Abstract
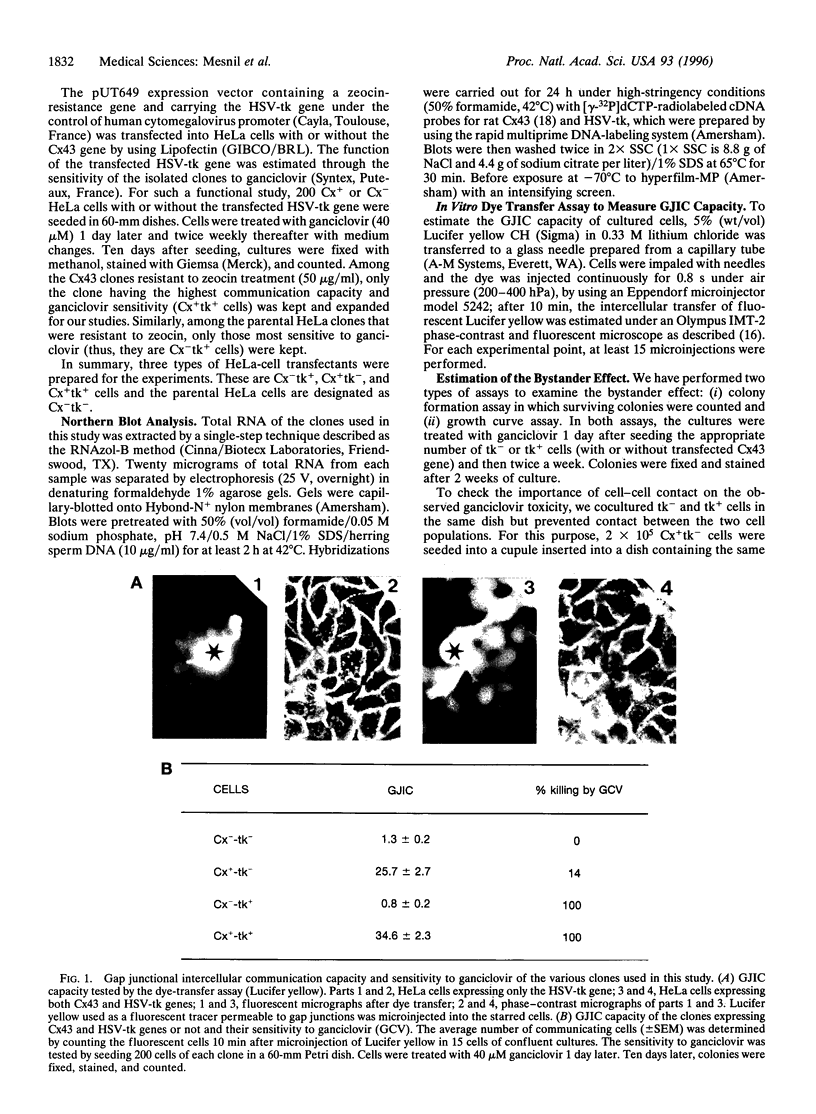
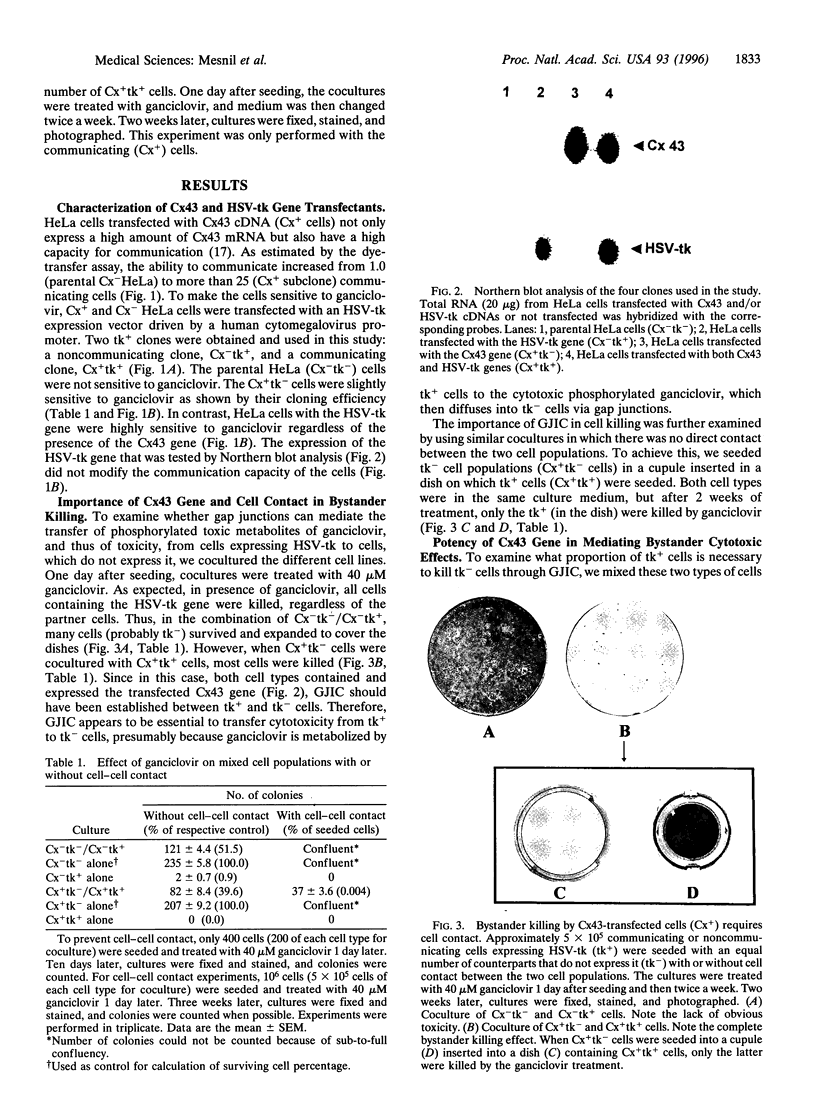
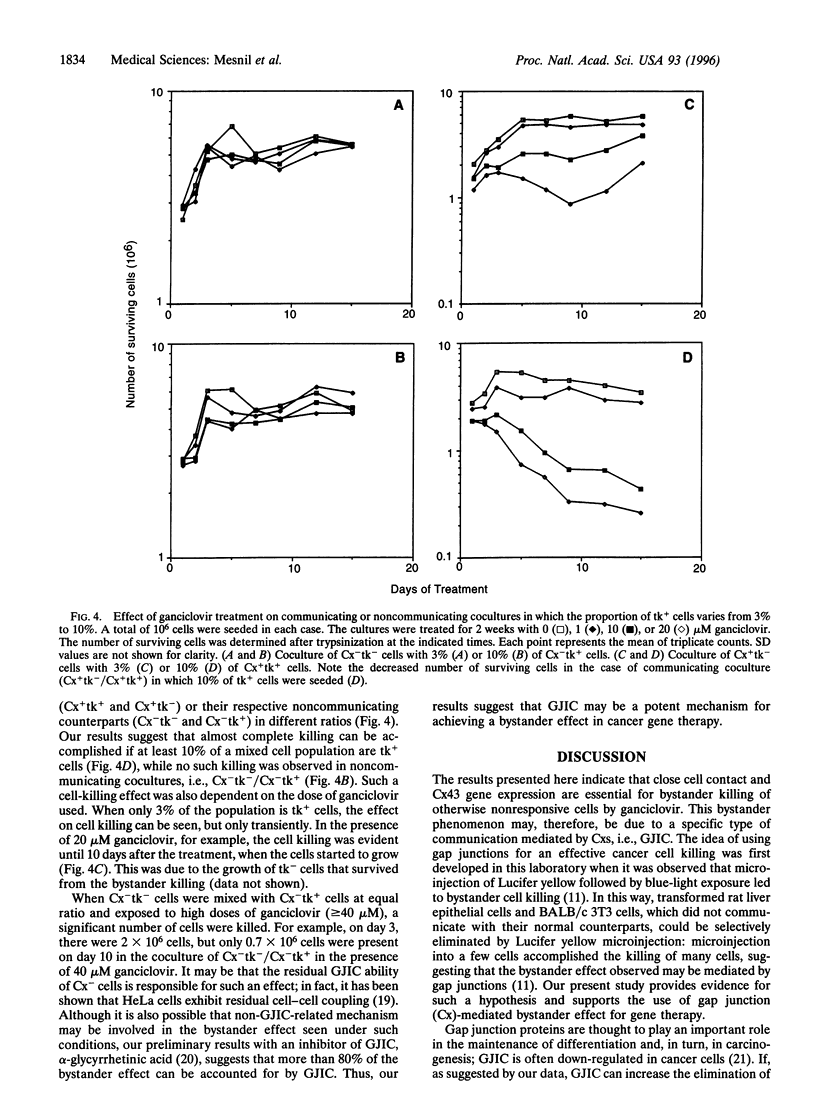
In gene therapy to treat cancer, typically only a fraction of the tumor cells can be successfully transfected with a gene. However, in the case of brain tumor therapy with the thymidine kinase gene from herpes simplex virus (HSV-tk), not only the cells transfected with the gene but also neighboring others can be killed in the presence of ganciclovir. Such a "bystander" effect is reminiscent of our previous observation that the effect of certain therapeutic agents may be enhanced by their diffusion through gap junctional intercellular communication (GJIC). Herein, we present the evidence, from in vitro studies, that gap junctions could indeed be responsible for such a gene therapy bystander effect. We used HeLa cells for this purpose, since they show very little, if any, ability to communicate through gap junctions. When HeLa cells were transfected with HSV-tk gene and cocultured with nontransfected cells, only HSV-tk-transfected HeLa cells (tk+) were killed by ganciclovir. However, when HeLa cells transfected with a gene encoding for the gap junction protein, connexin 43 (Cx43), were used, not only tk+ cells, but also tk- cells were killed, presumably due to the transfer, via Cx43-mediated GJIC, of toxic ganciclovir molecules phosphorylated by HSV-tk to the tk- cells. Such bystander effect was not observed when tk+ and tk- cells were cocultured without direct cell-cell contact between those two types of cells. Thus, our results give strong evidence that the bystander effect seen in HSV-tk gene therapy may be due to Cx-mediated GJIC.
Full text
PDF

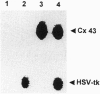
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beyer E. C. Gap junctions. Int Rev Cytol. 1993;137C:1–37. [PubMed] [Google Scholar]
- Beyer E. C., Paul D. L., Goodenough D. A. Connexin43: a protein from rat heart homologous to a gap junction protein from liver. J Cell Biol. 1987 Dec;105(6 Pt 1):2621–2629. doi: 10.1083/jcb.105.6.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi W. L., Parysek L. M., Warnick R., Stambrook P. J. In vitro evidence that metabolic cooperation is responsible for the bystander effect observed with HSV tk retroviral gene therapy. Hum Gene Ther. 1993 Dec;4(6):725–731. doi: 10.1089/hum.1993.4.6-725. [DOI] [PubMed] [Google Scholar]
- Charles A. C., Naus C. C., Zhu D., Kidder G. M., Dirksen E. R., Sanderson M. J. Intercellular calcium signaling via gap junctions in glioma cells. J Cell Biol. 1992 Jul;118(1):195–201. doi: 10.1083/jcb.118.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culver K. W., Ram Z., Wallbridge S., Ishii H., Oldfield E. H., Blaese R. M. In vivo gene transfer with retroviral vector-producer cells for treatment of experimental brain tumors. Science. 1992 Jun 12;256(5063):1550–1552. doi: 10.1126/science.1317968. [DOI] [PubMed] [Google Scholar]
- Davidson J. S., Baumgarten I. M., Harley E. H. Reversible inhibition of intercellular junctional communication by glycyrrhetinic acid. Biochem Biophys Res Commun. 1986 Jan 14;134(1):29–36. doi: 10.1016/0006-291x(86)90522-x. [DOI] [PubMed] [Google Scholar]
- Eckert R., Dunina-Barkovskaya A., Hülser D. F. Biophysical characterization of gap-junction channels in HeLa cells. Pflugers Arch. 1993 Aug;424(3-4):335–342. doi: 10.1007/BF00384361. [DOI] [PubMed] [Google Scholar]
- Elfgang C., Eckert R., Lichtenberg-Fraté H., Butterweck A., Traub O., Klein R. A., Hülser D. F., Willecke K. Specific permeability and selective formation of gap junction channels in connexin-transfected HeLa cells. J Cell Biol. 1995 May;129(3):805–817. doi: 10.1083/jcb.129.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman S. M., Abboud C. N., Whartenby K. A., Packman C. H., Koeplin D. S., Moolten F. L., Abraham G. N. The "bystander effect": tumor regression when a fraction of the tumor mass is genetically modified. Cancer Res. 1993 Nov 1;53(21):5274–5283. [PubMed] [Google Scholar]
- Goldberg G., Bertram J. S. Re: Z. Ram et al., In situ retroviral-mediated gene transfer for the treatment of brain tumors in rats. Cancer Res., 53: 83-88, 1993. Cancer Res. 1994 Jul 15;54(14):3947–3948. [PubMed] [Google Scholar]
- Krutovskikh V., Mazzoleni G., Mironov N., Omori Y., Aguelon A. M., Mesnil M., Berger F., Partensky C., Yamasaki H. Altered homologous and heterologous gap-junctional intercellular communication in primary human liver tumors associated with aberrant protein localization but not gene mutation of connexin 32. Int J Cancer. 1994 Jan 2;56(1):87–94. doi: 10.1002/ijc.2910560116. [DOI] [PubMed] [Google Scholar]
- Mesnil M., Krutovskikh V., Piccoli C., Elfgang C., Traub O., Willecke K., Yamasaki H. Negative growth control of HeLa cells by connexin genes: connexin species specificity. Cancer Res. 1995 Feb 1;55(3):629–639. [PubMed] [Google Scholar]
- Moolten F. L., Wells J. M. Curability of tumors bearing herpes thymidine kinase genes transferred by retroviral vectors. J Natl Cancer Inst. 1990 Feb 21;82(4):297–300. doi: 10.1093/jnci/82.4.297. [DOI] [PubMed] [Google Scholar]
- Ram Z., Culver K. W., Walbridge S., Blaese R. M., Oldfield E. H. In situ retroviral-mediated gene transfer for the treatment of brain tumors in rats. Cancer Res. 1993 Jan 1;53(1):83–88. [PubMed] [Google Scholar]
- Samejima Y., Meruelo D. 'Bystander killing' induces apoptosis and is inhibited by forskolin. Gene Ther. 1995 Jan;2(1):50–58. [PubMed] [Google Scholar]
- Simpson I., Rose B., Loewenstein W. R. Size limit of molecules permeating the junctional membrane channels. Science. 1977 Jan 21;195(4275):294–296. doi: 10.1126/science.831276. [DOI] [PubMed] [Google Scholar]
- Subak-Sharpe H., Bürk R. R., Pitts J. D. Metabolic co-operation between biochemically marked mammalian cells in tissue culture. J Cell Sci. 1969 Mar;4(2):353–367. doi: 10.1242/jcs.4.2.353. [DOI] [PubMed] [Google Scholar]
- Sáez J. C., Connor J. A., Spray D. C., Bennett M. V. Hepatocyte gap junctions are permeable to the second messenger, inositol 1,4,5-trisphosphate, and to calcium ions. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2708–2712. doi: 10.1073/pnas.86.8.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trosko J. E., Madhukar B. V., Chang C. C. Endogenous and exogenous modulation of gap junctional intercellular communication: toxicological and pharmacological implications. Life Sci. 1993;53(1):1–19. doi: 10.1016/0024-3205(93)90606-4. [DOI] [PubMed] [Google Scholar]
- Vile R. G., Nelson J. A., Castleden S., Chong H., Hart I. R. Systemic gene therapy of murine melanoma using tissue specific expression of the HSVtk gene involves an immune component. Cancer Res. 1994 Dec 1;54(23):6228–6234. [PubMed] [Google Scholar]
- Yamasaki H. Gap junctional intercellular communication and carcinogenesis. Carcinogenesis. 1990 Jul;11(7):1051–1058. doi: 10.1093/carcin/11.7.1051. [DOI] [PubMed] [Google Scholar]
- Yamasaki H., Katoh F. Novel method for selective killing of transformed rodent cells through intercellular communication, with possible therapeutic applications. Cancer Res. 1988 Jun 1;48(11):3203–3207. [PubMed] [Google Scholar]