Abstract
Background
This study was carried out to investigate the effect of the steaming process on chemical constituents, free radical scavenging activity, and antiproliferative effect of Vietnamese ginseng.
Methods
Samples of powdered Vietnamese ginseng were steamed at 120°C for various times and their extracts were subjected to chemical and biological studies.
Results
Upon steaming, contents of polar ginsenosides, such as Rb1, Rc, Rd, Re, and Rg1, were rapidly decreased, whereas less polar ginsenosides such as Rg3, Rg5, Rk1, Rk3, and Rh4 were increased as reported previously. However, ocotillol type saponins, which have no glycosyl moiety at the C-20 position, were relatively stable on steaming. The radical scavenging activity was increased continuously up to 20 h of steaming. Similarly, the antiproliferative activity against A549 lung cancer cells was also increased.
Conclusion
It seems that the antiproliferative activity is closely related to the contents of ginsenoside Rg3, Rg5, and Rk1.
Keywords: antiproliferation, ginsenoside, Panax vietnamensis, steaming, Vietnamese ginseng
1. Introduction
Panax spp. occur in the northern hemisphere and mostly in temperate regions. In 1973, a wild Panax species was found at Mount Ngoc Linh in Central Vietnam. The plant was then identified as Panax vietnamensis Ha et Grushv., a new Panax species and now commonly known as Vietnamese ginseng (VG), which is the most southern Panax plant discovered so far. It has been used by the Sedang ethnic group as a miraculous herbal medicine for enhancement of physical strength and treatment of many diseases with similar therapeutic indications as those of Panax ginseng [1]. VG contains not only protopanaxadiol (PPD) and protopanaxatriol (PPT) saponins such as ginsenoside Rb1, Rd, Re, Rg1, but also ocotillol saponins, such as majonoside R1, R2 (in high yield), and vina-ginsenoside R1 and R2 (Fig. 1) [1–5]. Majonoside R2 constitutes >5% of the dried weight of VG [2]. In addition, ocotillol saponins, especially majonoside R2 exert remarkable pharmacological effects on the central nervous system such as antistress, antidepressive, and anxiolytic activities, which distinguishes VG from other Panax species [6–11].
Fig. 1.
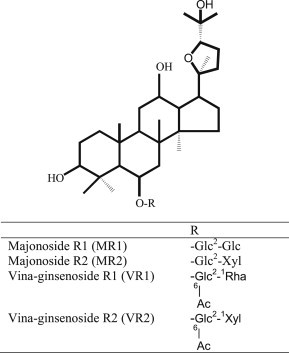
Structures of ocotillol saponins in Vietnamese ginseng.
P. ginseng, or Korean ginseng (KG), has been regarded as an important and valuable oriental herbal medicine for thousands of years. Recently, a new type of processed ginseng, named as Sun Ginseng (SG), was reported as a steamed ginseng at higher temperature than that used for the preparation of red ginseng [12]. SG contains a high yield of less polar ginsenosides, especially Rg3, Rg5, and Rk1, which showed a stronger anticancer activity. Increased pharmacological activities including antioxidant, vasodilating, and antitumor promoting activities have been reported for SG [12,13]. These active ginsenosides could be generated from ginsenoside Rb1, Rb2, Rc, and Rd via hydrolysis, dehydration, and deglycosylation during the steaming process [14].
This study aimed to investigate the influence of different durations of steaming on the saponin composition as well as the antiproliferative and antioxidant activities of processed VG.
2. Materials and methods
2.1. Materials and reagents
Vietnamese ginseng (VG) was collected in Quangnam Province, Vietnam in 2010. A voucher specimen was deposited at the herbarium of College of Pharmacy, Seoul National University, Seoul, Korea (SNUP-2012-A-01).
Perkin Elmer series 200 HPLC (Waltham, MA, USA) (high performance liquid chromatography) system equipped with evaporative light scattering detector (Alltech ELSD 2000, Alltech, Deerfield, IL, USA) and Phenomenex C18 column (250 mm × 4.6 mm. i.d., 5 μm, Torrance, CA, USA) were used for HPLC analysis. MicroTOF-Q II LC/MS (Bruker Daltonics, Bremen, Germany) was used for the LC/MS analysis.
A549 lung cancer cells line was purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). DMEM/F12 media, fetal bovine serum, penicillin/streptomycin antibiotics, and phosphate buffer saline (PBS) were purchased from Gibco (Grand Island, NY, USA). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchase from Amresco (Solon, OH, USA), and 2,2-diphenyl-1-picrylhydrazyl radicals (DPPH), DMSO were purchased from Sigma Aldrich (St. Louis, MO, USA). SpectraMax 340PC384 microplate reader (Molecular Devices, Sunnyvale, CA, USA) was used to measure the absorbance of the samples. HPLC solvents and other reagents were purchased from Duksan (Ansan, Korea). Ginsenoside standards were isolated and identified from KG and VG in our laboratory [2,12].
2.2. Sample preparation
Dried VG, including radix, rhizome, and hairy root, was ground and sieved to get the powder of 355–425 μm. A 150 mg portion of each powdered VG sample was put into stainless steel vessel with 1.5 mL of distilled water. The vessel was closed tightly and heated in an oven for 2 h, 4 h, 6 h, 8 h, 10 h, 12 h, 14 h, 16 h, 18 h, or 20 h at 120°C. After heating, the samples were lyophilized to yield a dried powder, which were extracted three times by ultrasonication at 65°C for 3 h, 1.5 h, and 1 h, using 10 mL, 10 mL, and 5 mL of methanol (MeOH), respectively. The combined extract was centrifuged and then made up to 25 mL with MeOH.
2.2.1. For HPLC analysis
A 2 mL of the MeOH extract of each sample was dried under nitrogen stream. The residue was dissolved in 1 mL of MeOH and then filtered through a 0.45 μm membrane filter prior to HPLC analysis.
2.2.2. For cell proliferation analysis
The MeOH extract of each sample was dried under nitrogen stream, then dissolved in DMEM/F12 media containing 0.1% DMSO to get various concentrations for the cell proliferation analysis.
2.2.3. For DPPH radical scavenging activity
The MeOH extract of each sample was used at the final concentration equivalent to 6 mg of dried VG powder in 1 mL of MeOH.
2.3. HPLC analysis
The reported method [15] was applied for the HPLC analysis of ginsenosides with a slight modification. Separation was achieved by using Phenomenex C18 column (250 mm × 4.6 mm. i.d., 5 μm) and the following gradient program with 5% acetonitrile (A) and 95% acetonitrile (B): 0–20 min (85–80% A); 20–45 min (80–52.5% A); 45–55 min (52.5–0% A); 55–65 min (0% A). Flow rate was set at 1 mL/min and injection volume was 20 μL. ELSD was set to a probe temperature of 80°C, and nebulizer gas (N2) flow was adjusted to 1.5 L/min.
2.4. Antiproliferative activity
A549 lung cancer cells were cultured in DMEM/F12 medium supplemented with 10% fetal bovine serum and 1% antibiotics in a humidified atmosphere of 5% CO2 at 37°C.
Antiproliferative activity was measured by a previously reported method [16]. A549 lung cancer cells at 104 cells/well were seeded in 96-well plates and incubated for 24 h. Then 200 μL of VG extract in DMEM/F12 media containing 0.1% DMSO were added to each well to make a final concentration of VG corresponding to 0.5 mg, 1 mg, and 3 mg of dried VG/mL of medium. After incubation for 24 h, the supernatant was removed and 50 μL of 4 mg/mL MTT in PBS was added to each well, and then incubated for 60 min. The supernatant was removed and 100 μL DMSO was added into each well, and then incubated for 30 min to dissolve the purple formazan crystal formed. The absorbance of each well was measured at 570 nm.
2.5. DPPH radical scavenging activity
The free radical scavenging activity was determined by measuring the reducing power of the stable radical DPPH [17]. The MeOH extract of VG was mixed with DPPH solution (0.25 mg/mL in MeOH). The amount of remaining DPPH was measured at 520 nm. Inhibition of DPPH in percent (%) was calculated by: I (%) = [1– (Si – Bi) / (C – Bi)] × 100, where Si, Bi, and C are the absorbance of sample with DPPH, of sample with MeOH, and of DPPH with MeOH, respectively.
2.6. Statistical analysis
The data are presented as the mean ± standard deviation. Data were analyzed by Student t test for comparing two groups using SPSS version 21.0. A p-value of <0.05 was considered statistically significant.
3. Results and discussion
3.1. Change in chemical composition by heat processing
It has been reported that the steaming process modifies the chemical composition of ginseng, in particular of ginsenosides. Reported chemical modification of ginsenosides includes an elimination of sugar at the C-20 position and further dehydration to form a new double bond (Fig. 2). Some acetylated ginsenosides were also reported. As a result, the contents of polar ginsenosides were decreased whereas those of less polar ginsenosides were increased [12,14,15,18–21].
Fig. 2.
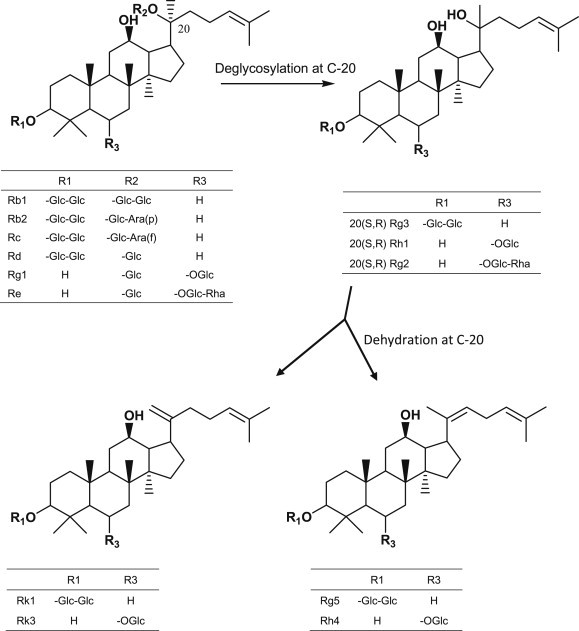
Typical modification of ginsenosides by heat processing.
This phenomenon was also observed in this study as demonstrated in the HPLC chromatogram (Fig. 3). Peak intensities of polar ginsenosides, which appeared prior to 45 min, were decreased, whereas those of less polar ginsenosides, which appeared after 45 min, were increased.
Fig. 3.
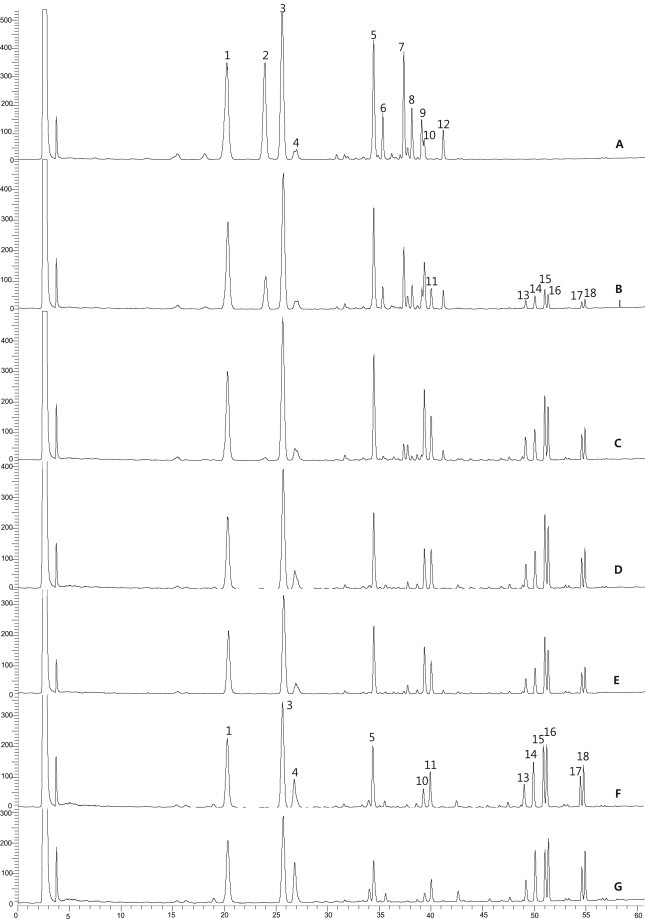
Typical HPLC-ELSD chromatograms of VG. Raw (A), 120°C for 2 h (B), 4 h (C), 8 h (D), 12 h (E), 16 h (F), 20 h (G). Peak identities: 1, MR1; 2, Rg1+Re; 3, MR2; 4, unknown 1; 5, VR1+VR2; 6, unknown 2; 7, Rb1; 8, Rc; 9, Rb2; 10, 20(S)-Rh1; 11, 20(R)-Rh1; 12, Rd; 13, Rk3; 14, Rh4; 15, 20(S)-Rg3; 16, 20(R)-Rg3; 17, Rk1; 18, Rg5.
In our HPLC condition, ginsenoside Rg1 and Re, as well as vina-ginsenoside R1 and R2 were not separated. Therefore, the total amount of ginsenoside Re and Rg1 was calculated as ginsenoside Rg1, and that of vina-ginsenoside R1 and R2 was calculated as vina-ginsenoside R2.
The contents of polar ginsenosides, such as Rb1, Rb2, Rc, Rd, Re, and Rg1, were rapidly decreased during steaming process (Fig. 4). The sum of the contents of these ginsenosides was 85.4 mg/g in dried VG, which decreased to 44.2 mg/g and 12.5 mg/g after 2 h and 4 h steaming, respectively. In particular, PPT ginsenosides, namely Rg1 and Re, were shown to be less stable than PPD ginsenosides. Only 39% and 4% of PPT ginsenosides remained after 2 h and 4 h steaming, respectively, whereas 59% and 20% of PPD ginsenosides remained after the same steaming condition.
Fig. 4.
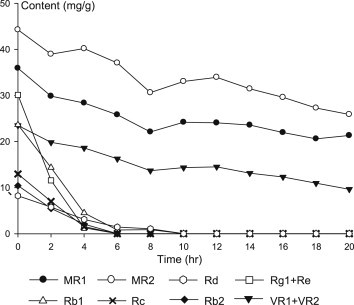
Changes in VG polar saponins upon steaming at 120°C.
However, ocotillol saponins including majonoside R1 and R2, and vina-ginsenoside R1 and R2 were stable until 20 h. This can be explained by the fact that ocotillol saponins have no heat-labile C-20 glycoside.
The content of most of less polar ginsenosides was rapidly increased up to 4 h, then slowly increased and reached maximum at 10–12 h. However, 20(S)-Rh1 reached its maximum at 4 h and decreased gradually, possibly by further dehydration at C-20 position to yield Rh4 or Rk3. The content of Rh4 was gradually increased even after 12 h (Fig. 5).
Fig. 5.
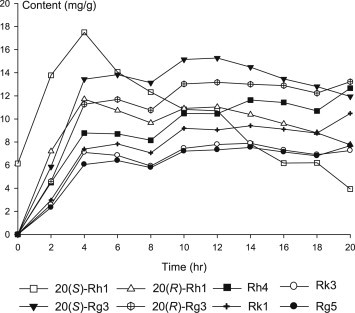
Formation of less polar ginsenosides upon steaming process.
Quantitative results are summarized in Table 1. Two unknown peaks were identified in HPLC chromatogram (Fig. 3). The contents of these unknown peaks were calculated by comparing their ELSD responses to those of MR2 and Rb1, respectively, as ELSD response is almost proportional to the amount of analyte.
Table 1.
Contents1) of Saponins in Processed Vietnamese Ginseng
| Peak No2) | Saponin | Molecular Formular | Steaming time |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Raw(0 h) | 2 h | 4 h | 6 h | 8 h | 10 h | 12 h | 14 h | 16 h | 18 h | 20 h | |||
| 1 | MR1 | C42H72O15 | 35.9 ± 7.0 | 29.9 ± 1.0 | 28.4 ± 1.8 | 25.8 ± 4.4 | 22.1 ± 0.7 | 24.2 ± 0.8 | 24.1 ± 1.0 | 23.6 ± 0.9 | 22.0 ± 1.7 | 20.6 ± 0.6 | 21.3 ± 0.6 |
| 2 | Rg1+Re3) | C42H72O14 | 30.1 ± 5.4 | 11.6 ± 0.4 | 1.3 ± 0.1 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| 3 | MR2 | C41H70O14 | 44.3 ± 6.9 | 39.0 ± 1.1 | 40.2 ± 2.4 | 37.1 ± 5.6 | 30.6 ± 0.4 | 33.1 ± 1.8 | 34.0 ± 1.9 | 31.4 ± 2.7 | 29.6 ± 2.4 | 27.3 ± 2.7 | 25.9 ± 0.8 |
| 4 | Unknown 14) | 5.4 ± 1.3 | 4.9 ± 0.2 | 6.2 ± 0.8 | 6.3 ± 0.8 | 5.6 ± 0.9 | 7.3 ± 0.1 | 8.0 ± 0.6 | 8.8 ± 0.7 | 9.8 ± 1.1 | 9.9 ± 1.7 | 12.4 ± 1.1 | |
| 5 | VR1+VR25) | C43H72O15 | 23.5 ± 3.2 | 19.8 ± 0.5 | 18.6 ± 1.6 | 16.3 ± 2.7 | 13.7 ± 0.2 | 14.3 ± 0.7 | 14.5 ± 0.9 | 13.1 ± 0.5 | 12.3 ± 1.0 | 11.0 ± 1.1 | 9.6 ± 0.4 |
| 6 | Unknown 24) | 11.7 ± 1.9 | 7.2 ± 0.5 | 1.0 ± 0.1 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | |
| 7 | Rb1 | C54H92O23 | 23.6 ± 3.5 | 14.4 ± 0.5 | 4.5 ± 0.7 | 0.8 ± 0.2 | 0.9 ± 0.4 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| 8 | Rc | C53H90O22 | 13.0 ± 2.3 | 7.0 ± 0.3 | 1.5 ± 0.2 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| 9 | Rb2 | C53H90O22 | 10.4 ± 1.9 | 5.5 ± 0.5 | 2.1 ± 0.2 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| 10 | 20(S)-Rh1 | C36H62O9 | 6.1 ± 1.4 | 13.8 ± 0.8 | 17.5 ± 0.7 | 14.1 ± 2.4 | 12.3 ± 1.2 | 10.8 ± 0.8 | 10.7 ± 0.6 | 7.8 ± 0.8 | 6.2 ± 0.5 | 6.2 ± 1.8 | 3.9 ± 0.4 |
| 11 | 20(R)-Rh1 | C36H62O9 | N.D.6) | 7.2 ± 0.4 | 11.7 ± 0.8 | 10.7 ± 1.8 | 9.7 ± 0.5 | 10.9 ± 0.7 | 11.0 ± 0.5 | 10.4 ± 0.4 | 9.6 ± 0.9 | 8.8 ± 0.6 | 7.8 ± 0.6 |
| 12 | Rd | C48H82O18 | 8.2 ± 1.2 | 5.8 ± 0.3 | 3.1 ± 0.3 | 1.4 ± 0.2 | 1.1 ± 0.5 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| 13 | Rk3 | C36H60O8 | N.D. | 2.6 ± 1.8 | 7.1 ± 0.7 | 6.8 ± 1.6 | 5.9 ± 0.7 | 7.5 ± 0.6 | 7.8 ± 0.6 | 7.9 ± 0.3 | 7.3 ± 0.5 | 6.9 ± 0.4 | 7.3 ± 0.3 |
| 14 | Rh4 | C36H60O8 | N.D. | 4.5 ± 0.3 | 8.8 ± 0.7 | 8.7 ± 1.8 | 8.2 ± 0.8 | 10.5 ± 0.6 | 10.4 ± 0.5 | 11.6 ± 0.5 | 11.4 ± 0.8 | 10.7 ± 1.4 | 12.7 ± 0.8 |
| 15 | 20(S)-Rg3 | C42H72O13 | N.D. | 5.9 ± 0.4 | 13.4 ± 0.7 | 13.8 ± 2.7 | 13.1 ± 0.5 | 15.1 ± 0.7 | 15.3 ± 1.0 | 14.5 ± 0.7 | 13.5 ± 1.0 | 12.8 ± 0.4 | 11.9 ± 0.3 |
| 16 | 20(R)-Rg3 | C42H72O13 | N.D. | 4.6 ± 0.3 | 11.3 ± 0.8 | 11.7 ± 2.4 | 10.8 ± 0.5 | 13.0 ± 0.9 | 13.2 ± 0.7 | 13.0 ± 0.9 | 12.9 ± 1.0 | 12.2 ± 0.8 | 13.2 ± 0.6 |
| 17 | Rk1 | C42H70O12 | N.D. | 2.3 ± 0.2 | 6.1 ± 0.4 | 6.4 ± 1.4 | 5.8 ± 0.6 | 7.2 ± 0.4 | 7.3 ± 0.4 | 7.5 ± 0.4 | 7.1 ± 0.6 | 6.8 ± 0.5 | 7.7 ± 0.6 |
| 18 | Rg5 | C42H70O12 | N.D. | 3.0 ± 0.2 | 7.4 ± 0.5 | 7.8 ± 1.9 | 7.1 ± 0.6 | 9.2 ± 0.5 | 9.0 ± 0.5 | 9.4 ± 0.7 | 9.1 ± 0.6 | 8.8 ± 0.8 | 10.5 ± 0.7 |
| Sum | 212.4 | 188.8 | 190.1 | 167.8 | 146.7 | 163.2 | 165.3 | 159.1 | 150.8 | 142.0 | 144.2 | ||
Results are expressed as mean ± SD (n = 3) as mg/(g dried VG).
Peak No. in Fig. 3.
Calculated as Rg1.
Concentration of unknown 1 and 2 were calculated by comparing ELSD responses to MR2 and Rb1, respectively.
Calculated as VR2.
N.D: not detected.
The total content of saponin in VG prior to steaming was 212.4 mg/g, which decreased to 144.2 mg/g after 20 h steaming (Table 1).
3.2. Antiproliferative and radical scavenging activities
Fig. 6 summarizes the change in antiproliferative activity of processed VG on A549 lung cancer cell line. The antiproliferative effect was rapidly increased upon steaming and reached its maximum at 12 h.
Fig. 6.
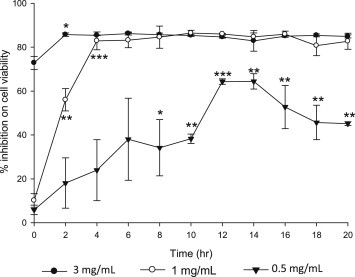
Anti-proliferative activity of VG on A549 lung cancer cells at different time of steaming. Results are expressed as mean ± SD (n = 3), *p < 0.05, **p < 0.01 and ***p < 0.001 compared with (a) raw sample (Student's t-test). Concentrations are expressed as a dry weight of VG in mL of final medium.
It is noteworthy that the antiproliferative activity seems to have a close relationship with the sum of the content of PPD-type less polar ginsenosides Rg3, Rg5, and Rk1 (Fig. 7), which is in accordance with the report that these less polar ginsenosides have stronger antiproliferative activity than their polar analogs [13,19,22,23]. Even though antiproliferative activity and the content of PPD-type less polar ginsenoside seem to have a close relationship, there might be other unknown factors that affect the activity as the curves of 0.5 mg/mL in Figs. 6, 7 are not all the same. PPT-type less polar ginsenosides Rh1, Rk3, and Rk4 were also increased by steaming; however, they have little antiproliferative effect [23]. Concentration of 3 mg/mL was too high for the test of antiproliferative activity as raw sample itself inhibited cell proliferation by 70% as shown in Fig. 6.
Fig. 7.
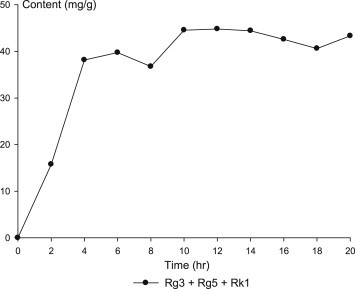
Changes in the sum content of ginsenoside Rg3, Rg5, and Rk1 during the steaming process.
DPPH radical scavenging activity, by contrast, continuously increased until 20 h (Fig. 8). This can be attributed by the fact that two activities are arisen from different chemical constituents. Antiproliferative activity arises from ginsenosides whereas radical scavenging activity is arisen mainly from phenolic compounds and Maillard reaction products [24].
Fig. 8.
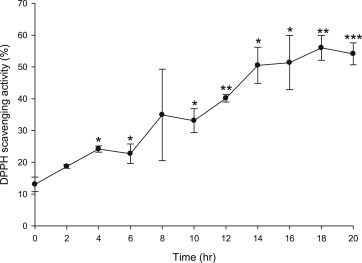
DPPH radical scavenging activity of VG at different time of steaming. Concentration: 6 mg of dried VG/mL. Result are expressed as mean ± SD (n = 3). * p < 0.05, **p < 0.01 and *** p < 0.001 compared with (a) raw sample (Student's t-test).
4. Conclusion
Steaming of Vietnamese ginseng at 120°C changed its saponin constituents and biological activities remarkably. Polar PPD and PPT ginsenosides transformed to their less polar analogs rapidly, whereas ocotillol saponins were stable upon steaming process. Antioxidant and antiproliferative activities are greatly increased by steaming. It seems that the antiproliferative activity of processed VG is closely related to the content of ginsenoside Rg3, Rg5, and Rk1.
Conflicts of interest
All contributing authors declare no conflicts of interest.
Acknowledgments
This work was supported by the grant from the Ministry of Education, Science, and Technology of Korea (No. 2012048796), Rural Development Administration of Korea (No. PJ008202022013), and Ministry of Science and Technology of The Socialist Republic of Vietnam (No. KC 10.25/11-15).
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Contributor Information
Jeong Hill Park, Email: hillpark@snu.ac.kr.
Minh Duc Nguyen, Email: ducng@hcm.vnn.vn.
References
- 1.Nguyen T.N. Study on Panax vietnamensis Ha et Grushv., Araliaceace. Botany, tissue culture-chemistry-biological properties. Herbal Polonica. 1989;35:1–229. [Google Scholar]
- 2.Duc N.M., Kasai R., Ohtani K., Ito A., Nguyen T.N., Yamasaki K., Tanaka O. Saponins from Vietnamese ginseng, Panax vietnamensis Ha et Grushv. Collected in central Vietnam. I. Chem Pharm Bull. 1993;41:2010–2014. doi: 10.1248/cpb.41.2010. [DOI] [PubMed] [Google Scholar]
- 3.Duc N.M., Kasai R., Ohtani K., Ito A., Nguyen T.N., Yamasaki K., Tanaka O. Saponins from Vietnamese Ginseng, Panax vietnamensis Ha et Grushv. Collected in central Vietnam. II. Chem Pharm Bull. 1994;42:115–122. doi: 10.1248/cpb.42.115. [DOI] [PubMed] [Google Scholar]
- 4.Duc N.M., Kasai R., Ohtani K., Ito A., Nguyen T.N., Yamasaki K., Tanaka O. Saponins from Vietnamese ginseng, Panax vietnamensis Ha et Grushv. collected in central Vietnam. III. Chem Pharm Bull. 1994;42:634–640. doi: 10.1248/cpb.42.634. [DOI] [PubMed] [Google Scholar]
- 5.Tran L.Q., Adnyana I., Tezuka Y., Nagaoka T., Tran Q., Kadota S. Triterpene saponins from Vietnamese ginseng (Panax vietnamensis) and their hepatocytoprotective activity. J Nat Prod. 2001;64:456–461. doi: 10.1021/np000393f. [DOI] [PubMed] [Google Scholar]
- 6.Huong N.T., Matsumoto K., Kasai R., Yamasaki K., Duc N.M., Nguyen T.N., Watanabe H. Crude saponin extracted from Vietnamese ginseng and its major constituent majonoside-R2 attenuate the psychological stress- and foot-shock stress-induced antinociception in mice. Pharmacol Biochem Behav. 1995;52:427–432. doi: 10.1016/0091-3057(95)00133-h. [DOI] [PubMed] [Google Scholar]
- 7.Huong N.T., Matsumoto K., Yamasaki K., Duc N.M., Nham N.T., Watanabe H. Effects of majonoside-R2 on pentobarbital sleep and gastric lesion in psychologically stressed mice. Pharmacol Biochem Behav. 1996;53:957–963. doi: 10.1016/0091-3057(95)02147-7. [DOI] [PubMed] [Google Scholar]
- 8.Huong N.T., Matsumoto K., Yamasaki K., Duc N.M., Duc N.M., Watanabe H. Majonoside-R2, a major constituent of Vietnamese ginseng, attenuates opioid-induced Antinociception. Pharmacol Biochem Behav. 1997;57:285–291. doi: 10.1016/s0091-3057(96)00348-6. [DOI] [PubMed] [Google Scholar]
- 9.Huong N.T., Matsumoto K., Yamasaki K., Watanabe H. Majonoside-R2 reverses social isolation stress-induced decrease in pentobarbital sleep in mice: possible involvement of neuroactive steroids. Life Sciences. 1997;61:395–402. doi: 10.1016/s0024-3205(97)00396-2. [DOI] [PubMed] [Google Scholar]
- 10.Huong N.T., Matsumoto K., Watanabe H. The antistress effect of majonoside-R2, a major saponin component of Vietnamese ginseng: neuronal mechanisms of action. Methods Find Exp Clin Pharmacol. 1998;20:65–76. doi: 10.1358/mf.1998.20.1.485634. [DOI] [PubMed] [Google Scholar]
- 11.Huong N.T., Murakami Y., Tohda M., Watanabe H., Matsumoto K. Social isolation stress-induced oxidative damage in mouse brain and its modulation by majonoside-R2, a Vietnamese ginseng saponin. Biol Pharm Bull. 2005;28:1389–1393. doi: 10.1248/bpb.28.1389. [DOI] [PubMed] [Google Scholar]
- 12.Kim W.Y., Kim J.M., Han S.B., Lee S.K., Kim N.D., Park M.K., Kim C.K., Park J.H. Steaming of ginseng at high temperature enhances biological activity. J Nat Prod. 2000;63:1702–1704. doi: 10.1021/np990152b. [DOI] [PubMed] [Google Scholar]
- 13.Kim Y.J., Kwon H.C., Ko H., Park J.H., Kim H.Y., Yoo J.H., Yang H.O. Anti-tumor activity of the ginsenoside Rk1 in human hepatocellular carcinoma cells through inhibition of telomerase activity and induction of apoptosis. Biol Pharm Bull. 2008;31:826–830. doi: 10.1248/bpb.31.826. [DOI] [PubMed] [Google Scholar]
- 14.Sun B.S., Xu M.Y., Li Z., Wang Y.B., Sung C.K. UPLC-Q-TOF-MS/MS analysis for steaming times-dependent profiling of steamed Panax quinquefolius and its ginsenosides transformations induced by repetitious steaming. J Ginseng Res. 2012;36:277–290. doi: 10.5142/jgr.2012.36.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwon S.W., Han S.B., Park I.H., Kim J.M., Park M.K., Park J.H. Liquid chromatographic determination of less polar ginsenosides in processed ginseng. J Chromatogr A. 2001;921:335–339. doi: 10.1016/s0021-9673(01)00869-x. [DOI] [PubMed] [Google Scholar]
- 16.Lee H., Kim J., Lee S.Y., Park J.H., Hwang G.S. Processed Panax ginseng, Sun Ginseng, decreases oxidative damage induced by tert-butyl hydroperoxide via regulation of antioxidant enzyme and anti-apoptotic molecules in HepG2 cells. J Ginseng Res. 2012;36:248–255. doi: 10.5142/jgr.2012.36.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bae H.M., Kim S.S., Cho C.W., Yang D.C., Ko S.K., Kim K.T. Antioxidant activities of ginseng seeds treated by autoclaving. J Ginseng Res. 2012;36:411–417. doi: 10.5142/jgr.2012.36.4.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang C.Z., Aung H.H., Ni M., Wu J.A., Tong R., Wicks S., He T.C., Yuan C.S. Red American ginseng: ginsenoside constituents and antiproliferative activities of heat-processed Panax quinquefolius roots. Planta Med. 2007;73:669–674. doi: 10.1055/s-2007-981524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toh D., Patel D., Chan E., Teo A., Neo S., Koh H. Anti-proliferative effects of raw and steamed extracts of Panax notoginseng and its ginsenoside constituents on human liver cancer cells. Chin Med. 2011;6:4. doi: 10.1186/1749-8546-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang D., Liao P., Zhu H., Chen K., Xu M., Zhang Y., Yang C. The processing of Panax notoginseng and the transformation of its saponin components. Food Chem. 2012;132:1808–1813. [Google Scholar]
- 21.Sun S., Wang C., Tong R., Li X., Fishbein A., Wang Q., He T., Du W., Yuan C. Effects of steaming the root of Panax notoginseng on chemical composition and anticancer activities. Food Chem. 2010;118:307–314. [Google Scholar]
- 22.Park I.H., Kwon S.W., Lee Y.J., Cho S.Y., Park M.K., Park J.H. Cytotoxic dammarane glycosides from processed ginseng. Chem Pharm Bull. 2002;50:538–540. doi: 10.1248/cpb.50.538. [DOI] [PubMed] [Google Scholar]
- 23.Nag S.A., Qin J.J., Wang W., Wang M.H., Wang H., Zhang R. Ginsenosides as anticancer agents: in vitro and in vivo activities, structure–activity relationships, and molecular mechanisms of action. Front Pharmacol. 2012;3:25. doi: 10.3389/fphar.2012.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamabe N., Song K.I., Lee W., Han I.-H., Lee J.H., Ham J., Kim S.-N., Park J.H., Kang K.S. Chemical and free radical-scavenging activity changes of ginsenoside Re by Maillard reaction and its possible use as a renoprotective agent. J Ginseng Res. 2012;36:256–262. doi: 10.5142/jgr.2012.36.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]


