Abstract
Dendritic cells (DCs) are highly potent stimulators of the immune system, and their contribution as such to the pathogenesis of corneal and ocular surface inflammatory disease has been well established. These vigorous antigen-presenting cells are reliant upon their effective migration from peripheral tissues (e.g., those of the ocular surface) to the lymphoid organs, where immune responses are triggered and can then cause disease. The chemokine receptor CCR7 expressed on DCs has emerged as the master mediator of this highly complex migratory process, and thus it is important in causing corneal and ocular surface inflammation. Furthermore, CCR7 has received considerable attention as a potential therapeutic target, as topically instilled antagonists of this receptor are quite effective therapeutically in a mouse model of ocular allergy. These findings and more are reviewed in the current article. In addition, the understanding regarding CCR7 function in mice and humans, and the biology of DCs that populate the ocular surface are also detailed herein. The involvement of DCs and their expression of CCR7 in corneal and ocular surface diseases such as in ocular allergy, dry eye disease, immune rejection and more, are also reviewed here.
Keywords: allergic conjunctivitis, CCR7, CD103, conjunctivitis, dendritic cells, dry eye disease, keratitis, ocular surface, ocular allergy, T cells
I. Introduction
Work in dendritic cell (DC) biology has expanded considerably in the last 5-7 years. The awarding of the 2011 Nobel Prize in Physiology or Medicine to the late Ralph Steinman for his contribution in the identification of these unique antigen-presenting cells indeed underscores this. The recent increase in information in DC biology has had a profound impact on the current understanding of immunity, inflammation, and disease. Similarly, the importance of DCs is indisputable in pathobiology of ocular inflammatory diseases, including those that involve the tissues of the ocular surface.
A key attribute of the DC machinery lies within their unequivocal potency for T cell stimulation, and the chemokine receptor CCR7 plays an essential role in this. In preclinical models of corneal and ocular surface inflammatory diseases, such as in ocular allergy and dry eye disease (DED), the role of antigen-charged DC from the cornea and ocular surface in activation of pathogenic T cells has been established.1-7 Furthermore, other such conditions that are associated with pathogenic T cells, including ocular involvement in graft-versus-host-disease (GVHD), keratolimbal allograft rejection, mucous membrane pemphigoid, and Stevens-Johnson syndrome, could implicate a role for DCs as well.
In animal models and in humans, the chemokine receptor CCR7 and C-C motif ligand (CCL)-19 and CCL21 interaction has emerged as one of the most —if not the most— important known chemokine systems in the migration of DCs from the affected tissue to the lymph node (LN) paracortex.7-13 This allows for encounter and consequent activation of cognate T cells to initiate/perpetuate adaptive immune responses and thus establishes a link for CCR7 and DCs with pathogenic T cell activation in corneal and ocular surface inflammatory diseases (Figure 1). As such, the involvement of CCR7 expression and function by DCs in ocular tissues has recently received considerable attention.2,7,14-22
Figure 1.
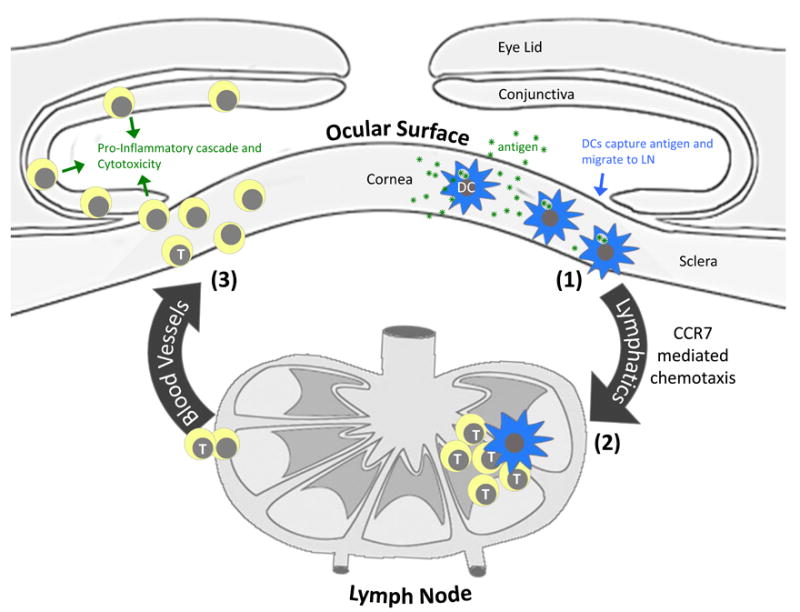
Contribution of DCs and their expression of CCR7 to the pathogenesis of corneal and ocular surface inflammatory disease. DCs that populate the tissues of the ocular surface initiate this pathway via (1) capture of antigen (e.g,, microbial products, allergens, autoantigen, alloantigen, etc.) and subsequent migration to regional lymph nodes via a CCR7 mediated process. (2) Pathogenic T cells are then activated in the LN via migratory DCs and access the ocular surface tissues by way of systemic circulation. (3) Pathogenic T cells, in turn, cause disease by promoting a proinflammatory cascade (e.g., secretion of cytokines, recruitment of myeloid cells, etc.), and causing cytotoxicity of ocular cells.
This article reviews recent literature that sheds light on how DC subsets that populate the cornea and conjunctiva utilize this chemokine receptor machinery to efficiently migrate to the LN in a highly coordinated fashion, and how this process contributes to the pathogenesis of corneal and ocular surface diseases, such as in ocular allergy. Furthermore, recent work validating the use of topical CCR7 antagonist as a therapeutic measure in ocular allergy is also reviewed.
II. Fundamentals of CCR7 and DC Migration
Appreciation for the role of CCR7 requires an understanding of certain fundamentals of DC biology. For example, it is important to know that DCs populate the interstitial tissues throughout the body in normal physiologic conditions, where they continuously sample antigen from their environment. During inflammation and exposure to ”danger signals,” such as Toll-like receptor signaling, DCs are stimulated to undergo phenotypic maturation, a process that is triggered in part via their loss of thrombospondin-1 expression.23 Maturation of DCs involves upregulation of the major histocompatibility complex (MHC) class II (or histocompatibility leukocyte antigen complex in humans) and CD80/CD86 costimulatory molecules. This maturation process prepares DCs to be able to prime/stimulate T cells, as MHC II is necessary for antigen presentation and CD80/CD86 are costimulatory molecules. Mature DCs also play a role in triggering secondary immune response, via activation of memory T cells also found in the LN.
However, maturation is merely an upstream event that is reliant upon CCR7-CCL19/21-mediated migration to the lymph node, where pools of naïve T cells and memory T cells are found. This is a highly complex chemotactic process that involves gaining access to terminal lymphatics, entry into the LN parenchyma, and trafficking to the T cell-rich paracortex. The sections below explain the manner by which this is accomplished.
A. CCR7 Ligands
CCR7 is a 7-transmembrane-spanning domain which signals through G protein-coupled receptors, with only known ligands including CCL19 and CCL21.24-42 Previously referred to as EBV-induced molecule 1 ligand chemokine (ELC), CCL19 is expressed by fibroblastic reticular cells in the paracortical regions of the LN where T cells are found.36,37 Migratory DCs also express CCL19 within the paracortical region, which is presented on the luminal side of high endothelial venules (HEV).38 Previously referred to as secondary lymphoid chemokine (SLC), CCL21 is the other CCR7 ligand.38-43 It is encoded by two functional variants in mice.40 One is CCL21-Leu (containing a leucine at position 65), which is expressed on lymphatic vessels in nonlymphoid tissues. The other is CCL21-Ser (containing a serine at this position), which is expressed in fibroblastic reticular cells of the LN paracortex, as well as by endothelial cells of HEV.40-43
B. Coordinated CCR7-Mediated Migration of DCs
How are the different CCR7 ligand sources coordinated to accomplish DC migration? Entry into the lymphatic vessels is facilitated by DC maturation. During an inflammatory response, DCs are activated to mature and upregulate their expression of CCR7. Concurrently in inflammation, endothelial cells of terminal lymphatic vessels of nonlymphoid tissues upregulate CCR7 ligands, and thus establish a chemotactic gradient by which CCR7 expressing DCs follow toward and into the lymphatic vessels (Figure 2).8,11,44 Lymphatic drainage, which carries these DCs, is eventually collected into afferent lymphatic vessels and subsequently discharged into the LN subscapular sinus (Figure 3A and B).45,46
Figure 2.

Antigen-charged DCs express CCR7 to gain access to terminal lymphatic vessels. DCs that populate peripheral tissues, such as the cornea and conjunctiva, will upregulate their expression of CCR7 in inflammation. This allows for chemotactic migration (green arrows) of such DCs toward CCR7 ligands expressed by endothelial cells of terminal lymphatic vessels, and subsequent entrance into the lymphatic system. Terminal lymphatics are not open-ended structures as depicted, and DCs must access them via passing by lymphatic endothelial cells.
Figure 3.

CCR7-mediated migration of DCs from peripheral tissues to the LN. (A) General migration path (green arrows) by which DCs migrate from peripheral tissues, e.g., ocular surface, to the LN paracortex. Black dashed box indicates the magnified region of interest. (B) Trafficking of DCs from the afferent lymphatic vessel, to the subcapsular sinus, and into the LN parenchyma. CCR7 ligand gradient indicated in red. Black dashed box indicates paracortical region, where T cells are activated. (C) Final chemotaxis toward the high endothelial venules (HEV). Inside the LN parenchyma, DCs follow a CCR7 ligand gradient toward HEV, where pools of naïve T cells are found. Fibroblastic reticular cells, endothelial cells of the HEV, and DCs themselves all contribute to this CCR7 ligand gradient. Naïve T cells and T regulatory cells also express CCR7, and gain access to the LN via CCR7 ligands found on the luminal side of the HEV.
DCs then make their way into the LN parenchyma (Figure 3B), which involves crossing through the cellular and collagen lining of the subcapsular sinus ”floor” by processes that are incompletely understood. Nevertheless, once within the LN parenchyma, yet another CCR7 ligand gradient is found emanating from fibroblastic reticular cells of the T cell-rich paracortex, which migrating DCs use to continue their chemotaxis (Figure 3C).36,39,46 Furthermore, by processes poorly understood, once inside in the paracortical region, migrating DCs themselves begin to express CCR7 ligands.38,39,47 This may serve to augment the chemokine gradient that draws additional DCs into the paracortex.
C. CCR7 Also Expressed by T Regulatory Cells
DCs are not the only immune cells that express and migrate to the LN with the help of CCR7. For example, naïve T cells also express CCR7 that allows these lymphocytes gain access to LN paracortex,8,48 albeit from systemic circulation as opposed to afferent lymphatic vessels (Figure 3C). This occurs through HEVs found within the paracortex, which also express CCL21 in addition to paracortical DCs. Furthermore, T cell expression of CCR7 is thought to help retention of these cells within the LN, so as to maximize the opportunity to encounter cognate antigen presented by DCs.
T regulatory cells (Treg) that are CD4+ CD25+ FoxP3+ also rely on their expression of CCR7 to gain access to and function within the LN.49 These cells work to suppress pathogenic T cell responses via a pathway referred to as immune tolerance. This is relevant for maintaining tissue homeostasis via staving off dysregulated immune responses (such as in autoimmunity or allergy), as well as suppressing unwarranted responses (such as occur following resolution of infection). Treg expression of CCR7 allows these modulatory cells to gain access to the LN from circulation and accomplishes this via HEVs. Furthermore, CCR7 recruits these Tregs in close proximity to immune synapses that occur between mature DCs and pathogenic T cells (Figure 3C).
III. Nonlymphoid Tissue DC Subsets that Populate the Ocular Surface
Given the recent appreciation for the existence of distinct DC subsets and their functionally divergent roles in shaping adaptive immune responses,1,50,56 recent attention focused on identifying DC subsets that populate ocular surface tissues, characterization of their respective functions, and examining whether migration to the LN is mediated through CCR7. Classical DCs under normal physiologic conditions can be divided into 1) those that populate lymphoid organs such as the spleen and LN, and 2) those that populate interstitial tissues of nonlymphoid organs. Relevant to the ocular surface, the nonlymphoid tissue DCs can be further subdivided into a) CD103+ CD11b- (i.e. CD103+) DCs, b) CD103-CD11b+ (i.e. CD11b+) DCs, and c) CD103+ CD11b+ DCs (Figure 4).1,52-56 The nonlymphoid tissue CD11b+, CD103+, and CD103+ CD11b+ DC subsets are of considerable interest. CD11b+ and CD103+ DCs have recently been shown to populate tissues of the ocular surface.1 Much is known about their ontogeny and development (Figure 4).1,52-56 For example, CD103+ DCs are derived exclusively from the bone marrow myeloid lineage referred to as common DC progenitors (CDP) and are hematopoietin Flt-3-dependent (Figure 4). CD103+ CD11b+ DCs are similarly derived, although they have only been reported to populate gut lamina propria (Figure 4). In contrast, CD11b+ DCs are heterogeneously contributed to by both CDPs and monocyte precursors, and are only partially Flt-3-driven (Figure 4). They also differ in their functional roles. For example, CD103+ DCs are crucial in cross presentation of viral antigens to CD8+ T cells.56-58 Recently, two independent reports indicated a role for CD11b+ DCs, or CD103+ CD11b+ DCs in mucosal IL-17-mediated responses.54,55
Figure 4.

Ontogeny and function of classical DCs that populate nonlymphoid tissues. Classical DCs that populate interstitial spaces of nonlymphoid tissues are derived from bone marrow precursors, including common DC progenitors (CDP) and/or monocytes. These include CD11b+, CD103+, and CD103+ CD11b+ DCs. This is in contrast to Langerhans cells, with precursors derived from a prenatal lineage.
Nonlymphoid CD11b+ and CD103+ DCs populate the conjunctiva, as recently shown by Khandelwal et al (Figure 5A).1 Also defined is the function of these DCs and the role of CCR7 in mediating migration to the LNs (reviewed below). The cornea is also populated with DCs,50 although their lineage and precise function remain unclear. Hattori et al reported a population of DCs in the stroma distinct from Langerhans cells (LC) that express langerin (Figure 5B).50 Langerin is a c-type lectin whose expression by classical DCs is a phenotypic marker consistent with CD103+ DCs. However, the DC population identified in the corneal stroma by Hattori et al co-expresses CD11b+50 and is thus inconsistent phenotypically with CD11b+ DCs seen elsewhere, which have no appreciable langerin expression. Future work is needed to determine whether these corneal DCs are from divergent lineages, or whether the corneal microenvironment simply leads to different integrin and/or lectin expression patterns.
Figure 5.

Classical DCs (CD11c+) that populate the cornea and conjunctiva. (A) Similar to the lung, the tissues of the mouse conjunctiva are populated by CD11b+ and CD103+ DCs. Cells from enzymatically digested tissues are analyzed via flow cytometry. Events are gated on CD11c+ I-A/I-E+ (MHC II), then on CD11c+ autofluorescent-, so that respective CD11b+ and CD103+ DC populations can be visualized. (This figure is adapted from Khandelwal et al doi: 10.1371/journal.pone.0064193.) (B) Characterization of Langerin-expressing DC in the cornea. Langerin-expressing DCs are LCs in the corneal epithelium, whereas ones in the corneal stroma are classical DCs that are likely of bone marrow origin. (This figure is adapted from Hattori et al, doi: 10.1167/iovs.10-6741.)
The cornea is also populated by other CD11c+ and CD11c- antigen-presenting cells,59-66 such as macrophages in the stroma.60-62 In addition, LCs are found in the corneal epithelium (Figure 5B),50,64,65 although these comprise only a fraction of intraepithelial DCs in mice and humans.50,64 This is in stark contrast with the epidermis in normal conditions, where the DC population is exclusively populated with LCs.66 Inflammatory DCs can also be found infiltrating in inflammation; this topic has been reviewed elsewhere.67
IV. CCR7-Mediated DC Migration in Ocular Allergy
Given the identification and appreciation for nonlymphoid DCs that populate the ocular surface, new questions have arisen. How do DCs contribute to the secondary allergic immune responses and ocular allergy? Does CCR7 govern the migration of these cells, and can CCR7 thus contribute to the pathogenesis of corneal and ocular surface inflammatory disease? We address these questions by reviewing recent work in the area of ocular allergy, such as the report by Schlereth et al.2 We also discuss work by Khandelwal et al, who recently showed in the mouse model that the CD11b+ DC subset plays the dominant role in the immunopathogenesis of ocular allergy.1
A. Clinical Forms
The different clinical forms of IgE-associated allergic eye disease has been reviewed elsewhere.7,68-71 Briefly, these include seasonal allergic conjunctivitis (SAC), perennial allergic conjunctivitis (PAC), vernal keratoconjunctivitis (VKC), and atopic keratoconjunctivitis (AKC). The less severe clinical forms, such as in SAC and PAC, are mediated predominantly by an immediate hypersensitivity response, although some aggressive cases may also present with a late-phase reaction. In contrast, the more severe/chronic forms of ocular allergy, such as in VKC and AKC, are predominantly eosinophil-mediated. Unlike SAC and PAC, chronic allergic disease significantly affects the cornea and can easily lead to corneal blindness in the absence of corticosteroid pharmacotherapy.
B. Ocular Allergy Immunopathogenesis
The T cell response is central to all phases of allergic immunity, including primary exposures that prime naïve T cells and lead to allergen sensitization, as well secondary allergen exposures that cause immediate hypersensitivity, and in some, subsequent late-phase and chronic disease.7,68-71Allergen sensitization is the process by which allergen exposure primes a host in a manner that results in the production of allergen-specific IgE. It involves activation of naïve T cells into T helper (h) 2 cells. Cytokines IL-4 and IL-13 are highly expressed by such Th2 cells, which in turn promote B cell differentiation into IgE secreting plasma cells. This IgE becomes bound by Fc receptors on resident mast cells, such as those in the conjunctiva. Thus, to secondary exposures, allergen becomes ligated to the Fab fragment of bound IgE, which causes Fc receptor cross-linking on the mast cell and membrane destabilization. This leads to their release of preformed histamine granules, which culminates in an immediate hypersensitivity response (Figure 6). 71
Figure 6.
Working model for the role of DCs in the immunopathogenesis of ocular allergy. DCs play numerous key roles in sensitized individuals. Through migration to the LN via CCR7 (1), DCs activate Th2 cells which, in turn, promote B cell production of IgE antibodies (2) relevant in subsequent immediate hypersensitivity reactions. DC activation of Th2 cells is also important in late phase/chronic responses, as Th2 promote differentiation (3), recruitment and activation of pathogenic eosinophils (4). (This figure is adapted from Saban et al, doi: 10.3109/02713683.2012.747617.)
Whereas the immediate hypersensitivity response subsides after 30 minutes following exposure, the late phase response may require 3-6 hours to become clinically evident, and may perpetuate for weeks, such as in chronic disease. Late-phase reactions are principally eosinophil-mediated, which is indeed driven by T cell responses as well.68-71 Through their IL-5 expression, Th2 cells are important in triggering eosinophil maturation in the bone marrow and the exit of eosinophils into systemic circulation (Figure 6). Furthermore, IL-4 and IL-13 from Th2 cells activate vascular endothelial cells, which promotes eosinophil recruitment (Figure 6). Lastly, IL-5 from Th2 cells then activates recruited eosinophils (Figure 6), which can then effect tissue damage via neurotoxin (EDN), peroxidase (EPO), and cationic protein (ECP).71
C. CCR7-Mediated DC Migration and T Cell Activation in Ocular Allergy
Given the central role of T cells in ocular allergy coupled with the appreciation of nonlymphoid DCs that populate the conjunctiva, Schlereth et al set out to examine the possible involvement and manner by which such DCs may contribute to secondary responses in ocular allergy.2 The authors accomplished this by using exogenously derived eGFP+ DCs that were injected subconjunctivally into sensitized mice, and subsequently administered a secondary exposure topically with fluorescent-labeled allergen (Texas Red-conjugated ovalbumin). This allowed them to identify DCs (eGFP+) within the LN that had captured allergen (Texas Red+) and had originated from the conjunctiva (Figure 7A). This is important, as DCs that populate the lymphoid tissues can also capture allergen, albeit it may be functionally distinct, particularly with respect to CCR7.2 Because of this, Texas Red+ eGFP+ DCs in the LNs of these mice had to be carefully isolated via flow cytometry, and the authors observed a markedly increased expression of CCR7 by these cells. This identification strongly suggested the importance for CCR7-mediated migration of DCs to secondary exposures in ocular allergy
Figure 7.

Schematic depiction of experiments that identified the role of CCR7-mediated migration of DCs on ocular allergy. (A) Exogenous eGFP+ DCs injected subconjunctivally into sensitized wild type mice capture allergen (red) from the ocular surface and express CCR7 (1) to migrate to the LN (2). Such migratory DCs are distinct from LN resident DCs (depicted in gray). Migratory DCs stimulate pathogenic T cells (3) which, in turn, cause disease. (B) Exogenous CCR7 knockout (-/-) DCs (green) injected subconjunctivally into sensitized wild type mice capture allergen (red) from the ocular surface. However, these DCs cannot express CCR7, and their migration to LN is thus inhibited. Activation of pathogenic T cells and consequent clinical disease is also inhibited. This schematic is a depiction of experiments performed by Schlereth et al, doi: 10.1016/j.ajpath.2012.02.015.
Functional evidence to support this conclusion came from the subsequent series of experiments, which used a similar approach. Schlereth et al examined secondary immune responses using exogenously derived DCs from wildtype vs CCR7-/- mice, subconjunctivally injected into mice adoptively transferred with allergen-primed T cells (Figure 7b).2 This experimental approach allows for clean interpretation of DC- T cell interactions, since adoptive transfer does not include IgE (or B cells) that would otherwise contribute to immediate hypersensitivity. The authors found that the secondary Th2 cell response in the LNs of mice receiving CCR7-/- DCs was markedly impaired compared to other mice that had received wildtype DCs (Figure 7A). These findings indicated that activation of adoptively transferred T cells by migratory DCs involves CCR7. Consistent with this, the clinical scores in these mice were decreased by significant levels, thereby linking the role of CCR7 and T cell responses with clinical disease. Schlereth et al verified that this pathway is also relevant in actively immunized and allergen-challenged mice by showing that topical application of blocking antibody against CCR7 leads to marked decrease of clinical scores. This experiment is further discussed in Section IV.D. Taken together, these data provided functional evidence supporting the conclusion that in secondary allergic T cell responses, DCs migrate to the LN to activate T cells using a CCR7-mediated process (Figure 7A).
D. Nonlymphoid Tissue CD11b+ DCs in Ocular Allergy
Appreciating the role of CCR7 in DC migration in ocular allergy, Khandelwal et al carried out a subsequent series of experiments to identify whether nonlymphoid CD11b+ or CD103+ DCs that populate the conjunctiva play a dominant role in triggering allergenreactive T cells.1 Similar to the approach taken by Schlereth et al,2 exogenously derived CD11b+ vs CD103+ DCs were subconjunctivally injected into adoptively transferred mice, and were subsequently challenged topically with allergen (Figure 8). Interestingly, clinical and T cell responses that followed were very different between the two groups. Whereas mice that received CD103+ DCs were unable to mount robust clinical signs of ocular allergy or T cell responses, mice that had received CD11b+ DCs showed vigorous responses in this regard (Figure 8). This experiment was performed using bone marrow-derived DCs, as well as purified nonlymphoid DC subsets from the lung, with the same results.1 These data indicated that CD11b+ DCs contribute in a pathogenic fashion to ocular allergy, whereas CD103+ DCs do not.
Figure 8.
CD11b+, but not CD103+, DCs are the dominant in triggering pathogenic T cells involved in ocular allergy. Exogenous CD11b+ or CD103+ DCs (or no DCS) were injected subconjunctivally in sensitized wild type mice. Host mice were challenged with allergen daily for 7 days, and clinical signs were scored at 20 minutes, and 6 and 24 hr post-challenge. (Taken from Khandelwal et al, Khandelwal et al doi: 10.1371/journal.pone.0064193.)
Thus, the collective works of Schlereth et al and Khandelwal et al indicate CD11b+ DCs that populate the conjunctiva to be the dominant subset in triggering pathogenic T cells in ocular allergy, and that this is accomplished in a CCR7-mediated fashion.1,2 Furthermore, these findings raise the possibility that a similar role for CCR7 may be relevant in mediating the migration of DCs in other corneal and ocular surface inflammatory diseases, including dry eye and other diseases.
V. CCR7 in Other Corneal and Ocular Surface Inflammatory Conditions
Other corneal and ocular surface inflammatory diseases likewise have a T cell involvement. Furthermore, there is evidence to support the role for CCR7-mediated migration of DC in immune rejection of allogeneic corneal transplants, as well as DED, as detailed below. Other corneal and ocular surface inflammatory diseases that involve pathogenic T cells include ocular GVHD, ocular Stevens-Johnson syndrome, and ocular mucous membrane pemphigoid; however, research is needed to determine whether such conditions involve CCR7-mediated migration of ocular surface DCs, as detailed below.
A. Dry Eye Disease
Multiple lines of evidence have led to the appreciation of DCs that populate the tissues of the ocular surface as important contributors to DED immunopathogenesis. This is predicated upon the appreciation that pathogenic T cells are central in the development and perpetuation of DED,3,4,72-81 which is a concept supported by a level of therapy seen clinically with Cyclosporine A treatment in certain DED patients.73 This is also supported by the numerous reports converging on the central T cell role (e.g., Th1 and Th17) in the desiccating induced-stress model in mice.3,4,74-81
There are multiple lines of evidence from independent laboratories supporting a role for nonlymphoid tissue DCs that populate the cornea and conjunctiva in DED pathogenesis. Regarding the cornea, Goyal et al identified in desiccating stress-induced mice the ingrowth of lymphatic vessels into the cornea that was associated with increased CD11b+ MHC II+ cells in the LN,82 and Lee et al showed a decrease in CD11c+ MHCII+ cells in the LN associated with TLR antagonist-mediated amelioration of clinical disease.83 With respect to nonlymphoid tissue DCs that populate the conjunctiva, Schaumburg et al reported that ablation of CD11b+ and CD11c+ cells in the conjunctiva (accomplished via liposome-encapsulated clodronate administration) led to a reduction in T cell recruitment and amelioration in clinical disease induced from desiccating stress.3
Given the evidence to support the role of nonlymphoid DCs of the ocular surface in DED pathogenesis, the possibility for CCR7 involvement in migration of such DCs in the disease process has been explored. Koldati et al observed that the majority of CD11b+ cells co-expressed CCR7 in cornea of desiccating stress-induced mice, and found an associated increase of CD11b+ MHCII+ CCR7+ cells in the LN of these mice.84 A role for CCR7 was also suggested in the model of spontaneous development of autoimmune Sjögren syndrome-associated ocular phenotype seen in thrombospondin-1 (TSP-1)-deficient mice.85 Contreras-Ruiz et al observed enhanced egress of antigen-bearing DCs to the LNs in such TSP1-/- mice, and found that treatment of DCs with TSP-1 decreased CCR7 expression as well as migration to the LN.86 Collectively, these reports point to the possibility of a CCR7-mediated process for the migration of DC to the LN for pathogenic T cell activation in DED. Future work is required to examine this process.
B. Immune Rejection
Immune rejection involving the tissues of the ocular surface can be seen in corneal transplantation, including penetrating keratoplasty and (perhaps less so in) posterior lamellar forms of keratoplasty. Immune rejection is also relevant in keratolimbal allografts used to reestablish a functional ocular surface in limbal stem cell disease. Indeed, the pathobiology of immune rejection involving tissues of the ocular surface is understood, albeit much of the work conducted in animal models has been focused on penetrating keratoplasty.87 It is known that DCs of the host (and in high-risk cases, donor-borne DCs15,23,88,89) migrate to the LN to trigger alloreactive T cells that consequently effect graft rejection.
There is a significant body of literature supporting the role for CCR7 expressed by DCs in immune rejection of corneal allografts. Irschick et al recently showed the expression of CCR7 by DCs in the human cornea and found that chemotaxis of these cells ex vivo were mediated by CCL19 and/or CCL21.16 In mice, Jin et al reported that CD11b+, CD11c+, and MHC II+ cells upregulate CCR7 in inflamed cornea and further observed that syngeneic hosts receiving corneal grafts charged with fluorescently tagged OVA possessed OVA+ CD11c+ CCR7+ DCs in ipsilateral LNs.17 This response was significantly inhibited with administration of CCL21 antibody blockade. Saban et al demonstrated that in vitro stimulation of TSP1-/- DCs have greater CCR7 expression compared to wildtype DCs.23 Furthermore, in the mouse corneal allotransplantation setting, TSP-1-/- donor-derived DCs showed increased migration to host LNs and increased sensitization of alloreactive host T cells.23 In another study, Hua et al used media conditioned with freshly excised LNs from corneal allografted hosts, and demonstrated with blocking antibodies that CCL19 and CCL21 mediated DC migration in transwell assays.21
Collectively, these reports converge on the idea that CCR7 mediates the migration of DCs to the regional LN in stimulating alloreactive T cells in immune rejection. It is important to note that Jin et al found that CCR7-/- corneal allografts in mice led to reduced host Treg function, perhaps suggesting a CCR7-dependent role for donor-derived DC mediated Treg activation.15 It is also noteworthy that a VEGF-R3-mediated mechanism in antigen-presenting cell migration to LNs in mice has also been reported.90
C. Other Corneal and Ocular Surface Inflammatory Diseases
Ocular involvement in GVHD, mucous membrane pemphigoid, and Stevens-Johnson syndrome are other corneal and ocular surface inflammatory conditions that involve pathogenic T cells. However, whether a role for CCR7-mediated migration contributes to the immunopathogenesis of these conditions requires further investigation. GVHD is a T cell-mediated disease, shown to infiltrate ocular tissues.91 Stevens-Johnson syndrome also involves recruitment of T cells,92 although antigen-antibody complexes may mostly mediate the pathology. Mucous membrane pemphigoid has also been shown to involve T cells, although pathology is thought to be associated with deposition of antibodies.93 Collectively, in all three of these conditions, the presence locally of infiltrated T cells and/or antibody deposition may be suggestive of corneal/conjunctival-borne antigen. This is significant, because it may provide the opportunity for CCR7-mediated migration of nonlymphoid tissue DCs charged with such antigens to initiate or perpetuate adaptive immune responses that contribute to these conditions. Future work in this area is needed to address this.
It is important to note that DCs do not solely contribute to ocular surface pathology. Indeed, activation of T cells in the context of infection is important for host microbial defense, such as in herpetic keratitis.94 In addition, several groups have reported that corneal DCs are involved in promoting wound healing responses.95,96
VI. Therapeutic Opportunity for CCR7 Antagonists in Treatment of Corneal and Ocular Surface Inflammatory Diseases
Targeting DCs therapeutically has finally become a possibility, and strategies in this regard may be useful in treating corneal and ocular surface inflammatory disease. Certainly, such efforts are increasing in other areas, such as in clinical autoimmune diseases and transplantation.97
Antagonizing CCR7 is an attractive approach if a strategy could be implemented in a manner that targets nonlymphoid tissue DCs directly, but not circulating Tregs in the blood stream. Indeed, topical instillation administered to the ocular surface may be ideal for this, and, thus, application of CCR7 antagonists could be suitable for treatment of corneal and ocular surface inflammatory diseases. It is noteworthy that antagonizing CCR7 has been considered in various immune-inflammatory conditions in entities, such as Crohn disease, rheumatoid arthritis, and atherosclerosis.97-100
A. Therapeutic Evidence for Topical CCR7 Antagonists at the Ocular Surface
DCs populate the conjunctiva,1,101 and recent work by Schlereth et al has provided evidence to indicate that targeting these cells via topical delivery of CCR7 antagonist has a significant therapeutic effect.2 This was demonstrated in a model of ocular allergy in which mice are sensitized systemically and then challenged topically.2 Blocking antibody against CCR7 was applied topically and clinical disease was compared to that in mice that received an isotype control. Mice were examined at 20 minutes, 6 hours, and 24 hours post-challenge, and repeated for 4 days. Strikingly, at all time points a clear and marked reduction was seen in the clinical scores of the CCR7-treated mice (Figure 9), thus highlighting the importance of CCR7 in ocular allergy and supporting the use of topical inhibition in targeting CCR7.
Figure 9.

Topically instilled CCR7 antagonist has a robust therapeutic effect against late-phase responses in ocular allergy. Sensitized mice were challenged daily for 4 days. Mice were treated topically with either CCR7 antagonists or an isotype control antibody. Representative pictures on day 3 and day 4 of challenge are shown. Data are taken from Schlereth et al, doi: 10.1016/j.ajpath.2012.02.015.
Furthermore, a closer look at the clinical responses revealed some interesting implications. Clinical indicators of immediate hypersensitivity were still evident in CCR7-antagonized mice, such as lid swelling, tearing, and chemosis (Figure 9). However, the most striking observation was that late-phase/chronic disease-associated-sequelae, e.g., corneal involvement, epitheliopathy and blepharitis, were markedly reduced in the CCR7-treated mice (Figure 9). This is consistent with the understanding that such manifestations are due to eosinophil and T cell involvement in ocular allergy,2,7,68-70 and that CCR7 is a key component in driving T cell responses.2 Furthermore, these data suggest that CCR7 antagonists may have a therapeutic role in late-phase and chronic ocular allergy (Figure 6), which is an area of unmet medical need.
B. CCR7 Expression by DCs in Human Ocular Tissues
Human expression of CCR7 by DCs in mediating migration of these cells to the LN has been appreciated for nearly two decades29,102 and has been further validated in clinical conditions such as Crohn disease, atherosclerosis, and rheumatoid arthritis.98-100 Several studies have indicated a clear relevance of CCR7 in human ocular tissues, such as the cornea, conjunctiva, and anterior segment.16,18,103 Irschick et al showed expression of CCR7 by DCs in the human cornea and found that chemotaxis of these cells were mediated by CCL19 and /or CCL21.16 Birke et al found dendritiform cells that labeled for CCR7 within the anterior surface of human iris tissues,103 and they postulated a possible role for CCR7-mediated chemotaxis via conventional outflow pathways. Lastly, Mathew et al showed increased CCR7+ cells in conjunctival biopsies of seasonal allergic conjunctivitis patients 6 hours following allergen provocation.22 Collectively, is it clear not only that CCR7-mediated migration of DCs is relevant in humans, but it appears to be clearly relevant in tissues of the human eye, including those of the ocular surface.
VII. Conclusion
CCR7 expression by DCs is a key player in corneal and ocular surface inflammatory diseases. It is widely appreciated that DCs populate the tissues of the ocular surface and that these cells are important contributors in the activation of pathogenic T cells involved in ocular allergy and DED. Furthermore, CCR7 is the primary chemokine receptor that drives DC migration to the LN and, in turn, activates pathogenic T cells. Thus, antagonizing CCR7 at the ocular surface has been considered as a therapeutic strategy in corneal and ocular surface inflammatory disease. Indeed, topical administration of CCR7 antagonists has been tested in a mouse model of ocular allergy, and results demonstrated robust efficacy. Future work is needed to determine if CCR7 antagonists can have a similar therapeutic effect in DED, immune rejection, and other chronic inflammatory disease of the cornea and ocular surface.
Acknowledgments
Funded by R01EY021798 (Saban DR)
Abbreviations
- AKC
Atopic keratoconjunctivitis
- CDP
Common DC progenitors
- DC
Dendritic cell
- DED
Dry eye disease
- ELC
EBV-induced molecule 1 ligand chemokine
- GVHD
Graft-versus-host-disease
- HEV
High endothelial venules
- LN
Lymph node
- MHC
Major histocompatibility complex
- PAC
Perennial allergic conjunctivitis
- SAC
Seasonal allergic conjunctivitis
- SLC
Secondary lymphoid chemokine
- Treg
T regulatory cell
- TSP-1
Thrombospondin-1
- VKC
Vernal keratoconjunctivitis
Footnotes
Disclosure/Conflict of Interest: Author is inventor on patent application.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Khandelwal P, Blanco-Mezquita T, Emami P, et al. Ocular mucosal CD11b+ and CD103+ mouse dendritic cells under normal conditions and in allergic immune responses. PLoS One. 2013;8:e64193. doi: 10.1371/journal.pone.0064193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schlereth S, Lee HS, Khandelwal P, Saban DR. Blocking CCR7 at the ocular surface impairs the pathogenic contribution of dendritic cells in allergic conjunctivitis. Am J Pathol. 2012;180:2351–60. doi: 10.1016/j.ajpath.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schaumburg CS, Siemasko KF, De Paiva CS, et al. Ocular surface APCs are necessary for autoreactive T cell-mediated experimental autoimmune lacrimal keratoconjunctivitis. J Immunol. 2011;187:3653–62. doi: 10.4049/jimmunol.1101442. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Chauhan SK, Soo Lee H, et al. Chronic dry eye disease is principally mediated by effector memory Th17 cells. Mucosal Immunol. 2013 Apr 10; doi: 10.1038/mi.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stern ME, Schaumburg CS, Pflugfelder SC. Dry eye as a mucosal autoimmune disease. Int Rev Immunol. 2013;32:19–41. doi: 10.3109/08830185.2012.748052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pflugfelder SC, Stern ME. Symposium Participants. Immunoregulation on the ocular surface: 2nd Cullen Symposium. Ocul Surf. 2009;7:67–77. doi: 10.1016/s1542-0124(12)70297-5. [DOI] [PubMed] [Google Scholar]
- 7.Saban DR, Calder V, Kuo CH, et al. New twists to an old story: novel concepts in the pathogenesis of allergic eye disease. Curr Eye Res. 2013;38:317–30. doi: 10.3109/02713683.2012.747617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forster R, Schubel A, Breitfeld D, et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 9.Scandella E, Men Y, Gillessen S, et al. Prostaglandin E2 is a key factor for CCR7 surface expression and migration of monocyte-derived dendritic cells. Blood. 2002;100:1354–61. doi: 10.1182/blood-2001-11-0017. [DOI] [PubMed] [Google Scholar]
- 10.Saeki H, Moore AM, Brown MJ, Hwang ST. Cutting edge: secondary lymphoid-tissue chemokine (SLC) and CC chemokine receptor 7 (CCR7) participate in the emigration pathway of mature dendritic cells from the skin to regional lymph nodes. J Immunol. 1999;162:2472–5. [PubMed] [Google Scholar]
- 11.Ohl L, Mohaupt M, Czeloth N, et al. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity. 2004;21:279–88. doi: 10.1016/j.immuni.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 12.Forster R, Davalos-Misslitz AC, Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol. 2008;8:362–71. doi: 10.1038/nri2297. [DOI] [PubMed] [Google Scholar]
- 13.Yanagihara S, Komura E, Nagafune J, et al. EBI1/CCR7 is a new member of dendritic cell chemokine receptor that is up-regulated upon maturation. J Immunol. 1998;161:3096–102. [PubMed] [Google Scholar]
- 14.Yu CR, Mahdi RM, Liu X, et al. SOCS1 regulates CCR7 expression and migration of CD4+ T cells into peripheral tissues. J Immunol. 2008;181:1190–8. doi: 10.4049/jimmunol.181.2.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin Y, Chauhan SK, Saban DR, Dana R. Role of CCR7 in facilitating direct allosensitization and regulatory T-cell function in high-risk corneal transplantation. Invest Ophthalmol Vis Sci. 2010;51:816–21. doi: 10.1167/iovs.09-3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Irschick UM, Mayer WJ, Kranebitter N, et al. Active in vitro reduction of antigen presenting cells in human corneal grafts using different chemokines. Curr Eye Res. 2010;35:176–83. doi: 10.3109/02713680903453502. [DOI] [PubMed] [Google Scholar]
- 17.Jin Y, Shen L, Chong EM, et al. The chemokine receptor CCR7 mediates corneal antigen-presenting cell trafficking. Mol Vis. 2007;13:626–34. [PMC free article] [PubMed] [Google Scholar]
- 18.Ebihara N, Yamagami S, Yokoo S, et al. Involvement of C-C chemokine ligand 2-CCR2 interaction in monocyte-lineage cell recruitment of normal human corneal stroma. J Immunol. 2007;178:3288–92. doi: 10.4049/jimmunol.178.5.3288. [DOI] [PubMed] [Google Scholar]
- 19.Cook WJ, Kramer MF, Walker RM, et al. Persistent expression of chemokine and chemokine receptor RNAs at primary and latent sites of herpes simplex virus 1 infection. Virol J. 2004;1:5. doi: 10.1186/1743-422X-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mackensen F, Metea CA, Planck SR, Rosenbaum JT. Endotoxin upregulates CCR7 and its ligands in the lymphatic-free mouse iris. Mol Vis. 2007;13:2209–13. [PubMed] [Google Scholar]
- 21.Hua J, Stevenson W, Dohlman TH, et al. The CCR7-CCL19/CCL21 axis mediates enhanced antigen-presenting cell trafficking in high-risk corneal transplantation. Invest Ophthalmol Vis Sci. 2013;54 ARVO E-Abstract 1289. [Google Scholar]
- 22.Mathew R, Mohd Zaki A, Galatowicz G, et al. CCR7 expression profiles in conjunctival biopsies from seasonal allergic conjunctivitis patients following challenge. Invest Ophthalmol Vis Sci. 2013;54 ARVO E-Abstract 2549. [Google Scholar]
- 23.Saban DR, Bock F, Chauhan SK, et al. Thrombospondin-1 derived from APCs regulates their capacity for allosensitization. J Immunol. 2010;185:4691–7. doi: 10.4049/jimmunol.1001133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagira M, Imai T, Hieshima K, et al. Molecular cloning of a novel human CC chemokine secondary lymphoid-tissue chemokine that is a potent chemoattractant for lymphocytes and mapped to chromosome 9p13. J Biol Chem. 1997;272:19518–24. doi: 10.1074/jbc.272.31.19518. [DOI] [PubMed] [Google Scholar]
- 25.Gunn MD, Tangemann K, Tam C, et al. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc Natl Acad Sci USA. 1998;95:258–63. doi: 10.1073/pnas.95.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hromas R, Kim CH, Klemsz M, et al. Isolation and characterization of Exodus-2, a novel C-C chemokine with a unique 37-amino acid carboxyl-terminal extension. J Immunol. 1997;159:2554–8. [PubMed] [Google Scholar]
- 27.Hedrick JA, Zlotnik A. Identification and characterization of a novel beta chemokine containing six conserved cysteines. J Immunol. 1997;159:1589–93. [PubMed] [Google Scholar]
- 28.Tanabe S, Lu Z, Luo Y, et al. Identification of a new mouse beta-chemokine, thymus-derived chemotactic agent 4, with activity on T lymphocytes and mesangial cells. J Immunol. 1997;159:5671–9. [PubMed] [Google Scholar]
- 29.Yoshida R, Imai T, Hieshima K, et al. Molecular cloning of a novel human CC chemokine EBI1-ligand chemokine that is a specific functional ligand for EBI1, CCR7. J Biol Chem. 1997;272:13803–9. doi: 10.1074/jbc.272.21.13803. [DOI] [PubMed] [Google Scholar]
- 30.Rossi DL, Vicari AP, Franz-Bacon K, et al. Identification through bioinformatics of two new macrophage proinflammatory human chemokines: MIP-3α and MIP-3β. J Immunol. 1997;158:1033–6. [PubMed] [Google Scholar]
- 31.Ngo VN, Tang HL, Cyster JG. Epstein-Barr virus–induced molecule 1 ligand chemokine is expressed by dendritic cells in lymphoid tissues and strongly attracts naive T cells and activated B cells. J Exp Med. 1998;188:181–91. doi: 10.1084/jem.188.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshida R, Nagira M, Kitaura M, et al. Secondary lymphoid-tissue chemokine is a functional ligand for the CC chemokine receptor CCR7. J Biol Chem. 1998;273:7118–22. doi: 10.1074/jbc.273.12.7118. [DOI] [PubMed] [Google Scholar]
- 33.Burgstahler R, Kempkes B, Steube K, Lipp M. Expression of the chemokine receptor BLR2/EBI1 is specifically transactivated by Epstein-Barr virus nuclear antigen 2. Biochem Biophys Res Commun. 1995;215:737–43. doi: 10.1006/bbrc.1995.2525. [DOI] [PubMed] [Google Scholar]
- 34.Schweickart VL, Raport CJ, Godiska R, et al. Cloning of human and mouse EBI1, a lymphoid-specific G-protein-coupled receptor encoded on human chromosome 17q12-q21.2. Genomics. 1994;23:643–50. doi: 10.1006/geno.1994.1553. [DOI] [PubMed] [Google Scholar]
- 35.Birkenbach M, Josefsen K, Yalamanchili R, et al. Epstein-Barr virus-induced genes: first lymphocyte-specific G protein-coupled peptide receptors. J Virol. 1993;67:2209–20. doi: 10.1128/jvi.67.4.2209-2220.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Link A, Vogt TK, Favre S, et al. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol. 2007;8:1255–65. doi: 10.1038/ni1513. [DOI] [PubMed] [Google Scholar]
- 37.Baekkevold ES, Yamanaka T, Palframan RT, et al. The CCR7 ligand elc (CCL19) is transcytosed in high endothelial venules and mediates T cell recruitment. J Exp Med. 2001;193:1105–12. doi: 10.1084/jem.193.9.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sallusto F, Palermo B, Lenig D, et al. Distinct patterns and kinetics of chemokine production regulate dendritic cell function. Eur J Immunol. 1999;29:1617–25. doi: 10.1002/(SICI)1521-4141(199905)29:05<1617::AID-IMMU1617>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 39.Gunn MD, Kyuwa S, Tam C, et al. Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J Exp Med. 1999;189:451–60. doi: 10.1084/jem.189.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vassileva G, Soto H, Zlotnik A, et al. The reduced expression of 6Ckine in the plt mouse results from the deletion of one of two 6Ckine genes. J Exp Med. 1999;190:1183–8. doi: 10.1084/jem.190.8.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carlsen HS, Haraldsen G, Brandtzaeg P, Baekkevold ES. Disparate lymphoid chemokine expression in mice and men: no evidence of CCL21 synthesis by human high endothelial venules. Blood. 106:444–6. doi: 10.1182/blood-2004-11-4353. [DOI] [PubMed] [Google Scholar]
- 42.Luther SA, Tang HL, Hyman PL, et al. Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc Natl Acad Sci USA. 2000;97:12694–9. doi: 10.1073/pnas.97.23.12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakano H, Gunn MD. Gene duplications at the chemokine locus on mouse chromosome 4: multiple strain-specific haplotypes and the deletion of secondary lymphoid-organ chemokine and EBI-1 ligand chemokine genes in the plt mutation. J Immunol. 2001;166:361–9. doi: 10.4049/jimmunol.166.1.361. [DOI] [PubMed] [Google Scholar]
- 44.Martin-Fontecha A, Sebastiani S, Höpken UE, et al. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J Exp Med. 2003;198:615–21. doi: 10.1084/jem.20030448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stoll S, Delon J, Brotz TM, Germain RN. Dynamic imaging of T cell-dendritic cell interactions in lymph nodes. Science. 2002;296(5574):1873–6. doi: 10.1126/science.1071065. [DOI] [PubMed] [Google Scholar]
- 46.Mori S, Nakano H, Aritomi K, et al. Mice lacking expression of the chemokines CCL21-ser and CCL19 (plt mice) demonstrate delayed but enhanced T cell immune responses. J Exp Med. 2001;193:207–18. doi: 10.1084/jem.193.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bajénoff M, Granjeaud S, Guerder S. The strategy of T cell antigen-presenting cell encounter in antigen-draining lymph nodes revealed by imaging of initial T cell activation. J Exp Med. 2003;198:715–24. doi: 10.1084/jem.20030167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sallusto F, Lenig D, Förster R, et al. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401(6754):708–12. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 49.Schneider MA, Meingassner JG, Lipp M, et al. CCR7 is required for the in vivo function of CD4+ CD25+ regulatory T cells. J Exp Med. 2007;204:735–45. doi: 10.1084/jem.20061405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hattori T, Chauhan SK, Lee H, et al. Characterization of Langerin-expressing dendritic cell subsets in the normal cornea. Invest Ophthalmol Vis Sci. 2011;52:4598–604. doi: 10.1167/iovs.10-6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bursch LS, Wang L, Igyarto B, et al. Identification of a novel population of Langerin+ dendritic cells. J Exp Med. 2007;204:3147–56. doi: 10.1084/jem.20071966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ginhoux F, Liu K, Helft J, et al. The origin and development of nonlymphoid tissue CD103+ DCs. J Exp Med. 2009;206:3115–30. doi: 10.1084/jem.20091756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bogunovic M, Ginhoux F, Helft J, et al. Origin of the lamina propria dendritic cell network. Immunity. 2009;31:513–25. doi: 10.1016/j.immuni.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Persson EK, Uronen-Hansson H, Semmrich M, et al. IRF4 transcription-factor-dependent CD103(+)CD11b(+) dendritic cells drive mucosal T helper 17 cell differentiation. Immunity. 2013;38:958–69. doi: 10.1016/j.immuni.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 55.Schlitzer A, McGovern N, Teo P, et al. IRF4 transcription factor-dependent CD11b+ dendritic cells in human and mouse control mucosal IL-17 cytokine responses. Immunity. 2013;38:970–83. doi: 10.1016/j.immuni.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Edelson BT, KC W, Juang R, et al. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8alpha+ conventional dendritic cells. J Exp Med. 2010;207:823–36. doi: 10.1084/jem.20091627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bedoui S, Whitney PG, Waithman J, et al. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat Immunol. 2009;10:488–95. doi: 10.1038/ni.1724. [DOI] [PubMed] [Google Scholar]
- 58.del Rio ML, Rodriguez-Barbosa JI, Kremmer E, Förster R. CD103- and CD103+ bronchial lymph node dendritic cells are specialized in presenting and cross-presenting innocuous antigen to CD4+ and CD8+ T cells. J Immunol. 2007;178:6861–6. doi: 10.4049/jimmunol.178.11.6861. [DOI] [PubMed] [Google Scholar]
- 59.Hamrah P, Liu Y, Zhang Q, Dana MR. The corneal stroma is endowed with a significant number of resident dendritic cells. Invest Ophthalmol Vis Sci. 2003;44:581–9. doi: 10.1167/iovs.02-0838. [DOI] [PubMed] [Google Scholar]
- 60.Hamrah P, Huq SO, Liu Y, et al. Corneal immunity is mediated by heterogeneous population of antigen-presenting cells. J Leukoc Biol. 2003;74:172–8. doi: 10.1189/jlb.1102544. [DOI] [PubMed] [Google Scholar]
- 61.Brissette-Storkus CS, Reynolds SM, Lepisto AJ, Hendricks RL. Identification of a novel macrophage population in the normal mouse corneal stroma. Invest Ophthalmol Vis Sci. 2002;43:2264–71. [PMC free article] [PubMed] [Google Scholar]
- 62.Chinnery HR, Humphries T, Clare A, et al. Turnover of bone marrow-derived cells in the irradiated mouse cornea. Immunology. 2008;125:541–8. doi: 10.1111/j.1365-2567.2008.02868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mayer WJ, Mackert MJ, Kranebitter N, et al. Distribution of antigen presenting cells in the human cornea: correlation of in vivo confocal microscopy and immunohistochemistry in different pathologic entities. Curr Eye Res. 2012;37:1012–8. doi: 10.3109/02713683.2012.696172. [DOI] [PubMed] [Google Scholar]
- 64.Mayer WJ, Irschick UM, Moser P, et al. Characterization of antigen-presenting cells in fresh and cultured human corneas using novel dendritic cell markers. Invest Ophthalmol Vis Sci. 2007;48:4459–67. doi: 10.1167/iovs.06-1184. [DOI] [PubMed] [Google Scholar]
- 65.Hamrah P, Zhang Q, Liu Y, Dana MR. Novel characterization of MHC class II-negative population of resident corneal Langerhans cell-type dendritic cells. Invest Ophthalmol Vis Sci. 2002;43:639–46. [PubMed] [Google Scholar]
- 66.Merad M, Ginhoux F, Collin M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat Rev Immunol. 2008;8:935–47. doi: 10.1038/nri2455. [DOI] [PubMed] [Google Scholar]
- 67.Segura E, Amigorena S. Inflammatory dendritic cells in mice and humans. Trends Immunol. 2013;34:440–5. doi: 10.1016/j.it.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 68.Leonardi A, Fregona IA, Plebani M, et al. Th1- and Th2-type cytokines in chronic ocular allergy. Graefes Arch Clin Exp Ophthalmol. 2006;244:1240–5. doi: 10.1007/s00417-006-0285-7. [DOI] [PubMed] [Google Scholar]
- 69.Stern ME, Siemasko KF, Niederkorn JY. The Th1/Th2 paradigm in ocular allergy. Curr Opin Allergy Clin Immunol. 2005;5:446–50. doi: 10.1097/01.all.0000182547.60595.64. [DOI] [PubMed] [Google Scholar]
- 70.Metz DP, Hingorani M, Calder VL, et al. T-cell cytokines in chronic allergic eye disease. J Allergy Clin Immunol. 1997;100(6 Pt 1):817–24. doi: 10.1016/s0091-6749(97)70279-3. [DOI] [PubMed] [Google Scholar]
- 71.Paul W. Fundamental immunology. 6 Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 72.Niederkorn JY, Stern ME, Pflugfelder SC, et al. Desiccating stress induces T cell-mediated Sjögren's Syndrome like lacrimal keratoconjunctivitis. J Immunol. 2006;176:3950–7. doi: 10.4049/jimmunol.176.7.3950. [DOI] [PubMed] [Google Scholar]
- 73.Sall K, Stevenson OD, Mundorf TK, Reis BL. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 Study Group. Ophthalmology. 2000;107:631–9. doi: 10.1016/s0161-6420(99)00176-1. [DOI] [PubMed] [Google Scholar]
- 74.Stern ME, Schaumburg CS, Siemasko KF, et al. Autoantibodies contribute to the immunopathogenesis of experimental dry eye disease. Invest Ophthalmol Vis Sci. 2012;53:2062–75. doi: 10.1167/iovs.11-9299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang X, Chen W, De Paiva CS, et al. Desiccating stress induces CD4+ T-cell-mediated Sjögren's syndrome-like corneal epithelial apoptosis via activation of the extrinsic apoptotic pathway by interferon-γ. Am J Pathol. 2011;179:1807–14. doi: 10.1016/j.ajpath.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rahimy E, Pitcher JD, 3rd, Pangelinan SB, et al. Spontaneous autoimmune dacryoadenitis in aged CD25KO mice. Am J Pathol. 2010;177:744–53. doi: 10.2353/ajpath.2010.091116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stern ME, Gao J, Schwalb TA, et al. Conjunctival T-cell subpopulations in Sjögren's and non-Sjögren's patients with dry eye. Invest Ophthalmol Vis Sci. 2002;43:2609–14. [PubMed] [Google Scholar]
- 78.Dohlman TH, Chauhan SK, Kodati S, et al. The CCR6/CCL20 axis mediates Th17 cell migration to the ocular surface in dry eye disease. Invest Ophthalmol Vis Sci. 2013;54:4081–91. doi: 10.1167/iovs.12-11216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.El Annan J, Goyal S, Zhang Q, et al. Regulation of T-cell chemotaxis by programmed death-ligand 1 (PD-L1) in dry eye-associated corneal inflammation. Invest Ophthalmol Vis Sci. 2010;51:3418–23. doi: 10.1167/iovs.09-3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.El Annan J, Chauhan SK, Ecoiffier T, et al. Characterization of effector T cells in dry eye disease. Invest Ophthalmol Vis Sci. 2009;50:3802–7. doi: 10.1167/iovs.08-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chauhan SK, El Annan J, Ecoiffier T, et al. Autoimmunity in dry eye is due to resistance of Th17 to Treg suppression. J Immunol. 2009;182:1247–52. doi: 10.4049/jimmunol.182.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Goyal S, Chauhan SK, Dana R. Blockade of prolymphangiogenic vascular endothelial growth factor C in dry eye disease. Arch Ophthalmol. 2012;130:84–9. doi: 10.1001/archophthalmol.2011.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee HS, Hattori T, Park EY, et al. Expression of Toll-like receptor 4 contributes to corneal inflammation in experimental dry eye disease. Invest Ophthalmol Vis Sci. 2012;53:5632–40. doi: 10.1167/iovs.12-9547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Koldati S, Chauhan SK, Chen Y, Dana R. Chemokine receptor expression on corneal CD11b+ cells in dry eye disease. Invest Ophthalmol Vis Sci. 2011;52 ARVO Abstract# 3762. [Google Scholar]
- 85.Turpie B, Yoshimura T, Gulati A, et al. Sjögren's syndrome-like ocular surface disease in thrombospondin-1 deficient mice. Am J Pathol. 2009;175:1136–47. doi: 10.2353/ajpath.2009.081058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Contreras-Ruiz L, Regenfuss B, Mir FA, et al. Conjunctival inflammation in thrombospondin-1 deficient mouse model of Sjögren's syndrome. PLoS One. 2013;8:e75937. doi: 10.1371/journal.pone.0075937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Niederkorn JY. Corneal transplantation and immune privilege. Int Rev Immunol. 2013;32:57–67. doi: 10.3109/08830185.2012.737877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hattori T, Saban DR, Emami-Naeini P, et al. Donor-derived, tolerogenic dendritic cells suppress immune rejection in the indirect allosensitization-dominant setting of corneal transplantation. J Leukoc Biol. 2012;91:621–7. doi: 10.1189/jlb.1011500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Huq S, Liu Y, Benichou G, Dana MR. Relevance of the direct pathway of sensitization in corneal transplantation is dictated by the graft bed microenvironment. J Immunol. 2004;173:4464–9. doi: 10.4049/jimmunol.173.7.4464. [DOI] [PubMed] [Google Scholar]
- 90.Chen L, Hamrah P, Cursiefen C, et al. Vascular endothelial growth factor receptor-3 mediates induction of corneal alloimmunity. Nat Med. 2004;10:813–5. doi: 10.1038/nm1078. [DOI] [PubMed] [Google Scholar]
- 91.Saito T, Shinagawa K, Takenaka K, et al. Ocular manifestation of acute graft-versus-host disease after allogeneic peripheral blood stem cell transplantation. Int J Hematol. 2002;75:332–4. doi: 10.1007/BF02982052. [DOI] [PubMed] [Google Scholar]
- 92.Kawasaki S, Nishida K, Sotozono C, et al. Conjunctival inflammation in the chronic phase of Stevens-Johnson syndrome. Br J Ophthalmol. 2000;84:1191–3. doi: 10.1136/bjo.84.10.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bernauer W, Wright P, Dart JK, et al. The conjunctiva in acute and chronic mucous membrane pemphigoid. An immunohistochemical analysis. Ophthalmology. 1993;100:339–46. doi: 10.1016/s0161-6420(93)31644-1. [DOI] [PubMed] [Google Scholar]
- 94.Frank GM, Buela KA, Maker DM, et al. Early responding dendritic cells direct the local NK response to control herpes simplex virus 1infection within the cornea. J Immunol. 2012;188:1350–9. doi: 10.4049/jimmunol.1101968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gao Y, Li Z, Hassan N, et al. NK cells are necessary for recovery of corneal CD11c+ dendritic cells after epithelial abrasion injury. J Leukoc Biol. 2013;94:343–51. doi: 10.1189/jlb.1212633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gao N, Yin J, Yoon GS, et al. Dendritic cell-epithelium interplay is a determinant factor for corneal epithelial wound repair. Am J Pathol. 2011;179:2243–53. doi: 10.1016/j.ajpath.2011.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Steinman RM. Some active areas of DC research and their medical potential. Eur J Immunol. 2010;40:2085–8. doi: 10.1002/eji.201040733. [DOI] [PubMed] [Google Scholar]
- 98.Schieffer B, Luchtefeld M. Emerging role of chemokine receptor 7 in atherosclerosis. Trends Cardiovasc Med. 2011;21:211–6. doi: 10.1016/j.tcm.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 99.Burman A, Haworth O, Hardie DL, et al. A chemokine-dependent stromal induction mechanism for aberrant lymphocyte accumulation and compromised lymphatic return in rheumatoid arthritis. J Immunol. 2005;174:1693–700. doi: 10.4049/jimmunol.174.3.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kawashima D, Oshitani N, Jinno Y, et al. Augmented expression of secondary lymphoid tissue chemokine and EBI1 ligand chemokine in Crohn's disease. J Clin Pathol. 2005;58:1057–63. doi: 10.1136/jcp.2004.024828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ohbayashi M, Manzouri B, Flynn T, et al. Dynamic changes in conjunctival dendritic cell numbers, anatomical position and phenotype during experimental allergic conjunctivitis. Exp Mol Pathol. 2007;83:216–23. doi: 10.1016/j.yexmp.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 102.Dieu MC, Vanbervliet B, Vicari A, et al. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med. 1998;188:373–86. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Birke K, Lütjen-Drecoll E, Kerjaschki D, Birke MT. Expression of podoplanin and other lymphatic markers in the human anterior eye segment. Invest Ophthalmol Vis Sci. 2010;51:344–54. doi: 10.1167/iovs.08-3307. [DOI] [PubMed] [Google Scholar]




