Summary
Little is known about the extracellular signaling factors that govern mammary stem cell behavior. Here, we identify CRIPTO and its cell-surface receptor GRP78 as regulators of stem cell behavior in isolated fetal and adult mammary epithelial cells. We develop a CRIPTO antagonist that promotes differentiation and reduces self-renewal of mammary stem cell-enriched populations cultured ex vivo. By contrast, CRIPTO treatment maintains the stem cell phenotype in these cultures and yields colonies with enhanced mammary gland reconstitution capacity. Surface expression of GRP78 marks CRIPTO-responsive, stem cell-enriched fetal and adult mammary epithelial cells, and deletion of GRP78 from adult mammary epithelial cells blocks their mammary gland reconstitution potential. Together, these findings identify the CRIPTO/GRP78 pathway as a developmentally conserved regulator of fetal and adult mammary stem cell behavior ex vivo, with implications for the stem-like cells found in many cancers.
Graphical Abstract

Highlights
-
•
CRIPTO/GRP78 signaling activates PI3K/AKT in fetal mammary epithelial cells ex vivo
-
•
Cell-surface GRP78 marks a CRIPTO-responsive adult mammary stem cell population
-
•
An antagonist, ALK4L75A-Fc, blocks soluble CRIPTO growth-factor-like activity
-
•
CRIPTO promotes and ALK4L75A-Fc inhibits mammary stem cell maintenance ex vivo
Little is known about the niche factors governing mammary stem cell function and if they are conserved throughout mammary gland development and homeostasis. Here, Gray and colleagues identify CRIPTO as a stem cell maintenance factor in fetal and adult mammary stem/progenitor cells and show that its cell-surface receptor, GRP78, is a mammary stem cell marker and determinant of CRIPTO responsiveness.
Introduction
Somatic stem cells govern the development, maintenance, and regeneration of tissues, and their dysregulation is associated with diverse pathologies, including cancer. Given the significance of these cells both biologically and therapeutically, it is critical to define factors and signaling mechanisms that dictate their behavior, including those that define niches capable of promoting the stem cell phenotype in normal and disease settings. However, few such factors have been elucidated, and progress toward this goal has been impeded by the fact that most somatic stem cells, including those of the mammary gland, are rare and difficult to isolate and propagate ex vivo.
The mammary epithelium consists principally of lineage-restricted basal keratin-14-positive (KRT14+) myoepithelial cells and keratin-8-positive (KRT8+) luminal epithelial cells (Mikaelian et al., 2006). Although recent reports indicate extensive self-renewal within each of these lineage-committed populations (Van Keymeulen et al., 2011), classic single-cell transplant experiments indicate the presence of rare transplantable bipotent mammary stem cells (MaSCs) in the mature mammary gland (Shackleton et al., 2006). These cells can be significantly enriched through the use of cell-surface marker combinations such as CD24 and CD49f (Stingl et al., 2006). However, the functional significance of such markers to stem cell biology is often unclear, and the resulting enrichment generally remains too low to discern core molecular determinants of the stem cell state from the population at large.
In an effort to circumvent these challenges, we recently characterized a highly enriched population of stem cells from murine embryonic mammary rudiments (Spike et al., 2012). The greater purity of these fetal mammary stem cells (fMaSC) relative to their adult counterparts makes them particularly useful in the study of MaSC biology. Interestingly, we found that fMaSCs share gene expression features with certain aggressive human breast cancers that are not shared between enriched populations of adult MaSCs and the same breast cancers. This distinction may reflect intrinsic differences between the fetal and adult MaSCs or differential heterogeneity in the stem cell-enriched populations used for profiling. Alternatively, this observation may be due to critical differences in the tissue contexts from which these cells are derived, a possibility consistent with prior reports indicating an important role for microenvironmental factors in establishing and maintaining the stem cell competence of both fetal and adult mammary cells (Makarem et al., 2013, Spike et al., 2012, Vaillant et al., 2011). However, the ability of specific factors to promote the MaSC phenotype has rarely been directly demonstrated.
CRIPTO (CR-1, TDGF1) is an oncofetal, GPI-anchored/secreted signaling protein that plays key roles as a stem cell regulator (Adewumi et al., 2007, Bianco et al., 2010, Hough et al., 2009, Miharada et al., 2011). CRIPTO is dynamically expressed during mammary gland development (Bianco et al., 2008, Kenney et al., 1995) and in breast cancer (Gong et al., 2007) but has not been previously examined in the context of MaSCs. CRIPTO promotes proliferation, invasion, and migration of mammary epithelial cell lines in vitro, whereas its overexpression in the mouse mammary gland promotes excessive ductal branching, hyperplasia, and latent tumor growth (Rangel et al., 2012). Although the relevant molecular and cellular mechanisms for these effects remain to be determined, CRIPTO impacts multiple signaling pathways associated with stem cells and oncogenesis, including transforming growth factor β (TGF-β), phosphatidylinositol 3-kinase (PI3K), WNT, and NOTCH (Rangel et al., 2012). Two distinct CRIPTO signaling activities have been best characterized: (1) a coreceptor function in which CRIPTO modulates signaling of TGF-β ligands such as NODAL that signal via SMAD2/3 activation and (2) a growth-factor-like activity in which soluble CRIPTO activates SRC/mitogen-activated protein kinase/PI3K pathways in a TGF-β pathway-independent manner (Bianco et al., 2002, Gray and Vale, 2012). Both of these signaling activities require CRIPTO binding to cell-surface GRP78, an HSP70 family member that localizes to the surfaces of stem cells and tumor cells (Gray and Vale, 2012, Kelber et al., 2009, Miharada et al., 2011, Shani et al., 2008).
Here, we investigate CRIPTO’s ability to regulate primary fetal and adult mammary stem/progenitor cells using a series of ex vivo culture and mammary gland reconstitution experiments. We develop an antagonist that selectively blocks the growth factor-like signaling of soluble CRIPTO without affecting its cell-surface coreceptor activity, and we use this reagent to demonstrate that soluble CRIPTO maintains the MaSC state ex vivo. We further identify cell-surface GRP78 as a determinant of CRIPTO responsiveness and critical functional marker in fetal and adult MaSCs.
Results
An ALK4-Based Antagonist Inhibits Growth-Factor-like Effects of Soluble CRIPTO on Mammary Epithelial Cells
We developed a CRIPTO antagonist in order to test if CRIPTO signaling is required for the maintenance of MaSCs. We took advantage of the CRIPTO binding properties of the extracellular domain (ECD) of the ACTIVIN/NODAL type I receptor ALK4 (Bianco et al., 2002, Gray and Vale, 2012, Yeo and Whitman, 2001) and the fact that a leucine to alanine substitution at amino acid 75 (L75A) disrupts ALK4/ACTIVIN binding without affecting ALK4 binding to CRIPTO (Kelber et al., 2008). Although the L75A mutation does not guarantee complete specificity of ALK4 ECD binding to CRIPTO, we reasoned that this substitution should increase the selectivity of their interaction. We previously showed that a kinase-deleted ALK4L75A protein blocks CRIPTO effects on ACTIVIN and NODAL signaling (Kelber et al., 2008), leading us to hypothesize that a soluble version of the ALK4L75A ECD would similarly block CRIPTO signaling. To test this, we fused an N-terminally Flag-tagged ALK4-ECDL75A to the Fc region of human immunoglobulin G (ALK4L75A-Fc). We purified the recombinant protein from conditioned media using affinity chromatography and confirmed its ability to bind CRIPTO in solution (Figures S1A–S1D and Supplemental Experimental Procedures available online).
Using the CRIPTO-responsive human mammary epithelial MCF10A cell line, we find that ALK4L75A-Fc effectively inhibits binding of soluble CRIPTO to intact cells and blocks CRIPTO-dependent augmentation of AKT phosphorylation (Figures 1A and 1B). This effect appears to be specific, because ALK4L75A-Fc only blocks CRIPTO-dependent AKT phosphorylation, whereas a PI3K inhibitor (LY 294002) blocks detection of both CRIPTO-induced and basal phospho-AKT (Figure 1B). As expected, and in contrast to CRIPTO-independent ACTIVIN A signaling, NODAL treatment induces SMAD2 phosphorylation in MCF10A cells stably transduced with CRIPTO lentivirus (MCF10A-CR), but not in control cells transduced with empty vector (MCF10A-V) (Figure 1C). Both NODAL and ACTIVIN A signal via ALK4, and SMAD2 phosphorylation in response to each ligand is blocked by the ALK4/5/7 kinase inhibitor SB 431542 (Figure 1C). ALK4L75A-Fc treatment, on the other hand, has no effect on ACTIVIN A-induced SMAD2 phosphorylation (Figure 1C), consistent with our previous demonstration that the ALK4L75A mutation blocks ACTIVIN A binding (Kelber et al., 2008). Surprisingly, however, ALK4L75A-Fc treatment also has no discernible effect on CRIPTO-dependent NODAL signaling in MCF10A-CR cells (Figure 1C) or on CRIPTO-dependent NODAL induction of a SMAD2-responsive luciferase reporter in 293T cells (Figure S1E). This failure of ALK4L75A-Fc to block CRIPTO coreceptor activity is consistent with our inability to detect ALK4L75A-Fc binding on the surface of intact CRIPTO-overexpressing 293T cells (Figure S1F).
Figure 1.
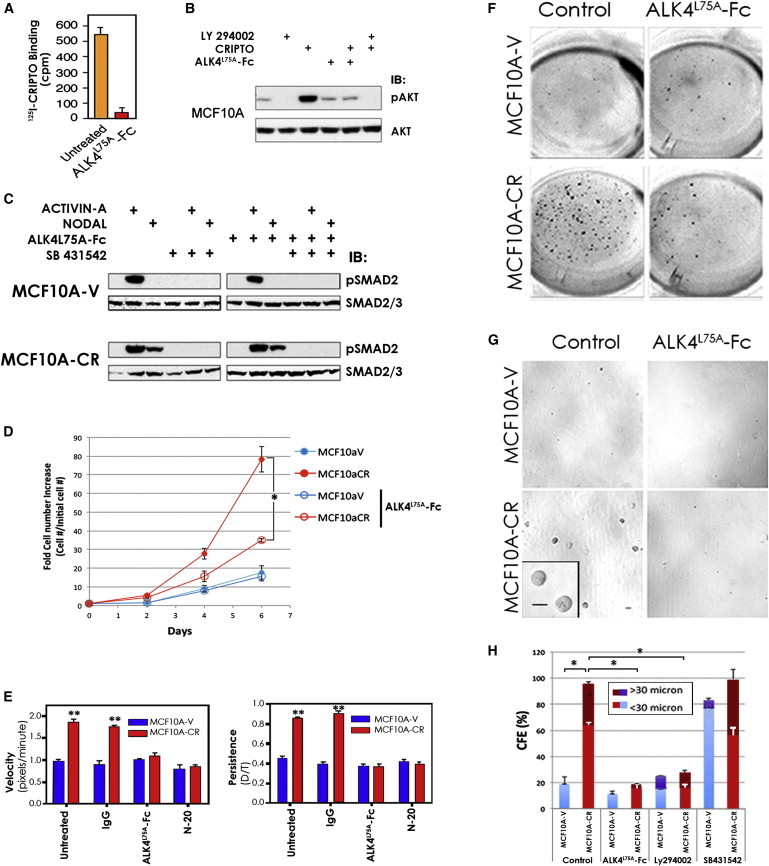
ALK4L75A-Fc Inhibits Growth-Factor-like Effects of CRIPTO on Immortalized Human Mammary Epithelial Cells
(A) ALK4L75A-Fc (10 μg/ml) inhibits binding of 125I-CRIPTO to native MCF10A cells. Data from one of two independent experiments using three technical replicates are shown, with SDs.
(B) Phospho-AKT (Ser 473) or total AKT western blots on lysates from MCF10A cells treated with soluble CRIPTO (300 ng/ml), ALK4L75A-Fc, and PI3K inhibitor LY 294002 (10 μM) as indicated.
(C) Phospho-SMAD2 and SMAD2/3 western blots on MCF10A cells stably transduced with CRIPTO-Flag virus (MCF10A-CR) or control virus (MCF10A-V) and treated as indicated with ACTIVIN A (1 nM), NODAL (30 nM), ALK4L75A-Fc, and the ALK4/5/7 inhibitor SB 431542 (10 μM).
(D) Time course showing that MCF10A-CR cells accumulate more rapidly than MCF10A-V cells in 2D culture. Treatment with ALK4L75A-Fc selectively inhibits growth of MCF10A-CR cells. ∗p < 0.01. Triplicate wells in a representative experiment are shown, with SDs.
(E) MCF10A-CR cells are more migratory than MCF10A-V cells. The CRIPTO-dependent increase in migrational velocity and persistence is blocked by ALK4L75A-Fc treatment or a GRP78 neutralizing antibody (N-20). ∗∗p < 0.01, two-way ANOVA. Data from ≥50 individual cells from three fields of view per condition in one experiment (of two performed) are shown, with SDs.
(F) Suspension cultures of MCF10A-V and MCF10A-CR cells 11 days after seeding in 24-well plates. MCF10A-CR cells grow more rapidly than MCF10A-V cells in suspension culture, and this growth advantage is inhibited by ALK4L75A-Fc treatment.
(G) MCF10A-CR cells produce larger and more plentiful colonies than MCF10A-V cells when grown in immobilizing methylcellulose suspension cultures, and ALK4L75A-Fc treatment blocks this CRIPTO-dependent growth. Bar represents 30 μm.
(H) Colony growth was significantly reduced by either ALK4L75A-Fc treatment or PI3K inhibition (LY 294002 10 μM), but not by treatment with SB 431542. ∗p < 0.05. Two independent experiments with duplicate wells in each are tabulated, with SDs. SB 431542 treatment increased colony number, but not mean size, of MCF10A-V cells.
See also Figures S1 and S2.
MCF10A-CR cells secrete CRIPTO (Figure S2A) and have elevated levels of PI3K/AKT signaling relative to MCF10A-V cells (Figure S2B), leading us to test if ALK4L75A-Fc inhibits autocrine/paracrine CRIPTO growth factor activity in these cells. We use live-cell time-course and time-lapse microscopy, respectively, to track proliferation and migration of MCF10A-CR cells and control cells (MCF10A-V). MCF10A-CR cells accumulate more rapidly than MCF10A-V cells (Figure 1D), consistent with our previous findings (Kelber et al., 2009), and these CRIPTO expressing cells also have enhanced migration relative to control cells (Figure 1E). Treatment with ALK4L75A-Fc inhibits the CRIPTO-dependent increases in growth (Figure 1D) and motility (Figure 1E; Figure S2C; Movies S1, S2, S3, and S4). The inhibitory effects of ALK4L75A-Fc treatment on CRIPTO-dependent migrational velocity and persistence are similar to those of GRP78 antibody (N-20) (Figure 1E; Figure S2C) that we previously showed blocks CRIPTO/GRP78 binding (Kelber et al., 2009).
We also analyzed the effects of CRIPTO signaling and antagonism on MCF10A cells in 3D culture in the presence or absence of the reconstituted basement membrane Matrigel. Although suspension cultures have been widely used to measure the production of multicellular mammary epithelial structures called “mammospheres” (Dontu et al., 2003), Matrigel-containing mammary epithelial cultures promote the formation of differentiated, polarized acinar structures from MCF10A cells and multilayered, multilineage organoids from primary mammary epithelial stem cells (Debnath et al., 2003, Spike et al., 2012). MCF10A-CR cells accumulate more rapidly than MCF10A-V cells in free-floating suspension cultures, where they exhibit a greater proportion of cells with 2N-4N DNA content indicative of cycling S phase cells (Figure 1F; Figures S2D and S2E). The growth promoting effects of CRIPTO under these conditions are blocked by treatment with ALK4L75A-Fc or LY 294002, but not SB 431542 (Figure S2D). We used methylcellulose immobilization of cells in suspension cultures to measure the size of individual spheres (Figures 1G and 1H). Consistent with the increased cell numbers observed in free-floating cultures, immobilized MCF10A-CR cells form larger, more numerous mammospheres than MCF10A-V cells, and the increased growth of the CRIPTO-expressing cells is again blocked by ALK4L75A-Fc and LY 294002, but not by SB 431542 (Figure 1H). CRIPTO overexpression similarly promotes MCF10A colony growth in Matrigel, and this is inhibited by treatment with ALK4L75A-Fc, whereas treatment of these cultures with NODAL neither augments CRIPTO-induced growth nor overcomes ALK4L75A-Fc -mediated inhibition of CRIPTO-induced growth (Figure S2F). Altogether, these results indicate that soluble CRIPTO promotes proliferation and migration of mammary epithelial cells and that this can be blocked by ALK4L75A-Fc.
CRIPTO/GRP78 Expression and Function in fMaSCs
Our data show that MCF10A cells respond to soluble CRIPTO signaling in multiple contexts, including 3D growth assays that are similar to those used previously to evaluate stem cell-like cellular behaviors (Dontu et al., 2003). We also previously demonstrated that soluble CRIPTO activates the PI3K/AKT pathway in MCF10A cells by binding to cell-surface GRP78 (Kelber et al., 2009). These findings, together with reports that CRIPTO and GRP78 cooperatively regulate stem cell function in other contexts, prompted us to test if CRIPTO and GRP78 are expressed in murine mammary tissue and if the CRIPTO/GRP78 pathway is operative in primary mammary stem/progenitor cells. We first investigated the pattern of CRIPTO and GRP78 expression in embryonic day 18.5 (E18.5) mouse mammary rudiments, because they provide a rich source of robust stem cell activity (Spike et al., 2012). We find GRP78 to be expressed throughout the mammary rudiment, with the most intense and distinct staining coinciding with the burgeoning, arborized mammary epithelium, demarcated here by costaining with antibodies to KRT8 (Figures 2A and 2B). By contrast, CRIPTO staining is more diffuse and distributed throughout the rudiment, with significant staining in the fetal mammary stroma and areas within and adjacent to the GRP78-positive and KRT8-positive epithelium. Interestingly, we observe intense CRIPTO staining at or near the termini of the arborized mammary rudiment, where it overlaps with both the condensed mesenchyme adjacent to the epithelium and epithelial cells that stain prominently for GRP78 (Figures 2A and 2B). CRIPTO mRNA was detected in fMaSCs but is more highly expressed in a variety of other cell populations isolated from the fetal mammary microenvironment, including putative adipose precursor cells that resist centrifugation during rudiment processing (“Fat”), myeloid cells (Lin+CD11b+F4/80+), and non-myeloid lineage-positive cells (CD11b−F4/80−) (Figure 2C). CRIPTO message is also present in other stromal cells (fStromal; Lin−CD24low) that likely include mammary tissue fibroblasts (Figure 2C). Costaining of the mammary rudiment for the macrophage marker F4/80 confirms colocalization of CRIPTO protein with not only macrophages but also other nonmacrophage stromal components adjacent to the fetal mammary epithelium (Figure 2D). Thus, CRIPTO is expressed in multiple cell types within the MaSC microenvironment, leading us to reason that soluble secreted CRIPTO may govern fMaSC behavior as an autocrine/paracrine growth factor.
Figure 2.
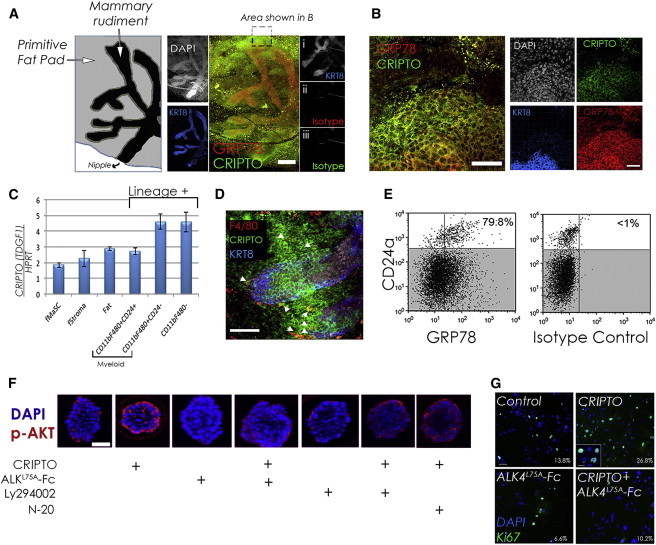
Expression of CRIPTO and GRP78 in the Fetal Mammary Rudiment and Responsiveness of fMaSCs to Soluble CRIPTO
(A and B) Whole-mount E18.5 fetal mammary rudiment demarcated with immunofluorescence staining for KRT8 (blue) or DAPI (white) and costained for CRIPTO (green) and GRP78 (red) (right). (A, i–iii) control rudiment stained with KRT8 and isotype-matched controls for GRP78 and CRIPTO. Scale bar represents 200 μm (A) or 50 μm (B).
(C) CRIPTO is expressed in the fMaSC population and stromal cells as measured by RT-PCR. A total of 150 rudiments were pooled and assayed in technical triplicates, with SDs.
(D) CRIPTO staining colocalizes with F4/80 staining (arrows) adjacent to mammary epithelium demarcated by KRT8 staining (blue).
(E) Flow cytometric analysis of fetal mouse mammary cells stained with CD24, GRP78, and isotype control antibodies as indicated. The percentage of the CD24high population also positive for GRP78 is shown.
(F) Phospho-AKT staining in serum-starved, fMaSC-derived organoids treated as indicated with soluble CRIPTO (300 ng/ml), ALK4L75A-Fc (10 μg/ml), LY 294002 (10 μM), or N-20 (2 μg/ml). Scale bar represents 50 μm.
(G) 2D fMaSC cultures were treated with CRIPTO (400 ng/ml) and/or ALK4L75A-Fc (10 μg/ml) as indicated and stained with Ki67 antibody and DAPI. Scale bar represents 50 μm, and inset represents 10 μm.
Our previous studies indicating that CRIPTO signals via cell-surface GRP78 led us to test if GRP78 is expressed on the surface of fMaSCs (Lin−CD24high) and if these cells respond to soluble CRIPTO in a GRP78-dependent manner. We analyzed dissociated cells from E18.5 mammary rudiments by flow cytometry and discovered that >80% of the fMaSC population is GRP78 positive (Figure 2E). Furthermore, when we treat fMaSC-derived Matrigel colonies with soluble CRIPTO, we observe increased AKT phosphorylation that is blocked by treatment with ALK4L75A-Fc or LY 294002 (Figure 2F). This increase in AKT phosphorylation is also blocked by N-20, indicating that CRIPTO signals via cell-surface GRP78 in these cells (Figure 2F). Soluble CRIPTO treatment also increases the proportion of Ki67+ cells relative to untreated cells in 2D culture, and this effect was once again blocked by ALK4L75A-Fc treatment (Figure 2G). Thus, multiple cell types within the fetal mammary rudiment produce CRIPTO, and it can act on cells within the fMaSC population.
CRIPTO/GRP78 Signaling Promotes Maintenance of fMaSCs Ex Vivo
To explore the consequences of CRIPTO signaling in MaSCs further, we examined its effects on growth, self-renewal, and differentiation in fMaSC cultures. We previously reported that fMaSCs grown in media that promotes their differentiation give rise to a high proportion of bilineage organoids containing both KRT8+KRT14− luminal cells and KRT14+KRT8− myoepithelial cells by 7–12 days of culture (Spike et al., 2012). At 5 days of culture, organoids grown in this differentiation promoting media typically contain a fraction of cells coexpressing KRT8 and KRT14 (Figure 3A, top left, yellow arrows, and Figure 3B). Supplementation of these cultures with CRIPTO or ALK4L75A-Fc has opposing effects on the fraction of organoids containing double-positive (KRT8+KRT14+) cells and on the amount of double-positive area per organoid (Figure 3A, left panels, and Figure 3B; Figures S3A and S3B). Specifically, CRIPTO treatment increases the proportion of double-positive area per organoid while ALK4L75A-Fc treatment decreases it, suggesting that CRIPTO signaling promotes maintenance of a multipotent phenotype. However, rapid cellular differentiation under these culture conditions prevented us from confirming stem cell activity of resultant colonies by mammary reconstitution analyses (data not shown; Spike et al., 2012).
Figure 3.
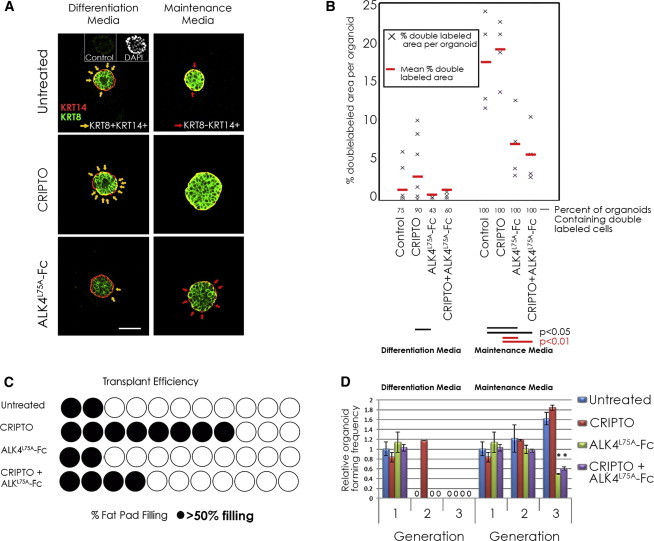
CRIPTO Promotes Maintenance of fMaSCs
(A) Sphere-bisecting confocal cross sections of day 5, fMaSC-derived colonies grown in Matrigel and stained with KRT8 (green) and KRT14 (red). Colonies were grown in differentiation media or maintenance media with soluble CRIPTO (0.33 μg/ml) or ALK4L75A-Fc (10 μg/ml) as indicated. Yellow arrows indicate KRT8+KRT14+ double-positive cells. Red arrows indicate lineage-committed KRT14 single-positive cells. Control represents the same isotype. Scale bar represents 100 μm.
(B) KRT8 and KRT14 staining was quantified from images and is represented as the percent KRT8+KRT14+ double-labeled area per organoid. The percentage of organoids containing KRT8+KRT14+ double-labeled cells is also indicated. Results of three independent representative experiments are shown.
(C) Mammary gland repopulation efficiency of first-generation colonies grown in Matrigel-containing maintenance media culture with soluble CRIPTO and/or ALK4L75A-Fc. Each circle represents one recipient mammary gland. Shaded circles represent ducts extending through >50% of the fat pad.
(D) Colony-forming efficiencies (>100 μm) at first, second, and third passage for fMaSCs grown for 7 days per passage in differentiation or maintenance media with CRIPTO and/or ALK4L75A-Fc. Results of three independent experiments (maintenance media) and a representative experiment (differentiation media) were quantified. Triplicate wells were quantified in each. Error bars represent SDs. ∗p ≤ 0.01.
See also Figure S3.
To address this issue, we developed culture conditions that are better able to produce mammary epithelial colonies including bilineage organoids that also give rise to mammary outgrowths following transplantation. CRIPTO and ALK4L75A-Fc also have opposite effects on the stem cell phenotype of fMaSC-derived colonies grown in this stem cell maintenance media (Figure 3A, right panels, and Figure 3B). Endogenous CRIPTO is implicated as a stem cell factor in these cultures by the fact that ALK4L75A-Fc treatment alone increases the number of keratin single-positive cells discernible per organoid (Figure 3A, right panels, red arrows). ALK4L75A-Fc also significantly increases the amount of single keratin staining area (either KRT14+KRT8− or KRT8+KRT14−) relative to KRT8+KRT14+ double-positive area per organoid (Figure 3B; Figure S3B), although some double-positive cells are evident in all organoids in maintenance media following 5 days of culture (Figures 3A and 3B; Figure S3B).
Although our data indicate that CRIPTO signaling correlates with KRT8+KRT14+ double positivity, the precise relationship between keratin expression and differentiation status in these cultures is uncertain. It is therefore important that CRIPTO signaling also has functional stem cell relevance, because treatment with soluble CRIPTO enhanced the ability of fMaSC-derived colonies grown for 5 days in this media to reconstitute mammary glands upon transplantation relative to untreated controls and this CRIPTO effect was attenuated by ALK4L75A-Fc (Figure 3C). Despite the clear effects of CRIPTO signaling on keratin expression and transplantation, treatment with CRIPTO and/or ALK4L75A-Fc does not significantly affect overall colony number and size in 5-day cultures (Figure 3D). However, when cells are serially passaged for three rounds under stem cell maintenance conditions, ALK4L75A-Fc treatment substantially reduces colony-forming efficiency and the growth of large colonies (>100 μm) is almost completely abolished (Figure 3D). Conversely, addition of CRIPTO can support a round of serial passage of fMaSC-derived cells, even under conditions that strongly favor differentiation (Figure 3D). We note that clonogenicity of fetal mammary cells was recently shown to be enhanced by unidentified factors secreted by 3T3 fibroblasts in coculture (Makarem et al., 2013). We find that 3T3 cells express CRIPTO protein (Figure S3D) and that their ability to enhance clonogenicity of fetal mammary cells in coculture can be partially blocked by treatment with either ALK4L75A-Fc or CRIPTO antibodies (Figure S3E). This result is consistent with our results using recombinant proteins and further highlights the potential for non-cell-autonomous CRIPTO regulation of fMaSCs.
GRP78 Is a Critical Functional Marker and Cell-Surface Determinant of CRIPTO-Responsive MaSCs
We examined CRIPTO and GRP78 expression in the adult mammary gland and identified marked immunoreactivity for both proteins that overlaps with the morphologically distinct mammary ductal epithelium and adjacent adipose tissue (Figure 4A). Immunofluorescent colocalization studies confirm overlapping CRIPTO and GRP78 staining in these cell types, including elevated staining in adipose and a subset of mammary epithelial cells (Figure 4B). To determine whether adult MaSCs require GRP78, we used CRE-mediated recombination and excision of GRP78 alleles together with mammary gland reconstitution experiments. In these experiments, cells harboring CRE-expressing virus failed to reconstitute mammary ductal trees in each of nine transplant attempts (zero out of nine), while an equivalent number of cells transduced with control virus robustly repopulate the gland (three out of three) (Figure 4A). Furthermore, cells isolated from control outgrowths exhibit cell-surface GRP78 and contain both the luminal and myoepithelial fractions (Figure S4C). In contrast, we only occasionally recover a low number of presumptive mammary epithelial cells from transplants of GRP78Fl/Fl cells infected with CRE virus. These cells appear restricted to the mature luminal fraction, lack detectable cell-surface GRP78, and do not give rise to colonies when cultured in vitro (Figure S4A; data not shown). These data indicate that GRP78 is required for MaSC activity as measured by transplantation assays.
Figure 4.

GRP78 Is Required for MaSC Activity and Marks a CRIPTO-Responsive Stem Cell-Enriched Population of Adult Mouse Mammary Epithelial Cells
(A) Immunohistochemical localization of CRIPTO and GRP78 in adult mouse mammary tissue. Inset: secondary antibody alone (2nd).
(B) CRIPTO and GRP78 proteins colocalize in stromal areas including adipose (f, fat) and in epithelial structures (e), where a subset of cells display elevated staining for both markers (white arrows).
(C) Repopulation rate of de-epithelialized mammary glands inoculated with 500 GRP78-floxed epithelial cells transduced with Ad-CRE/GFP- or Ad-GFP and sorted for GFP. Each circle represents one recipient mammary gland. Filled circles indicate >50% fat pad filling.
(D) Flow cytometric analysis of wild-type adult mouse mammary cells stained for CD24 and GRP78 (lineage excluded) indicating the presence of cell-surface GRP78 in adult mammary epithelia and demarcating the GRP78high and GRP78low populations (left). Antibodies to CD49f were also incorporated (right), and cells positive for GRP78 and CD24 were gated to generate a population density graph (red lines) superimposed on the CD24/CD49f staining. The standard positions of the CFC, MYO, and MRU populations (Stingl et al., 2006) are indicated.
(E) Colonies grown from single cells sorted into individual wells in 96-well plates. Luminal, organoid, and myoepithelial morphologies were distinguishable (left). The number of each type of clonal outgrowth deriving from single GRP78low and GRP78high cells in one representative experiment (of three similar experiments) was quantified according to morphology (right).
(F) Confocal micrographs showing the keratin distribution observed in representative colonies grown from cells sorted using CD24 and GRP78. GRP78low cells predominantly expressed either KRT8 or KRT14, while GRP78high cultures frequently exhibited KRT8+KRT14+ outgrowths. Representative organoid (i), luminal (ii), and myoepithelial (iii) colonies are shown. Color panels show individual fluorophores separately for the organoid (i, insets). Scale bars represent 100 μm.
(G) Mammary gland repopulation efficiencies of GRP78high and GRP78low cells represented as in (C) (the data fit a single-hit model; likelihood ratio test p = 0.938; ELDA).
(H) GRP78high cells are selectively responsive to soluble CRIPTO or ALK4L75A–Fc treatment when serially passaged in Matrigel culture. Results of two independent experiments conducted in triplicate are shown, with SDs. Black bracket p < 0.05; red bracket p < 0.01.
(I) Model depicting cooperativity of soluble CRIPTO (sCR) and cell-surface GRP78 in promoting mammary stem cell maintenance. Soluble CRIPTO reinforces a robust primitive epithelial stem cell state, whereas loss of soluble CRIPTO signaling leaves cells vulnerable to differentiation cues and eventual loss of stem cell characteristics such as bipotency (yellow cells) and self-renewal (circular arrow).
See also Figure S4.
Next, we sought to examine the role of cell-surface GRP78 as an adult MaSC marker and as a MaSC regulator specifically in its capacity as a mediator of CRIPTO signaling. We isolated CD24-positive mammary epithelial cells with either high (GRP78high) or low (GRP78low) levels of cell-surface GRP78 from wild-type mice (Figure 4D, left panel). A substantial portion of adult mammary epithelial cells was GRP78high (19.4% ± 3.4% of the CD24-positive fraction), though the proportion was considerably lower than in fetal mammary epithelia (Figure 3D). In costained samples, we observe that while the GRP78high population overlaps partially with the previously reported luminal colony-forming cell (CFC) and myoepithelial (MYO) populations, it overlaps most precisely with the stem cell-enriched CD24medCD49fhigh mammary repopulating unit (MRU) fraction (Figure 4D, right panel) (Stingl et al., 2006). While cells from both the GRP78high and GRP78low fractions produce clonal outgrowths in Matrigel culture, those derived from the GRP78high population are much more likely to give rise to colonies containing multiple cell layers (Figure 4E; organoids) and to stain positive for both KRT14 and KRT8 (Figure 4F; Figure S4B). In these assays, the most frequently observed and morphologically distinguishable colony types include those that appear luminal, composed of a single peripheral cell layer surrounding an open lumen, small myoepithelial colonies (usually < 16 cells), and distinctive, multilayered organoids containing both luminal and myoepithelial cell types (Figures 4E and 4F) (Spike et al., 2012, Stingl et al., 2006). Importantly, these morphologically distinct colony types are produced in cultures both when single cells were plated in individual wells (Figure 4E) and when cells are plated at single-cell densities (1,000 cells per 0.32 cm2) (Figure 4F; data not shown) (Spike et al., 2012). Thus, the substantially higher proportion of organoids and KRT14+KRT8+ double-positive colonies formed from the GRP78high population (Figures 4E and 4F; Figure S4B) suggests that the GRP78high population is enriched for bipotent stem/progenitor cells. In contrast, GRP78low cells predominantly give rise to small myoepithelial (KRT14+KRT8−) or monolayered luminal (KRT14−KRT8+) outgrowths (Figures 4E and 4F; Figure S4B).
As an independent criterion for assessing the stem cell content of the GRP78high population, we transplanted CD24medGRP78high and CD24medGRP78low mammary epithelial cell populations into cleared mammary fat pads at limiting dilutions. The results clearly indicate that the CD24medGRP78high cells reconstitute mammary glands more efficiently the CD24medGRP78low cells (Figure 4G; Figures S4C–S4E). We estimate the frequency of mammary gland-reconstituting units to be approximately 1/26 in the GRP78high cells (90% confidence interval [CI], 1/15–45.4; extreme limiting dilution analysis [ELDA]) versus approximately 1/152 in the GRP78low population (90% CI, 1/67.0–346.2; ELDA) (Figure 4G; Figure S4E). This correlates with a significant enrichment (p < 0.01; ELDA) of stem cell activity in the GRP78high fraction relative to the GRP78low cells (Figure 4G; Figures S4C–S4E). Outgrowths from transplanted GRP78high cells resemble endogenous mammary glands in FACS analyses including the presence of GRP78high cells within the CD24 expressing mammary epithelium (Figure S4F). Significantly, the self-renewal potential of these cells is evident in their capacity to reconstitute fully arborized mammary epithelial outgrowths in tertiary transplants that respond normally to pregnancy (Figure S4G). We also identify a diffuse, small, and variable population of GRP78 “superpositive” (GRP78high+) cells with intermediate performance in stem cell assays (Figures S4D and S4E).
Because CRIPTO signaling promotes serial passage of fMaSCs ex vivo (Figure 3D), we tested the effects of this pathway on cultures of adult GRP78high and GRP78low mammary epithelial populations. Consistent with its greater enrichment for cells with a bipotent stem cell phenotype (Figures 4E–4G; Figure S4B), the GRP78high population exhibits greatly enhanced potential for serial colony formation relative to GRP78low cells, especially over increasing serial passage number in Matrigel (Figure 4H; Figure S4H). Furthermore, serial passage of GRP78high cells is significantly enhanced by CRIPTO treatment (Figure 4H) and, similar to what was observed with fMaSCs, serial colony formation of adult GRP78high cells is also inhibited by ALK4L75A-Fc treatment, suggesting that endogenous soluble CRIPTO maintains the self-renewal potential of these cells. By contrast, GRP78low cells are largely refractory to treatment with CRIPTO or ALK4L75A-Fc (Figure 4H), consistent with a requirement for surface GRP78 for CRIPTO signaling (Kelber et al., 2009). Together, these data support a role for soluble CRIPTO in maintaining the stem cell phenotype of GRP78high mammary epithelial cells.
The data are consistent with a model (Figure 4I) in which surface GRP78 is a functional MaSC marker. In this model, the most undifferentiated MaSC state depends on endogenous CRIPTO signaling, because treatment with ALK4L75A-Fc leads to loss of multipotency and eventual rundown of self-renewal in serial passage. In addition, a subset of cells, at least in the adult context, lacks endogenous CRIPTO but can respond to exogenous soluble CRIPTO with increased serial clonogenicity or self-renewal. Thus, according to this model, CRIPTO treatment shifts cells to a more primitive, stable, or competent stem cell state while ALK4L75A-Fc treatment makes them susceptible to commitment and differentiation.
Discussion
Extracellular signaling factors play critical roles in regulating the balance between stem cell self-renewal and differentiation (Jones and Wagers, 2008, Spradling et al., 2001). In the present study, we identify CRIPTO as one such factor that promotes multipotency and maintains gland reconstitution potential of mammary stem/progenitor cells ex vivo. We also show that the CRIPTO cell-surface receptor GRP78 is required for MaSC activity and CRIPTO responsiveness. Thus, CRIPTO and GRP78 are shared determinants of both fetal and adult mammary stem/progenitor cells pointing to a developmentally conserved role for the CRIPTO/GRP78 pathway in maintenance of the MaSC state.
ALK4L75A-Fc Identifies Soluble CRIPTO as a MaSC Factor
ALK4L75A-Fc selectively targets soluble CRIPTO and blocks CRIPTO-induced PI3K/AKT signaling, indicating that CRIPTO’s growth factor-like activity is essential for maintenance of MaSC properties in the contexts we analyzed. Miharada et al. (2011) similarly showed that soluble CRIPTO regulates hematopoietic stem cells (HSC) via the PI3K/AKT pathway independent of SMAD2/3 signaling. However, although our results uncover a critical role for soluble CRIPTO growth-factor-like signaling in regulating MaSC behavior, they do not exclude a role for CRIPTO coreceptor function. Indeed, NODAL, which requires CRIPTO or a related coreceptor, may act on fMaSCs during development in vivo, because it is expressed in fetal mammary stroma (Spike et al., 2012).
ALK4L75A-Fc treatment alone is sufficient to cause MaSC differentiation and loss of self-renewal potential ex vivo, suggesting that endogenously secreted CRIPTO sustains the clonogenicity and multipotency of mammary epithelial cells in maintenance media and in 3T3 fibroblast cocultures. Consistent with this, CRIPTO treatment inhibits lineage commitment of cells in organoid culture and increases their serial colony formation and transplantation capacity. Therefore, CRIPTO acts as an extrinsic, soluble regulator of MaSC function in vitro, raising the possibility that it may function similarly as an autocrine/paracrine stem cell factor in vivo. In support of such a role, we show that GRP78 is prominently expressed throughout the ductal epithelium within the fetal mammary rudiment while CRIPTO is concentrated near the termini of the epithelial tree and the mesenchyme surrounding these termini where stem cell activity may be focused (Kenney et al., 2001). CRIPTO expression is low in fMaSCs but higher in a variety of nonepithelial cells of the mammary rudiment such as fat cells and macrophage/myeloid cells that could mark potential niche sources of secreted CRIPTO. Adipose tissue has been previously reported to express CRIPTO (Andersson et al., 2008) and constitutes the natural environment for growth and development of the mammary ductal epithelium (Veltmaat et al., 2003). Myeloid cells, including macrophages, may also provide soluble CRIPTO for stem cell regulation, because they comprise important niche components in other systems and are required for effective mammary reconstitution (Ehninger and Trumpp, 2011, Gyorki et al., 2009).
Cell-Surface GRP78 Is a Developmentally Conserved Marker of Fetal and Adult MaSCs
Our data identify cell-surface GRP78 as an enrichment marker of mammary stem/progenitor cells. CRIPTO and GRP78 are coexpressed in the adult mammary gland, and cell-surface GRP78 correlates with the stem cell-surface markers CD24 and CD49f (Stingl et al., 2006). Bipotent stem/progenitor cells, though rare in the adult gland, are relatively plentiful in the sorted GRP78high population. GRP78high cells are also selectively responsive to CRIPTO signaling, because CRIPTO and ALK4L75A-Fc had opposing effects on their serial colony growth and neither treatment significantly affected the GRP78low population. Interestingly, the percentage of GRP78high cells is greater in the fetal than in the adult mammary gland, in agreement with the larger percentage of stem cells in the fetal mammary gland than in the adult (Spike et al., 2012). However, despite its ability to augment serial colony formation in cultures derived from GRP78high adult cells, CRIPTO treatment only has a modest effect on serial colony formation of fMaSCs in maintenance media. It is possible that CRIPTO growth-factor-like signaling is maximally activated in newly isolated fetal, but not adult, stem cells. This would explain the profound ability of the CRIPTO antagonist ALK4L75A-Fc to inhibit fMaSC maintenance despite the lack of a more profound effect of CRIPTO treatment on these cells. This activity appears to be more rapidly lost in stronger differentiation-promoting contexts, unveiling a clear effect of exogenous CRIPTO in sustaining a limited capacity for serial passage.
CRIPTO/GRP78 Signaling as a General Regulator of the Stem Cell State
CRIPTO is an established regulator of embryonic stem cells and induced pluripotent stem cells (Bianco et al., 2010), whereas GRP78 is a necessary mediator of CRIPTO signaling in human embryonic stem cells (Kelber et al., 2009) and promotes survival and proliferation of the murine inner cell mass cells that are precursors of murine embryonic stem cells (Luo et al., 2006). In the adult, GRP78 was recently reported to be essential for maintenance of adult intestinal stem cells (Heijmans et al., 2013) and conditional deletion of GRP78 in the hematopoietic system suppressed PI3K activity and decreased HSC number (Wey et al., 2012a, Wey et al., 2012b). Furthermore, CRIPTO was shown to be a hypoxic niche-associated factor that maintains the stem cell state and transplantability of GRP78high HSCs cultured ex vivo (Miharada et al., 2011). Although the mechanistic basis of CRIPTO/GRP78 signaling is still emerging, it has been shown that cell-surface localization of GRP78 often correlates with its overall expression level and occurs under stress conditions in which GRP78 is induced (Zhang et al., 2010). Therefore, although the stem cell niche remains poorly defined in the mammary gland, our results suggest that conditions known to increase the levels of GRP78 and/or CRIPTO such as hypoxia, nutrient deprivation, and endoplasmic reticulum stress could impact stem cell maintenance, expansion, and induction during normal mammary gland development and in homeostasis. Similarly, other signals from within the MaSC microenvironment likely intersect with CRIPTO and GRP78. For instance, Wnt signaling induces CRIPTO expression (Morkel et al., 2003) and has been reported to promote MaSC self-renewal in vitro (Zeng and Nusse, 2010).
Our recent demonstration that fMaSCs share a high degree of similarity with certain human breast cancers (Spike et al., 2012) is consistent with other evidence suggesting that tumor initiation, aggressiveness, and recurrence correlate with developmentally primitive states that are associated with cellular plasticity and increased stem cell disposition (Ben-Porath et al., 2008, Mizuno et al., 2010, Spike and Wahl, 2011). The results presented here show that CRIPTO/GRP78 signaling increases proliferation and maintains the potency of MaSCs ex vivo and raise the possibility that this pathway may be involved in promoting stem cell-like states in cancer, especially because expression of CRIPTO and GRP78 has been correlated with breast cancer progression and decreased patient survival (Dong et al., 2008, Gong et al., 2007, Lee, 2007). The stress-related cues that increase the expression of CRIPTO and GRP78 are prevalent in cancer and might specifically promote stem cell properties via increased CRIPTO/GRP78 signaling (Miharada et al., 2011, Zhang et al., 2010). In this regard, the ALK4L75A-Fc CRIPTO antagonist described here would have potential as a stem cell-directed cancer therapeutic.
Experimental Procedures
See Supplemental Information for additional details.
Reagents
ALK4L75A-Fc was produced in 293T cells and purified by sequential protein-A and Flag affinity chromatography from conditioned media. Recombinant mouse CRIPTO (R&D Systems) was iodinated as previously described (Kelber et al., 2009). The following antibodies were used: CRIPTO (Abcam), GRP78 (N-20, Santa Cruz Biotechnology), KRT14 (AF-64, Covance), KRT8 (Troma-1, DSHB), F4/80 (BM8, eBioscience), Ki67 (B56, BD). Polyclonal rabbit antibodies directed against amino acids 19–39 of human GRP78 or amino acids 81–97 of mouse CRIPTO were produced in-house (see Supplemental Information). The A3-luciferase reporter assay has been described previously (Gray et al., 2003, Kelber et al., 2008). GRP78 floxed mice were provided by Amy Lee (University of Southern California).
Media
MCF10A media was composed of Dulbecco’s modified Eagle’s medium/F12 with 5% horse serum, 10 μg/ml insulin, 20 ng/ml epidermal growth factor, 100 ng/ml cholera toxin, 0.5 μg/ml hydrocortisone, and 10 μg/ml ciprofloxacin. Maintenance media was composed of MCF10A with 1× B27 supplement (Invitrogen). Differentiation media was composed of Epicult-B mouse media containing B supplement (Stem Cell Technologies), recombinant human epidermal growth factor, recombinant human basic fibroblast growth factor, and heparin, as previously described (Spike et al., 2012), with ciprofloxacin 10 μg/ml.
Cell Preparation and Culture
All animal care and procedures were approved and monitored by an Institutional Animal Care and Use Committee. MCF10A cells were cultured free floating in CnT-27 media or immobilized in MCF10A media with 2% Matrigel or 1% methylcellulose. Primary cells were prepared, sorted, cultured, and stained as previously described (Spike et al., 2012) using the indicated media. 3T3 cocultures were adapted from Makarem et al. (2013).
Assays
Proliferation in 2D was quantified on a Celigo microplate cytometer (Cyntellect). Migrational dynamics were tracked using time-lapse microscopy and Metamorph Imaging software on 2D cultures on Matrigel. DNA content was measured by Hoechst staining. mRNA abundance was measured using Superscript III (Life Technologies) and TaqMan assays Mm03024051_g1 and Mm01324427_m1 (Applied Biosciences). Mammary transplantation analyses were conducted as previously described (Spike et al., 2012). Organoid transplants used 20 organoids each by dilution. Western blotting and quantitation of cell-surface proteins by intact cell ELISA have also been previously described (Gray et al., 2003, Kelber et al., 2008).
Statistics
Data are presented as means and SDs with p values derived from pairwise Student’s t tests, except where otherwise noted. ELDA (http://bioinf.wehi.edu.au/software/elda) was used to estimate stem cell frequencies.
Acknowledgments
We thank Y. Zheng, L. Mack, J. Fitzpatrick, and J. Nguyen for technical assistance and equipment, J. Green and L. Bilezijkian for careful reading of the manuscript and helpful comments, and S. Ganley and D. Doan for administrative assistance. This work was supported by the Clayton Medical Research Foundation, The Breast Cancer Research Foundation, DoD Award number W81XWH-10-1-0891, Cancer Center Core grant P30 CA014195, and the Leona M. and Harry B. Helmsley Charitable Trust (#2012PG-MED002). This manuscript is dedicated to Wylie Vale in remembrance of his wisdom, humor, and irrepressible enthusiasm for science.
Published: April 3, 2014
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/3.0/).
Supplemental Information includes Supplemental Experimental Procedures, four figures, and four movies and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2014.02.010.
Contributor Information
Benjamin T. Spike, Email: bspike@salk.edu.
Peter C. Gray, Email: gray@salk.edu.
Supplemental Information
References
- Adewumi O., Aflatoonian B., Ahrlund-Richter L., Amit M., Andrews P.W., Beighton G., Bello P.A., Benvenisty N., Berry L.S., Bevan S., International Stem Cell Initiative Characterization of human embryonic stem cell lines by the International Stem Cell Initiative. Nat. Biotechnol. 2007;25:803–816. doi: 10.1038/nbt1318. [DOI] [PubMed] [Google Scholar]
- Andersson O., Korach-Andre M., Reissmann E., Ibáñez C.F., Bertolino P. Growth/differentiation factor 3 signals through ALK7 and regulates accumulation of adipose tissue and diet-induced obesity. Proc. Natl. Acad. Sci. USA. 2008;105:7252–7256. doi: 10.1073/pnas.0800272105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Porath I., Thomson M.W., Carey V.J., Ge R., Bell G.W., Regev A., Weinberg R.A. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat. Genet. 2008;40:499–507. doi: 10.1038/ng.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco C., Adkins H.B., Wechselberger C., Seno M., Normanno N., De Luca A., Sun Y., Khan N., Kenney N., Ebert A. Cripto-1 activates nodal- and ALK4-dependent and -independent signaling pathways in mammary epithelial Cells. Mol. Cell. Biol. 2002;22:2586–2597. doi: 10.1128/MCB.22.8.2586-2597.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco C., Strizzi L., Mancino M., Watanabe K., Gonzales M., Hamada S., Raafat A., Sahlah L., Chang C., Sotgia F. Regulation of Cripto-1 signaling and biological activity by caveolin-1 in mammary epithelial cells. Am. J. Pathol. 2008;172:345–357. doi: 10.2353/ajpath.2008.070696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco C., Rangel M.C., Castro N.P., Nagaoka T., Rollman K., Gonzales M., Salomon D.S. Role of Cripto-1 in stem cell maintenance and malignant progression. Am. J. Pathol. 2010;177:532–540. doi: 10.2353/ajpath.2010.100102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnath J., Muthuswamy S.K., Brugge J.S. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30:256–268. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- Dong D., Ni M., Li J., Xiong S., Ye W., Virrey J.J., Mao C., Ye R., Wang M., Pen L. Critical role of the stress chaperone GRP78/BiP in tumor proliferation, survival, and tumor angiogenesis in transgene-induced mammary tumor development. Cancer Res. 2008;68:498–505. doi: 10.1158/0008-5472.CAN-07-2950. [DOI] [PubMed] [Google Scholar]
- Dontu G., Abdallah W.M., Foley J.M., Jackson K.W., Clarke M.F., Kawamura M.J., Wicha M.S. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehninger A., Trumpp A. The bone marrow stem cell niche grows up: mesenchymal stem cells and macrophages move in. J. Exp. Med. 2011;208:421–428. doi: 10.1084/jem.20110132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y.P., Yarrow P.M., Carmalt H.L., Kwun S.Y., Kennedy C.W., Lin B.P., Xing P.X., Gillett D.J. Overexpression of Cripto and its prognostic significance in breast cancer: a study with long-term survival. Eur. J. Surg. Oncol. 2007;33:438–443. doi: 10.1016/j.ejso.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Gray P.C., Vale W. Cripto/GRP78 modulation of the TGF-β pathway in development and oncogenesis. FEBS Lett. 2012;586:1836–1845. doi: 10.1016/j.febslet.2012.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray P.C., Harrison C.A., Vale W. Cripto forms a complex with activin and type II activin receptors and can block activin signaling. Proc. Natl. Acad. Sci. USA. 2003;100:5193–5198. doi: 10.1073/pnas.0531290100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyorki D.E., Asselin-Labat M.L., van Rooijen N., Lindeman G.J., Visvader J.E. Resident macrophages influence stem cell activity in the mammary gland. Breast Cancer Res. 2009;11:R62. doi: 10.1186/bcr2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijmans J., van Lidth de Jeude J.F., Koo B.K., Rosekrans S.L., Wielenga M.C., van de Wetering M., Ferrante M., Lee A.S., Onderwater J.J., Paton J.C. ER stress causes rapid loss of intestinal epithelial stemness through activation of the unfolded protein response. Cell Rep. 2013;3:1128–1139. doi: 10.1016/j.celrep.2013.02.031. [DOI] [PubMed] [Google Scholar]
- Hough S.R., Laslett A.L., Grimmond S.B., Kolle G., Pera M.F. A continuum of cell states spans pluripotency and lineage commitment in human embryonic stem cells. PLoS ONE. 2009;4:e7708. doi: 10.1371/journal.pone.0007708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D.L., Wagers A.J. No place like home: anatomy and function of the stem cell niche. Nat. Rev. Mol. Cell Biol. 2008;9:11–21. doi: 10.1038/nrm2319. [DOI] [PubMed] [Google Scholar]
- Kelber J.A., Shani G., Booker E.C., Vale W.W., Gray P.C. Cripto is a noncompetitive activin antagonist that forms analogous signaling complexes with activin and nodal. J. Biol. Chem. 2008;283:4490–4500. doi: 10.1074/jbc.M704960200. [DOI] [PubMed] [Google Scholar]
- Kelber J.A., Panopoulos A.D., Shani G., Booker E.C., Belmonte J.C., Vale W.W., Gray P.C. Blockade of Cripto binding to cell surface GRP78 inhibits oncogenic Cripto signaling via MAPK/PI3K and Smad2/3 pathways. Oncogene. 2009;28:2324–2336. doi: 10.1038/onc.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney N.J., Huang R.P., Johnson G.R., Wu J.X., Okamura D., Matheny W., Kordon E., Gullick W.J., Plowman G., Smith G.H. Detection and location of amphiregulin and Cripto-1 expression in the developing postnatal mouse mammary gland. Mol. Reprod. Dev. 1995;41:277–286. doi: 10.1002/mrd.1080410302. [DOI] [PubMed] [Google Scholar]
- Kenney N.J., Smith G.H., Lawrence E., Barrett J.C., Salomon D.S. Identification of Stem Cell Units in the Terminal End Bud and Duct of the Mouse Mammary Gland. J. Biomed. Biotechnol. 2001;1:133–143. doi: 10.1155/S1110724301000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A.S. GRP78 induction in cancer: therapeutic and prognostic implications. Cancer Res. 2007;67:3496–3499. doi: 10.1158/0008-5472.CAN-07-0325. [DOI] [PubMed] [Google Scholar]
- Luo S., Mao C., Lee B., Lee A.S. GRP78/BiP is required for cell proliferation and protecting the inner cell mass from apoptosis during early mouse embryonic development. Mol. Cell. Biol. 2006;26:5688–5697. doi: 10.1128/MCB.00779-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarem M., Kannan N., Nguyen L.V., Knapp D.J., Balani S., Prater M.D., Stingl J., Raouf A., Nemirovsky O., Eirew P., Eaves C.J. Developmental changes in the in vitro activated regenerative activity of primitive mammary epithelial cells. PLoS Biol. 2013;11:e1001630. doi: 10.1371/journal.pbio.1001630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miharada K., Karlsson G., Rehn M., Rörby E., Siva K., Cammenga J., Karlsson S. Cripto regulates hematopoietic stem cells as a hypoxic-niche-related factor through cell surface receptor GRP78. Cell Stem Cell. 2011;9:330–344. doi: 10.1016/j.stem.2011.07.016. [DOI] [PubMed] [Google Scholar]
- Mikaelian I., Hovick M., Silva K.A., Burzenski L.M., Shultz L.D., Ackert-Bicknell C.L., Cox G.A., Sundberg J.P. Expression of terminal differentiation proteins defines stages of mouse mammary gland development. Vet. Pathol. 2006;43:36–49. doi: 10.1354/vp.43-1-36. [DOI] [PubMed] [Google Scholar]
- Mizuno H., Spike B.T., Wahl G.M., Levine A.J. Inactivation of p53 in breast cancers correlates with stem cell transcriptional signatures. Proc. Natl. Acad. Sci. USA. 2010;107:22745–22750. doi: 10.1073/pnas.1017001108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morkel M., Huelsken J., Wakamiya M., Ding J., van de Wetering M., Clevers H., Taketo M.M., Behringer R.R., Shen M.M., Birchmeier W. Beta-catenin regulates Cripto- and Wnt3-dependent gene expression programs in mouse axis and mesoderm formation. Development. 2003;130:6283–6294. doi: 10.1242/dev.00859. [DOI] [PubMed] [Google Scholar]
- Rangel M.C., Karasawa H., Castro N.P., Nagaoka T., Salomon D.S., Bianco C. Role of Cripto-1 during epithelial-to-mesenchymal transition in development and cancer. Am. J. Pathol. 2012;180:2188–2200. doi: 10.1016/j.ajpath.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackleton M., Vaillant F., Simpson K.J., Stingl J., Smyth G.K., Asselin-Labat M.L., Wu L., Lindeman G.J., Visvader J.E. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- Shani G., Fischer W.H., Justice N.J., Kelber J.A., Vale W., Gray P.C. GRP78 and Cripto form a complex at the cell surface and collaborate to inhibit transforming growth factor beta signaling and enhance cell growth. Mol. Cell. Biol. 2008;28:666–677. doi: 10.1128/MCB.01716-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spike B.T., Wahl G.M. p53, Stem Cells, and Reprogramming: Tumor Suppression beyond Guarding the Genome. Genes Cancer. 2011;2:404–419. doi: 10.1177/1947601911410224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spike B.T., Engle D.D., Lin J.C., Cheung S.K., La J., Wahl G.M. A mammary stem cell population identified and characterized in late embryogenesis reveals similarities to human breast cancer. Cell Stem Cell. 2012;10:183–197. doi: 10.1016/j.stem.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling A., Drummond-Barbosa D., Kai T. Stem cells find their niche. Nature. 2001;414:98–104. doi: 10.1038/35102160. [DOI] [PubMed] [Google Scholar]
- Stingl J., Eirew P., Ricketson I., Shackleton M., Vaillant F., Choi D., Li H.I., Eaves C.J. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- Vaillant F., Lindeman G.J., Visvader J.E. Jekyll or Hyde: does Matrigel provide a more or less physiological environment in mammary repopulating assays? Breast Cancer Res. 2011;13:108. doi: 10.1186/bcr2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Keymeulen A., Rocha A.S., Ousset M., Beck B., Bouvencourt G., Rock J., Sharma N., Dekoninck S., Blanpain C. Distinct stem cells contribute to mammary gland development and maintenance. Nature. 2011;479:189–193. doi: 10.1038/nature10573. [DOI] [PubMed] [Google Scholar]
- Veltmaat J.M., Mailleux A.A., Thiery J.P., Bellusci S. Mouse embryonic mammogenesis as a model for the molecular regulation of pattern formation. Differentiation. 2003;71:1–17. doi: 10.1046/j.1432-0436.2003.700601.x. [DOI] [PubMed] [Google Scholar]
- Wey S., Luo B., Lee A.S. Acute inducible ablation of GRP78 reveals its role in hematopoietic stem cell survival, lymphogenesis and regulation of stress signaling. PLoS ONE. 2012;7:e39047. doi: 10.1371/journal.pone.0039047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wey S., Luo B., Tseng C.C., Ni M., Zhou H., Fu Y., Bhojwani D., Carroll W.L., Lee A.S. Inducible knockout of GRP78/BiP in the hematopoietic system suppresses Pten-null leukemogenesis and AKT oncogenic signaling. Blood. 2012;119:817–825. doi: 10.1182/blood-2011-06-357384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo C., Whitman M. Nodal signals to Smads through Cripto-dependent and Cripto-independent mechanisms. Mol. Cell. 2001;7:949–957. doi: 10.1016/s1097-2765(01)00249-0. [DOI] [PubMed] [Google Scholar]
- Zeng Y.A., Nusse R. Wnt proteins are self-renewal factors for mammary stem cells and promote their long-term expansion in culture. Cell Stem Cell. 2010;6:568–577. doi: 10.1016/j.stem.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Liu R., Ni M., Gill P., Lee A.S. Cell surface relocalization of the endoplasmic reticulum chaperone and unfolded protein response regulator GRP78/BiP. J. Biol. Chem. 2010;285:15065–15075. doi: 10.1074/jbc.M109.087445. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


