Abstract
The current state of knowledge on how copper metallochaperones support the maturation of cuproproteins is reviewed. Copper is needed within mitochondria to supply the CuA and intramembrane CuB sites of cytochrome oxidase, within the trans-Golgi network to supply secreted cuproproteins and within the cytosol to supply superoxide dismutase 1 (Sod1). Subpopulations of copper-zinc superoxide dismutase also localize to mitochondria, the secretory system, the nucleus and, in plants, the chloroplast, which also requires copper for plastocyanin. Prokaryotic cuproproteins are found in the cell membrane and in the periplasm of gram-negative bacteria. Cu(I) and Cu(II) form tight complexes with organic molecules and drive redox chemistry, which unrestrained would be destructive. Copper metallochaperones assist copper in reaching vital destinations without inflicting damage or becoming trapped in adventitious binding sites. Copper ions are specifically released from copper metallochaperones upon contact with their cognate cuproproteins and metal transfer is thought to proceed by ligand substitution.
Keywords: P1-type ATPase, cytochrome oxidase, Cox17, CopZ, Atx1, Ccs1
Introduction
As cofactors for enzymes, copper ions are required for processes ranging from oxidative phosphorylation, mobilization of iron, connective tissue cross-linking, pigment formation, neuropeptide amidation, catecholamine synthesis, and antioxidant defense. Copper has additional biological roles that may be distinct from serving as a catalytic moiety in cuproenzymes, for example, in the innate immune response, in the modulation of synaptic transmission, and in angiogenesis. A consequence of these vital functions is that copper deficiency has profound clinical outcomes often associated with neurodegeneration. Dietary intake of copper salts generally exceeds tissue demands, so homeostatic mechanisms exist to modulate uptake and facilitate export through the bile.
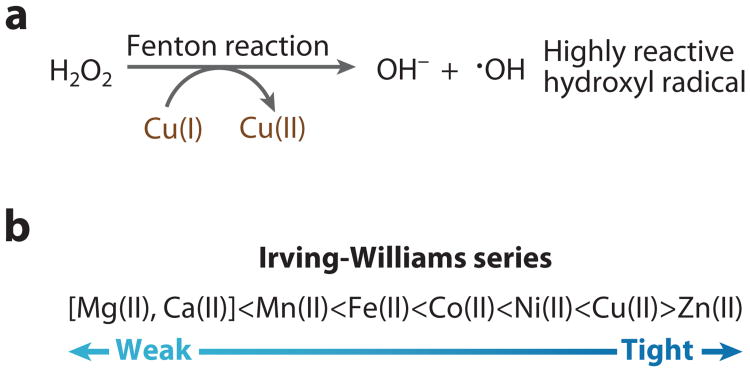
Impairment in biliary copper excretion results in liver and brain copper overload in patients with Wilson's disease. Copper's ability to accept and donate single electrons make it an ideal redox cofactor, but copper ions are also complicit in the Fenton reaction and hence capable of driving the generation of deleterious hydroxyl radicals (Figure 1a). Cu(II) is located at the top of the Irving-Williams series, and Cu(I) is also highly competitive, and hence, all copper ions have the potential to displace less competitive metals from metalloproteins (Figure 1b). For all of these reasons, cellular copper pools must be tightly balanced to sustain a sufficent supply while minimizing toxic effects.
Figure 1.
(a) Copper catalyzes production of hydroxyl radicals via the Fenton reaction.
(b) Copper has a tendency to form stable complexes relative to other essential divalent metals, and hence it is at the top of the Irving-Williams series.
(b) Monovalent copper is also competitive, forming tight protein complexes.
In the 1990s, a class of protein, designated copper metallochaperones, was discovered that functions as intracellular Cu(I) ion shuttles distributing Cu(I) ions to specific partner proteins, thereby overcoming a high copper chelation capacity of the cytoplasm. In many cells, the abundance of Cu(I)-buffering metallothionein and glutathione (GSH) molecules results in a cytoplasm depleted of free copper ions. The discoveries of the Atx1 and Ccs1 metallochaperones and the elucidation of their structures were reviewed in the 2001 edition of the Annual Review of Biochemistry (1). This review focuses on subsequent advances, including the mechanisms of copper transfer, copper metallochaperone-dependent and -independent maturation of superoxide dismutase and the assembly of the copper sites of cytochrome oxidase.
The Copper Proteome
Attempts have been made to define the copper proteome (cuproproteome) using genomic sequence data (2, 3). Ten distinct Cu-containing proteins have been identified in prokaryotes, but wide fluctuations exist in the distribution of these proteins within species (2). Analyses of prokaryotic and archean genomes indicated that nearly 72% of bacteria and 31% of archean species utilize copper (2). The cuproproteome distribution may actually be wider if novel copper proteins exist. Bioinformative searches using 39 Cu-binding motif signatures revealed additional putative Cu-binding proteins in Bacteria and Archaea (3). Over 70% of the putative cuproproteins identified in prokaryotes have homologs in Archaea and Eukaryotes. The most frequently utilized cuproprotein is cytochrome c oxidase. The second most common cuproprotein is NADH dehydrogenase-2 (2). Secretory cuproenzymes include dopamine-β-hydroxylase, tyrosinase, ceruloplasmin, lysyl oxidase, and amine oxidases. Certain bacterial phyla (Thermotogae, Chlorobi, Lactobacillales, and Mollicutes) lack known copper enzymes or copper metallochaperones. However, most of these species contain copper effluxers likely as a defense against the deleterious effect of copper ions in the cytoplasm.
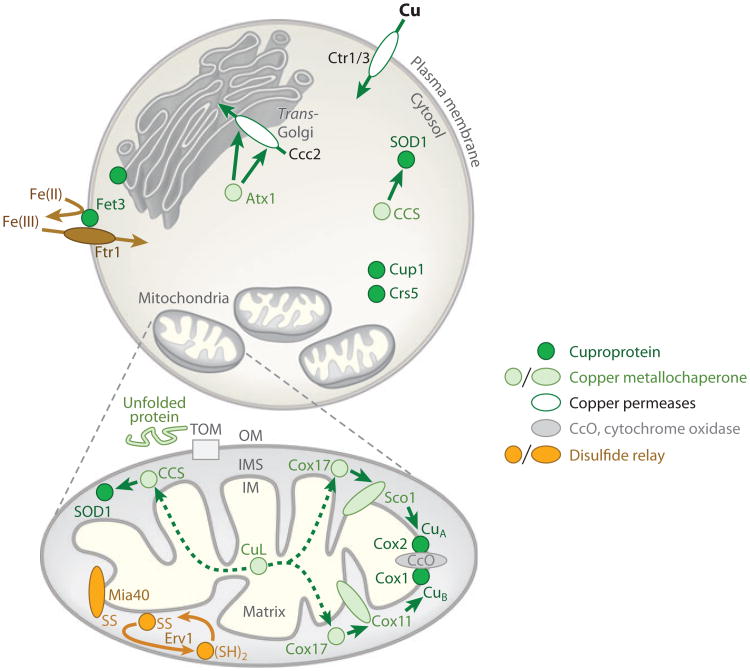
Compartmentalization of the eukaryotic cuproproteome is well established (Figure 2). Cuproenzymes are localized within the plasma membrane and the periplasm of gram-negative bacteria. Within plants, copper is required in six compartments, including the cytoplasm, endoplasmic reticulum, mitochondria, chloroplast stroma, thylakoid lumen, and apoplast (4). Copper redistribution between compartments can occur upon transitions to copper deficiency (5).
Figure 2.
Copper is passed (green arrows) to cuproproteins (dark green circles), including metallothioneins Crs5 and Cup1, in for example Saccharomyces cerevisiae, by copper metallochaperones (pale green circles, or ovals if membrane associated). Copper permeases (white ovals) import copper into the cell or deliver Cu(I) to compartments, such as the trans-Golgi network where secreted cuproproteins (such as Fet3) acquire copper. Fet3 oxidizes ferrous ions (curved brown arrow) to provide substrate for high-affinity iron import by Ftr1. P1-type ATPases (such as Ccc2) have one or more soluble N-terminal domains, which engage in regulatory interactions with copper metallochaperones (such as Atx1), but additional Cu(I) donation (hence two arrows) may supply the trans-membrane regions for Cu(I) transport. Metallation of the cytochrome c oxidase complex (CcO) in mitochondria (OM, outer membrane of mitochondria) involves a pathway via Cox11 for the CuB site of the Cox1 subunit, embedded in the inner membrane (IM), and via Sco1 for the CuA site of Cox2, protruding into the intermembrane space (IMS). Cox17 supplies both pathways. Nuclear encoded mitochondrial proteins, such as Cox17 and Ccs1, are imported across the OM unfolded (pale green line) via the TOM translocase then captured in the IMS, following introduction of disulfide bonds (SS) through the actions of Mia40. A sulfhydryl oxidase Erv1 generates a reactive disulfide on Mia40. A small copper ligand (CuL) supplies Cu(I) (dashed arrows) to the IMS copper metallochaperones.
Cuproprotein Metallation Reactions
In general, metallation reactions are expected to occur during protein biosynthesis, or shortly thereafter, because many metal cofactors influence protein stability. On cytoplasmic ribosomes, protein biosynthesis and chain elongation are coupled to chaperone-mediated protein folding for many proteins. Nascent polypeptides are protected within the ribosome and can only fold as they emerge from the exit tunnel. Subsequently, molecular chaperones bind nascent polypeptides and actively guide the polypeptides in folding through cycles of binding and release. Whereas cytosolic metalloproteins are likely metallated at this stage, because many cuproenzymes are either compartmentalized or secreted their folding and metallation must occur after translation and translocation. Secretory cuproenzymes pass through the endoplasmic reticulum lumen, where folding reactions commence, and maturation continues during transit through the Golgi apparatus. Copper metallation occurs within Golgi vesicles.
Proteins discriminate between metal ions imperfectly, at least in vitro, yet biology seems to achieve precision in metallation reactions. Metallation is influenced by preferences in coordination geometry and in polarizability of ligand donor atoms. Computational chemistry studies infer that the specificity of a metal for a set of ligands in a protein cavity depends mainly on the metal's natural abundance in the biological locality, tailored by the properties of the protein and metal(6). For example, two structurally related cyanobacterial proteins, MncA and CucA, are the predominant manganese- and copper-containing proteins in the periplasm but have similar metal-binding sites (7). The mangano-protein prefers to bind Cu(II), or Cu(I), or Zn(II) rather than Mn(II) in vitro (7). However, precise metallation is achieved by differential compartmentalization during the metallation reactions. The mangano-protein, MncA, is dependent on incorporation of Mn(II) within the cytoplasm, where copper and zincare highly restricted. Metallated MncA is then secreted into the periplasm as a holoenzyme via the Tat pathway. In contrast, metallation of the copper protein, CucA, occurs after export of the nascent chain via the Sec system. Another strategy for precision of metallation is through the actions of metallochaperones.
Copper Metallochaperones for Copper Transporters
Bacterial copZ was discovered in the nucleotide sequences adjacent to a gene encoding a copper-transporting P1-type ATPase (8), whereas yeast Atx1 was cloned as a suppressor of oxygen sensitivity in strains lacking superoxide dismutase (9). Studies of these two small proteins (69 residues for CopZ and 73 for Atx1) established the concept of copper metallochaperones. Atx1 and CopZ are similar in amino acid sequence to the N-terminal regions of metal-transporting P1-type ATPases, including the one (CopA) from the same operon as CopZ, human Menkes and Wilson ATPases and the P-type ATPase from yeast, Ccc2. All of these proteins have a metal-binding motif MXCXXC (where X is any amino acid) associated with the first loop of a βαββαβ (ferredoxin) structural fold. The single βαββαβ-fold of Atx1 or CopZ is replicated once (CopA), or twice (Ccc2), or six (Menkes and Wilson) times in the cytosolic regions of the ATPases (1). Copper metallochaperones that are variants of this structural theme occur in plants and Archeae. CopZ or Atx1-like copper metallochaperones are not obligatory partners for copper-transporting P1-type ATPases since some genomes encode the ATPases but no homologs of these metallochaperones (10).
Yeast mutants deficient in Atx1 are deficient in iron uptake owing to impaired copper supply to the multicopper oxidase Fet3 (Figure 2). Extracellular Fet3 activity is needed to generate ferric substrate for the high-affinity iron importer Ftr1. As noted above, copper metallation of secreted proteins occurs within Golgi vesicles, and this is true of Fet3. The Ccc2 ATPase transports copper into this compartment, and thus Atx1 is phenotypically linked to the activity of the ATPase. Evidence that Atx1 forms associations with the N-terminal region of Ccc2 and that the human homolog Atox1 (sometimes called Hah1) forms associations with the Menkes or Wilson ATPases was obtained from coimmunoprecipitations and Cudependent two-hybrid interactions, whereas copper transfer between pairs of recombinant proteins was observed in vitro. A model was proposed in which these copper metallochaperones donate metal ions to the N-terminal regions of the ATPases via a sequence of ligand-exchange reactions. At the time of the 2001 Annual Review of Biochemistry article (1), solution structures had been obtained for apo-Atx1, Cu(I)-Atx1, as well as for the apo and Cu(I) forms of one domain of Ccc2 (Ccc2a), plus an X-ray crystal structure was known for the homodimer of Cu(I)-Hah1, among others. The homodimer structure was used to generate models of docked heterodimers (11). Solution structural studies have now examined these anticipated heterodimers.
Visualization of Adducts with Cytosolic Domains of P1-Type ATPases
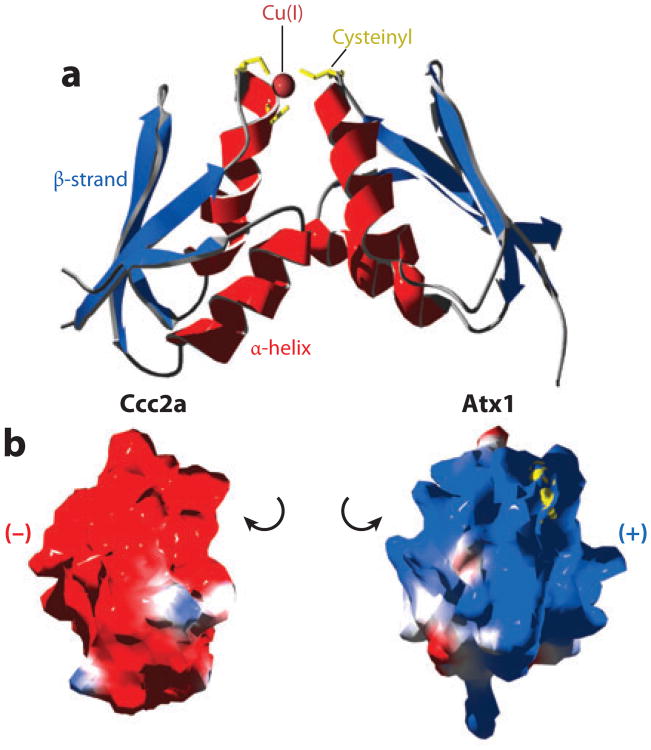
A solution structure of an Atx1-Cu(I)-Ccc2a heterodimer has been generated (Figure 3) (12). The interaction is dependent upon copper, with the first cysteine of each protein (Cys15 of Atx1 and Cys13 of Ccc2a) being essential for complex formation (12). This seems reasonable because Cys15 of Atx1 is the more solvent accessible of its cysteine pair. A collection of lysine residues (residues 24, 28, 62, and 65) confer complementary surface charge to a negative surface of Ccc2a. Conversion of these lysines to glutamate impairs Atx1 activity as assessed by loss of yeast two-hybrid interactions with Ccc2a and poor ferrous 55Fe uptake by yeast strains carrying these Atx1 variants (13). Thus, electrostatics enables encounter complexes to form between the copper metallochaperone and the P1-type ATPase (Figure 3). However, the encounter complex must be stabilized by coincident coordination of copper through cysteine residues from both partners if the transient complex is to persist for a sufficient length of time to enable detection by NMR. The complex is also predicted to be transient in vivo as Atx1 is largely found in the cytoplasm rather than associated with Golgi membrane preparations. A possible sequence for the exchange of metal-binding ligands between the two proteins was proposed with Cys13 of Ccc2a first invading a digonally coordinated Cu(I)-Atx1 site after which the second Cys of Ccc2a replaces the second Cys of Atx1. An expectation is that the heterodimer dissociates upon formation of a bisthiolate Cu(I) complex with Ccc2a (Figure 4), although there is only a small change in free energy associated with the transfer of Cu(I) from Atx1 to Ccc2a (14).
Figure 3.
(a) NMR solution structural model [Protein Data Bank (PDB) code 2GGP] of a heterodimer of the S. cerevisiae copper metallochaperone Atx1 and one of two N-terminal cytosolic domains of its cognate P1-type copper-transporting ATPase Ccc2. Cysteinyl (yellow) thiol coordinated Cu(I) (sphere) stabilizes a transient complex between similar βαββαβ (ferredoxin)-structural folds (red, α-helix; blue, β-strand). (b) The interacting faces of Ccc2a and Atx1 have complementary (red, negative; blue, positive) electrostatic potentials.
Figure 4.
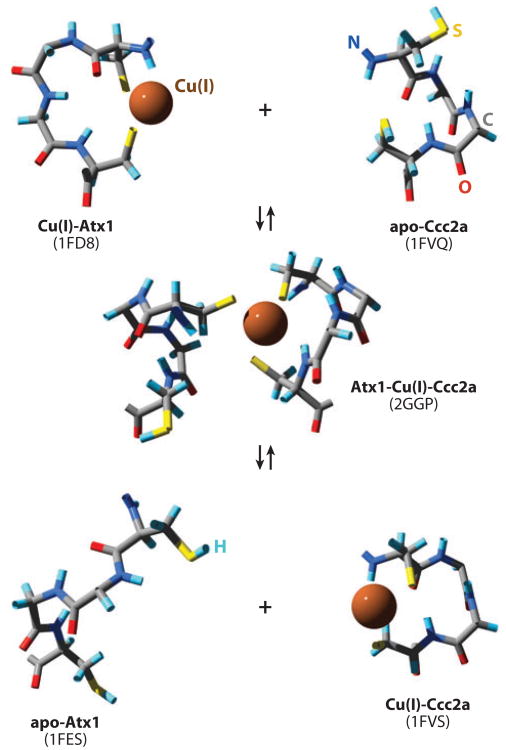
Visualization of the Cys-Xaa-Xaa-Cys motifs taken from NMR solution structures (Protein Data Bank codes indicated) of the apo-, Cu(I)-, and heterodimeric forms of Atx1 and Ccc2a to represent the transfer of Cu(I) from Atx1 to Ccc2a via ligand-exchange reactions.
The Transfer of Copper
Copper transfer from Atx1 to Ccc2a in vitro was unaffected by the presence of a 50-fold molar excess of GSH, which was added to chelate any released Cu(I) (14). The off rate from Cu(I)-Atx1 to solvent is thought to be negligible, such that transfer to Ccc2a can only occur by direct protein contact and not via free solution. Presumably, the off rate for copper is enhanced in the heterodimer owing to conformation changes induced in Atx1 by its partner. This predicted switch in the Cu(I)-Atx1 off rate has not been measured, although structural data have suggested what might trigger a change. Loop 5 of Atx1 contains a lysine residue (Lys65), which is conspicuously conserved in many homologs from other species. Lys65 is thought to serve as a counter ion, balancing the negative charge of a metal site in which Cu(I) is bound to two thiolates and shielding the site. In adducts with Ccc2a, this residue is displaced away from the metal-binding site (12). Thus, movement of Lys65 in adducts opens the Atx1 metal site for ligand invasion by Ccc2a. The metal-binding site of apo-Atx1 is disordered, whereas that of apo-Ccc2a is structured (12), perhaps also contributing a small free-energy change to encourage metal release from the former to the latter.
The bacterial copper metallochaperones, CopZ from Enterococcus hirae (8, 15) and from Bacillus subtilis (16, 17), plus ScAtx1 from Synechocystis PCC 6803 (18), have a tendency to multimerize when bound to copper. These copper metallochaperone homodimers are presumed to dissociate to allow heterodimers to form with the ATPases. In the bacterial systems, the electrostatics are reversed relative to yeast Atx1-Cu(I)-Ccc2a, with CopZ and ScAtx1 presenting negative faces to positive ones on the respective P-type ATPase domains. Bioinformatics initially suggested that the CopA P-type ATPase of B. subtilis may import copper; however, 15N chemical shift differences attributed to CopZ-Cu(I)-CopA showed that the metal-binding region of CopZ adopts an apo-like form and that CopA adopts a Cu(I)-like form in the heterodimer, more consistent with a vector for copper transfer from the copper metallochaperone to the ATPase (17). In support of this prediction, ΔcopA B. subtilis was subsequently shown to be hypersensitive to elevated copper and deficient in copper export not import (19). Indeed, there is now uncertainty about whether any P1-type ATPases import copper, although this was first proposed for CopA from E. hirae and remains the simplest model for cyanobacterial CtaA on the basis of the phenotypes of ΔctaA strains.
Copper metallochaperones can direct Cu(I) to bona fide destinations but also keep it away from aberrant ones. Zinc-transporting P1-type ATPases, such as ZntA and ZiaA, also possess βαββαβ-folds plus CXXC metal-binding sites, akin to those of copper transporters (20, 21). In ZiaA, from the cyanobacterium Synechocystis PCC 6803, the metal site has been shown to have a preference for Cu(I) over Zn(II). However, the N-terminal domain of ZiaA does not form heterodimers with the copper metallochaperone Cu(I)-ScAtx1 (22). Electrostatics repulses encounter complexes between ZiaA and ScAtx1, while complexes between ScAtx1 and related copper transporters CtaA and PacS are not repulsed. This mechanism provides a kinetic barrier to inhibit aberrant association of Cu(I) with ZiaA (21). Whereas most bacteria have no cytoplasmic membranous compartments and no known copper-requiring enzymes in the cytoplasm, cyanobacteria, such as Synechocystis PCC 6803, are peculiar in possessing thylakoid membranes housing plastocyanin and a caa3-type cytochrome oxidase. CtaA, PacS, and ScAtx1 act positively with respect to thylakoid cuproproteins, and crucially, the ATPases are nonredundant (22, 23). PacS has been localized to thylakoid membranes (24), suggesting a model in which CtaA imports copper at plasma membranes while PacS loads it into thylakoids, analogous to the supply of copper to cuproproteins within the Golgi in eukaryotes. This model, however, relies upon CtaA acting to import copper, which has not been demonstrated for a P1-type ATPase. A credible alternative model is that CtaA transports copper into thylakoids but at a different stage in their biogenesis than when PacS is active. For example, copper must be supplied twice to tyrosinase in mammals, transiently by the Menkes ATPase in Golgi and again by the Menkes ATPase in specialized melanosome compartments derived from the Golgi (25). Copper binding is necessary to stabilize tyrosinase in the Golgi, but its folding state, coupled with the ionic conditions in the earlier Golgi network, is thought to lead to release of the initial copper atoms.
Copper Donation to Membrane Sites
Because there is only a small free-energy change associated with metal transfer from copper metallochaperones to the N-terminal regions of P1-type ATPase, it has been widely assumed that irreversible membrane translocation driven by the hydrolysis of ATP is what shifts the equilibrium by removing the Cu(I)-ATPase product. However, there is no direct evidence that copper acquired by an N-terminal metal-binding domain of a P1-type ATPase is transferred to trans-membrane regions for transport. CopZ from Archaeoglobus fulgidus interacts with, and can donate metal to, soluble βαββαβ-domains of CopA from the same organism (26). Importantly, Cu(I)-CopZ can also activate ATPase activity of isolated CopA, even when the ATPase is missing its own soluble βαββαβ-domain or when Cu-binding Cys residues have been converted to Ala. In contrast, the purified Cu(I)-bound βαββαβ-domain of CopA, expressed without the rest of the ATPase, does not activate the variant CopA missing the same domain. Under nonturnover conditions (in the absence of Mg2+-ATP), irreversible copper transfer can be detected between Cu(I)-CopZ and the membrane metal-binding sites of CopA (26). It is possible that the transfer of metal from copper metallochaperones to the N-terminal regions of P1-type ATPases serves regulatory roles and that separate donation events to the metal sites located in the membranous regions might be a common requirement.
The metal-binding domain of CopA interacts with the nucleotide-binding domain of the ATPase, and this interaction is impaired upon Cu(I) binding or nucleotide binding (27, 28). Thus, Cu(I) donation to the N-terminal region could serve to regulate Cu(I) transport by modulating this internal interaction within the ATPase. It is notable that CopZ from A. fulgidus is atypical in structure, having an extra 130-residue N-terminal domain that contains both zinc and a 2Fe2S cluster (29).
The cytosolic domain of the Wilson ATPase containing its ATP-binding site also interacts with the N-terminal βαββαβ-regions in vitro, and these interactions are also inhibited by Cu(I) binding (30). Donation of copper to the N-terminal regions may thus expose the ATP-binding site to assist catalytic turnover in response to increased abundance of Cubound metallochaperone. Both the Menkes and WilsonATPases also undergo metal-dependent shifts in their cellular localization when copper becomes elevated, and the N-terminal βαββαβ-regions of the Menkes ATPase are known to influence trafficking (31–33).
The Vmax of CopA from A. fulgidus was greater with Cu(I)-CopZ rather than Cu(I) alone (26). The dynamics of copper release from human Atox1 have been investigated by using the Cu(I) chelator bicinchonic acid (BCA), which forms a Cu(I) BCA2 complex (34). Transfer progresses via formation of an Atox1-Cu(I)-BCA intermediate before ligand invasion by a second molecule of BCA. Lack of release to GSH presumably implies that the thiol of GSH is unable to invade the Atox1 Cu(I) site in the same manner as BCA. BCA acquires copper more rapidly from Cu(I)-Atox1 than it does from buffer alone because Cu(I)-dithiothreitol complexes dissociate more slowly than the copper metallochaperone. The rate of release to BCA is further enhanced using Atox1 mutant proteins in which alanine residues are substituted for either the shielding lysine or the methionine of the MXCXXC motif. In both cases, rapid release from mutant Atox1 occurs because the second molecule of BCA more readily displaces Atox1 from the intermediate adduct relative to wild-type Atox1 (34). Copper metallochaperones can thus make copper swiftly accessible to the correct substrates capable of ligand invasion, presumably including membranous metal-binding sites of P1-type ATPases.
Copper Acquisition by Atx1-Like Copper Metallochaperones
Yeast mutants missing any of the diverse types of copper permeases still retain functional copper metallochaperones, implying that there is no single preferred donor (35). Furthermore, because the permeases are so varied, it seems improbable that they can all support specific interactions with copper metallochaperones. The electron crystallographic structure of the Ctr1 copper pore may reveal a cytosolic domain with a candidate docking site for Atx1 (36). Copper transfer between the cytosolic regions of Ctr1 and Atx1 does occur in vitro, although the estimated affinities of the two proteins are similar at about 10−19 M(37, 38). Constitutive overexpression of competing Cu(I)-binding proteins, such as the metallothioneins Crs5 and Cup1, in the cytosol does not interfere with Cu(I) acquisition by copper metallochaperones. One of the burning questions is how do cells prioritize copper supply? An intriguing notion is that this could be achieved if some, but not all, copper metallochaperones gained access to metal ions released at cuproprotein turnover. Atx1 targets would be subordinate if Atx1 solely obtained copper from de novo import, and the primacy of cytochrome oxidase could be sustained provided its copper metallochaperones had preferential access to recycled copper.
Handling Copper in a More Oxidizing Periplasm
A five-stranded β-barrel protein, CusF, involved in the efflux of surplus copper from the periplasm of gram-negative bacteria, such as Escherichia coli, has been added to the catalog of copper metallochaperones. CusF has an unusual copper site, involving a histidine imidazole, two methionine thioethers, plus a π-interaction from the aromatic ring of tryptophan (39–41). The methionine thioether group appears as a Cu ligand in sites located within more oxidizing environments, for example, in copper permeases such as Ctr1 (42). The E. coli CusBCA proteins are thought to form a complex that straddles the inner and outer membranes to couple the inner-membrane (IM) proton motive force to copper efflux across the outer membrane. It is predicted that the substrate is acquired in the periplasm rather than the cytoplasm. By using selenomethionine derivatives to distinguish the metal sites, it was possible to follow movement of copper from Cu(I)-SeMet-CusF to apo-CusB by X-ray absorption spectroscopy (43). The proteins have closely matched affinities, leading to a suggestion that metal transfer might be driven by removal of the Cu(I)-CusB product through CusBCA-mediated efflux, analogous to the arguments proposed for the driving force for copper transfer from Atx1 to Ccc2.
Copper Metallochaperones for Cytochrome Oxidase
Unlike Atx1 and Ccs1 that donate Cu(I) directly to the target molecule, Cox17-mediated Cu(I) donation to cytochrome c oxidase (CcO) subunits involves two accessory factors Cox11 and Sco1, which function in the metallation of the CuB site in Cox1 and CuA site in Cox2, respectively. Both Cox11 and Sco1 are IM-associated proteins with Cu(I)-binding globular domains protruding into the intermembrane space (IMS). Cox17-mediated Cu(I) transfer to Cox11 and Sco1 is the initial event, followed by the subsequent transfer to Cox1 and Cox2, respectively. Cu(I) donation to Cox11 and Sco1 is believed to impart specificity to the Cu(I) transfer to CcO subunits during CcO biogenesis. One difference between the Cu(I) metallation of Cox1 and Cox2 is that the CuA site in Cox2 is localized 8 Å above the IM, whereas the CuB site in Cox1 is buried 13 Å below the membrane surface (44).
Cox17
Cox17 was cloned from Saccharomyces cerevisiae from a CcO deficiency mutant. Cells lacking Cox17 were respiratory deficient and unable to propagate on nonfermentable carbon sources (45). Mitochondria isolated from the mutant cells contained both mitochondrial and nuclear CcO subunits, precluding a role for Cox17 in the expression or import of the subunit polypeptides. The mutant cells lacked the CcO-specific heme a, but not heme b found in other respiratory complexes or cytochrome c (45). Insight into the role of Cox17 in CcO biogenesis was gleaned by the observation that cox17Δ cells regained an ability to propagate on glycerol and ethanol medium with the addition of 0.4% copper salts. Because the mutant cells contained a functional Sod1 superoxide dismutase, the role of Cox17 is specific to CcO and/or mitochondria. Mice lacking Cox17 die during early embryogenesis at a stage similar to mice lacking the copper transporter Ctr1 (46, 47). Prior to embryonic death, CcO activity was severely impaired in COX17 -/- mice. Depletion of COX17 mRNA, using siRNA, in HeLa cells resulted in an induced CcO deficiency (48). Steady-state levels of Cox1 and Cox2 were diminished following the knockdown, but the other respiratory complexes retained the same magnitude of abundance as in wild-type (WT) cells.
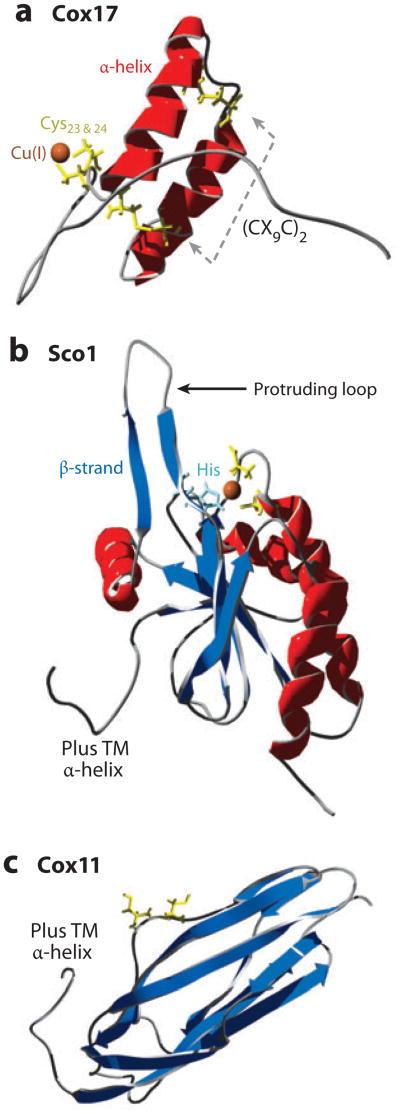
Cox17 is a small hydrophilic protein of 63 residues in humans. It contains a conserved twin CX9C motif that forms a helical hairpin configuration (Figure 5). This helical hairpin is also referred as a CHCH (coiled-coil1 helix1 coiled-coil2 helix2) motif (49). The twin CX9C structural motif is found in numerous IMS proteins; several of these, including Cox19, Cox23, and Pet191, are important in CcO biogenesis. The Cox12 structural subunit of CcO projecting into the IMS also contains the twin CX9C motif. The cysteines within the twin CX9C motif are present in two disulfides, stabilizing the helical hairpin of Cox17 (Figure 5a) (50, 51). The redox couple of the double disulfide configuration of Cox17 to the fully reduced state has a midpoint potential of −294 mV, consistent with the dual disulfide molecule being the likely species in vivo, considering that the IMS redox potential is approximately −255 mV unlike the more-reducing potential of the cytoplasm (−296 mV) (52, 53). In support of the disulfide configuration of Cox17 within the IMS is the observation that other Cys-rich helical hairpin proteins within the IMS with a twin CX3C motif form heterohexameric complexes in which disulfides are essential to stabilize both the structure and function of the complex (55). Although Cox17 likely exists with twin disulfides, Cox17 is functional without either of the two disulfides (54).
Figure 5.
Copper metallochaperones for CcO. (a) Disulfide bonds between two pairs of cysteine residues (yellow) on antiparallel α-helices (red) create a helical hairpin of Cox17 [Protein Data Bank (PDB) code 2RNB]. Cu(I) is coordinated to a pair of cysteine residues. (b) Sco1 is tethered to the inner membrane (IM) (hydrophobic α-helix not shown) with a thioredoxin fold (β-strand, dark blue; histidine, pale blue) in the intermembrane space (PDB 2GQM). (c) Cox11 is tethered to the IM (hydrophobic α-helix not shown) with an immunoglobulin-like β-barrel in the IMS (PDB 1SPO).
Cu(I) binding to Cox17 occurs through two vicinal cysteine residues in a digonal Cu(I)-thiolate complex (Figures 5a and 6) (50, 51, 55). The mononuclear Cu(I)-Cox17 has been characterized only after in vitro Cu(I) reconstitution. Recombinant expression of Cox17 in E. coli leads to the accumulation of a Cu-Cox17 oligomeric, tetranuclear Cu(I) complex in cells cultured in Cu-supplemented medium (56). Recombinant expression in bacteria without copper supplementation results in the isolation of metal-free protein. This is presumably due to the absence of available cytosolic copper ions in E. coli (57). The polycopper protein is in a dimer/tetramer equilibrium, with the Cu(I)-thiolate cluster likely existing at the dimer interface (56). The tetracopper cluster conformer requires multiple cysteine residues to be in the reduced thiolate state. Cox17 purified from the IMS is largely devoid of bound Cu(I) and is monomeric (58). Cu-reconstituted human Cox17 shows a S-Cu-S angle of 130° (55). The bent coordination may permit an exogenous thiolate to provide a third ligand. The Cu(I) thiolate center is charge stabilized by two adjacent Lys residues. Lys20 may mimic the stabilizing Lys65 in the Cu-Atx1 complex. In addition, a hydrophobic patch, formed by ends of the C-terminal helix and the N-terminal segment, orients the Cys thiolates for Cu(I) binding. The Cu(I) center is solvent accessible yet avidly bound with a Kd value of 6.4 × 10−15 M (59). The Cu(I)-binding affinity must be poised for transfer to target proteins Sco1 and Cox11. Another well-defined digonal Cu(I)-thiolate complex exists in the E. coli CueR transcriptional regulator (57). This MerR-based regulator exhibits an essentially linear S-Cu-S bond angle of 176° with short Cu-S bond distances of 2.13 Å. Eight residues separate the two coordinating cysteines. The linearity of the Cu(I) coordination results in a Cu(I) Kd near 10−21 M. In contrast, the bent coordination of Cu(I) by the vicinal Cys residues in Cox17 reduces the binding affinity perhaps to permit transfer.
Figure 6.
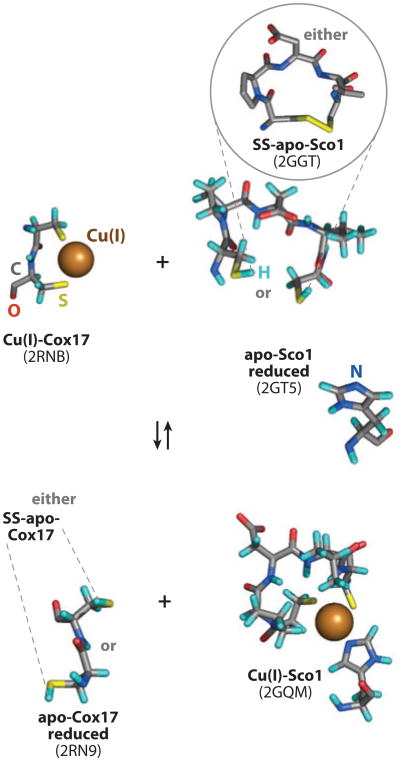
Visualization of metal-binding motifs taken from structures (Protein Data Bank codes indicated) of the apo and Cu(I) forms of Cox17 and Sco1 to represent the transfer of Cu(I) from Cox17 to Sco1 and, potentially, to represent the coincident transfer of two electrons when apo-Sco1 is oxidized.
The disulfides in Cox17 are introduced during import into the IMS. The IMS import of small cysteine-rich polypeptides with either twin CX9C or twin CX3C structural motifs is mediated by a disulfide relay system involving the mitochondrial intermembrane space assembly (MIA) machinery consisting of at least Mia40 and Erv1 (Figure 2) (60–64). Transit of these proteins, as unfolded polypeptides through the TOM translocase in the OM, results in transient capture of the imported molecules by Mia40 through an intermolecular disulfide. Mia40 contains two structural disulfide bonds formed by its twin CX9C motif as well as a N-terminal labile disulfide. The reactive disulfide lies within a CPC sequence motif and has a reduction potential of −200 mV (65). The nonoptimal stereochemistry of the disulfide with a single residue spacer likely contributes to its instability. The sulfhydryl oxidase Erv1 catalyzes formation of a reactive disulfide in Mia40 that is responsible for transient capture of imported protein with reduced thiolates (60, 61, 66, 67). The second Cys of the CPC motif is the active Cys forming the transient disulfide with Cox17 and other target proteins (65). Protein release from the Mia40 complex is facilitated by disulfide exchange reactions resulting in disulfides in the imported proteins. The oxidative folding of Cox17 and other Mia40 targets traps the proteins within the IMS. It remains unclear whether the Cu(I)-binding Cys23 and Cys24 residues of Cox17 are also initially oxidized. If a disulfide forms with Cys23 and Cys24, reduction would be required for Cu(I) binding. Information is lacking on what redox couples exist within the IMS. GSH reductase and thioredoxin reductase are not known to be present in this compartment.
Cox17 is also present in the yeast cytoplasm, initially encouraging a hypothesis that Cox17 may shuttle Cu(I) to mitochondria (58). However, restricting Cox17 to mitochondria through tethering to the IM retained normal CcO biogenesis (68). Import of Cox17 through the TOM translocase occurs as an unfolded and, presumably, apopolypeptide. The source of copper for metallation of Cox1 and Cox2 during CcO biogenesis arises from a mitochondrial matrix copper pool. A significant fraction of mitochondrial copper is localized within the matrix as a soluble, low-molecular-weight ligand complex (CuL), which is conserved from yeast to humans. Information is lacking on whether copper within the CuL complex is imported as ionic Cu(I) or as a CuL complex. The ligand appears to be present in the apo state within the cytoplasm, so an attractive postulate is that formation of the CuL complex occurs within the cytoplasm and the complex is imported into the mitochondrial matrix through an unknown carrier. Heterologous expression of two Cu(I)-binding proteins in the mitochondrial matrix was shown to titrate out the matrix Cu(I)-ligand complex and induce a respiratory deficiency (69, 70). These effects are reversed by supplementation of cell cultures with CuSO4. Attenuation of mitochondrial copper in Podospora anserina by a heterologous Cu(I)-binding protein also induces a CcO deficiency (71). In addition, the presence of the matrix-targeted Crs5 in yeast results in diminished Sod1 protein levels within the IMS and impaired activity of an IM-tethered hSod1.
Cu-Cox17- and Sco1-Mediated Cu(I) Transfer to Cox2
The recipient of Cu(I) from Cu-Cox17 is Sco1 in yeast, and a combination of Sco1 and Sco2 in mammalian cells. Overexpression of Sco1 restored respiratory function in yeast lacking Cox17, thereby linking Cox17 and Sco1 (72). Sco1 participates in the formation of the mixed valent CuA site in Cox2. In the absence of Sco1, yeast are stalled in CcO biogenesis, and the complex is degraded (73, 74). Yeast also possess Sco2, but cells lacking Sco2 are not respiratory deficient. Both human Sco1 and Sco2 proteins are required for CcO biogenesis and have nonoverlapping functions (75). Mutations in either gene results in a fatal encephalomyopathy, although the clinical presentation differs between hSCO1 and hSCO2 patients (76, 77). In addition to the CcO deficiency, patient tissues show a tissue-dependent copper deficiency (78).
Sco1 and Sco2 are tethered to the IM by a single trans-membrane helix with a globular domain protruding into the IMS containing a single Cu(I) site (79, 80). Cu(I) is trigonally coordinated by two cysteinyl residues within a CPDVC sequence motif plus a histidine imidazole (Figures 5b and 6) (80). Mutation of the Cys or His residues abrogates Cu(I) binding, resulting in a nonfunctional CcO (79, 80). The Cu(I) ion is partially shielded by the first Cys and the adjacent two residues (81). The structures of the metal-free human Sco1 and Cu1Sco1 complex are similar with the exception of one protruding loop that adopts two different conformations (81) (see the loop marked by an arrow in Figure 5b). In the apo conformation, the Cu(I)-binding region is more disordered, and the conformer, frozen in the crystal structure of hSco1, contained the His rotated out of position for planar Cu(I) coordination (82–84). The movement of the protruding loop orients the Cu(I) binding His residue for metal binding. The dynamic properties of this loop suggest it could influence interactions involved in Cu(I) transfer (81).
Human Sco2 shows greater conformational dynamics than hSco1 (85). These proteins have thioredoxin folds, raising questions about whether Sco proteins may function in redox reactions. Recent evidence suggests that hSco2 acts as a thioldisulfide oxidoreductase in CcO biogenesis, whereas hSco1 may be the dominant Cu(I) donor to Cox2 (86). Thioredoxins typically contain a cis-Pro in juxtaposition to the redox active cysteinyl residues. The cis-Pro precludes metal ion binding (87). The corresponding positions in Sco1 and Sco2 are the Cu(I) ligand His, consistent with their Cu(I)-binding function. If Sco2 is primarily an oxidoreductase, its function remains dependent on the Cys and His residues. Substrate recognition by protein disulfide isomerases, which share the thioredoxin fold (88), may also be dissimilar to partner recognition by copper metallochaperones with the former being dominated by hydrophobic interactions. The reduction potential of the Cys residues in hSco1 and hSco2 are −280 mV and −310 mV, respectively. Considering the reduction potential of the IMS is approximately −255 mV (52), it is predicted that Sco proteins may exist with at least some Cys residues oxidized and therefore not poised for Cu(I) binding. Sco1 and Sco2 in fibroblasts do have a mixture of oxidized and reduced Cys residues (86).
Direct Cu(I) transfer from Cox17 to Sco1 has been shown in vitro and in vivo. This likely occurs through a highly transient interaction of the two molecules (59). The interaction presumably imparts specificity to the Cu(I) transfer step. The specificity of the transfer reaction was demonstrated by using mutant proteins (89). Cys57 forms a disulfide with Cys26 in the twin CX9C motif of Cox17. Whereas a C57S substitution maintains function, a C57Y substitution abrogates Cox17 function (45, 54). The nonfunctional C57Y Cox17 mutant failed to mediate Cu(I) transfer to Sco1 in both in vivo and in vitro assays despite binding Cu(I) normally (89). However, the mutant retained the ability to transfer Cu(I) to its second target Cox11. Sco1 P174L is an allele of patients with CcO deficiency (90–92). Pro174 is adjacent to the second Cys in the Cu-binding CXXXC motif in Sco1, and this mutant has impaired Cu(I) acquisition from Cox17 (59, 92).Atransient interaction persists between P174L Sco1 and Cox17, but KCu is weaker, perhaps too weak to allow Cu(I) acquisition from a, now relatively tighter, Cu(I) site in Cox17 (59).
Recently, Cu-Cox17 was shown to effectively transfer both Cu(I) and reducing equivalents to oxidized apo-Sco1, leading to Cu-Sco1 formation and fully oxidized Cox17 (93). The Cu-free conformer of Cox17 failed to reduce oxidized Sco1, suggesting that Cu(I) is necessary for the disulfide exchange mechanism. The ability of Cu-Cox17 to mediate Cu(I) transfer to oxidized Sco1 abrogates a necessary mechanism to maintain reduced thiolates in Sco1. Although Cu-Cox17 can also transfer Cu(I) to Sco2 with reduced thiolates (94), it fails to transfer to oxidized Sco2 (93).
The Cu(I) ions transferred from Cox17 to Sco1 appear to be donated subsequently to Cox2 as an intermediate step in CcO assembly (Figure 2). The Sco1-mediated metallation of Cox2 likely occurs after extrusion of the Cox2-soluble domain into the IMS by the Cox18 translocase. Information on the Sco1-mediated metallation of Cox2 in eukaryotes is limited, although a group of conserved residues on the dynamic protruding loop in Sco1 was shown to be important for function with Cox2 (95). Sco proteins are widely distributed phylogenically. In prokaryotes, Sco1 is often codistributed with CcO and linked to the CuA center in Cox2 (96). However, Sco1 in Rhodobacter capulatus is associated with a cbb3 CcO lacking a CuA site (97). The Cu-binding function of this Sco protein, designated SenC, is required for maturation of the cbb3 CcO. One scenario is that SenC is the Cu(I) donor to the CuB center. The B. subtilis Sco1 is implicated in formation of the CuA site in CcO. The intriguing aspect of the Bacillus Sco1 is its dramatic preference for Cu(II) over Cu(I) (98). The marked stability of the Cu(II)-Sco1 complex may necessitate a conversion step to an intermediate state of Cu(I)-Sco1 compatible with Cu transfer (99).
Cox17 is restricted to eukaryotes. A bioinformatic search for genes associated with Sco1 in bacteria revealed a protein of unknown function with a conserved H(M)X10MX21HXM sequence motif present in both gram-negative and gram-positive organisms (100). The protein, designated DR1885, has a single-candidate trans-membrane helix followed by a globular domain folded into a Greek key β-barrel conformation capable of binding a single Cu(I) ion with a Met3His ligand set. The protein is analogous to other bacterial periplasmic Cu-binding proteins that use Met-rich motifs to coordinate Cu(I). The surface-exposed Cu(I) site makes it accessible for Cu(I) transfer. A homologous protein in Thermus, designated PCuAC for the periplasmic CuA chaperone, was shown to bind Cu(I) with a subpicomolar affinity (101). Cu-PCuAC was able to transfer Cu(I) in vitro to the reduced, soluble CuA domain of CcO subunit II of Thermus without the assistance of Sco1 (101). Successive Cu(I) transfer steps assembled the binuclear CuA center in subunit II. No transfer occurred when the Cys residues in subunit II were oxidized, unless reduced apo-Sco1 was present, leading to the oxidation of Sco1. Thus, Thermus Sco1 functions as an oxidoreductase, rather than a Cu(I) donor, to form the CuA center in CcO. The direct Cu(I) transfer from Cu-PCuAC to subunit II may involve ligand-exchange steps with the two Cys residues in the CuA center. Cu(I) coordination by a Met3 His protein is preferred over a Cys-based donor like Cox17 in the more oxidizing periplasm. The DR1885/PCAC family of proteins may functionally replace Cox17 in prokaryotes as Cu(I) donors to CuA centers that face the periplasmic space. A clear prediction is that bacterial cells lacking DR1885/PCuAC may be impaired in CcO biogenesis.
The implications of Sco1 having a redox role rather than a Cu(I) transfer function in Thermus are unclear. Maintaining Sco1 in the reduced state competent for a redox function would require reducing equivalents in the periplasm. In yeast, Sco1 has a Cu(I) transfer function, and it remains to be demonstrated whether it possesses a redox function. In humans, Sco2 is implicated in a redox function (86).
Cox11-Mediated CuB Site Formation
Copper metallation of the CuB site in Cox1 requires Cox11 (Figure 2). S. cerevisiae lacking Cox11 have impaired CcO activity and less Cox1 (102). CcO isolated from Rhodobacter sphaeroides cox11Δ cells lacked CuB but contained both hemes, and the environment of the heme in the CuB-heme a3 site was altered (103). The CuA site in R. sphaeroides CcO was unaffected in cells lacking Cox11. Thus, the absence of Cox11 appears to specifically preclude CuB site formation.
Cox11 is tethered to the IM by a single trans-membrane helix with a C-terminal domain protruding into the IMS (104, 105). The Cu(I)-binding cysteinyl residues lie within this C-terminal domain. The structure of the globular domain of the Cox11 homolog from Sinorhizobium meliloti adopts an immunoglobulin-like β-barrel fold (Figure 5c) (106). Removal of the trans-membrane domain of yeast Cox11 yields a soluble protein that dimerizes upon Cu(I) binding (104). The Cu(I) sites in each monomer are closely juxtaposed as the dimeric complexes can form a binuclear Cu(I) thiolate cluster at the dimer interface. Mutations of two Cys residues within a CXC sequence motif abrogate Cu(I) binding and formation of active CcO. A third functionally important Cys is spatially removed from the CXC motif, yet when mutated, this Cys results in substoichiometric Cu(I) binding.
CuB site formation occurs in Cox1 prior to addition of the Cox2 or Cox3 subunits. The CuB-heme a3 binuclear site forms in an early assembly intermediate of Cox1 that is stabilized by the Shy1 assembly factor. Cox11 forms a transient interaction with Shy1, which may be important to coordinate CuB site formation. This proposition is supported by the observation that accumulation of the nonfunctional CcO complexes in Rhodobacter and Paracoccus surf1Δ (Shy1 homolog) cells is compromised in both CuB and heme a3 (107, 108).
The membrane-embedded CuB site in Cox1 consists of three His ligands lying within a 12-helical bundle. In the absence of Cox2, a partially occluded channel forms from the IMS side of Cox1, where the heme a and Cu(I) moieties enter. The putative physical transfer from Cu-Cox11 to the buried CuB site in Cox1 may involve the third Cys residue that was removed from the CXC motif. This Cys is predicted to be juxtaposed to the IM and may mediate the Cu(I) transfer. However, it is not clear whether it would mediate Cu(I) transfer through the channel or laterally through an accessible port of the helical bundle. Cox11 appears to transiently occlude the Cox1 channel, as mutant alleles lacking the Cu-binding CXC residues confer resistance to the pro-oxidant heme a3:Cox1 assembly intermediate (109).
Summary of the Key Steps in Copper Metallation of CcO
Copper-transfer reactions are initiated within the IMS by Cu-Cox17, which acquires Cu(I) from a small molecule (Figure 2). Cox17 transfers Cu(I) ions to IM-associated Sco1 and Cox11. Cu-Cox11 is the donor to the CuB site in Cox1, and this site is formed in a Cox1 assembly intermediate that may contain only the Cox5a and Cox6 peripheral CcO subunits. The assembly intermediate is fully inserted within the IM and is associated with CcO assembly factors, including Shy1. Upon hemylation of the two heme a centers and formation of CuB, Cox1 is poised for the addition of Cox2. It is not clear whether the binuclear CuA site in Cox2 is formed in an isolated Cox2 or as Cox2 is associated with metallated Cox1. Sco1 mediates CuA site formation, and this reaction may occur as a ternary complex of Cu-Cox17, Sco1, and Cox2. Once the redox copper and heme cofactors are added to Cox1 and Cox2, the remaining subunits are added for final CcO maturation.
Copper Metallochaperone for Superoxide Dismutase
The copper metallochaperone for Cu,Zn-superoxide dismutase is Ccs1, a 27-kDa three-domain polypeptide (Figure 2) (110, 111). Two of the domains, domains 1 and 3, bind Cu(I), whereas the central domain is a key domain for interaction with Sod1 (112, 113). The physiological significance of Ccs1 has been defined in multiple species. Yeast lacking Ccs1 is devoid of Sod1 enzymatic activity unless cells are cultured with high levels of copper (114, 115). Drosophila melanogaster deficient in Ccs1 lacks Sod1 activity and shows exquisite sensitivity to redox cycling of paraquat (116). Mice with a targeted disruption of CCS have a markedly attenuated level of Sod1 activity and exhibit no apparent radio-copper incorporation into Sod1 (117). Thus, a strong correlation exists that Ccs1 is required for Sod1 activation.
Role of Ccs1 in Superoxide Dismutase 1 Activation
Cu-Ccs1 induces the activation of monomeric reduced Sod1, facilitating both Cu(I) transfer and disulfide bond formation (118). Active Sod1 requires binding of both Zn(II) and Cu and formation of an intrasubunit disulfide bond between Cys57 and Cys146 (118, 119). Sod1 is unusual in possessing such an essential disulfide bond in the reducing cytoplasm. The reduction potential of the Sod1 disulfide is −230 mV, whereas the potential in the cytoplasm is approximately −290 mV. The stability of the Sod1 disulfide may arise from the low solvent accessibility of Cys146 in the disulfide pair as well as from the stabilization imparted by Sod1 dimerization. The copper center in Sod1 is sunken within an active site channel that superoxide anions enter for dismutation (120, 121). In the absence of the metal cofactors and upon reduction of the disulfide, the Sod1 dimer is destabilized and exists as an inactive monomer (118, 122). Metal binding and/or disulfide bond formation stabilizes the β-barrel and loop conformations within a dimeric enzyme.
Domain Structure of Ccs1
The N-terminal domain 1 of Ccs1 is an Atx1-like βαββαβ-fold capable of binding a single Cu(I) ion (123). Domain 1 of yeast Ccs1 is required for Sod1 activation in Cu-limited cells, but this domain is not essential under normal growth conditions (114). In the absence of domain 1, no radiocopper incorporation into mouse Sod1 was observed in cultured mouse fibroblasts, but copper salts restored limited active Sod1 (124). Drosophila and Anopheles gambiae Ccs1 proteins lack domain 1, yet these proteins activate Sod1 efficiently (116). Human mutant Ccs1 with Cys substitutions in the MXCXXC motif and Cu(I) bound to domain 3 retain the in vitro activation function of Sod1 (123).
Domain 2 adopts an eight-stranded β-barrel structure analogous to Sod1 (125, 126). The dimeric domain 2 in human Ccs1 contains a Zn(II) site similar to Sod1 but lacks the ligand set for Cu coordination. In contrast, yeast Ccs1 domain 2 lacks both the Zn and Cu centers. Domain 2 is important for docking with Sod1 during the activation reaction. Mutations that compromise Ccs1 dimerization also abrogate both Ccs1 and Sod1 interaction and Ccs1-mediated Sod1 activation (111, 124).
Domain 3 is a short C-terminal segment containing a critical CXC motif that is unstructured in the yeast apo-Ccs1 protein (125), but domain 3 is ordered in the co-structure of yeast Ccs1 and a mutant Sod1 that stabilizes the intermolecular complex (113). Ccs1 lacking the domain 3 CXC motif fails to transfer Cu(I) or to induce disulfide formation in Sod1 (114, 123, 124). The binding of Ccs1 to Sod1 involves both domains 2 and 3, and binding is retained if the Cys residues are mutated (111). Whereas the domain 3 CXC motif may suggest digonal Cu(I)-thiolate coordination, EXAFS analyses of the Cu(I) complex clearly showed a polycopper cluster. The cluster formed with only domain 3 Cys residues is consistent with a binuclear Cu(I)-thiolate center bridging two Ccs1 molecules (123). Polycopper cluster formation is lost when the domain 3 Cys residues are mutated.
The Sequence of Events in Ccs1-Mediated Activation of Superoxide Dismutase 1
The Ccs1-Sod1 complex is transient and becomes detectable with reduced Sod1 lacking a Cusite or in mutant Sod1 lacking one Cys of the disulfide pair (112, 118, 127). In the absence of bound Cu in Sod1, complex formation between Ccs1 and Sod1is dependent on the disulfide being reduced. A loop in Sod1 containing Cys57, one of the disulfide-forming residues, is more flexible when the disulfide bond is reduced (128). Ccs1 is postulated to recognize a partially folded nascent conformation of Sod1 through mobile loop elements (127). Ccs1 interaction with Sod1 is abrogated when the loops become ordered in the copper-bound, disulfide-bonded fold. The Ccs1 interaction with Sod1 is independent of Zn(II) binding to Sod1. Because Zn binding to Sod1 stabilizes a folded conformation (129), Ccs1 must be competent to interact with a partially folded Sod1 conformer, but this structure has reduced thiolates and an empty copper site. The activation process involves copper insertion and disulfide bond formation.
Cu site formation appears to be concurrent with disulfide bond formation. Sod1 mutants blocked in Cu site formation remain largely in the reduced state in yeast containing Ccs1 (127). Likewise, in vitro studies revealed that reduced Zn-Sod1 protein is inefficiently converted to the oxidized state aerobically in the absence of Ccs1 (118). Sod1 oxidation requires the intact CXC motif in Ccs1 (130). The addition of Cu-Ccs1 to Zn-Sod1 fails to induce disulfide bond formation anaerobically, but this process is facile with the addition of air (118). A putative intermediate in the oxidation reaction is an intermolecular disulfide between Cys57 of Sod1 and Cys229 of Ccs1 (113). Formation of this intermolecular disulfide may resolve to the C57-C146 disulfide by a disulfide exchange reaction.
Ccs1-Independent Activation of Superoxide Dismutase 1
Although Ccs1 has a key role in Sod1 activation, it is now clear that a secondary mechanism exists for Sod1 activation independent of Ccs1 in multicellular enkaryotes. The Caenorhabditis elegans genome completely lacks Ccs1 (131). Mice lacking Ccs1 have 10% to 30% WT Sod1 activity in various tissues (132). Likewise, Ccs1-null flies show residual Sod1 activity such that the phenotype of the null flies is less severe compared to flies lacking Sod1 (116). Human Sod1 is capable of being partially activated independently of Ccs1 when expressed in yeast or flies (116, 133). This Ccs1-independent pathway of Sod1 activation does not require Atx1 or Cox17 (133).
Yeast Sod1 differs from human and fly Sod1 in depending on Ccs1 for activation (115, 131). Two C-terminal Pro residues in yeast Sod1 preclude Ccs1-independent activation. Replacement of the yeast Pro residues restores Ccs1-independent activation, and insertion of Pro residues at the corresponding positions in human Sod1 abrogates Ccs1-independent activation (133). The Ccs1-independent pathway requires GSH, although the role of GSH in Cu insertion and/or disulfide bond formation is unclear. Because Sod1 proteins lacking the C-terminal Pro residues are Cu activated in ccs1Δ yeast cells (116, 131), there must be some bioavailable Cu(I) complexes in the yeast cytosol. The identity of the bioavailable Cu(I) complex is a major unresolved question. Cu metallation of Sod1 by such complexes is less efficient than by Cu-Ccs1, but once it occurs, disulfide bond formation also proceeds. The facile oxidation of worm Sod1 in the absence of Ccs1 implies a secondary pathway (131). Disulfide bond formation is typically a facilitated process, but sulfhydryl oxidases are not known in the cytoplasm.
Activation of Superoxide Dismutase 1 in the Mitochondrial Intermembrane Space by Ccs1
Ccs1 is key to activation of Sod1 within the mitochondrial IMS, where 1% to 5% of Sod1 resides (Figure 2). The presence of Sod1 within the IMS is largely dependent on Ccs1 (134). In the absence of Ccs1, only minimal levels of Sod1 are apparent in the IMS (131). The limiting factor for the import of Ccs1 into the IMS is Mia40 (135–137). Overexpression of Mia40 in yeast or mammalian cells enhances IMS levels of Ccs1, and depletion of Mia40 has the opposite effect (136, 137). Import and capture of Ccs1 in the IMS are likely achieved through disulfide bonding to Mia40, followed by transfer of the disulfide to Ccs1 either in domain 1 or 3 (137). Two factors question whether oxidative folding of Ccs1 is the major mechanism of its retention within the IMS. First, it is unclear whether disulfide bonding within the CXXC in domain 1 or CXC in domain 3 would be sufficient to trap Ccs1 within the IMS as the crosslinks would not stabilize disperse segments of the Ccs1 polypeptide to sterically block diffusion back out through the TOM translocase. Second, the stability of those candidate disulfides may be low because of unsatisfactory spacing between Cys residues (138). This also supports a transient disulfide introduced in domain 1 of Ccs1 upon IMS import. However, retention of Ccs1 in the IMS must involve general folding of domains 1 and 2. Because the Zn site in domain 2 is not conserved in all Ccs1 orthologs, metal binding is not an obvious trigger for IMS folding.
If transient disulfides form in domain 1 of Ccs1, it is unlikely this disulfide is transferred initially to imported Sod1 as Cu insertion must precede disulfide bond formation in Sod1. It is conceivable that Cu(I) insertion into Sod1 is mediated by domain 3 of Ccs1 and that the disulfide is subsequently transferred from the Ccs1 domain 1. Sod1 is not an apparent substrate of the Mia40/Erv1 system (137), so disulfide bond formation in Sod1 must occur through a Ccs1-dependent pathway as in the cytoplasm. Human Sod1 contains two additional Cys residues (C6 and C111), and neither of these is involved in a disulfide bond, yet retention of human Sod1 in the mitochondria of cultured cells is dependent on both (136). Cys111 is near the dimer interface, and the distance between the two Cys side chains is about 10 Å. Cys111 was reported to be part of a nonnative Cu(II)-binding site, leading to speculation that it may mediate Cu insertion into the active site (139).
Key Features of Ccs1-Mediated Superoxide Dismutase 1 Activation
Ccs1 binds Cu(I) in either domains 1 or 3. Domain 1 binding must necessitate transfer to the domain 3 CXC motif for subsequent transfer to Sod1. Cu-charged Ccs1 interacts with newly synthesized Sod1 polypeptides prior to completion of its native fold. The transient interaction is mediated by domains 2 and 3 of Ccs1. Zn(II) binding to Sod1 can occur either prior to Ccs1 complex formation or concomitantly. Cu(I) transfer occurs from Cu(I) associated in the domain 3 CXC motif. The only characterized Cu(I)-bound state in domain 3 is the binuclear Cu(I) thiolate cluster bridging a Ccs1 dimer. A second candidate Cu(I) donor is a mononuclear digonal Cu(I) complex, but this state has not been reported to date. Cu(I) transfer to Sod1 is accompanied by disulfide bond formation in an oxygen- or superoxide-dependent manner. Major unresolved questions are the mechanism by which the disulfide bond is formed and the sequence of Cu(I)-ligand exchange. The major questions in the Ccs1-independent pathway of Sod1 activation include the identity of the bioavailable copper pool(s) used in Sod1 metallation and the mechanism of disulfide bond formation.
Additional Targets for Copper Metallochaperones
Atx1 is implicated in copper supply to amine oxidase 1 (Cao1) in Schizosaccharomyces pombe, which has been localized (as a green fluorescent protein fusion) to the cytosol rather than the Golgi or some other internal membranous compartment (140). Of the copper metallochaperones, only disruption of Atx1 caused a loss of enzymatic activity, and a yeast two-hybrid interaction has been detected between Atx1 and Cao1. Arabidopsis thaliana has a second Atx1, CCH, with an extra C-terminal domain that is inhibitory to its interactions with the P1-type ATPases HMA5 (4, 141–143). CCH is expressed in the vicinity of xylem and phloem elements, and one hypothesis is that the C-terminal region enables movement through plasmodesmata, suggesting a role for CCH in systemic Cu trafficking.
Mutants of E. hirae missing CopZ show low expression of the cop operon, including copA and copB, plus hypersensitivity to copper resulting from insufficient copper-exporting P1-type ATPase activity (8). If CopZ solely donated Cu(I) ions to a copper exporter, then intracellular copper levels would be elevated in ΔcopZ, and copper-responsive expression of the cop operon would be predicted to become higher. An alternate target for CopZ has been suggested. Transcriptional regulation of the cop operon is mediated by the DNA-binding repressor CopY. Purified CopY contains one Zn(II) ion, which can be displaced by two copper ions to form a Cu(I)-thiolate cluster (144, 145). Binding of CopY to nucleotide sequences from the cop operator promoter region is impaired by the replacement of zinc with copper. The second βαββαβ-domain of the Menkes ATPases expressed in isolation is incapable of donating Cu(I) to CopY, although a variant in which four residues are replaced with lysines does donate (145). This supports the idea of direct protein contact mediated by complementary electrostatic surfaces on CopZ and CopY. As with analogous Cu(I) transfer experiments, the metal might not attain a similar equilibrium by exchanging via free solution owing to its kinetic properties, notably the copper metallochaperone off rate for Cu(I), that is unique to the heterodimer interface.
Atox1 can affect the transcriptional control of cyclin D1 and mouse embryonic fibroblast proliferation (146). A yeast two-hybrid interaction also occurs between Ccs1 and the β-secretase (BACE1) that cleaves the amyloid precursor protein and generates the Aβ peptides that aggregate in Alzheimer's disease senile plaques. Ccs1 and BACE1 can be coimmunoprecipitated in extracts from rat brains (147). A short cytosolic tail of BACE1 contains three Cys residues that bind Cu(I), and this same short region associates with domain 1 of Ccs1. Fluorescent fusions of the two proteins track together along axons of neuronal cells, heading toward the synapse, implying that Ccs1 forms a stable association with secretory vesicles containing BACE1. Perhaps the intermolecular interaction between domain 1 of Ccs1 and BACE1's tail is structurally similar to transient intramolecular interactions with domain 3 of Ccs1. Some detected copper metallochaperone interactions might not be physiological, but aberrations linked to grave pathologies demand consideration.
Summary Points.
The switch between cuprous and cupric ions is exploited in oxidoreductases but can also catalyze deleterious redox chemistry, for example via the Fenton reaction.
Cu(II) is at the top of the Irving-Williams series, and Cu(I) also has a tendency to form tight thiol complexes; hence copper is liable to outcompete other metals in metalloproteins.
The cytosol contains numerous potential copper-binding sites, and the estimated available copper concentration is negligible, probably subfemtomolar.
Copper is passed to cuproproteins by ligand-exchange reactions from copper metallochaperones to avoid copper becoming kinetically trapped, or engaging in deleterious interactions while en route with docking between a metallochaperone and its partner, presumed to encourage the release of copper from the metallochaperone.
Solution structural studies have visualized transient complexes between N-terminal domains of P1-type ATPases and Atx1-type copper metallochaperones to reveal changes predicted to aid Cu(I) release and to reveal interacting surfaces with complementary electrostatics.
Interactions between Atx1-type copper metallochaperones and N termini of P1-type ATPase are regulatory, and Cu(I) can be separately donated to ATPase membrane sites.
Cox17, Sco1, and Cox11 in the mitochondrial IMS form two copper supply pathways for CuA and CuB, sites of cytochrome oxidase.
Ccs1 introduces an essential disulfide bond into Sod1 and supplies Cu(I) to Sod1 both in the cytosol and in the mitochondrial IMS, although several Sod1 species can acquire Cu(I) and the critical disulfide without Ccs1 provided GSH is present.
Future Issues.
Studies over the coming years should aim to further visualize heterodimer complexes between Ccs1 and Sod1, the mitochondrial copper metallochaperones for cytochrome oxidase and their partners, along with CopZ and membranous regions of P1-type ATPases.
The effect that docking between a metallochaperone and its partner has on KOFF for Cu(I) from the metallochaperone needs to quantified, perhaps by stopped-flow methods.
In addition to determining the sequence of Cu(I)-ligand exchange, proton and electron migration that prepares recipient Cys residues for Cu(I) awaits a full description, as does the coupling of Cu(I) transfer from Ccs1 to Sod1 with disulfide bond formation in Sod1.
Additional twin CX9C proteins within the IMS contribute to CcO biogenesis, including Cox19, Cox23, and Pet191, but their actions remain to be defined.
The sources of copper for each metallochaperone remain to be unequivocally defined, the low-molecular-weight mitochondrial matrix copper ligand needs to be structurally characterized, and mechanisms that prioritize different cuproprotein destinations are yet to be discovered.
Acknowledgments
The authors are supported by the Biotechnology and Biological Sciences Research Council (BB/E001688/1) to N.J.R. and grant ES 03817 from the National Institutes of Environmental Health Sciences, NIH to D.R.W.
Glossary
- Atx1
the yeast copper metallochaperone for P1-type ATPases
- Ccs1
a copper metallochaperone for Cu-Zn superoxide dismutase
- CopZ
a bacterial protein similar to Atx1 and Atox1
- βαββαβ-fold
ferredoxin-fold metal-binding domains of P1-type ATPases and some copper metallochaperones
- Atox1
the human copper metallochaperone for P1-type ATPases
- Cox17
a eukaryotic copper metallochaperone for Sco1 and Cox11
- Cox11
a copper metallochaperone for the CuB site of cytochrome oxidase
- Sco1
a copper metallochaperone for the CuA site of cytochrome oxidase
Footnotes
Disclosure Statement: The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
Nigel J. Robinson, Email: N.J.Robinson@ncl.ac.uk.
Dennis R. Winge, Email: dennis.winge@hsc.utah.edu.
Literature Cited
- 1.Huffman DL, O'Halloran TV. Function, structure, and mechanism of intracellular copper trafficking proteins. Annu Rev Biochem. 2001;70:677–701. doi: 10.1146/annurev.biochem.70.1.677. [DOI] [PubMed] [Google Scholar]
- 2.Ridge PG, Zhang Y, Gladyshev VN. Comparative genomic analyses of copper transporters and cuproproteomes reveal evolutionary dynamics of copper utilization and its link to oxygen. PLoS ONE. 2008;3:e1378. doi: 10.1371/journal.pone.0001378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andreini C, Banci L, Bertini I, Rosato A. Occurrence of copper proteins through the three domains of life: a bioinformatic approach. J Proteome Res. 2008;7:209–16. doi: 10.1021/pr070480u. [DOI] [PubMed] [Google Scholar]
- 4.Burkhead JL, Reynolds KA, Abdel-Ghany SE, Cohu CM, Pilon M. Copper homeostasis. New Phytol. 2009;182:799–816. doi: 10.1111/j.1469-8137.2009.02846.x. [DOI] [PubMed] [Google Scholar]
- 5.Merchant SS, Allen MD, Kropat J, Moseley JL, Long JC, et al. Between a rock and a hard place: trace element nutrition in Chlamydomonas. Biochim Biophys Acta. 2006;1763:578–94. doi: 10.1016/j.bbamcr.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Dudev T, Lim C. Metal binding affinity and selectivity in metalloproteins: insights from computational studies. Annu Rev Biophys. 2008;37:97–116. doi: 10.1146/annurev.biophys.37.032807.125811. [DOI] [PubMed] [Google Scholar]
- 7.Tottey S, Waldron KJ, Firbank SJ, Reale B, Bessant C, et al. Protein-folding location can regulate manganese-binding versus copper- or zinc-binding. Nature. 2008;455:1138–42. doi: 10.1038/nature07340. [DOI] [PubMed] [Google Scholar]
- 8.Odermatt A, Solioz M. Two trans-acting metalloregulatory proteins controlling expression of the copper-ATPases of Enterococcus hirae. J Biol Chem. 1995;270:4349–54. doi: 10.1074/jbc.270.9.4349. [DOI] [PubMed] [Google Scholar]
- 9.Lin SJ, Culotta VC. The ATX1 gene of Saccharomyces cerevisiae encodes a small metal homeostasis factor that protects cells against reactive oxygen toxicity. Proc Natl Acad Sci USA. 1995;92:3784–88. doi: 10.1073/pnas.92.9.3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Gladyshev VN. Comparative genomics of trace elements: emerging dynamic view of trace element utilization and function. Chem Rev. 2009;109:4828–61. doi: 10.1021/cr800557s. [DOI] [PubMed] [Google Scholar]
- 11.Wernimont AK, Huffman DL, Lamb AL, O'Halloran TV, Rosenzweig AC. Structural basis for copper transfer by the metallochaperone for the Menkes/Wilson disease proteins. Nat Struct Biol. 2000;7:766–71. doi: 10.1038/78999. [DOI] [PubMed] [Google Scholar]
- 12.Banci L, Bertini I, Cantini F, Felli IC, Gonnelli L, et al. The Atx1-Ccc2 complex is a metal-mediated protein-protein interaction. Nat Chem Biol. 2006;2:367–68. doi: 10.1038/nchembio797. [DOI] [PubMed] [Google Scholar]
- 13.Portnoy ME, Rosenzweig AC, Rae T, Huffman DL, O'Halloran TV, Culotta VC. Structure-function analyses of the ATX1 metallochaperone. J Biol Chem. 1999;274:15041–45. doi: 10.1074/jbc.274.21.15041. [DOI] [PubMed] [Google Scholar]
- 14.Huffman DL, O'Halloran TV. Energetics of copper trafficking between the Atx1 metallochaperone and the intracellular copper transporter, Ccc2. J Biol Chem. 2000;275:18611–14. doi: 10.1074/jbc.C000172200. [DOI] [PubMed] [Google Scholar]
- 15.Wimmer R, Herrmann T, Solioz M, Wuthrich K. NMR structure and metal interactions of the CopZ copper chaperone. J Biol Chem. 1999;274:2597–603. doi: 10.1074/jbc.274.32.22597. [DOI] [PubMed] [Google Scholar]
- 16.Zhou L, Singleton C, Le Brun NE. High Cu(I) and low proton affinities of the CXXC motif of Bacillus subtilis CopZ. Biochem J. 2008;413:459–65. doi: 10.1042/BJ20080467. [DOI] [PubMed] [Google Scholar]
- 17.Banci L, Bertini I, Ciofi-Baffoni S, Del Conte R, Gonnelli L. Understanding copper trafficking in bacteria: interaction between the copper transport protein CopZ and the N-terminal domain of the copper ATPase CopA from Bacillus subtilis. Biochemistry. 2003;42:1939–49. doi: 10.1021/bi027096p. [DOI] [PubMed] [Google Scholar]
- 18.Banci L, Bertini I, Ciofi-Baffoni S, Kandias NG, Robinson NJ, et al. The delivery of copper for thylakoid import observed by NMR. Proc Natl Acad Sci USA. 2006;103:8320–25. doi: 10.1073/pnas.0600142103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Radford DS, Kihlken MA, Borrelly GP, Harwood CR, Le Brun NE, Cavet JS. CopZ from Bacillus subtilis interacts in vivo with a copper exporting CPx-type ATPase CopA. FEMS Microbiol Lett. 2003;220:105–12. doi: 10.1016/S0378-1097(03)00095-8. [DOI] [PubMed] [Google Scholar]
- 20.Banci L, Bertini I, Ciofi-Baffoni S, Finney LA, Outten CE, O'Halloran TV. A new zinc-protein coordination site in intracellular metal trafficking: solution structure of the apo and Zn(II) forms of ZntA (46–118) J Mol Biol. 2002;323:883–97. doi: 10.1016/s0022-2836(02)01007-0. [DOI] [PubMed] [Google Scholar]
- 21.Banci L, Bertini I, Ciofi-Baffoni S, Poggi L, Vanarotti M, et al. NMR structural analysis of the soluble domain of ZiaA-ATPase and the basis of selective interactions with copper metallochaperone Atx1. J Biol Inorg Chem. 2010;15:87–98. doi: 10.1007/s00775-009-0568-7. [DOI] [PubMed] [Google Scholar]
- 22.Tottey S, Rondet SA, Borrelly GP, Robinson PJ, Rich PR, Robinson NJ. A copper metallochaperone for photosynthesis and respiration reveals metal-specific targets, interaction with an importer, and alternative sites for copper acquisition. J Biol Chem. 2002;277:5490–97. doi: 10.1074/jbc.M105857200. [DOI] [PubMed] [Google Scholar]
- 23.Tottey S, Rich PR, Rondet SAM, Robinson NJ. Two Menkes-type ATPases supply copper for photosynthesis in Synechocystis PCC 6803. J Biol Chem. 2001;276:19999–20004. doi: 10.1074/jbc.M011243200. [DOI] [PubMed] [Google Scholar]
- 24.Kanamaru K, Kashiwagi S, Mizuno T. Acopper-transporting P-type ATPase found in the thylakoid membrane of the cyanobacterium Synechococcus species PCC7942. Mol Microbiol. 1994;13:369–77. doi: 10.1111/j.1365-2958.1994.tb00430.x. [DOI] [PubMed] [Google Scholar]
- 25.Setty SR, Tenza D, Sviderskaya EV, Bennett DC, Raposo G, Marks MS. Cell-specific ATP7A transport sustains copper-dependent tyrosinase activity in melanosomes. Nature. 2008;454:1142–46. doi: 10.1038/nature07163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.González-Guerrero M, Arguello JM. Mechanism of Cu+-transporting ATPases: soluble Cu+ chaperones directly transfer Cu+ to transmembrane transport sites. Proc Natl Acad Sci USA. 2008;105:5992–97. doi: 10.1073/pnas.0711446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu CC, Rice WJ, Stokes DL. Structure of a copper pump suggests a regulatory role for its metal-binding domain. Structure. 2008;16:976–85. doi: 10.1016/j.str.2008.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gonzalez-Guerrero M, Hong D, Arguello JM. Chaperone-mediated Cu+ delivery to Cu+ transport ATPases: requirement of nucleotide binding. J Biol Chem. 2009;284:20804–11. doi: 10.1074/jbc.M109.016329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sazinsky MH, LeMoine B, Orofino M, Davydov R, Bencze KZ, et al. Characterization and structure of a Zn2+ and [2Fe-2S]-containing copper chaperone from Archaeoglobus fulgidus. J Biol Chem. 2007;282:25950–59. doi: 10.1074/jbc.M703311200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsivkovskii R, MacArthur BC, Lutsenko S. The Lys1010-Lys1325 fragment of the Wilson's disease protein binds nucleotides and interacts with the N-terminal domain of this protein in acopper-dependent manner. J Biol Chem. 2001;276:2234–42. doi: 10.1074/jbc.M003238200. [DOI] [PubMed] [Google Scholar]
- 31.Petris MJ, Mercer JFB, Culvenor JG, Lockhart P, Gleeson PA, Camakaris J. Ligand-regulated transport of the Menkes copper P-type ATPase efflux pump from the Golgi apparatus to the plasma membrane: a novel mechanism of regulated trafficking. EMBO J. 1996;15:6084–95. [PMC free article] [PubMed] [Google Scholar]
- 32.Voskoboinik I, Strausak D, Greenough M, Brooks H, Petris M, et al. Functional analysis of the N-terminal CXXC metal-binding motifs in the human Menkes copper-transporting P-type ATPase expressed in cultured mammalian cells. J Biol Chem. 1999;274:22008–12. doi: 10.1074/jbc.274.31.22008. [DOI] [PubMed] [Google Scholar]
- 33.Guo Y, Nyasae L, Braiterman LT, Hubbard AL. NH2-terminal signals in ATP7B Cu-ATPase mediate its Cu-dependent anterograde traffic in polarized hepatic cells. Am J Physiol Gastrointest Liver Physiol. 2005;289:G904–16. doi: 10.1152/ajpgi.00262.2005. [DOI] [PubMed] [Google Scholar]
- 34.Hussain F, Olson JS, Wittung-Stafshede P. Conserved residues modulate copper release in human copper chaperone Atox1. Proc Natl Acad Sci USA. 2008;105:11158–63. doi: 10.1073/pnas.0802928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Portnoy ME, Schmidt PJ, Rogers RS, Culotta VC. Metal transporters that contribute copper to metallochaperones in Saccharomyces cerevisiae. Mol Genet Genomics. 2001;265:873–82. doi: 10.1007/s004380100482. [DOI] [PubMed] [Google Scholar]
- 36.De Feo CJ, Aller SG, Siluvai GS, Blackburn NJ, Unger VM. Three-dimensional structure of the human copper transporter hCTR1. Proc Natl Acad Sci USA. 2009;106:4237–42. doi: 10.1073/pnas.0810286106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiao Z, Wedd AG. A C-terminal domain of the membrane copper pump Ctr1 exchanges copper(I) with the copper chaperone Atx1. Chem Commun. 2002;6:588–89. doi: 10.1039/b111180a. [DOI] [PubMed] [Google Scholar]
- 38.Xiao Z, Loughlin F, George GN, Howlett GJ, Wedd AG. C-terminal domain of the membrane copper transporter Ctr1 from Saccharomyces cerevisiae binds four Cu(I) ions as a cuprous-thiolate polynuclear cluster: sub-femtomolar Cu(I) affinity of three proteins involved in copper trafficking. J Am Chem Soc. 2004;126:3081–90. doi: 10.1021/ja0390350. [DOI] [PubMed] [Google Scholar]
- 39.Loftin IR, Franke S, Blackburn NJ, McEvoy MM. Unusual Cu(I)/Ag(I) coordination of Escherichia coli CusF as revealed by atomic resolution crystallography and X-ray absorption spectroscopy. Protein Sci. 2007;16:2287–93. doi: 10.1110/ps.073021307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loftin IR, Franke S, Roberts SA, Weichsel A, Heroux A, et al. A novel copper-binding fold for the periplasmic copper resistance protein CusF. Biochemistry. 2005;44:10533–40. doi: 10.1021/bi050827b. [DOI] [PubMed] [Google Scholar]
- 41.Xue Y, Davis AV, Balakrishnan G, Stasser JP, Staehlin BM, et al. Cu(I) recognition via cation-pi and methionine interactions in CusF. Nat Chem Biol. 2008;4:107–9. doi: 10.1038/nchembio.2007.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davis AV, O'Halloran TV. A place for thioether chemistry in cellular copper ion recognition and trafficking. Nat Chem Biol. 2008;4:148–51. doi: 10.1038/nchembio0308-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bagai I, Rensing C, Blackburn NJ, McEvoy MM. Direct metal transfer between periplasmic proteins identifies a bacterial copper chaperone. Biochemistry. 2008;47:11408–14. doi: 10.1021/bi801638m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsukihara T, Aoyama H, Yamashita E, Tomizaki T, Yamaguchi H, et al. Structures of metal sites of oxidized bovine heart cytochrome c oxidase at 2.8 A. Science. 1995;269:1069–74. doi: 10.1126/science.7652554. [DOI] [PubMed] [Google Scholar]
- 45.Glerum DM, Shtanko A, Tzagoloff A. Characterization of COX17, a yeast gene involved in copper metabolism and assembly of cytochrome oxidase. J Biol Chem. 1996;271:14504–9. doi: 10.1074/jbc.271.24.14504. [DOI] [PubMed] [Google Scholar]
- 46.Takahashi Y, Kako K, Kashiwabara SI, Takehara A, Inada Y, et al. Mammalian copper chaperone Cox17 has an essential role in activation of cytochrome c oxidase and embryonic development. Mol Cell Biol. 2002;22:7614–21. doi: 10.1128/MCB.22.21.7614-7621.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee J, Prohaska JR, Thiele DJ. Essential role for mammalian copper transporter Ctr1 in copper homeostasis and embryonic development. Proc Natl Acad Sci USA. 2001;98:6842–47. doi: 10.1073/pnas.111058698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oswald C, Krause-Buchholz U, Rödel G. Knockdown of human COX17 affects assembly and supramolecular organization of cytochrome c oxidase. J Mol Biol. 2009;389:470–79. doi: 10.1016/j.jmb.2009.04.034. [DOI] [PubMed] [Google Scholar]
- 49.Banci L, Bertini I, Ciofi-Baffoni S, Tokatlidis K. The coiled coil-helix-coiled coil-helix proteins may be redox proteins. FEBS Lett. 2009;583:1699–702. doi: 10.1016/j.febslet.2009.03.061. [DOI] [PubMed] [Google Scholar]
- 50.Abajian C, Yatsunyk LA, Ramirez BE, Rosenzweig AC. Yeast Cox17 solution structure and copper(I) binding. J Biol Chem. 2004;279:53584–92. doi: 10.1074/jbc.M408099200. [DOI] [PubMed] [Google Scholar]
- 51.Arnesano F, Balatri E, Banci L, Bertini I, Winge DR. Folding studies of Cox17 reveal an important interplay of cysteine oxidase and copper binding. Structure. 2005;13:713–22. doi: 10.1016/j.str.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 52.Hu J, Dong L, Outten CE. The redox environment in the mitochondrial intermembrane space is maintained separately from the cytosol and matrix. J Biol Chem. 2008;283:29126–34. doi: 10.1074/jbc.M803028200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Palumaa P, Zovo K. Modulation of redox switches of copper chaperone Cox17 by Zn(II) ions, determined by new ESI MS based approach. Antioxid Redox Signal. 2008;11:985–95. doi: 10.1089/ars.2008.2262. [DOI] [PubMed] [Google Scholar]
- 54.Heaton D, Nittis T, Srinivasan C, Winge DR. Mutational analysis of the mitochondrial copper metallochaperone Cox17. J Biol Chem. 2000;275:37582–87. doi: 10.1074/jbc.M006639200. [DOI] [PubMed] [Google Scholar]
- 55.Banci L, Bertini I, Ciofi-Baffoni S, Janicka A, Martinelli M, et al. A structural-dynamical characterization of human Cox17. J Biol Chem. 2008;283:7912–20. doi: 10.1074/jbc.M708016200. [DOI] [PubMed] [Google Scholar]
- 56.Heaton DN, George GN, Garrison G, Winge DR. The mitochondrial copper metallochaperone Cox17 exists as an oligomeric polycopper complex. Biochem. 2001;40:743–51. doi: 10.1021/bi002315x. [DOI] [PubMed] [Google Scholar]
- 57.Changela A, Chen K, Xue Y, Holschen J, Outten CE, et al. Molecular basis of metal-ion selectivity and zeptomolar sensitivity by CueR. Science. 2003;301:1383–87. doi: 10.1126/science.1085950. [DOI] [PubMed] [Google Scholar]
- 58.Beers J, Glerum DM, Tzagoloff A. Purification, characterization, and localization of yeast Cox17p, a mitochondrial copper shuttle. J Biol Chem. 1997;272:33191–96. doi: 10.1074/jbc.272.52.33191. [DOI] [PubMed] [Google Scholar]
- 59.Banci L, Bertini I, Ciofi-Baffoni S, Leontari I, Martinelli M, et al. Human Sco1 functional studies and pathological implications of the P174L mutant. Proc Natl Acad Sci USA. 2007;104:15–20. doi: 10.1073/pnas.0606189103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rissler M, Wiedemann N, Pfannschmidt S, Gabriel K, Guiard B, et al. The essential mitochondrial protein Erv1 cooperates with Mia40 in biogenesis of intermembrane proteins. J Mol Biol. 2005;353:485–92. doi: 10.1016/j.jmb.2005.08.051. [DOI] [PubMed] [Google Scholar]
- 61.Chacinska A, Pfannschmidt S, Wiedemann N, Kozjak V, Sanjuan Szklarz LK, et al. Essential role of Mia40 in import and assembly of mitochondrial intermembrane space proteins. EMBO J. 2004;23:3735–46. doi: 10.1038/sj.emboj.7600389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Allen S, Balabanidou V, Sideris DP, Lisowsky T, Tokatidis K. Erv1 mediates the Mia40-dependent protein import pathway and provides a functional link to the respiratory chain by shuttling electrons to cytochrome c. J Mol Biol. 2005;353:937–44. doi: 10.1016/j.jmb.2005.08.049. [DOI] [PubMed] [Google Scholar]
- 63.Tokatlidis K. A disulfide relay system in mitochondria. Cell. 2005;121:965–67. doi: 10.1016/j.cell.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 64.Terziyska N, Grumbt B, Bien M, Neupert W, Herrmann JM, Hell K. The sulfhydryl oxidase Erv1 is a substrate of the Mia40-dependent protein translocation pathway. FEBS Lett. 2007;581:1098–102. doi: 10.1016/j.febslet.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 65.Banci L, Bertini I, Cefaro C, Ciofi-Baffoni S, Gallo A, et al. MIA40 is an oxidoreductase that catalyzes oxidative protein folding in mitochondria. Nat Struct Mol Biol. 2009;16:198–206. doi: 10.1038/nsmb.1553. [DOI] [PubMed] [Google Scholar]
- 66.Mesecke N, Terziyska N, Kozany C, Baumann F, Neupert W, et al. A disulfide relay system in the intermembrane space of mitochondria that mediates protein import. Cell. 2005;121:1059–69. doi: 10.1016/j.cell.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 67.Terziyska N, Lutz T, Kozany C, Mokranjac D, Mesecke N, et al. Mia40, a novel factor for protein import into the intermembrane space of mitochondria is able to bind metal ions. FEBS Lett. 2005;579:179–84. doi: 10.1016/j.febslet.2004.11.072. [DOI] [PubMed] [Google Scholar]
- 68.Maxfield AB, Heaton DN, Winge DR. Cox17 is functional when tethered to the mitochondrial inner membrane. J Biol Chem. 2004;279:5072–80. doi: 10.1074/jbc.M311772200. [DOI] [PubMed] [Google Scholar]
- 69.Cobine PA, Ojeda LD, Rigby KM, Winge DR. Yeast contain a non-proteinaceous pool of copper in the mitochondrial matrix. J Biol Chem. 2004;279:14447–55. doi: 10.1074/jbc.M312693200. [DOI] [PubMed] [Google Scholar]
- 70.Cobine PA, Pierrel F, Bestwick ML, Winge DR. Mitochondrial matrix copper complex used in metallation of cytochrome oxidase and superoxide dismutase. J Biol Chem. 2006;281:36552–59. doi: 10.1074/jbc.M606839200. [DOI] [PubMed] [Google Scholar]
- 71.Scheckhuber CQ, Grief J, Boilan E, Luce K, Debacq-Chainiaux F, et al. Age-related cellular copper dynamics in the fungal ageing model Podospora anserina and in ageing human fibroblasts. PLoS ONE. 2009;4:e4919. doi: 10.1371/journal.pone.0004919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Glerum DM, Shtanko A, Tzagoloff A. SCO1 and SCO2 act as high copy suppressors of a mitochondrial copper recruitment defect in Saccharomyces cerevisiae. J Biol Chem. 1996;271:20531–35. doi: 10.1074/jbc.271.34.20531. [DOI] [PubMed] [Google Scholar]
- 73.Schulze M, Rödel G. SCO1, a yeast nuclear gene essential for accumulation of mitochondrrial cytochrome c oxidase subunit II. Mol Gen Genet. 1988;211:492–98. doi: 10.1007/BF00425706. [DOI] [PubMed] [Google Scholar]
- 74.Krummeck G, Rödel G. Yeast SCO1 protein is required for a post-translational step in the accumulation of mitochondrial cytochrome c oxidase subunits I and II. Curr Genet. 1990;18:13–15. doi: 10.1007/BF00321109. [DOI] [PubMed] [Google Scholar]
- 75.Leary SC, Kaufman BA, Pellechia G, Gguercin GH, Mattman A, et al. Human SCO1 and SCO2 have independent, cooperative functions in copper delivery to cytochrome c oxidase. Hum Mol Genet. 2004;13:1839–48. doi: 10.1093/hmg/ddh197. [DOI] [PubMed] [Google Scholar]
- 76.Shoubridge EA. Cytochrome c oxidase deficiency. Am J Med Genet. 2001;106:46–52. doi: 10.1002/ajmg.1378. [DOI] [PubMed] [Google Scholar]
- 77.Stiburek L, Vesela K, Hansikova H, Hulkova H, Zeman J. Loss of function of Sco1 and its interaction with cytochrome c oxidase. Am J Physiol Cell Physiol. 2009;296:C1218–26. doi: 10.1152/ajpcell.00564.2008. [DOI] [PubMed] [Google Scholar]
- 78.Leary SC, Cobine PA, Kaufman BA, Guercin GH, Mattman A, et al. The human cytochrome c oxidase assembly factors SCO1 and SCO2 have regulatory roles in the maintenance of cellular copper homeostasis. Cell Metab. 2007;5:9–20. doi: 10.1016/j.cmet.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 79.Rentzsch N, Krummeck-Weiß G, Hofer A, Bartuschka A, Ostermann K, Rödel G. Mitochondrial copper metabolism in yeast: mutational analysis of Sco1p invovled in the biogenesis of cytochrome c oxidase. Curr Genet. 1999;35:103–8. doi: 10.1007/s002940050438. [DOI] [PubMed] [Google Scholar]
- 80.Nittis T, George GN, Winge DR. Yeast Sco1, a protein essential for cytochrome c oxidase function is a Cu(I)-binding protein. J Biol Chem. 2001;276:42520–26. doi: 10.1074/jbc.M107077200. [DOI] [PubMed] [Google Scholar]
- 81.Banci L, Bertini I, Calderone V, Ciofi-Baffoni S, Mangani S, et al. A hint for the function of human Sco1 from different structures. Proc Natl Acad Sci USA. 2006;103:8595–600. doi: 10.1073/pnas.0601375103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Williams JC, Sue C, Banting GS, Yang H, Glerum DM, et al. Crystal structure of human SCO1: implications for redox signaling by a mitochondrial cytochrome c oxidase “assembly” protein. J Biol Chem. 2005;280:15202–11. doi: 10.1074/jbc.M410705200. [DOI] [PubMed] [Google Scholar]
- 83.Abajian C, Rosenzweig AC. Cystal structure of yeast Sco1. J Biol Inorg Chem. 2006;11:459–66. doi: 10.1007/s00775-006-0096-7. [DOI] [PubMed] [Google Scholar]
- 84.Banci L, Bertini I, Calderone V, Ciofi-Baffoni S, Mangani S, et al. A hint for the function of human Sco1 from different strucures. Proc Natl Acad Sci USA. 2006;103:8595–600. doi: 10.1073/pnas.0601375103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Banci L, Bertini I, Ciofi-Baffoni S, Gerothanassis IP, Leontari I, et al. A structural characterization of human SCO2. Structure. 2007;15:1132–40. doi: 10.1016/j.str.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 86.Leary SC, Sasarman F, Nishimura T, Shoubridge EA. Human SCO2 is required for the synthesis of CO II and as a thiol-disulphide oxidoreductase for SCO1. Hum Mol Genet. 2009;18:2230–40. doi: 10.1093/hmg/ddp158. [DOI] [PubMed] [Google Scholar]
- 87.Su D, Berndt C, Fomenko DE, Holmgren A, Gladyshev VN. Conserved cis-proline precludes metal binding by the active site thiolates in members of the thioredoxin family of proteins. Biochemistry. 2007;46:6903–10. doi: 10.1021/bi700152b. [DOI] [PubMed] [Google Scholar]
- 88.Riemer J, Bulleid N, Herrmann JM. Disulfide formation in the ER and mitochondria: two solutions to a common process. Science. 2009;324:1284–87. doi: 10.1126/science.1170653. [DOI] [PubMed] [Google Scholar]
- 89.Horng YC, Cobine PA, Maxfield AB, Carr HS, Winge DR. Specific copper transfer from the Cox17 metallochaperone to both Sco1 and Cox11 in the assembly of yeast cytochrome c oxidase. J Biol Chem. 2004;279:35334–40. doi: 10.1074/jbc.M404747200. [DOI] [PubMed] [Google Scholar]
- 90.Valnot I, Osmond S, Gigarel N, Mehaye B, Amiel J, et al. Mutations of the SCO1 gene in mitochondrial cytochrome c oxidase deficiency with neonatal-onset hepatic failure and encephalopathy. Am J Hum Genet. 2000;67:1104–9. doi: 10.1016/s0002-9297(07)62940-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Paret C, Lode A, Krause-Buchholz U, Rödel G. The P(174)L mutation in the human hSCO1 gene affects the assembly of cytochrome c oxidase. Biochem Biophys Res Commun. 2000;279:341–47. doi: 10.1006/bbrc.2000.3949. [DOI] [PubMed] [Google Scholar]
- 92.Cobine PA, Pierrel F, Leary SC, Sasarman F, Horng YC, et al. The P174L mutation in human Sco1 severely compromises Cox17-dependent metallation but does not impair copper binding. J Biol Chem. 2006;281:12270–76. doi: 10.1074/jbc.M600496200. [DOI] [PubMed] [Google Scholar]
- 93.Banci L, Bertini I, Ciofi-Baffoni S, Hadjiloi T, Martinelli M, Palumaa P. Mitochondrial copper(I) transfer from Cox17 to Sco1 is coupled to electron transfer. Proc Natl Acad Sci USA. 2008;105:6803–8. doi: 10.1073/pnas.0800019105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Horng YC, Leary SC, Cobine PA, Young FB, George GN, et al. Human Sco1 and Sco2 function as copper-binding proteins. J Biol Chem. 2005;280:34113–22. doi: 10.1074/jbc.M506801200. [DOI] [PubMed] [Google Scholar]
- 95.Rigby K, Cobine PA, Khalimonchuk O, Winge DR. Mapping the functional interaction of Sco1 and Cox2 in cytochrome oxidase biogenesis. J Biol Chem. 2008;283:15015–22. doi: 10.1074/jbc.M710072200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Banci L, Bertini I, Cavallaro G, Rosato A. The functions of Sco proteins from genome-based analysis. J Proteome Res. 2007;6:1568–79. doi: 10.1021/pr060538p. [DOI] [PubMed] [Google Scholar]
- 97.Swem DL, Swem LR, Setterdahl A, Bauer CE. Involvement of SenC in assembly of cytochrome c oxidase in Rhodobacter capsulatus. J Bacteriol. 2005;187:8081–87. doi: 10.1128/JB.187.23.8081-8087.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Davidson DE, Hill BC. Stability of oxidized, reduced and copper bound forms of Bacillus subtilis Sco. Biochim Biophys Acta. 2009;1794:275–81. doi: 10.1016/j.bbapap.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 99.Cawthorn TR, Poulsen BE, Davidson DE, Andrews D, Hill BC. Probing the kinetics and thermodynamics of copper(II) binding to Bacillus subtilis Sco, a protein involved in the assembly of the Cu(A) center of cytochrome c oxidase. Biochemistry. 2009;48:4448–54. doi: 10.1021/bi802288m. [DOI] [PubMed] [Google Scholar]
- 100.Banci L, Bertini I, Ciofi-Baffoni S, Katsari E, Katsaros N, et al. A copper(I) protein possibly involved in the assembly of CuA center of bacterial cytochrome c oxidase. Proc Natl Acad Sci USA. 2005;102:3994–99. doi: 10.1073/pnas.0406150102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Abriata LA, Banci L, Bertini I, Ciofi-Baffoni S, Gkazonis P, et al. Mechanism of Cu(A) assembly. Nat Chem Biol. 2008;4:599–601. doi: 10.1038/nchembio.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tzagoloff A, Capitanio N, Nobrega MP, Gatti D. Cytochrome oxidase assembly in yeast requires the product of COX11, a homolog of the P. denitrificans protein encoded by ORF3. EMBO J. 1990;9:2759–64. doi: 10.1002/j.1460-2075.1990.tb07463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hiser L, Di Valentin M, Hamer AG, Hosler JP. Cox11 is required for stable formation of the CuB and magnesium centers of cytochrome c oxidase. J Biol Chem. 2000;275:619–23. doi: 10.1074/jbc.275.1.619. [DOI] [PubMed] [Google Scholar]
- 104.Carr HS, George GN, Winge DR. Yeast Cox11, a protein essential for cytochrome c oxidase assembly, is a Cu(I) binding protein. J Biol Chem. 2002;277:31237–42. doi: 10.1074/jbc.M204854200. [DOI] [PubMed] [Google Scholar]
- 105.Carr HS, Maxfield AB, Horng YC, Winge DR. Functional analysis of the domains of Cox11. J Biol Chem. 2005;280:22664–69. doi: 10.1074/jbc.M414077200. [DOI] [PubMed] [Google Scholar]
- 106.Banci L, Bertini I, Cantini F, Ciofi-Baffoni S, Gonnelli L, Mangani S. Solution structure of Cox11: a novel type of β-immunoglobulin-like fold involved in CuB site formation of cytochrome c oxidase. J Biol Chem. 2004;279:34833–39. doi: 10.1074/jbc.M403655200. [DOI] [PubMed] [Google Scholar]
- 107.Smith D, Gray J, Mitchell L, Antholine WE, Hosler JP. Assembly of cytochrome c oxidase in the absence of the assembly protein Surf1p leads to loss of the active site heme. J Biol Chem. 2005;280:17652–56. doi: 10.1074/jbc.C500061200. [DOI] [PubMed] [Google Scholar]
- 108.Bundschuh FA, Hoffmeier K, Ludwig B. Two variants of the assembly factor Surf1 target specific terminal oxidases in Paracoccus denitrificans. Biochim Biophys Acta. 2008;1777:1336–43. doi: 10.1016/j.bbabio.2008.05.448. [DOI] [PubMed] [Google Scholar]
- 109.Khalimonchuk O, Bird A, Winge DR. Evidence for a pro-oxidant intermediate in the assembly of cytochrome oxidase. J Biol Chem. 2007;282:17442–49. doi: 10.1074/jbc.M702379200. [DOI] [PubMed] [Google Scholar]
- 110.Culotta VC, Klomp LWJ, Strain J, Casareno RLB, Krems B, Gitlin JD. The copper chaperone for superoxide dismutase. J Biol Chem. 1997;272:23469–72. doi: 10.1074/jbc.272.38.23469. [DOI] [PubMed] [Google Scholar]
- 111.Schmidt PJ, Kunst C, Culotta VC. Copper activation of superoxide dismutase 1 (SOD1) in vivo. J Biol Chem. 2000;275:33771–76. doi: 10.1074/jbc.M006254200. [DOI] [PubMed] [Google Scholar]
- 112.Lamb AL, Torres AS, O'Halloran TV, Rosenzweig AC. Heterodimer formation between superoxide dismutase and its copper chaperone. Biochemistry. 2000;39:14720–27. doi: 10.1021/bi002207a. [DOI] [PubMed] [Google Scholar]
- 113.Lamb AL, Torres AS, O'Halloran TV, Rosenzweig AC. Heterodimeric structure of superoxide dismutase in complex with its metallochaperone. Nat Struct Biol. 2001;8:751–55. doi: 10.1038/nsb0901-751. [DOI] [PubMed] [Google Scholar]
- 114.Schmidt PJ, Rae TD, Pufahl RA, Hamma T, Strain J, et al. Multiple protein domains contribute to the action of the copper chaperone for superoxide dismutase. J Biol Chem. 1999;274:23719–25. doi: 10.1074/jbc.274.34.23719. [DOI] [PubMed] [Google Scholar]
- 115.Rae RD, Schmidt PJ, Pufahl RA, Culotta VC, O'Halloran TV. Undetectable intracellular free copper: the requirement of a copper chaperone for superoxide dismutase. Science. 1999;284:805–7. doi: 10.1126/science.284.5415.805. [DOI] [PubMed] [Google Scholar]
- 116.Kirby K, Jensen LT, Binnington J, Hilliker AJ, Ulloa J, et al. Instability of superoxide dismutase 1 of Drosophila in mutants deficient for its cognate copper chaperone. J Biol Chem. 2008;283:35393–401. doi: 10.1074/jbc.M807131200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wong PC, Waggoner D, Subramaniam JR, Tessarollo L, Bartnikas TB, et al. Copper chaperone for superoxide dismutase is essential to activate mammalian Cu/Zn superoxide dismutase. Proc Natl Acad Sci USA. 2000;97:2886–91. doi: 10.1073/pnas.040461197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Furukawa Y, Torres AS, O'Halloran TV. Oxygen-induced maturation of SOD1: a key role for disulfide formation by the copper chaperone CCS. EMBO J. 2004;23:2872–81. doi: 10.1038/sj.emboj.7600276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fridovich I. Superoxide anion radical (), superoxide dismutases and related matters. J Biol Chem. 1997;272:18515–17. doi: 10.1074/jbc.272.30.18515. [DOI] [PubMed] [Google Scholar]
- 120.Tainer JA, Getzoff ED, Beem KM, Richardson JS, Richardson DC. Determination and analysis of the 2 A-structure of copper, zinc superoxide dismutase. J Mol Biol. 1982;160:181–217. doi: 10.1016/0022-2836(82)90174-7. [DOI] [PubMed] [Google Scholar]
- 121.Tainer JA, Getzoff ED, Richardson JS, Richardson DC. Structure and mechanism of copper, zinc superoxide dismutase. Nature. 1983;306:284–87. doi: 10.1038/306284a0. [DOI] [PubMed] [Google Scholar]
- 122.Forman HJ, Fridovich I. On the stability of bovine superoxide dismutase. The effects of metals. J Biol Chem. 1973;248:2645–49. [PubMed] [Google Scholar]
- 123.Stasser JP, Siluvai GS, Barry AN, Blackburn NJ. A multinuclear copper(I) cluster forms the dimerization interface in copper-loaded human copper chaperone for superoxide dismutase. Biochemistry. 2007;46:11845–56. doi: 10.1021/bi700566h. [DOI] [PubMed] [Google Scholar]
- 124.Caruano-Yzermans AL, Bartnikas TB, Gitlin JD. Mechanisms of the copper-dependent turnover of the copper chaperone for superoxide dismutase. J Biol Chem. 2006;281:13581–87. doi: 10.1074/jbc.M601580200. [DOI] [PubMed] [Google Scholar]
- 125.Lamb AL, Wernimont AK, Pufahl RA, O'Halloran TV, Rosenzweig AC. Crystal structure of the copper chaperone for superoxide dismutase. Nat Struct Biol. 1999;6:724–29. doi: 10.1038/11489. [DOI] [PubMed] [Google Scholar]
- 126.Lamb AL, Wernimont AK, Pufahl RA, O'Halloran TV, Rosenzweig AC. Crystal structure of the second domain of the human copper chaperone for superoxide dismutase. Biochemistry. 2000;39:1589–95. doi: 10.1021/bi992822i. [DOI] [PubMed] [Google Scholar]
- 127.Winkler DD, Schuermann JP, Cao X, Holloway SP, Borchelt DR, et al. Structural and biophysical properties of the pathogenic SOD1 variant H46R/H48Q. Biochemistry. 2009;48:3436–47. doi: 10.1021/bi8021735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Banci L, Bertini I, Cantini F, D'Onofrio M, Viezzoli MS. Structure and dynamics of copper-free SOD: the protein before binding copper. Protein Sci. 2002;11:2479–92. doi: 10.1110/ps.0210802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Potter SZ, Zhu H, Shaw BF, Rodriguez JA, Doucette PA, et al. Binding of a single zinc ion to one subunit of copper-zinc superoxide dismutase apoprotein substantially influences the structure and stability of the entire homodimeric protein. J Am Chem Soc. 2007;129:4575–83. doi: 10.1021/ja066690+. [DOI] [PubMed] [Google Scholar]
- 130.Proescher JB, Son M, Elliott JL, Culotta VC. Biological effects of CCS in the absence of SOD1 enzyme activation: implications for disease in a mouse model for ALS. Hum Mol Genet. 2008;17:1728–37. doi: 10.1093/hmg/ddn063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jensen LT, Culotta VC. Activation of CuZn superoxide dismutases from Caenorhabditis elegans does not require the copper chaperone CCS. J Biol Chem. 2005;280:41373–79. doi: 10.1074/jbc.M509142200. [DOI] [PubMed] [Google Scholar]
- 132.Subramaniam JR, Lyons WE, Liu J, Bartnikas TB, Rothstein J, et al. Mutant SOD1 causes motor neuron disease independent of copper chaperone-mediated copper loading. Nat Neurosci. 2002;5:301–7. doi: 10.1038/nn823. [DOI] [PubMed] [Google Scholar]
- 133.Carroll MC, Girouard JB, Ulloa JL, Subramaniam JR, Wong PC, et al. Mechanisms for activating Cu- and Zn-containing superoxide dismutase in the absence of the CCS Cu chaperone. Proc Natl Acad Sci USA. 2004;101:5964–69. doi: 10.1073/pnas.0308298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Field LS, Furukawa Y, O'Halloran TV, Culotta VC. Factors controlling the uptake of yeast copper/zinc superoxide dismutase into mitochondria. J Biol Chem. 2003;278:28052–59. doi: 10.1074/jbc.M304296200. [DOI] [PubMed] [Google Scholar]
- 135.Son M, Puttaparthi K, Kawamata H, Rajendran B, Boyer PJ, et al. Overexpression of CCS in G93 A-SOD1 mice leads to accelerated neurological deficits with severe mitochondrial pathology. Proc Natl Acad Sci USA. 2007;104:6072–77. doi: 10.1073/pnas.0610923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kawamata H, Manfredi G. Different regulation of wild-type and mutant Cu,Zn superoxide dismutase localization in mammalian mitochondria. Hum Mol Genet. 2008;17:3303–17. doi: 10.1093/hmg/ddn226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Reddehase S, Grumbt B, Neupert W, Hell K. The disulfide relay system of mitochondria is required for the biogenesis of mitochondrial Ccs1 and Sod1. J Mol Biol. 2009;385:331–38. doi: 10.1016/j.jmb.2008.10.088. [DOI] [PubMed] [Google Scholar]
- 138.Zhang R, Snyder GH. Factors governing selective formation of specific disulfides in synthetic variants of α-conotoxin. Biochemistry. 1991;30:11343–48. doi: 10.1021/bi00111a021. [DOI] [PubMed] [Google Scholar]
- 139.Liu H, Zhu H, Eggers DK, Nersissian AM, Faull KF, et al. Copper2+ binding to the surface residue cysteine 111 of His46Arg human copper-zinc superoxide dismutase, a familial amyotrophic lateral sclerosis mutant. Biochemistry. 2000;39:8125–32. doi: 10.1021/bi000846f. [DOI] [PubMed] [Google Scholar]
- 140.Peter C, Laliberte J, Beaudoin J, Labbe S. Copper distributed by Atx1 is available to copper amine oxidase 1 in Schizosaccharomyces pombe. Eukaryot Cell. 2008;7:1781–94. doi: 10.1128/EC.00230-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Puig S, Penarrubia L. Placing metal micronutrients in context: transport and distribution in plants. Curr Opin Plant Biol. 2009;12:299–306. doi: 10.1016/j.pbi.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 142.Puig S, Mira H, Dorcey E, Sancenon V, Andres-Colas N, et al. Higher plants possess two different types of ATX1-like copper chaperones. Biochem Biophys Res Commun. 2007;354:385–90. doi: 10.1016/j.bbrc.2006.12.215. [DOI] [PubMed] [Google Scholar]
- 143.Andres-Colas N, Sancenon V, Rodriguez-Navarro S, Mayo S, Thiele DJ, et al. The Arabidopsis heavy metal P-type ATPase HMA5 interacts with metallochaperones and functions in copper detoxification of roots. Plant J. 2006;45:225–36. doi: 10.1111/j.1365-313X.2005.02601.x. [DOI] [PubMed] [Google Scholar]
- 144.Cobine P, Wickramasinghe WA, Harrison MD, Weber T, Solioz M, Dameron CT. The Enterococcus hirae copper chaperone CopZ delivers copper(I) to the CopY repressor. FEBS Lett. 1999;445:27–30. doi: 10.1016/s0014-5793(99)00091-5. [DOI] [PubMed] [Google Scholar]
- 145.Cobine PA, George GN, Jones CE, Wickramasinghe WA, Solioz M, Dameron CT. Copper transfer from the Cu(I) chaperone, CopZ, to the repressor, Zn(II)CopY: metal coordination environments and protein interactions. Biochemistry. 2002;41:5822–29. doi: 10.1021/bi025515c. [DOI] [PubMed] [Google Scholar]
- 146.Itoh S, Kim HW, Nakagawa O, Ozumi K, Lessner SM, et al. Novel role of antioxidant-1 (Atox1) as a copper dependent transcription factor involved in cell proliferation. J Biol Chem. 2008;283:9157–67. doi: 10.1074/jbc.M709463200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Angeletti B, Waldron KJ, Freeman KB, Bawagan H, Hussain I, et al. BACE1 cytoplasmic domain interacts with the copper chaperone for superoxide dismutase-1 and binds copper. J Biol Chem. 2005;280:17930–37. doi: 10.1074/jbc.M412034200. [DOI] [PubMed] [Google Scholar]