 1989. Measuring obesity: classification and distribution of anthropometric data. [Google Scholar]
- 22.Hur M., Kim Y., Song H.R., Kim J.M., Choi Y.I., Yi H. Effect of genetically modified poplars on soil microbial communities during the phytoremediation of waste mine tailings. Appl Environ Microbiol. 2011;77:7611–7619. doi: 10.1128/AEM.06102-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chunlab, Technical note_454 amplicon primer design [Internet]. 2013 [cited 2013 Mar 5]. Available from: http://oklbb.ezbiocloud.net/content/1001.
- 24.Eddy S.R. Accelerated Profile HMM Searches. PLoS Comput Biol. 2011;7:e1002195. doi: 10.1371/journal.pcbi.1002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim O.S., Cho Y.J., Lee K., Yoon S.H., Kim M., Na H., Park S.C., Jeon Y.S., Lee J.H., Yi H. Introducing EzTaxon-e: a prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int J Syst Evol Microbiol. 2012;62:716–721. doi: 10.1099/ijs.0.038075-0. [DOI] [PubMed] [Google Scholar]
- 26.Edgar R.C., Haas B.J., Clemente J.C., Quince C., Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27:2194–2200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schloss P.D., Westcott S.L., Ryabin T., Hall J.R., Hartmann M., Hollister E.B., Lesniewski R.A., Oakley B.B., Parks D.H., Robinson C.J. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chunlab, Sequence data for influence of Panax Ginseng on gut microbiota [Internet]. 2013 [cited 2013 May 5]. Available from: http://www.ebi.ac.uk/ena/data/view/PRJEB4531.
- 29.Mueller S., Saunier K., Hanisch C., Norin E., Alm L., Midtvedt T., Cresci A., Silvi S., Orpianesi C., Verdenelli M.C. Differences in fecal microbiota in different European study populations in relation to age, gender, and country: a cross-sectional study. Appl Environ Microbiol. 2006;2:1027–1033. doi: 10.1128/AEM.72.2.1027-1033.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yatsunenko T., Rey F.E., Manary M.J., Trehan I., Dominguez-Bello M.G., Contreras M., Magris M., Hidalgo G., Baldassano R.N., Anokhin A.P. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwon D.H., Bose S., Song M.Y., Lee M.J., Lim C.Y., Kwon B.S., Kim H.J. Efficacy of Korean Red Ginseng by single nucleotide polymorphism in obese women: randomized, double-blind, placebo-controlled trial. J Ginseng Res. 2012;36:176–189. doi: 10.5142/jgr.2012.36.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cho Y.H., Ahn S.C., Lee S.Y., Jeong D.W., Choi E.J., Kim Y.J., Lee J.G., Lee Y.H., Shin B.C. Effect of Korean Red Ginseng on insulin sensitivity in non-diabetic healthy overweight and obese adults. Asia Pac J Clin Nutr. 2013;22:365–371. doi: 10.6133/apjcn.2013.22.3.04. [DOI] [PubMed] [Google Scholar]
- 33.Wang H.Y., Qi L.W., Wang C.Z., Li P. Bioactivity enhancement of herbal supplements by intestinal microbiota focusing on ginsenosides. Am J Chin Med. 2011;39(6):1103–1115. doi: 10.1142/S0192415X11009433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wakabayashi C., Hasegawa H., Murata J., Saiki I. In vivo antimetastatic action of ginseng protopanaxadiol saponins is based on their intestinal bacterial metabolites after oral administration. Oncol Res. 1997;9:411–417. [PubMed] [Google Scholar]
- 35.Turnbaugh P.J., Ley R.E., Hamady M., Fraser-Liggett C.M., Knight R., Gordon J.I. The human microbiome project. Nature. 2007;449:804–810. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee J., Lee E., Kim D., Lee J., Yoo J., Koh B. Studies on absorption, distribution and metabolism of ginseng in humans after administration. J Ethnopharmaol. 2009;122:143–148. doi: 10.1016/j.jep.2008.12.012. [DOI] [PubMed] [Google Scholar]


