Abstract
Background
Hemorrhage is associated with ischemic complications in cardiac patients. The nature of this relationship in surgical patients is unknown.
Methods and Results
We examined the association between major perioperative hemorrhage and stroke or myocardial infarction among adults who underwent surgery from 2005 through 2009 at centers participating in the National Surgical Quality Improvement Program. We excluded patients with emergent, trauma-related, transplantation, cardiac, or neurological operations. Major hemorrhage was defined as bleeding necessitating transfusion of >4 U of packed red blood cells or whole blood. Stroke was defined as focal brain dysfunction lasting ≥24 hours from a vascular cause. A diagnosis of myocardial infarction required new ECG Q waves. Outcomes were assessed from surgery until 30 days afterward. Among 651 775 patients who underwent surgery, 5233 (0.80%) experienced major hemorrhage, 1575 (0.24%) developed Q-wave myocardial infarction, and 1321 (0.20%) suffered a stroke. In Cox proportional hazards analyses controlling for vascular risk factors, illness severity, and type of surgery, hemorrhage was independently associated with subsequent stroke (hazard ratio, 2.5; 95% confidence interval, 1.9 –3.3) and subsequent Q-wave myocardial infarction (hazard ratio, 2.7; 95% confidence interval, 2.1–3.4). Interaction terms revealed no significant variation in these associations by age, sex, or type of surgery. Our results were robust across multiple sensitivity analyses.
Conclusions
Major perioperative hemorrhage is associated with subsequent stroke and myocardial infarction in patients undergoing noncardiac, nonneurological surgery. This suggests the need for randomized trials to guide perioperative use of antiplatelet drugs, which affect the risk of both bleeding and vascular events.
Keywords: hemorrhage, myocardial infarction, risk factors, stroke, surgery
Stroke and myocardial infarction (MI) are serious complication of surgery. Perioperative stroke and MI are associated with a mortality rate of 12% to 25%1,2 compared with 2% mortality after uncomplicated major surgery.3 MI occurs after ≈1.5% of operations,1 and perioperative stroke affects up to 7 of every 1000 surgical patients.2-4 With >200 million operations occurring globally each year,5 perioperative stroke and MI cause significant morbidity and mortality and therefore represent important targets for improving surgical outcomes.
Rates of stroke and MI may be reduced by optimizing perioperative use of antiplatelet agents such as aspirin and clopidogrel. Antiplatelet drugs are frequently stopped before surgery because of concerns about bleeding,6,7 but their cessation has been associated with increased risk of stroke and MI.8-10 Two recent randomized trials of perioperative aspirin therapy lacked power to detect meaningful differences in rates of MI, stroke, and bleeding.11,12 Therefore, decisions about discontinuing antiplatelet agents before surgery must rely on clinical judgment and guidelines based largely on observational data.
Current guidelines recommend balancing the risks of ischemic and hemorrhagic complications when making decisions about perioperative antiplatelet therapy.13,14 This assumes that bleeding does not affect the risk of ischemia and that patients can be identified who are at higher risk of 1 complication than the other. However, hemorrhage has been associated with higher rates of recurrent MI in patients with acute coronary syndrome,15,16 and risk factors for stroke and hemorrhage overlap in patients with atrial fibrillation.17 Robust data are unavailable on the relationship between hemorrhage and the risk of stroke or MI in the general surgical population. An association between perioperative bleeding and stroke or MI would raise the possibility that ischemic events precipitated by bleeding could in certain cases outweigh the antithrombotic benefits of antiplatelet agents. Therefore, we examined the relationship between perioperative hemorrhage and stroke or MI in a large cohort of patients undergoing surgery.
Methods
Design
We assessed the relationship between major perioperative hemorrhage and stroke or MI in a cohort of patients prospectively assembled from 2005 through 2009 by the American College of Surgeons National Surgical Quality Improvement Program (NSQIP). NSQIP has developed a national registry to promote quality improvement by providing comparisons of risk-adjusted surgical outcomes among centers with a range of surgical volume and subspecialty expertise.18 The number of participating centers grew from 121 in 2005 to 237 in 2009. Our analysis involved only deidentified data from these centers and was therefore exempt from evaluation by our institutional review boards. This report of our study is consistent with guidelines from the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Consortium.19
Patients
The NSQIP cohort comprises a systematic sample of all patients undergoing surgery under general, spinal, or epidural anesthesia and all patients undergoing endovascular repair of abdominal aortic aneurysms. Trauma and transplant cases are excluded. For our analysis, we excluded pediatric patients (age <18 years). We used the NSQIP designations of surgical type to exclude patients undergoing cardiac surgery because they face distinct, procedure-specific risks of perioperative stroke and MI.20,21 We also excluded patients undergoing neurological surgery because they often have postoperative neurological deficits from causes other than ischemia, thereby potentially clouding the diagnosis of perioperative stroke. In addition, we excluded patients undergoing carotid endarterectomy because aspirin has a proven role in these patients.22 Finally, because our underlying interest lay in decisions about perioperative antiplatelet therapy, we excluded cases of emergency surgery, for which such decisions are not applicable.
Measurements
Trained reviewers at centers participating in NSQIP collect data by a variety of methods, including medical chart review, discussions with healthcare providers, and telephone interviews with patients. Data are abstracted and coded by the use of standardized definitions. All reviewers undergo a uniform training program, and data from each center are subject to audit. The most recent audit showed >98% interrater agreement for all assessed variables.18 This high rate of interrater agreement has been confirmed in independent reviews.23,24 Prior work has shown outcome measures in the NSQIP database to be more reliable than commonly used administrative data.25
To control for potential confounders in the relationship between bleeding and stroke or MI, we analyzed previously reported risk factors for perioperative bleeding,26 stroke,20 and MI27 using relevant variables as defined in the NSQIP database.18 Prior transient ischemic attack was defined as a transient focal neurological deficit of sudden onset reflecting a cerebral vascular distribution. Prior stroke was defined similarly but required more persistent deficits. Coronary heart disease reflected MI within the 6 months before surgery, angina within the month before surgery, or any history of percutaneous coronary intervention. The definition of congestive heart failure required evidence of heart failure within the month before surgery. Hypertension was defined as blood pressure persistently >140/90 mm Hg or antihypertensive therapy for >1 month before surgery. A diagnosis of diabetes mellitus required treatment with oral hypoglycemic agents or insulin. Renal insufficiency was determined by a preoperative creatinine level >2 mg/dL or a history of dialysis.27 We defined anemia as preoperative hematocrit <36% in women and <39% in men based on World Health Organization criteria.28 Chronic obstructive pulmonary disease encompassed emphysema or chronic bronchitis resulting in functional disability, hospitalization, chronic bronchodilator therapy, or a diagnostic pulmonary function test. A diagnosis of peripheral vascular disease required rest pain, ischemic ulceration, or revascularization or amputation. Bleeding disorders included known clotting deficiencies such as thrombocytopenia and long-term anticoagulation therapy that was not stopped before surgery. Tobacco use was determined by self-report of active cigarette use in the year before surgery. Patients’ overall degree of morbidity was categorized according to the American Society of Anesthesiologists’ illness severity classification.29
To account for the type of surgery when examining the relationship between perioperative bleeding and stroke or MI, we reviewed Current Procedural Terminology codes and case descriptions to identify high-risk cases, defined as intraperitoneal, intrathoracic, or suprainguinal vascular surgery based on the validated classification from the Revised Cardiac Risk Index.27 In sensitivity analyses, we used alternative classifications of surgical type based on other widely accepted definitions of high-risk surgery30,31 and the NSQIP classification of cases as head and neck, general, gynecologic, ophthalmologic, oral, orthopedic, plastic, thoracic, urologic, or vascular surgery.
Our exposure of interest was major hemorrhage requiring transfusion of >4 U of packed red blood cells (PRBCs) or whole blood based on the NSQIP definition18 and consistent with the quantity of blood loss in typical major surgical hemorrhages.32 Our primary outcomes were stroke and MI during surgery or within 30 days after surgery. NSQIP defines perioperative MI as acute transmural infarction manifesting in new Q waves on an ECG and perioperative stroke as focal brain dysfunction lasting ≥24 hours from a vascular cause.18 This definition of stroke encompasses intracranial hemorrhage,18 but we considered it a reliable surrogate of ischemic stroke because hemorrhagic strokes make up only 1% of perioperative strokes.33,34
Statistical Analyses
Descriptive statistics with exact binomial confidence intervals were used to report the proportion of patients with major hemorrhage, stroke, and MI. Kaplan–Meier survival statistics and the log-rank test were used to compare cumulative rates of stroke or MI within 30 days of surgery among patients with and without major hemorrhage. Patients were censored at the time of stroke, MI, or death, and bleeding events occurring on the same day or afterward were not included as exposures in our analysis. We used multivariable Cox proportional hazards models to examine the independent association between hemorrhage and subsequent stroke or MI. On the basis of previously published reports,20,26,27 the following covariates were included a priori as potential confounders in our models: age, sex, race, coronary heart disease, prior stroke or transient ischemic attack, congestive heart failure, peripheral vascular disease, hypertension, diabetes mellitus, renal insufficiency, chronic obstructive pulmonary disease, anemia, bleeding disorders, smoking, American Society of Anesthesiologists classification, and type of surgery. Because our goal was to isolate the relationship between hemorrhage and stroke or MI, not to develop a parsimonious prediction model, all covariates were left in place regardless of statistical significance. We examined interaction terms to determine whether the association between bleeding and stroke or MI varies by age, sex, or type of surgery. To test the validity of the proportional hazards assumption, we also examined hemorrhage as a time-dependent covariate and visually inspected regressions of Schoenfeld residuals over time.
To explore causality, we used data on the specific number of units of PRBCs or whole blood administered intraoperatively to determine whether a dose-response relationship exists between the severity of bleeding and the risk of stroke or MI. To address the possibility that some diagnoses of hemorrhage represented transfusions for anemia in the absence of major bleeding, we performed a sensitivity analysis that excluded patients with preoperative anemia because they are more likely to receive transfusions in the absence of bleeding, and we defined major hemorrhage only by the receipt of >4 U of PRBCs or whole blood intraoperatively, a setting in which this quantity of transfusion is highly likely to be related to hemorrhage.35
Unless otherwise specified, the threshold of statistical significance was a 2-sided α of 0.05. All analyses were performed with Stata SE (Version 11, StataCorp, College Station, TX).
Results
From cases included in NSQIP from 2005 through 2009, we excluded 97 940 emergency surgeries, 8709 neurological surgeries, 3395 cardiac surgeries, 1374 surgeries on patients < 18 years of age, and 179 carotid endarterectomies. We excluded 36 observations (0.006%) with missing values for sex and 2214 observations (0.3%) with missing American Society of Anesthesiologists status. Sensitivity analyses were performed with diagnoses of anemia or renal insufficiency assigned to all or none of the patients with missing values for preoperative hematocrit (15%) or preoperative creatinine (21%) levels to assess the range of possible bias from nonrandom missing laboratory values. There were no missing values for the other variables used in this analysis.
Of the 651 775 patients who were eligible for our analysis (Table 1), 1575 patients (0.24%; 95% confidence interval [CI], 0.23–0.25) experienced a perioperative Q-wave MI, 1321 patients (0.20%; 95% CI, 0.19–0.21) suffered a perioperative stroke, and 2855 patients (0.44%; 95% CI, 0.42–45) developed the composite end point of stroke or Q-wave MI. The rate of stroke or Q-wave MI ranged from 0.08% (95% CI, 0.07–0.09) among younger patients (age <75 years) without vascular risk factors to 2.9% (95% CI, 2.5–3.3) among patients with ≥5 risk factors. Perioperative bleeding occurred in 5233 patients (0.80%; 95% CI, 0.78–0.83), of whom 34% returned to the operating room after their initial surgery. Bleeding preceded vascular events by at least 1 day in most (71%) of the 184 patients with both perioperative bleeding and stroke or Q-wave MI.
Table 1.
Baseline Characteristics of Patients Undergoing Surgery, Overall and Stratified by Major Perioperative Hemorrhage
| Overall (n=651 775) | Hemorrhage (n=5233) | No Hemorrhage (n=646 542) | |
|---|---|---|---|
| Age, y | 56 (17) | 64 (14) | 56 (17) |
| Female sex, % | 58.6 | 42.2 | 58.8 |
| Race, % | |||
| White | 72.4 | 74.9 | 72.4 |
| Black | 9.9 | 11.4 | 9.9 |
| Hispanic | 6.7 | 4.6 | 6.7 |
| Asian | 1.9 | 2.0 | 1.9 |
| Other | 9.1 | 7.2 | 9.1 |
| ASA class | 2.4 (0.7) | 3.1 (0.7) | 2.4 (0.7) |
| Surgical category, % | |||
| General | 75.1 | 51.5 | 75.3 |
| Vascular | 12.5 | 40.9 | 12.3 |
| Orthopedic | 5.4 | 3.1 | 5.4 |
| Gynecologic | 2.6 | 1.2 | 2.6 |
| Urologic | 1.8 | 1.9 | 1.8 |
| Head and neck | 1.1 | 0.4 | 1.1 |
| Plastic | 1.0 | 0.2 | 1.0 |
| Thoracic | 0.5 | 0.8 | 0.5 |
| Coronary heart disease, % | 6.1 | 14.9 | 6.0 |
| Stroke or TIA, % | 6.5 | 11.5 | 6.4 |
| Congestive heart failure, % | 0.8 | 2.8 | 0.7 |
| Hypertension, % | 46.5 | 67.9 | 46.3 |
| Diabetes mellitus, % | 15.0 | 21.8 | 14.9 |
| Renal insufficiency, % | 3.3 | 9.4 | 3.3 |
| Peripheral vascular disease, % | 5.1 | 14.1 | 5.0 |
| COPD, % | 4.5 | 11.5 | 4.5 |
| Anemia, % | 24.5 | 58.1 | 24.2 |
| Bleeding disorder, % | 5.0 | 14.6 | 4.9 |
| Tobacco use, % | 20.3 | 24.5 | 20.3 |
ASA indicates American Society of Anesthesiologists; TIA, transient ischemic attack; and COPD, chronic obstructive pulmonary disease. Values are mean (SD) when appropriate.
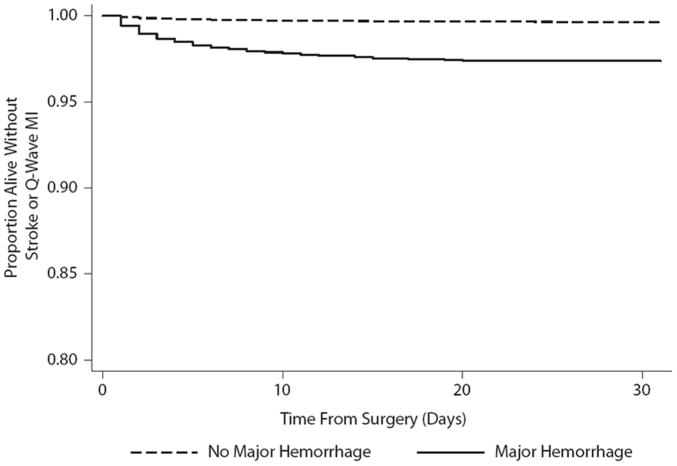
In survival analysis, the cumulative rate of stroke or Q-wave MI after major hemorrhage (2.62%; 95% CI, 2.21–3.11) was significantly higher than the rate among patients without hemorrhage (0.42%; 95% CI, 0.41–0.44; P<0.001 by the log-rank test; the Figure). In Cox proportional hazards analysis, hemorrhage was independently associated with subsequent stroke (hazard ratio, 2.5; 95% CI, 1.9 –3.3), subsequent Q-wave MI (hazard ratio, 2.7; 95% CI, 2.1–3.4), and a composite of subsequent stroke or Q-wave MI (hazard ratio, 2.6; 95% CI, 2.2–3.1). There appeared to be a dose-response relationship between the severity of bleeding and the risk of subsequent stroke or Q-wave MI (hazard ratio for each unit of intraoperative PRBCs or whole blood transfused, 1.11; 95% CI, 1.10 –1.13). Interaction terms did not reveal significant variation in the association between hemorrhage and subsequent stroke or Q-wave MI by age, sex, or type of surgery. There was no evidence of a violation of the proportional hazards assumption in any of our models. Our results were robust across multiple sensitivity analyses (Table 2).
Figure.
Kaplan–Meier survival curves demonstrating the proportion of patients alive without stroke or Q-wave myocardial infarction (MI) after surgery stratified by the presence of major perioperative hemorrhage (P<0.001 by the log-rank test for the difference between survival curves).
Table 2.
Results of Proportional Hazards Analysis and Sensitivity Analyses of the Association Between Major Perioperative Hemorrhage and Subsequent Stroke or Q-Wave Myocardial Infarction
| HR for Subsequent Stroke or Q-Wave MI (95% CI) | |
|---|---|
| Major perioperative hemorrhage | |
| Unadjusted analysis* | 4.1 (3.4–4.9) |
| Primary adjusted analysis† | 2.6 (2.2–3.1) |
| Sensitivity analysis of possible misclassification of major hemorrhage‡ | 4.2 (3.0–5.7) |
| Sensitivity analysis of missing preoperative creatinine values§ | 2.6 (2.2–3.1) |
| Sensitivity analysis of missing preoperative hematocrit values∥ | 2.6 (2.2–3.2) |
| Sensitivity analysis of surgical classifications¶ | 2.4 (2.0–2.8) |
HR indicates hazard ratio; MI, myocardial infarction; and CI, confidence interval.
This model controlled for only age, sex, and race. Patients were censored at the time of stroke, MI, or death, and bleeding events occurring on the same day or afterward were not included as exposures in our analyses. A diagnosis of major perioperative hemorrhage required intraoperative or postoperative administration of >4 U of packed red blood cells or whole blood.
This model controlled for age, sex, race, coronary heart disease, prior stroke or transient ischemic attack, congestive heart failure, peripheral vascular disease, hypertension, diabetes mellitus, renal insufficiency, chronic obstructive pulmonary disease, anemia, bleeding disorders, smoking, American Society of Anesthesiologists classification, and type of surgery. Missing creatinine (15%) and hematocrit (21%) values were assumed to be normal.
Patients with preoperative anemia were excluded, and a diagnosis of major hemorrhage required intraoperative administration of >4 U of packed red blood cells.
Patients with missing preoperative creatinine values were assumed to have renal insufficiency.
Patients with missing preoperative hematocrit values were assumed to have anemia.
Instead of the definition of high-risk cases from the Revised Cardiac Risk Index,27 alternative classifications of surgical type were used. Results shown are from an analysis using the National Surgical Quality Improvement Program classification of surgical type; analyses using other classifications yielded nearly identical results.30,31
Discussion
In a large cohort of surgical patients, we found a strong association between major perioperative hemorrhage and subsequent stroke and MI. This association partly reflects shared risk factors such as older age and medical comorbidities. However, the relationship persisted after such confounders were controlled for, and there appeared to be a dose-response relationship between the severity of bleeding and the risk of stroke or MI, suggesting that perioperative hemorrhage may play a causative role in vascular events.
Our results should be interpreted in light of the limitations of this study. First, we lacked information on perioperative antiplatelet drugs and cannot comment on their effects on the risks of bleeding, stroke, and MI. Randomized clinical trials are required to clearly delineate the risks and benefits of perioperative antiplatelet therapy. Second, the NSQIP classification of perioperative stroke includes hemorrhagic and ischemic stroke, thereby potentially clouding our definition of perioperative ischemic events. However, this is unlikely to have significantly affected our results because perioperative strokes are predominantly ischemic, with intracranial hemorrhages accounting for only 1% of perioperative strokes.33,34 Third, the rate of MI in our study was lower than in prior analyses,1 indicating that we likely underestimated the total number of MIs because the stringent NSQIP definition limited this end point to severe infarction manifesting in ECG Q waves. Our findings may have differed if we had had access to more sensitive measures of MI such as biomarkers of myocardial necrosis. Fourth, we lacked certain data on patients’ intraoperative courses—such as data on blood pressure, use of vasopressor therapy, or platelet transfusions—that may have shed additional light on the relationship between perioperative hemorrhage and stroke or MI. Fifth, we were unable to account for clustering within individual surgical centers, and such an analysis may have produced different results. Finally, our results may be confounded by the adverse effects of blood transfusions given for anemia in the absence of major bleeding. However, although clinicians commonly administer 1 to 2 U of PRBCs for anemia, a single transfusion of >4 U is unlikely to occur outside the setting of major hemorrhage.32,35 To further guard against misclassification of hemorrhage, we performed sensitivity analyses limited to large intraoperative transfusions in patients without baseline anemia, and our results remained unchanged. In addition, recent observational and randomized studies have not found a higher rate of cardiac or neurological complications in surgical patients receiving 1 to 2 U of PRBCs for anemia,35,36 which suggests that our results are not due simply to confounding from blood transfusions.
Our findings highlight the need for randomized clinical trials to determine the optimal strategies for perioperative antiplatelet therapy. Current practice depends on clinical judgments about the relative risks of bleeding and ischemia.13,14,37 However, if perioperative bleeding independently increases the risk of stroke or MI, perioperative antiplatelet drugs may in some cases cause excessive bleeding that in turn increases thrombotic risk, thus outweighing the beneficial antithrombotic effects of the drugs. On the other hand, despite their tendency to increase surgical bleeding,6,7 antiplatelet drugs have benefits that may outweigh the deleterious effects of hemorrhage. This is likely the case for some patients with recently implanted coronary stents8 but remains unknown for other groups such as those with prior stroke or coronary heart disease without coronary stents.14 Two recent trials of perioperative aspirin therapy were underpowered to fully resolve these uncertainties.11,12 However, >200 million patients worldwide undergo surgery each year, and a substantial number face the morbidity and mortality of perioperative MI and stroke, so adequately powered randomized trials of cheap and readily available drugs such as aspirin are both necessary and feasible.
Conclusions
Major surgical hemorrhage appears to independently increase the risk of ischemic perioperative complications such as stroke and MI. This suggests that surgical bleeding should be factored into clinical assessments of the risk of serious perioperative cardiac and neurological complications. In addition, the possibility of a link between perioperative hemorrhage and ischemia highlights the importance of a tightly coordinated multidisciplinary approach to decisions about the perioperative use of antiplatelet therapy. Finally, awareness that surgical patients with major hemorrhage are vulnerable to ischemic complications may promote more vigilant perioperative care of patients with surgical bleeding and improve timely recognition and treatment of perioperative stroke and MI.
CLINICAL PERSPECTIVE.
Perioperative stroke and myocardial infarction (MI) cause significant morbidity and mortality. Patients with vascular risk factors often continue to receive antiplatelet drugs during the perioperative period to reduce their risk of stroke and myocardial infarction. However, these drugs also increase the risk of surgical bleeding, and bleeding has been associated with a higher risk of ischemic complications in patients with acute coronary syndrome. Therefore, we investigated the association between perioperative hemorrhage and stroke or myocardial infarction in a large cohort of surgical patients prospectively assembled by the National Surgical Quality Improvement Program. In multivariable Cox proportional hazards analyses that controlled for vascular risk factors, type of surgery, and illness severity, we found that major perioperative hemorrhage was associated with higher risks of subsequent stroke (hazard ratio, 2.5; 95% confidence interval, 1.9 –3.3), Q-wave myocardial infarction (hazard ratio, 2.7; 95% confidence interval, 2.1–3.4), and a composite of stroke or Q-wave myocardial infarction (hazard ratio, 2.6; 95% confidence interval, 2.2–3.1). These results underscore the importance of randomized clinical trials to define optimal strategies of perioperative antiplatelet use. In the meantime, clinical assessments of the risk of perioperative cardiac and neurological complications should account for surgical bleeding. Furthermore, our results highlight the importance of tightly coordinated multidisciplinary perioperative care of patients with vascular risk factors, especially in regard to perioperative antiplatelet treatment. Finally, clinicians should recognize that surgical patients with major hemorrhage may be more vulnerable to ischemic complications and may benefit from vigilant perioperative care and close observation to identify complications as soon as they arise.
Footnotes
Disclaimer: The American College of Surgeons National Surgical Quality Improvement Program and the hospitals participating in it are the source of the data used here; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
Disclosures
None.
References
- 1.Devereaux PJ, Xavier D, Pogue J, Guyatt G, Sigamani A, Garutti I, Leslie K, Rao-Melacini P, Chrolavicius S, Yang H, Macdonald C, Avezum A, Lanthier L, Hu W, Yusuf S. Characteristics and short-term prognosis of perioperative myocardial infarction in patients undergoing noncardiac surgery: a cohort study. Ann Intern Med. 2011;154:523–528. doi: 10.7326/0003-4819-154-8-201104190-00003. [DOI] [PubMed] [Google Scholar]
- 2.Mashour GA, Shanks AM, Kheterpal S. Perioperative stroke and associated mortality after noncardiac, nonneurologic surgery. Anesthesiology. 2011;114:1289–1296. doi: 10.1097/ALN.0b013e318216e7f4. [DOI] [PubMed] [Google Scholar]
- 3.Bateman BT, Schumacher HC, Wang S, Shaefi S, Berman MF. Perioperative acute ischemic stroke in noncardiac and nonvascular surgery: incidence, risk factors, and outcomes. Anesthesiology. 2009;110:231–238. doi: 10.1097/ALN.0b013e318194b5ff. [DOI] [PubMed] [Google Scholar]
- 4.Parikh S, Cohen JR. Perioperative stroke after general surgical procedures. N Y State J Med. 1993;93:162–165. [PubMed] [Google Scholar]
- 5.Weiser TG, Regenbogen SE, Thompson KD, Haynes AB, Lipsitz SR, Berry WR, Gawande AA. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet. 2008;372:139–144. doi: 10.1016/S0140-6736(08)60878-8. [DOI] [PubMed] [Google Scholar]
- 6.Sethi GK, Copeland JG, Goldman S, Moritz T, Zadina K, Henderson WG. Implications of preoperative administration of aspirin in patients undergoing coronary artery bypass grafting: Department of Veterans Affairs Cooperative Study on Antiplatelet Therapy. J Am Coll Cardiol. 1990;15:15–20. doi: 10.1016/0735-1097(90)90168-o. [DOI] [PubMed] [Google Scholar]
- 7.Herman CR, Buth KJ, Kent BA, Hirsch GM. Clopidogrel increases blood transfusion and hemorrhagic complications in patients undergoing cardiac surgery. Ann Thorac Surg. 2010;89:397–402. doi: 10.1016/j.athoracsur.2009.10.051. [DOI] [PubMed] [Google Scholar]
- 8.Kaluza GL, Joseph J, Lee JR, Raizner ME, Raizner AE. Catastrophic outcomes of noncardiac surgery soon after coronary stenting. J Am Coll Cardiol. 2000;35:1288–1294. doi: 10.1016/s0735-1097(00)00521-0. [DOI] [PubMed] [Google Scholar]
- 9.Biondi-Zoccai GGL, Lotrionte M, Agostoni P, Abbate A, Fusaro M, Burzotta F, Testa L, Sheiban I, Sangiorgi G. A systematic review and meta-analysis on the hazards of discontinuing or not adhering to aspirin among 50,279 patients at risk for coronary artery disease. Eur Heart J. 2006;27:2667–2674. doi: 10.1093/eurheartj/ehl334. [DOI] [PubMed] [Google Scholar]
- 10.Broderick JP, Bonomo JB, Kissela BM, Khoury JC, Moomaw CJ, Alwell K, Woo D, Flaherty ML, Khatri P, Adeoye O, Ferioli S, Kleindorfer DO. Withdrawal of antithrombotic agents and its impact on ischemic stroke occurrence. Stroke. 2011;42:2509–2514. doi: 10.1161/STROKEAHA.110.611905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oscarsson A, Gupta A, Fredrikson M, Jarhult J, Nystrom M, Pettersson E, Darvish B, Krook H, Swahn E, Eintrei C. To continue or discontinue aspirin in the perioperative period: a randomized, controlled clinical trial. Br J Anaesth. 2010;104:305–312. doi: 10.1093/bja/aeq003. [DOI] [PubMed] [Google Scholar]
- 12.Mantz J, Samama CM, Tubach F, Devereaux PJ, Collet J-P, Albaladejo P, Cholley B, Nizard R, Barre J, Piriou V, Poirier N, Mignon A, Schlumberger S, Longrois D, Aubrun F, Farese ME, Ravaud P, Steg PG. Impact of preoperative maintenance or interruption of aspirin on thrombotic and bleeding events after elective non-cardiac surgery: the multicentre, randomized, blinded, placebo-controlled, STRATAGEM trial. Br J Anaesth. 2011;107:899–910. doi: 10.1093/bja/aer274. [DOI] [PubMed] [Google Scholar]
- 13.O’Riordan JM, Margey RJ, Blake G, O’Connell PR. Antiplatelet agents in the perioperative period. Arch Surg. 2009;144:69–76. doi: 10.1001/archsurg.144.1.69. [DOI] [PubMed] [Google Scholar]
- 14.Douketis JD, Berger PB, Dunn AS, Jaffer AK, Spyropoulos AC, Becker RC, Ansell J. The perioperative management of antithrombotic therapy: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. (8) 2008;133:299S–339S. doi: 10.1378/chest.08-0675. [DOI] [PubMed] [Google Scholar]
- 15.Manoukian SV, Feit F, Mehran R, Voeltz MD, Ebrahimi R, Hamon M, Dangas GD, Lincoff AM, White HD, Moses JW, King SB, 3rd, Ohman EM, Stone GW. Impact of major bleeding on 30-day mortality and clinical outcomes in patients with acute coronary syndromes: an analysis from the ACUITY Trial. J Am Coll Cardiol. 2007;49:1362–1368. doi: 10.1016/j.jacc.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 16.Feldman D, Minutello R, Bergman G, Moussa I, Wong S. Combined effect of anemia and bleeding on long-term outcomes following PCI with drug-eluting stents in real world clinical practice. Circulation. 2009;1120:S448–S449. [Google Scholar]
- 17.Poli D, Testa S, Antonucci E, Grifoni E, Paoletti O, Lip GYH. Bleeding and stroke risk in a real-world prospective primary prevention cohort of patients with atrial fibrillation. Chest. 2011;140:918–924. doi: 10.1378/chest.10-3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American College of Surgeons. [January 5, 2012];Data User Guide: American College of Surgeons National Surgical Quality Improvement Program. www.acsnsqip.org/main/puf/PufRequestHomepage.jsp.
- 19.STROBE checklist for cross-sectional studies. STROBE Statement Web site; [January 5, 2012]. http://www.strobe-statement.org. [Google Scholar]
- 20.Selim M. Perioperative stroke. N Engl J Med. 2007;356:706–713. doi: 10.1056/NEJMra062668. [DOI] [PubMed] [Google Scholar]
- 21.Hillis LD, Smith PK, Anderson JL, Bittl JA, Bridges CR, Byrne JG, Cigarroa JE, Disesa VJ, Hiratzka LF, Hutter AM, Jr, Jessen ME, Keeley EC, Lahey SJ, Lange RA, London MJ, Mack MJ, Patel MR, Puskas JD, Sabik JF, Shahian DM, Selnes O, Trost JC, Winniford MD. 2011 ACCF/AHA guideline for coronary artery bypass graft surgery: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the American Association for Thoracic Surgery, Society of Cardiovascular Anesthesiologists, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2011;58:e123–e210. doi: 10.1016/j.jacc.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 22.Engelter S, Lyrer P. Antiplatelet therapy for preventing stroke and other vascular events after carotid endarterectomy. Stroke. 2004;35:1227–1228. doi: 10.1161/01.STR.0000125710.17337.8b. [DOI] [PubMed] [Google Scholar]
- 23.Davis CL, Pierce JR, Henderson W, Spencer CD, Tyler C, Langberg R, Swafford J, Felan GS, Kearns MA, Booker B. Assessment of the reliability of data collected for the Department of Veterans Affairs National Surgical Quality Improvement Program. J Am Coll Surg. 2007;204:550–560. doi: 10.1016/j.jamcollsurg.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 24.Shiloach M, Frencher SK, Jr, Steeger JE, Rowell KS, Bartzokis K, Tomeh MG, Richards KE, Ko CY, Hall BL. Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210:6–16. doi: 10.1016/j.jamcollsurg.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 25.Best WR, Khuri SF, Phelan M, Hur K, Henderson WG, Demakis JG, Daley J. Identifying patient preoperative risk factors and postoperative adverse events in administrative databases: results from the Department of Veterans Affairs National Surgical Quality Improvement Program. J Am Coll Surg. 2002;194:257–266. doi: 10.1016/s1072-7515(01)01183-8. [DOI] [PubMed] [Google Scholar]
- 26.Mehta RH, Sheng S, O’Brien SM, Grover FL, Gammie JS, Ferguson TB, Peterson ED. Reoperation for bleeding in patients undergoing coronary artery bypass surgery. Circ Cardiovasc Qual Outcomes. 2009;2:583–590. doi: 10.1161/CIRCOUTCOMES.109.858811. [DOI] [PubMed] [Google Scholar]
- 27.Lee TH, Marcantonio ER, Mangione CM, Thomas EJ, Polanczyk CA, Cook EF, Sugarbaker DJ, Donaldson MC, Poss R, Ho KKL, Ludwig LE, Pedan A, Goldman L. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043–1049. doi: 10.1161/01.cir.100.10.1043. [DOI] [PubMed] [Google Scholar]
- 28.Kulier A, Levin J, Moser R, Rumpold-Seitlinger G, Tudor IC, Snyder-Ramos SA, Moehnle P, Mangano DT. Investigators of the Multicenter Study of Perioperative Ischemia Research Group, Ischemia Research and Education Foundation. Impact of preoperative anemia on outcome in patients undergoing coronary artery bypass graft surgery. Circulation. 2007;116:471–479. doi: 10.1161/CIRCULATIONAHA.106.653501. [DOI] [PubMed] [Google Scholar]
- 29.ASA Physical Status Classification System. American Society of Anesthesiologists; [January 5, 2012]. Web site. http://www.asahq.org/clinical/physicalstatus.htm. [Google Scholar]
- 30.Bernard AC, Davenport DL, Chang PK, Vaughan TB, Zwischenberger JB. Intraoperative transfusion of 1 U to 2 U packed red blood cells is associated with increased 30-day mortality, surgical-site infection, pneumonia, and sepsis in general surgery patients. J Am Coll Surg. 2009;208:931–937. doi: 10.1016/j.jamcollsurg.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 31.American College of Cardiology Foundation, American Heart Association Task Force on Practice Guidelines, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Angiography, Interventions, Society for Vascular Medicine, Society for Vascular Surgery. Fleisher LA, Beckman JA, Brown KA, Calkins H, Chaikof EL, Fleischmann KE, Freeman WK, Froehlich JB, Kasper EK, Kersten JR, Riegel B, Robb JF. 2009 ACCF/AHA focused update on perioperative beta blockade incorporated into the ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery. J Am Coll Cardiol. 2009;54:e13–e118. doi: 10.1016/j.jacc.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 32.Borzecki AM, Kaafarani H, Cevasco M, Hickson K, Macdonald S, Shin M, Itani KM, Rosen AK. How valid is the AHRQ Patient Safety Indicator “postoperative hemorrhage or hematoma”? J Am Coll Surg. 2011;212:946–953. doi: 10.1016/j.jamcollsurg.2010.09.033. [DOI] [PubMed] [Google Scholar]
- 33.Limburg M, Wijdicks EF, Li H. Ischemic stroke after surgical procedures: clinical features, neuroimaging, and risk factors. Neurology. 1998;50:895–901. doi: 10.1212/wnl.50.4.895. [DOI] [PubMed] [Google Scholar]
- 34.Likosky DS, Marrin CA, Caplan LR, Baribeau YR, Morton JR, Weintraub RM, Hartman GS, Hernandez F, Jr, Braff SP, Charlesworth DC, Malenka DJ, Ross CS, O’Connor GT Northern New England Cardiovascular Disease Study Group. Determination of etiologic mechanisms of strokes secondary to coronary artery bypass graft surgery. Stroke. 2003;34:2830–2834. doi: 10.1161/01.STR.0000098650.12386.B3. [DOI] [PubMed] [Google Scholar]
- 35.Glance LG, Dick AW, Mukamel DB, Fleming FJ, Zollo RA, Wissler R, Salloum R, Meredith UW, Osler TM. Association between intraoperative blood transfusion and mortality and morbidity in patients undergoing noncardiac surgery. Anesthesiology. 2011;114:283–292. doi: 10.1097/ALN.0b013e3182054d06. [DOI] [PubMed] [Google Scholar]
- 36.Carson JL, Terrin ML, Noveck H, Sanders DW, Chaitman BR, Rhoads GG, Nemo G, Dragert K, Beaupre L, Hildebrand K, Macaulay W, Lewis C, Cook DR, Dobbin G, Zakriya KJ, Apple FS, Horney RA, Magaziner J. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365:2453–2462. doi: 10.1056/NEJMoa1012452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chassot PG, Marcucci C, Delabays A, Spahn DR. Perioperative antiplatelet therapy. Am Fam Physician. 2010;82:1484–1489. [PubMed] [Google Scholar]