Introduction
For nearly a century, digital rectal exam (DRE) was the only tool available to aid in tissue sampling for diagnosis of prostate cancer (CaP)1. With the advent of ultrasound in the 1980’s, physicians had a new modality for directing biopsy needles in real-time. Originally developed by Stamey, the ultrasound-guided, trans-rectal sextant method became widely adopted2.Since that time, additional samples are taken (usually totaling 12) and local anesthesia has been added, but otherwise the random, systematic procedure of the 1980s has remained largely unchanged. ‘Saturation’ biopsy has been advocated, but may increase detection of insignificant cancers, and it typically requires general anesthesia.
Thus, CaPis the only important solid malignancy diagnosed by blind biopsy of the organ, i.e., without tumor visualization. Some 50% of cancers detected by this methodmay not be of clinical significance 3.In addition, systematic biopsies are poor at sampling lesions in the anterior, midline, and apex of the prostate. This can lead to under-diagnosis of important lesions in these regions. Further, almost one third of currently detected cancers are re-classified from original biopsy Gleason score to a higher score on final pathology 4.
Groundwork for a change in the above schema was established with the observation that some CaP lesions could be visualized with magnetic resonance imaging 5.As MRI usage became widely disseminated, and as the technology improved, the value of MRI to diagnose (and stage) CaP became increasingly apparent.The advent of MRI coincided with decreasing volume of CaP at diagnosis6. In an earlier time, when CaPusually presented as a palpable mass, ultrasound imaging could detect many lesions.Today, due to early PSA screening, most newly-diagnosedCaPis non-palpable, and ultrasound usuallyfails to visualize a lesion.Thus, use of MRI to identify suspicious prostate lesions fills an important void, helping to identify regions of interest and enable targeted biopsy 7.
Advent of MRI for Diagnosis of Prostate Cancer (CaP)
Among the first to show that CaP could be imaged by MRI was Hricakin 1983 5. Subsequent advances in magnet strength and the availability of multi-parametricstudies have now made MRI the imaging modality of choice for diagnosis ofCaP(Figure 1). The established parameters of today’s multi-parametric MRI are T2 weighted images (T2WI), dynamic contrast enhancement (DCE), and diffusion-weighted imaging (DWI).As the limitations of PSA testing to diagnose prostate cancer have become increasingly apparent, the importance of a visual representation of the tumor has become compelling.Accurate imaging ofCaP and the offshoot, targeted biopsy, containthe seeds for a major change in the current management of the disease.
Figure 1.

Prostate MRI c. 19835. Among the first published MRI images were those above, obtained with a 0.35T coil. In the transverse scan (A), the prostate (P) is enlarged and the Foley catheter (arrow) in the prostatic urethra is displaced posteriorly to the left by adenomatous tissue. Seminal vesicles are seen inferior to the bladder (s). In the sagittal scan (B), air (A) and urine (U) level can be seen in the bladder. At the time, magnet strength was not capable of demonstrating zonal anatomy or small cancers.From Hricak H, Williams RD, Spring DB, et al. Anatomy and pathology of the male pelvis by magnetic resonance imaging. AJR. American journal of roentgenology. Dec 1983;141(6):1101-1110; with permission.
Current Use of MRI for Diagnosis of Prostate Cancer (CaP)
Either pelvic phased array (PPA) orendorectal coils (ERC) may be used when performing mp-MRI of the prostate. ERC may improve definition of the prostate capsule, but does not appear critical for characterization of intra-prostatic lesions. Thus, because of patient discomfort and increased procedure time, the endorectal approach is not routinely used for diagnostic purposes. Likewise, to identify regions of interest and guide biopsy, spectroscopy adds little and is not generally used.3 T magnets provide higher signal-to-noise ratios and shorter acquisition times than 1.5 T; both have been used successfully to define cancer within the prostate 8.
Multi-parametric MRI (mpMRI)
Multi-parametric MRI incorporates several different imaging modalities: T2-weighted imaging (T2WI), diffusion weighted imaging (DWI), and dynamic contrast enhancement (DCE) to best assess potential lesions in the prostate. Figure 2 shows an example of CaP visualized in all three modalities.
Figure 2.

CaP visualized by multi-parametric MRI (mp-MRI). Arrows point to lesion.(A) T2-weighted image, (B)diffusion weighted imaging (DWI), (C)dynamic contrast enhancement (DCE), (D) whole mount specimen obtained by radical prostatectomy, showing cancer13.From Natarajan S, Marks LS, Margolis DJ, et al. Clinical application of a 3D ultrasound-guided prostate biopsy system. Urologic oncology. May-Jun 2011;29(3):334-342; with permission.
T2-weighted imaging (T2WI) produces an anatomic image based on the “transverse” relaxation time after magnetically aligning a tissue to an external magnetic field. T2WI provides the best tissue contrast for the detection, localization, and staging of CaP, which has “shorter” T2 than normal tissue. However, other processes such as inflammation and prostatic hyperplasia can also shorten T2, and additional parameters are necessary to increase the specificity of T2WI.
Diffusion weighted imaging (DWI) provides a measure of the “Brownian” motion of water molecules and is an essential component of mp-MRI. At body temperature, the mobility of water is primarily dependent on the molecular environment such as cell size and microstructure. DWI is a good indicator for CaPbecause free motion of water is generally restricted within cancerous tissue. The slope of change of the received signal, based on the degree of diffusion weighting, is called the apparent diffusion coefficient (ADC) and creates quantitative maps of molecular mobility. By measuring the hydrodynamic environment of tissue using DWI, the specificity of CaP detection is improved compared to T2WI alone 9. In creating the UCLA score for MRI suspicion, DWI is doubly weighted, as discussed below.
Dynamic Contrast Enhancment (DCE) uses T1-shortening contrast to evaluate tumor vascularity 10 and adds value to diagnosis of suspicious lesions 11. For this method, rapidly repeated imaging is performed during the dynamic administration of intravenous contrast. Increased micro-vascular density and breakdown of capillary walls within tumors can lead to increased contrast arrival (“wash-in”) and dispersion (“wash-out”).
MRI-identified ‘regions of interest’ are scored to help determine the likelihood of cancer in that area. Different scoring systems have been proposed, but all rely on the above three parameters. The UCLA scoring system is shown in Table 1. Image Score is determined by assigning an image-grade number (left column) to each parameter; ADC value is assigned double weighting. For example, if a region of interest was moderately dark on T2WI (i.e., a grade 3), had an ADC value of 0.7 mm2/s (i.e., a grade 4), and had a DCE showing moderately abnormal enhancement (i.e., a grade 3), the overall score would be 3+8+3/4 = 3.5. The score is rounded up if the region of interest is in the peripheral zone, in this case giving it a score of4, and rounded down if the region is in the transition zone, in this case giving it a score of 3. The higher the score the more likely cancer will be present in the region of interest 12. The “PI-RADS” scoring system, which is similar to the UCLA scoring system, has been recently proposed as an industry standard 8.
Table 1.
UCLA Scoring System for Assigning Level of Suspicion to Regions of Interest (ROI) Found in the Prostate on Multi-Parametric MRI (mpMRI)*
| Image grade |
T2-weighted imaging (T2WI) |
Apparent diffusion coefficient (ADC) |
Dynamic contrast enhancement (DCE) |
|---|---|---|---|
| 1 | Normal | >1.2 × 10−3 mm2/s | Normal |
| 2 | Faint decreased signal | 1.0-1.2 × 10−3 mm2/s | Mildly abnormal enhancement |
| 3 | Moderately dark nodule | 0.8-1.0 × 10−3 mm2/s | Moderately abnormal enhancement |
| 4 | Intensely dark nodule | 0.6-0.8 × 10−3 mm2/s | Highly abnormal enhancement |
| 5 | Dark nodule with mass effect |
<0.6 × 10−3 mm2/s | Profoundly abnormal enhancement |
The higher the score, the greater the level of suspicion. Regions of interest with scores of 1 and 2 are no more likely to contain cancer than normal tissue and are not usually targeted. A score of 5 indicates cancer in most cases. From Sonn GA, Natarajan S, Margolis DJ, et al. Targeted biopsy in the detection of prostate cancer using an office based magnetic resonance ultrasound fusion device. The Journal of urology. Jan 2013;189(1):86-91; with permission.
Image Fusion
Image fusion is the process of combining information from two or more images into a single image (Figure 3), with the intent that the resulting image provides more information than any input image alone. Image fusion, as an aid to prostate biopsy targeting, refers to the superimposition of prostatic images---stored MRI images and real-time ultrasoundimages---to create a 3D reconstruction upon which biopsy work is performed. The fused image result gives the operator the tumor-detecting value of MRI with the ease of use of ultrasound.Fusion devices (Table 2), allow the operator to electronically bring MRI to the US biopsy suite, to fuse MRI and US images into a 3D reconstruction, and under real-time ultrasound guidance, to aim the biopsy needle at suspicious regions of interest seen on MRI. Performance of the biopsy is operationally similar to that performed by urologists for several decades.
Figure 3.
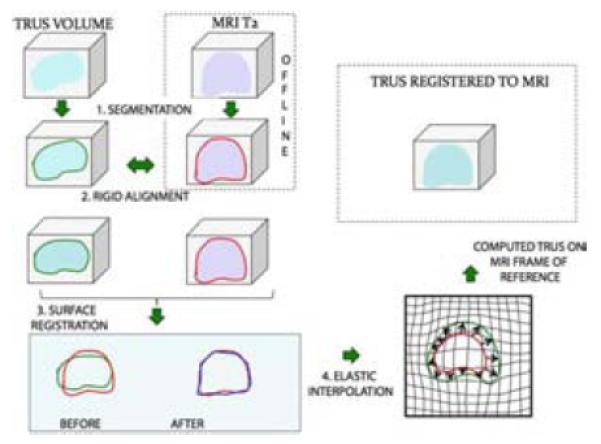
Process of MRI-US Fusion. MR and TRUS images are outlined or segmented (1) and then rigidly aligned (2). Fusion then proceeds involving a surface registration (3), and elastic (non-rigid) interpolation (4). Finally, the registered, or superimposed images are produced on a monitor, where targeted biopsy is performed. The target is derived from the MRI; the biopsy aiming is via real-time ultrasound13.From Natarajan S, Marks LS, Margolis DJ, et al. Clinical application of a 3D ultrasound-guided prostate biopsy system. Urologic oncology. May-Jun 2011;29(3):334-342; with permission.
Table 2.
MRI/ultrasound fusion devices approved by US Food and Drug Administration
| Manufacturer/ trade name |
Us image acquisition |
Biopsy route |
Tracking mechanism |
Year of FDA Approval |
Comments |
|---|---|---|---|---|---|
| Philips /UroNav | Manual US sweep from base to apex |
Transrectal | External magnetic field generator |
2005 | Prospective targeting, integrated with existing ultrasound device, freehand manipulation |
| Eigen/Artemis | Manual rotation along fixed axis |
Transrectal | Mechanical arm with encoders |
2008 | Prospective targeting, stabilized TRUS probe |
| Koelis/Urostation | Automatic US probe rotation, |
Transrectal | Real-time TRUS- TRUS registration |
2010 | Retrospective targeting, real-time elastic registration |
|
Hitachi/HI-RVS (real-
time virtual sonography) |
Real-time biplanar TRUS |
Transrectal or transperineal |
External magnetic field generator |
2010 | Prospective targeting, integrated with existing ultrasound device |
| BioJet/Jetsoft/GeoScan | Manual US sweep in sagittal |
Transrectal or transperineal |
Mechanical arm with encoders; uses stepper |
2012 | Prospective targeting, rigid registration |
From Marks L, Young S, Natarajan S. MRI-ultrasound fusion for guidance of targeted prostate biopsy. Curr Opin Urol. Jan 2013;23(1):43-50; with permission.
Methods of MRI-guided Biopsy
Three methods of MRI fusionfor targeting prostate biopsy are currently employed: direct in-bore fusion, cognitive fusion, or device fusion.
Direct MRI-guided biopsy occurs within an MRI tube (“in-bore”), wherein the operator compares a previously obtained MRI scan with one just acquired to guide the biopsy needle.An endorectal coil and the prone position are used for this method of targeted biopsy (Figure 4). A repeat scan is taken after needle insertion to confirm localization. In-bore fusion relies on an MRI scan before, during, and after a biopsy which is performed in the tube itself.In-bore biopsies are usually obtained only from the region of interest seen on MRI. Systematic biopsies are usually not performed due to the extra time in-bore needed to obtain the additional cores. Since systematic biopsies are not performed, small, insignificant cancers are found less often than when the entire gland is sampled throughout 14. However, a number of significant cancers may be outside the targets, leading to a concern of missing some cancers when only the MRI-target is sampled 12.
Figure 4.

In-Bore MRI guided biopsy is performed prone; patient undergoes a diagnostic MRI in advance of biopsy; the images are then processed and delineated; patient subsequently returns to MR facility for procedure, which involves fusing the diagnostic MRI with the 2nd MRI used to guide biopsy. Courtesy of Invivo Corporation, Gainesville, FL; with permission.
Cognitive fusion relies on an ultrasound operator’s ability to guide a biopsy needle based on an impression gleaned from a 2D MRI image.Cognitive fusion requires an experienced ultrasonographer, but otherwise is a relatively fast procedure and requires no special training or instrumentation. However, cognitive fusion does not permit quantification of targeting accuracy and is subject to interpretation of the anatomy by the operator. In a recent study from Europe, the tumor-detection rate of cognitive fusion was similar to that obtained by device fusion; and both were better than blind, systematic sampling 15. The use of cognitive fusion in biopsy site tracking, as for men undergoing active surveillance, has not been evaluated.
Device fusion employsa 3D rendering apparatus, which allows apreviously acquired MRI scan to be superimposed on real-time ultrasound images, forming a digital reconstruction on a computer monitor. This digital overlay of an MRI image onto ultrasound, allows the operator to obtain both systematic and targeted biopsies; in addition, biopsy sites are recorded for later repeat targeting, if necessary, as during active surveillance. An online video explaining the procedure and rationale behind targeted prostate biopsy using MRI-US fusion, has been made available (YouTube: “UCLA Biopsy”).Five fusion devices are currently approved by the U.S. FDA (Table 2).
MRI-US Fusion Devices
Image fusion for prostate biopsy (MRI-US) was first described in 2002 by radiation therapists, who used it to obtain tissue from two men with rising PSA levels after treatment for CaP16. However, it was the work of Aaron Fenster and colleagues at Robarts Research Institute in Canada17 and that from the NCI-Philips collaboration at National Institutes of Health18 that gave rise to the commercial devices nowavailable.Five instruments are currently approved by the U.S. FDA (Table 2). The present discussion focuses on Artemis (Eigen/Hitachi) and UroNav (Invivo/Philips), which have been extensively studiedand are manufactured in the U.S.
All image fusion devices for targeted prostate biopsy are combinations of hardware and softwareto permit the acquisition, storage, and reconstruction of real-time ultrasound images.All employ a tracking mechanism, a video processor, and a computer with monitor. Stored MR images are thus fused or superimposed on real-time ultrasound images, allowing users to target-biopsy regions of interest identified on the MRI. In addition, 3D maps of lesion locations and biopsy sites are created and stored for future use. Movement of the patient, or movement of the prostate within the patient, affects the image registration; on-the-fly repeat registration is provided by motion-compensation software.
The Invivo/UroNavsystem was developed under a collaborative agreement between Philips, the parent of Invivo, and the National Cancer Institute, beginning in 2006. UroNav is a modification of the PercuNav, introduced by Philips as a “GPS for medical instruments” a few years earlier. Tracking is performed within an electromagnetic field, created over the patient by a small generator. In 2008, Xu and colleagues described the initial evaluation of a UroNav prototype, which was found to be accurate in phantom studies and in 20 patients 19. Since that time, more than 1000 men have undergone targeted prostate biopsy at the NCI with the UroNav device or precursor devices. In one recent publication from that group, more than 80% of men with highly suspicious MRI lesions were found to have prostate cancer when fusion biopsy was performed using the UroNav18. The UroNav system is shown in Figure 5.
Figure 5. UroNav Fusion Device.
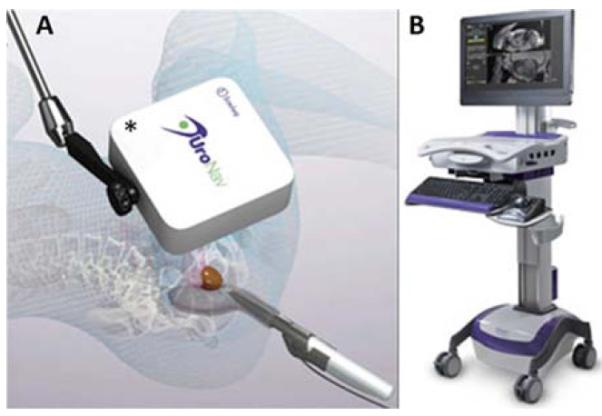
Originally developed in a collaboration between Phillips and the National Cancer Institute, the UroNav system uses an external magnetic field generator (A) for tracking a biopsy needle’s position in 3D space which is recorded at an imaging terminal (B). Courtesy of Invivo Corporation, Gainesville, FL; with permission.
The Artemis device (Eigen, Grass Valley, California, USA) was approved by the U.S. FDA in April 2008 and has been studied since early 2009 at the University of California, Los Angeles (UCLA) 13. The current model of the device is shown in Figure 6. A developmental agreement between Eigen and the Hitachi Corporation was established in 2013.The Artemis device uses a mechanical arm for both TRUS scanning and biopsy needle placement. The probe position is tracked by angle-sensing devices (encoders) within each arm joint 17. Function of the encoders is shown in Figure 7.
Figure 6. Artemis Fusion Device.

Originally developed at Robarts Research Institute in Canada, the Artemis device gained FDA-approved in 2008. It is manufactured by Eigen (Grass Valley, Ca). The Artemis device uses a mechanical arm with built-in encoders to track biopsy location. During atransrectal ultrasound scan, 2D images are digitized with a frame grabber and reconstructed into a 3D image.A model of the prostate is then generated from the 3D image; biopsy, tracking of the biopsy site, and MRI fusion are then performed on the reconstructed model. Courtesy of Eigen, Grass Valley, CA; with permission.
Figure 7. Tracking Arm Encoders.
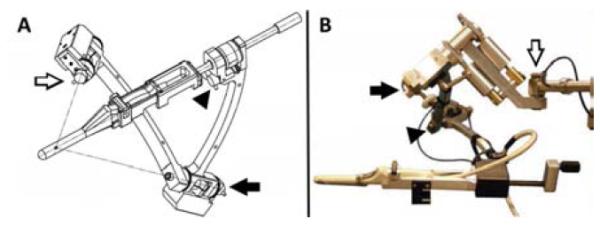
(A) Prototype created at Robarts Research Institute, (B) Working model in current use. Arrows denote location of the 3 encoders. As the TRUS transducer and cradle are moved, encoders (arrows) in the tracking mechanism measure the angles between linkages, and software calculates the transducer tip position and orientation in real-time17.From Bax J, Cool D, Gardi L, et al. Mechanically assisted 3D ultrasound guided prostate biopsy system. Med Phys. Dec 2008;35(12):5397-5410; with permission.
A summary of fusion biopsies performed with the Artemis device at UCLA Clark Urology Center is provided in Table 3. In the period March 2010 – January 2013, 501 men underwent fusion biopsy, involving nearly 8000 individual biopsy cores (5645 systematic and 2336 targeted)12,13,20.Targeted cores were more likely to contain cancer than systematic cores (18% vs. 8%), and most cancers found in targeted cores were significant ones (defined as Gleason score > 6 or cancer length > 4 mm) 21. Fusion biopsy in the clinic is performed under local anesthesia and is a 15-20 minute outpatient procedure. Antibiotic prophylaxis with a quinolone and a 3rd generation cephalosporin is used; with this regimen, among the 501 patients, only 2 episodes of sepsis have been encountered. Advantages of targeted biopsy are discussed below.
Table 3.
Summary of MRI Targeted Biopsy at University of California, Los Angeles (March 2010-January 2013)
| Patient Characteristics | All | Active Surveillance |
Prior Negative Biopsy |
Biopsy Naïve |
|---|---|---|---|---|
| No. of Patients | 501 | 229 | 150 | 122 |
| Median Age (yrs) | 65 | 65 | 65 | 65 |
| Median PSA (ng/ml) | 5.7 | 4.4 | 7.9 | 6 |
|
Median Prostate Volume
(cc) |
49 | 46 | 58 | 47 |
| No. with Cancer (%) | 221 (52%) | 148 (65%) | 51 (34%) | 64 (52%) |
| No. with Gleason ≥ 7 | 135 (27%) | 62 (27%) | 31 (21%) | 42 (34%) |
Value of Targeted Biopsy
Targeted prostate biopsy via MRI-US fusion has proven particularly valuable in two clinical settings.
Prior Negative Biopsy
As seen in Table 3, many men seeking targeted prostate biopsy are men who have undergone previous conventional biopsy, which fails to disclose a cancer. Typically, serum PSA levels continue to increase, and anxiety brings such men to look for alternatives.In a recent report detailing experience with this group of men20, we found that approximately one-third of the group (36/105) harbored CaP not detected by prior conventional biopsy (Figure 8). Most were significant cancers, as defined above. Some men had as many as 6-8 prior negative biopsies performed conventionally over the decade before their fusion biopsy.One had previously undergone two negative sets of saturation biopsies, before an anterior cancer was disclosed by MRI and diagnosed by targeted biopsy (Images in the patient example below).
Figure 8. Biopsy Results for Patients with Prior Negative Systematic Biopsies.
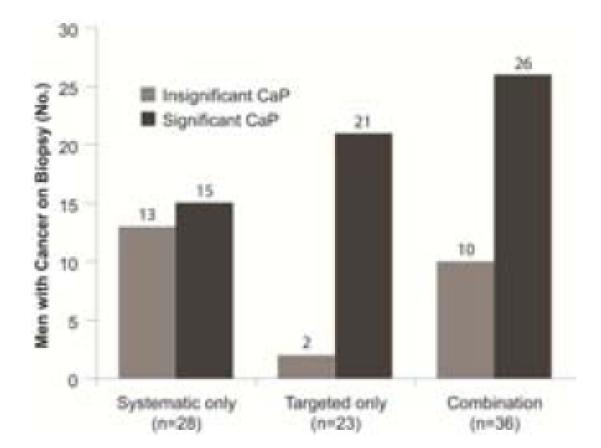
This chart shows the number of subjects diagnosed with significant cancers (dark grey) and insignificant cancers (light grey), depending on biopsy method. Clinically significant cancer was defined as Gleason >6 or >4 mm maximal core length 20.Targeted biopsy detected more significant cancers and fewer insignificant cancers than systematic biopsy. 15 patients were diagnosed only by systematic biopsy, i.e., cancer was present in areas where the MRI showed no abnormality. The false negative rate of MRI is not yet known. From Sonn GA, Chang E, Natarajan S, et al. Value of Targeted Prostate Biopsy Using Magnetic Resonance-Ultrasound Fusion in Men with Prior Negative Biopsy and Elevated Prostate-specific Antigen. Eur Urol. Mar 17 2013; with permission.
Figure 8 also shows the importance of obtaining both systematic and targeted biopsies.Significant cancer was diagnosed in 21 men by targeted biopsy alone. However, another 5 men with significant cancer (total 26) were diagnosed only by systematic biopsy and not by targeted biopsy. Thus, five significant cancers were found in areas of the prostate that appeared normal on MRI, i.e. the MRI was falsely negative in these areas. An explanation for falsely negative MR images is not yet clear, but others have made similar observations 22. An observation from several years of Artemis experience is that the biopsy map built into the Artemis software appears to provide better systematic spacing of the cores than ultrasound guidance alone.
Conventional TRUS biopsies may fail to detect CaP, especially when the tumor is located at the apical and anterior aspects of the prostate 23,24.When MRI reveals a lesion in one of these areas, targeted biopsy may be performed as usual. Saturation biopsy, regarded by many as a gold standard, increases the detection rate but also increases the numbers of insignificant cancers found 25.Targeted biopsy may reduce the risk of delayed diagnosis for patients with significant cancer, while providing increased reassurance to men whose targeted biopsy is negative.
If focal therapy becomes a treatment option, MRI-US fusion may become the method of choice for patient selection26. In prior studies, perineal template mapping biopsies have been used. However, perineal mapping biopsies are more invasive, expensive, time-consuming, and morbid than fusion biopsy27.
A 70 year old Caucasian male presented with a PSA value of 8.7 ng/ml, a prostate volume of 38.5 ml, and history of two prior negative conventional biopsies over the past 5 years.His MRI showed a highly suspicious lesion at the anterior central mid-gland (MRI score of 5). Fusion biopsy using the Artemis devicerevealed 3 cancerous cores from the lesion(Figure 9), including one in which the cancer occupied a length of 11 mm. Gleason score was 4+3=7.All systematic cores were negative for cancer. The patient underwent robotic assisted radical prostatectomy; a dominant tumor nodule of 1.9 cm was found in the anterior prostate; final pathology revealed Gleason 4+3=7 CaP.
Figure 9.

Patient with two prior negative biopsies wasfound to have a suspicious regionon MRI (Image Score =5) and underwent targeted confirmatory biopsy via MRI-US fusion. Gleason 3+4=7 CaP was found in the anterior target. A – T2-weighted MR image, B – Colorized apparent diffusion coefficient (ADC) MR image, C- Ultrasound showing prostate contour with areas of suspicion outlined (large grade 5 target, arrow), D – 3D reconstructed model of prostate showing targets and biopsy cores (tan lines).
Active Surveillance
A second important use for targeted prostate biopsy is in men undergoing active surveillance for presumed low-risk CaP.This management modality continues to be under-utilized, at least in part because of the uncertainties of conventional biopsy 28.MRI-US fusion biopsy provides a degree of reassurance beyond that provided by conventional biopsy.Further, confirmatory biopsy via MRI-US fusion, performed after conventional biopsy had indicated a low-risk lesion, has allowed exclusion of men who would be more appropriately managed with active intervention. An example of such a case is shown below.
While active surveillance of CaP has proven to be safe for low risk patients 29, participation in such programs remains low 30, with the majority of recently diagnosed men electing active treatment at the outset 31. Targeted prostate biopsy may improve patient selection for AS by more accurately identifying those at lower risk. In addition, the ability to accurately return to a previous biopsy site makes fusion biopsy an ideal modality for active surveillance follow up.
A 64 year old Caucasian male, presented with a serum PSA levelof 14.4ng/ml and a prostate volume of 55.2 ml. He was considered for active surveillance on the basis of an outside conventional biopsy revealing 1 mm of Gleason 3+3cancer. Subsequent MRI showed 2 suspicious lesions, one at the left base (MRI Score of 5) and the other at the right central gland (MRI Score of 3). The confirmatory biopsy was performed using the MRI-fusion techniqueand showed four cores of Gleason 4+4 from the grade 5 lesion with the cancer lengths measuring 3.5, 7, 10, and 15 mm. A 12 core systematic biopsy, performed at the same session, revealedtwo positive cores, both with only small microfoci (3 mm of Gleason 3+3=6; 1 mm ofGleason 3+4=7).If only a systematic biopsy had been performed, this patient mayhave remained in active surveillance despite the presence of significant CaP.Based onthe results from the MRI-targeted cores, the patient underwent robotic assisted radical prostatectomy. Final pathology showed a tumor of estimated volume of 11cc and Gleason 4+4 cancer.
Summary
The advent of multi-parametric MRI---which includes T2 weighted imaging (T2WI), diffusion-weighted imaging (DWI), and dynamic contrast enhancement (DCE)---has provided a method for visualizing prostate cancer.The MR imagesmay be used to guide prostate biopsy via image fusion, to enable targeted biopsy of suspicious areas within the MRI tube, or more efficiently, by MRI-ultrasound co-registration (fusion).MRI-US fusion allows prostate biopsy to be performed quickly, on an outpatient basis, using the transrectal technique familiar over the past several decades. The following conclusions represent a consensus from the initial 5 years experience, using various MRI-US fusion methods:
Targeted biopsies are several times more sensitive for detection of prostate cancer than non-targeted, systematic biopsies.
Targeted biopsies detect more significant prostate cancers and fewer insignificant cancers than conventional biopsies.
The false negative rate of MRI is not yet known, but is a concern; a negative MRI should not be used as a reason to defer biopsy.
Two groups that will especially benefit from targeted prostate biopsy are men with ‘low-risk’ lesions in active surveillance and men with elevated PSA levels and previous negative conventional biopsies.
Synopsis.
The advent of multi-parametric MRI---which includes T2 weighted imaging (T2WI), diffusion-weighted imaging (DWI), and dynamic contrast enhancement (DCE)---has provided a method for visualizing prostate cancer. The MR images may be used to guide prostate biopsy via image fusion, to enable targeted biopsy of suspicious areas, within the MRI tube or more efficiently, by MRI-ultrasound co-registration (fusion). MRI-US fusion allows prostate biopsy to be performed quickly, on an outpatient basis, using the transrectal technique familiar over the past several decades. The following conclusions represent a consensus from the initial 5 years experience, using various MRI-US fusion methods:
Targeted biopsies are several times more sensitive for detection of prostate cancer than non-targeted, systematic biopsies.
Targeted biopsies detect more significant prostate cancers and fewer insignificant cancers than conventional biopsies.
The false negative rate of MRI is not yet known, but is a concern; a negative MRI should not be used as a reason to defer biopsy.
Two groups that will especially benefit from targeted prostate biopsy are men with ‘low-risk’ lesions in active surveillance and men with elevated PSA levels and previous negative conventional biopsies.
KEY POINTS.
Reliable Imaging of prostate cancer within the organ has been elusive to date; however, over the past few years, use of multi-parametric MRI has begun to allow visualization of many organ-confined prostate cancers. The new imaging modality and its offshoot, targeted biopsy, offer the promise of a major transformation in management of this disease.
By aiming a biopsy needle at MRI regions of interest, a physician can now obtain tissue directly from suspicious lesions, i.e., targeted prostate biopsy, rather than by blindly sampling the organ.
Use of MRI images to guide prostate biopsy is accomplished by image-fusion and may be performed in one of three ways:by direct in-bore MRI-MRI fusion; by cognitive fusion, using US guidance to sample suspicious areas on MRI; and by MRI-US fusion using a device made for the purpose.
MRI-US fusion devices, such as the Artemis (Eigen-Hitachi) or UroNav (Invivo-Philips), allow the urologist to use sophisticated MRI images to guide prostate biopsy in an outpatient clinic setting; the procedure is contextually similar to that performed by most urologists for the past several decades.
Targeted prostate biopsy, via MRI-US fusion, (1) allows diagnosis of serious tumors not found with conventional biopsy; (2) helps to avoid detection of insignificant tumors; (3) provides a method for repeat biopsy of specific tumor-bearing sites for men in active surveillance; and (4) creates an opportunity for study of focal therapy.
Figure 10.
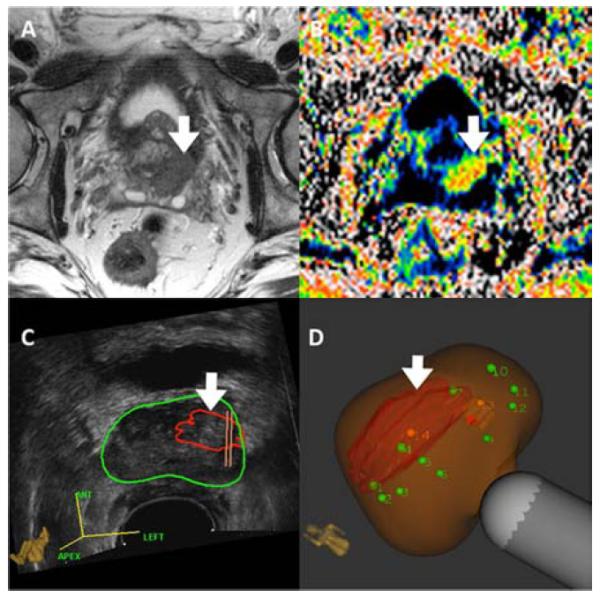
mpMRI(Panels A& B) and Artemis images (Panels C & D) of prostate from 64y.o. Caucasian male, who was enrolled in active surveillance on the basis of a microfocal lesion on conventional biopsy. Panel A shows T2WI. Panel B shows DWI. Panel C shows the lesion outlined in red, superimposed on the ultrasound image of the prostate (green circle).Panel D shows the lesion after MRI-US fusion; green dots are sites for systematic biopsy.Cancer was found only on targeted, but not systematic biopsies. Defining tumor burden in men with apparent ‘low risk’ CaP is an important use of targeted biopsy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
The project described was supported by Award Number R01CA158627 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. Additional support was provided by UCLA Clinical and Translational Sciences Institute Grant No. UL1TR000124, the Beckman Coulter Foundation, the Jean Perkins Foundation, the Steven C. Gordon Family Foundation, and the Nancy E. Barry and Letitia P. Rees Foundaton.
References
- 1.Silletti JP, Gordon GJ, Bueno R, Jaklitsch M, Loughlin KR. Prostate biopsy: past, present, and future. Urology. 2007 Mar;69(3):413–416. doi: 10.1016/j.urology.2007.01.096. [DOI] [PubMed] [Google Scholar]
- 2.Hodge KK, McNeal JE, Terris MK, Stamey TA. Random systematic versus directed ultrasound guided transrectal core biopsies of the prostate. The Journal of urology. 1989 Jul;142(1):71–74. doi: 10.1016/s0022-5347(17)38664-0. discussion 74-75. [DOI] [PubMed] [Google Scholar]
- 3.Cooperberg MR, Broering JM, Kantoff PW, Carroll PR. Contemporary trends in low risk prostate cancer: risk assessment and treatment. J Urol. 2007 Sep;178(3):S14–19. doi: 10.1016/j.juro.2007.03.135. Pt 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.King CR, McNeal JE, Gill H, Presti JC., Jr. Extended prostate biopsy scheme improves reliability of Gleason grading: implications for radiotherapy patients. Int J Radiat Oncol Biol Phys. 2004 Jun 1;59(2):386–391. doi: 10.1016/j.ijrobp.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 5.Hricak H, Williams RD, Spring DB, et al. Anatomy and pathology of the male pelvis by magnetic resonance imaging. AJR. American journal of roentgenology. 1983 Dec;141(6):1101–1110. doi: 10.2214/ajr.141.6.1101. [DOI] [PubMed] [Google Scholar]
- 6.Stamey TA, Caldwell M, McNeal JE, Nolley R, Hemenez M, Downs J. The prostate specific antigen era in the United States is over for prostate cancer: what happened in the last 20 years? J Urol. 2004 Oct;172(4):1297–1301. doi: 10.1097/01.ju.0000139993.51181.5d. Pt 1. [DOI] [PubMed] [Google Scholar]
- 7.Marks L, Young S, Natarajan S. MRI-ultrasound fusion for guidance of targeted prostate biopsy. Curr Opin Urol. 2013 Jan;23(1):43–50. doi: 10.1097/MOU.0b013e32835ad3ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barentsz JO, Richenberg J, Clements R, et al. ESUR prostate MR guidelines 2012. European radiology. 2012 Apr;22(4):746–757. doi: 10.1007/s00330-011-2377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hambrock T, Somford DM, Huisman HJ, et al. Relationship between Apparent Diffusion Coefficients at 3.0-T MR Imaging and Gleason Grade in Peripheral Zone Prostate Cancer. Radiology. 2011 Dec 14; doi: 10.1148/radiol.11091409. [DOI] [PubMed] [Google Scholar]
- 10.Collins DJ, Padhani AR. Dynamic magnetic resonance imaging of tumor perfusion. Approaches and biomedical challenges. IEEE engineering in medicine and biology magazine : the quarterly magazine of the Engineering in Medicine & Biology Society. 2004 Sep-Oct;23(5):65–83. doi: 10.1109/memb.2004.1360410. [DOI] [PubMed] [Google Scholar]
- 11.Hara N, Okuizumi M, Koike H, Kawaguchi M, Bilim V. Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) is a useful modality for the precise detection and staging of early prostate cancer. Prostate. 2005 Feb 1;62(2):140–147. doi: 10.1002/pros.20124. [DOI] [PubMed] [Google Scholar]
- 12.Sonn GA, Natarajan S, Margolis DJ, et al. Targeted biopsy in the detection of prostate cancer using an office based magnetic resonance ultrasound fusion device. The Journal of urology. 2013 Jan;189(1):86–91. doi: 10.1016/j.juro.2012.08.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Natarajan S, Marks LS, Margolis DJ, et al. Clinical application of a 3D ultrasound-guided prostate biopsy system. Urologic oncology. 2011 May-Jun;29(3):334–342. doi: 10.1016/j.urolonc.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoeks CM, Schouten MG, Bomers JG, et al. Three-Tesla Magnetic Resonance-Guided Prostate Biopsy in Men With Increased Prostate-Specific Antigen and Repeated, Negative, Random, Systematic, Transrectal Ultrasound Biopsies: Detection of Clinically Significant Prostate Cancers. European urology. 2012 Feb 1; doi: 10.1016/j.eururo.2012.01.047. [DOI] [PubMed] [Google Scholar]
- 15.Puech P, Rouviere O, Renard-Penna R, et al. Prostate Cancer Diagnosis: Multiparametric MR-targeted Biopsy with Cognitive and Transrectal US-MR Fusion Guidance versus Systematic Biopsy--Prospective Multicenter Study. Radiology. 2013 Apr 11; doi: 10.1148/radiol.13121501. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan I, Oldenburg NE, Meskell P, Blake M, Church P, Holupka EJ. Real time MRI-ultrasound image guided stereotactic prostate biopsy. Magn Reson Imaging. 2002 Apr;20(3):295–299. doi: 10.1016/s0730-725x(02)00490-3. [DOI] [PubMed] [Google Scholar]
- 17.Bax J, Cool D, Gardi L, et al. Mechanically assisted 3D ultrasound guided prostate biopsy system. Med Phys. 2008 Dec;35(12):5397–5410. doi: 10.1118/1.3002415. [DOI] [PubMed] [Google Scholar]
- 18.Pinto PA, Chung PH, Rastinehad AR, et al. Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. The Journal of urology. 2011 Oct;186(4):1281–1285. doi: 10.1016/j.juro.2011.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu S, Kruecker J, Turkbey B, et al. Real-time MRI-TRUS fusion for guidance of targeted prostate biopsies. Comput Aided Surg. 2008 Sep;13(5):255–264. doi: 10.1080/10929080802364645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sonn GA, Chang E, Natarajan S, et al. Value of Targeted Prostate Biopsy Using Magnetic Resonance-Ultrasound Fusion in Men with Prior Negative Biopsy and Elevated Prostate-specific Antigen. Eur Urol. 2013 Mar 17; doi: 10.1016/j.eururo.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmed HU, Hu Y, Carter T, et al. Characterizing clinically significant prostate cancer using template prostate mapping biopsy. J Urol. 2011 Aug;186(2):458–464. doi: 10.1016/j.juro.2011.03.147. [DOI] [PubMed] [Google Scholar]
- 22.Hadaschik BA, Kuru TH, Tulea C, et al. A novel stereotactic prostate biopsy system integrating pre-interventional magnetic resonance imaging and live ultrasound fusion. The Journal of urology. 2011 Dec;186(6):2214–2220. doi: 10.1016/j.juro.2011.07.102. [DOI] [PubMed] [Google Scholar]
- 23.Wright JL, Ellis WJ. Improved prostate cancer detection with anterior apical prostate biopsies. Urol Oncol. 2006 Nov-Dec;24(6):492–495. doi: 10.1016/j.urolonc.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Moussa AS, Meshref A, Schoenfield L, et al. Importance of additional "extreme" anterior apical needle biopsies in the initial detection of prostate cancer. Urology. 2010 May;75(5):1034–1039. doi: 10.1016/j.urology.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Zaytoun OM, Moussa AS, Gao T, Fareed K, Jones JS. Office based transrectal saturation biopsy improves prostate cancer detection compared to extended biopsy in the repeat biopsy population. J Urol. 2011 Sep;186(3):850–854. doi: 10.1016/j.juro.2011.04.069. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed HU, Hindley RG, Dickinson L, et al. Focal therapy for localised unifocal and multifocal prostate cancer: a prospective development study. The lancet oncology. 2012 Jun;13(6):622–632. doi: 10.1016/S1470-2045(12)70121-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmed HU, Freeman A, Kirkham A, et al. Focal therapy for localized prostate cancer: a phase I/II trial. J Urol. 2011 Apr;185(4):1246–1254. doi: 10.1016/j.juro.2010.11.079. [DOI] [PubMed] [Google Scholar]
- 28.Jacobs BL, Zhang Y, Schroeck FR, et al. Use of Advanced Treatment Technologies Among Men at Low Risk of Dying From Prostate Cancer. JAMA. 2013 Jun 26;309(24):2587–2595. doi: 10.1001/jama.2013.6882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tosoian JJ, Trock BJ, Landis P, et al. Active surveillance program for prostate cancer: an update of the Johns Hopkins experience. J Clin Oncol. 2011 Jun 1;29(16):2185–2190. doi: 10.1200/JCO.2010.32.8112. [DOI] [PubMed] [Google Scholar]
- 30.Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol. 2010 Mar 1;28(7):1117–1123. doi: 10.1200/JCO.2009.26.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cooperberg MR, Carroll PR, Klotz L. Active surveillance for prostate cancer: progress and promise. J Clin Oncol. 2011 Sep 20;29(27):3669–3676. doi: 10.1200/JCO.2011.34.9738. [DOI] [PubMed] [Google Scholar]


