Abstract
Donor lung utilization rates are persistently low primarily due to donor lung dysfunction. We hypothesized that a treatment that enhances the resolution of pulmonary edema by stimulating the rate of alveolar fluid clearance would improve donor oxygenation and increase donor lung utilization. We conducted a randomized, blinded, placebo-controlled trial of aerosolized albuterol (5 mg q4h) versus saline placebo during active donor management in 506 organ donors. The primary outcome was change in oxygenation (PaO2/FiO2) from enrollment to organ procurement. The albuterol (n=260) and placebo (n=246) groups were well matched for age, gender, ethnicity, smoking, and cause of brain death. The change in PaO2/FiO2 from enrollment to organ procurement did not differ between treatment groups (p=0.54) nor did donor lung utilization (albuterol 29% vs. placebo 32%, p=0.44). Donors in the albuterol vs. placebo group were more likely to have the study drug dose reduced (13% vs. 1%, p<0.001) or stopped (8% vs. 0%, p<0.001) for tachycardia. In summary, treatment with high dose inhaled albuterol during the donor management period did not improve donor oxygenation or increase donor lung utilization but did cause tachycardia. High dose aerosolized albuterol should not be used in donors to enhance the resolution of pulmonary edema.
Keywords: Lung transplantation, donor management, clinical trials, albuterol, pulmonary edema
INTRODUCTION
Compared to liver and kidney utilization rates of greater than 85%, the donor lung utilization rate in the U.S. is approximately 20% (1), and the demand for donor lungs far exceeds the available supply (2–4). The most common reasons for failure to utilize donor lungs are donor hypoxemia and/or pulmonary infiltrates. Since pulmonary edema is a common, reversible cause of pulmonary infiltrates and hypoxemia in patients with brain injury (5), strategies to treat pulmonary edema in organ donors could lead to higher rates of donor lung utilization.
Aerosolized beta-2 adrenergic agonists increase the rate of alveolar fluid clearance and reduce pulmonary edema in animal and human lungs (6). Our research group reported that the majority of human donor lungs that are rejected for transplantation have significant pulmonary edema and respond to beta-2 adrenergic agonists with increased rates of alveolar fluid clearance (7, 8). Beta-2 adrenergic agonists also have potent anti-inflammatory and endothelial and lung epithelial protective effects that may be beneficial in the brain-dead organ donor (9–11).
Based on this compelling preclinical evidence, we designed a blinded, randomized clinical trial to test the hypothesis that administration of an aerosolized beta-2 agonist (albuterol, also known as salbutamol) in brain-dead organ donors would improve donor oxygenation and static compliance of the respiratory system by enhancing clearance of pulmonary edema and would thereby improve donor lung procurement rates. In the subset of enrolled donors whose lungs were not utilized for transplantation, we tested the secondary hypothesis that aerosolized albuterol would reduce pulmonary edema and enhance the rate of alveolar fluid clearance in the excised lung.
METHODS
Trial design
The study was a prospective, randomized, double-blinded, placebo-controlled clinical trial of aerosolized albuterol compared to aerosolized placebo (saline) control. Donors were enrolled from April 23, 2007 until targeted enrollment was completed on April 23, 2011 (ClinicalTrials.gov NCT00310401) at hospitals served by the California Transplant Donor Network (CTDN) throughout Northern California and parts of Nevada. Detailed methods have been previously published (12). A summary is provided here.
Donors
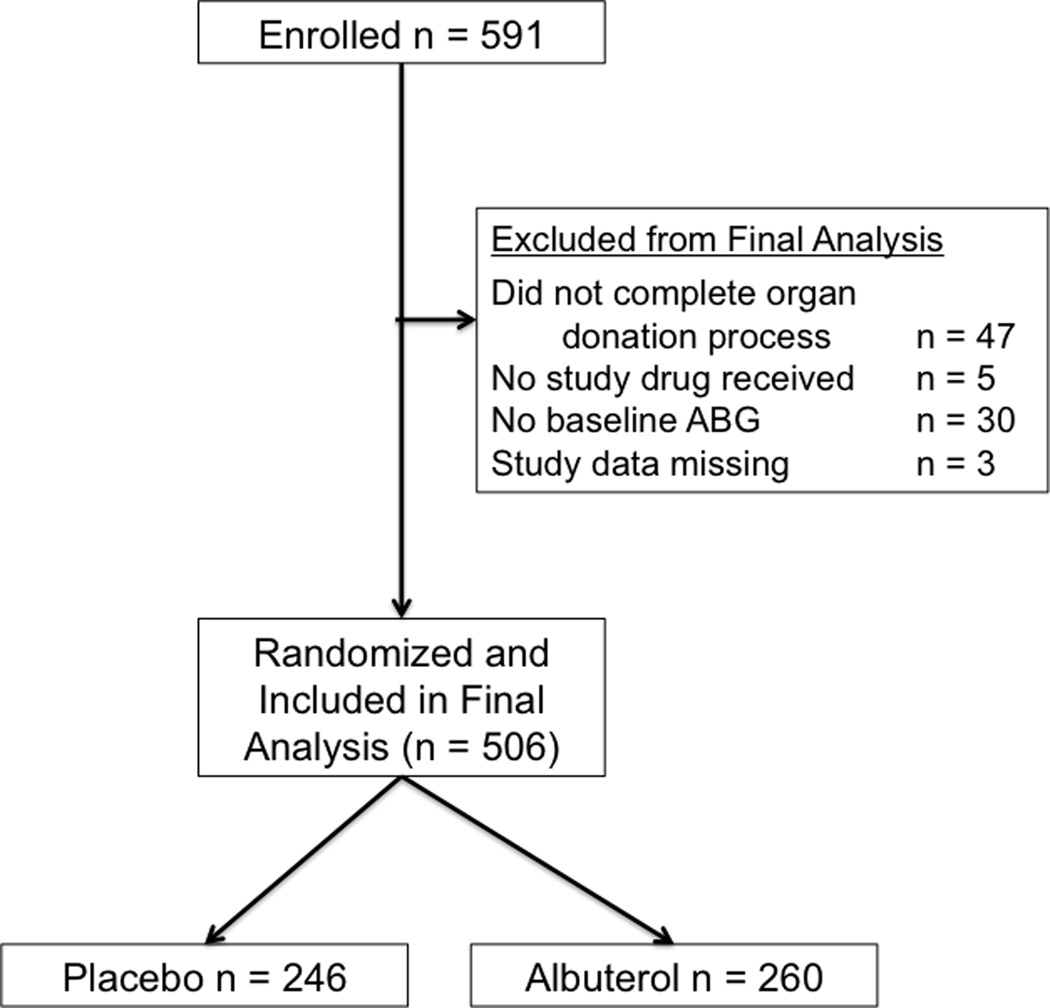
The study was approved by the Medical Board of the CTDN. All brain-dead organ donors managed by the CTDN during the study period were eligible for enrollment. Donation after cardiac death (DCD) donors were not included. Exclusion criteria included age less than 14 years or lack of legal next-of-kin authorization for organ donation for purposes of both transplantation and research as previously described (13). Additional exclusion criteria included failure to receive at least one dose of study drug after enrollment and failure to complete the donation process as defined by surgical recovery of at least one solid organ for transplantation (Figure 1). For measurement of physiologic endpoints in the excised lung, the lungs had to be rejected for transplantation and released by the coroner or medical examiner. In addition, a qualified surgeon had to be available to recover the lungs at the time of organ procurement.
Figure 1.
Flow diagram of donor enrollment
Randomization and treatment groups
Randomization was conducted by the University of California Investigational Pharmacy at the time of study drug preparation in a 1:1 ratio between study drug and placebo in permuted blocks of 8 using computer generated random numbers. The albuterol group received 5.0 mg of albuterol sulfate (dissolved in saline) by nebulization through the ventilator circuit every 4 hours from the time of study enrollment until organ procurement. This dose was chosen because it is within the usual dosing range for mechanically ventilated patients and should lead to alveolar concentrations that are on the plateau of the dose response curve for enhanced alveolar fluid clearance in the ex vivo human lung (14, 15). The placebo group received an equivalent volume of identical-appearing nebulized, preservative-free saline every 4 hours. To maintain blinding, study kits containing identically labeled placebo or study drug were distributed to each transplant coordinator and coordinators were instructed to utilize the study kits sequentially as they enrolled donors in the field. All transplant coordinators, respiratory therapists and others caring for the donor as well as study coordinators who recorded clinical data including clinical outcomes were blinded to treatment group, and there were no unblinding events. If during the aerosolization, the heart rate increased by >30 beats per minute (bpm), the aerosol was stopped. If during the next scheduled aerosolization the heart rate again increased by >30 bpm, the aerosol was stopped and subsequent study drug doses were reduced to 2.5 mg albuterol sulfate or equivalent volume of saline. Any subsequent increase in heart rate of >30 bpm was treated with discontinuation of study drug for the remainder of the study. Study drug was also discontinued in the event of sustained atrial or ventricular arrhythmias or development of more than four new premature ventricular contractions per minute during aerosolization.
Primary and secondary clinical outcomes
The primary outcome was the change in PaO2/FiO2 ratio from enrollment to organ procurement or to 72 hours, whichever occurred first. Secondary clinical outcomes included the change in static compliance of the respiratory system (as calculated from measurement of the plateau pressure during an end-inspiratory pause), the change in chest radiographic score (16) from enrollment to organ procurement, and the donor lung utilization rate. In a prespecified analysis, donor lung utilization was also analyzed after excluding donors who were deemed unlikely to be lung donors at enrollment (defined as > 50 pack year smoking, age > 65, positive serologies, or underlying chronic lung disease). Recipient outcomes including 30-day and one-year recipient survival were analyzed for lung and other solid organs including liver, kidney and heart using data collected by the United Network for Organ Sharing (UNOS) and the Organ Procurement and Transplantation Network (OPTN). The OPTN data system includes data on all donor, wait-listed candidates, and transplant recipients in the US, submitted by the members of the Organ Procurement and Transplantation Network (OPTN), and has been described elsewhere (17). The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services provides oversight to the activities of the OPTN contractor. The UCSF Committee on Human Research approved the use of UNOS/OPTN data for this study.
Physiologic endpoints
Lungs that were not used for clinical transplantation were recovered without perfusion and transported to UCSF at 4°C for physiologic evaluation. Evaluations included measurement of the lung weight, bronchoalveolar lavage on an isolated lobe for cell count and differential and measurement of the rate of alveolar fluid clearance as previously described (7).
Other study procedures
Arterial blood gases, static compliance of the respiratory system and chest radiograph were obtained at enrollment and immediately prior to organ procurement. Donors were managed by standard CTDN protocols (18). In addition, it was recommended (but not mandated) that donors be ventilated with 10 cc/kg tidal volume based on predicted body weight (19) to minimize ventilator-associated lung injury.
Power analysis
The trial was planned with early termination opportunities for efficacy based on the method of O’Brien and Fleming (20). The targeted final sample size of 500 donors (250 treated with albuterol and 250 treated with placebo) had a power of 80% to detect an increase in donor oxygenation, expressed as a mean increase in PaO2/FiO2 of 37.5. The standard deviation of the PaO2/FiO2 ratio was assumed to be 150 based on our prior studies in the organ donor population. Two interim analyses for efficacy were planned at total sample sizes of 100 and 300 with one-sided type I error rates of <0.0001, 0.0038. The type I error rate for the final analysis was 0.0212. Sample size computation was conducted using alpha spending approach (21) and later confirmed with PASS 2008 (22). An additional planned review of data quality was conducted when at least 50 donors were enrolled in each group. At the two interim analyses, a t-test was used to compare the primary outcome between the two treatment groups.
Statistical analysis
The baseline donor information was summarized with the mean and standard deviation for each treatment group, and the group difference was assessed with t-test for the continuous variables and chi-squared test for the categorical variables. Median and quartiles were used to summarize continuous variables that were heavily skewed; Wilcoxon rank sum tests were used in place of t-tests with the skewed data. Treatment comparisons on pulmonary physiologic variables were conducted with analysis of covariance models to account for baseline differences. The recipients’ survival data were analyzed using Cox proportional hazard (PH) models. To account for the repeated data structure due to shared donors, generalized estimating equations with robust variance estimator (23) were used. Because albuterol might only be effective in donors with substantial pulmonary edema, as a secondary analysis we analyzed the same outcomes excluding donors who had a baseline PaO2/FiO2 ratio of > 300 mmHg. SPSS version 20 (IBM Corporation, Armonk, New York) and R 2.15.1 (R Core Team, Vienna, Austria) were used for statistical analysis.
RESULTS
A total of 591 donors met inclusion criteria and were enrolled (Figure 1). The study was stopped when targeted enrollment was reached. 85 donors were excluded from the final analysis for prespecified reasons including donors who ultimately had no organs recovered (n = 47), lack of a baseline arterial blood gas for calculation of the primary outcome (n = 30), failure to receive any doses of study drug (n = 5) and all study data missing (n = 3). Among the remaining 506 donors, 260 were randomized to albuterol therapy and 246 were randomized to placebo.
Donor characteristics at enrollment are summarized in Table 1. The donors were well matched for baseline demographics, cause of brain death and smoking history. A diagnosis of chronic lung disease other than remote childhood asthma was infrequent (48/506, 9.5%), and did not differ significantly between treatment groups. The majority of these diagnoses were asthma (n = 40) or chronic obstructive pulmonary disease (n = 4). Donors who were not included in the final analysis (n = 85) were older (49 ± 15 vs. 42 ± 16 years, p < 0.001), more likely to be male (79% vs. 63%, p = 0.005) and less likely to have head trauma (28% vs. 41%, p = 0.065) as the cause of brain death compared to donors who were included.
Table 1.
Baseline Donor Characteristics
| Placebo (N = 246) |
Albuterol (N = 260) |
|
|---|---|---|
| Age (yrs) | 42 ± 15 | 43 ± 16 |
| Male | 161 (65%) | 158 (61%) |
| Race/ethnicity | ||
| -Caucasian | 145 (59%) | 159 (61%) |
| -Hispanic | 60 (24%) | 52 (20%) |
| -African American | 20 (8%) | 31 (12%) |
| -Asian | 19 (8%) | 16 (6%) |
| Current smoker | 112 (46%) | 106 (41%) |
| Cause of brain injury | ||
| -head trauma | 106 (43%) | 102 (39%) |
| -CVA/bleed | 95 (39%) | 111 (43%) |
| -anoxic injury | 43 (18%) | 40 (15%) |
| -other | 2 (1%) | 7 (3%) |
| Current lung disease | 17 (7%) | 31 (12%) |
| Time from clinical brain stem herniation to enrollment (h) | 26 ± 22 | 26 ± 24 |
| Albuterol treatment prior to enrollment | 59 (24%) | 59 (23%) |
| Tidal volume (ml/kg) | 8.9 ± 1.7 | 8.7 ± 1.8 |
| Positive end expiratory pressure (cm H2O) | 5.7 ± 1.9 | 5.9 ± 2.2 |
| Creatinine (mg/dL) | 1.28 ± 0.96 | 1.34 ± 1.20 |
Data as mean ± SD or N (%)
Table 2 summarizes study drug treatment during the study. The time from the enrollment arterial blood gas to the pre-procurement arterial blood gas ranged from 5 to 122 hours (mean 34 ± 15 hours) and donors received a mean of 9 ± 3 doses of study drug. The albuterol group was more likely than the control group to have the study drug dose reduced due to tachycardia (13% vs. 1%, p < 0.001) or to have the study drug held due to tachycardia (8% vs. 0%, p < 0.001). No other tachyarrhythmias were observed in either group.
Table 2.
Clinical data in the placebo versus albuterol groups
| Placebo (N = 246) |
Albuterol (N = 260) |
P value | |
|---|---|---|---|
| Time from enrollment to organ procurement (h) | 42 ± 15 | 38 ± 14 | 0.002 |
| Baseline to final ABG time (h) | 37 ± 16 | 33 ± 14 | 0.002 |
| Doses of study drug received | 9 ± 3 | 8 ± 3 | <0.001 |
| Percent of expected doses eceived | 86% | 87% | 0.41 |
| Open label albuterol | 15 (6%) | 11 (4%) | 0.34 |
| Donors with dose reduction for tachycardia | 3 (1%) | 35 (13%) | <0.001 |
| Donors with study drug stopped for tachycardia | 0 (0%) | 21 (8%) | <0.001 |
Data as mean ± SD or N (%)
Ventilatory and blood gas parameters at baseline and at study completion are summarized in Table 3. The primary outcome, change in oxygenation as measured by change in the PaO2/FiO2 ratio from enrollment to procurement was not different between treatment groups (Table 3). There were also no differences in other donor outcomes including change in alveolar-arterial oxygen difference, static compliance of the respiratory system or the chest radiographic score. Furthermore, no differences were observed in primary or secondary outcomes in those donors with a baseline PaO2/FiO2 ratio of ≤ 300 mmHg (data not shown).
Table 3.
Pulmonary physiologic results in the placebo versus albuterol groups
| Placebo (N = 246) | Albuterol (N = 260) | Treatment effect on markers |
|||||
|---|---|---|---|---|---|---|---|
| Variable | Baseline | End of Study |
P1 | Baseline | End of Study |
P1 | P2 |
| PaO2/FiO2 ratio (mmHg) | 317 ± 146 | 369 ± 135 | <0.001 | 312 ± 150 | 367 ± 138 | <0.001 | 0.98 |
| A-a gradient (mmHg) | 283 ± 162 | 247 ± 145 | <0.001 | 297 ± 169 | 256 ± 145 | <0.001 | 0.75 |
| Plateau pressure (cm H2O) | 20.4 ± 4.4 | 19.7 ± 5.1 | 0.032 | 21.1 ± 4.9 | 20.3 ± 4.1 | 0.002 | 0.43 |
| Tidal volume (ml) | 703 ± 132 | 718 ± 135 | 0.022 | 688 ± 126 | 718 ± 130 | <0.001 | 0.20 |
| Positive end expiratory pressure (cmH2O) | 5.70 ± 1.86 | 5.73 ± 1.88 | 0.82 | 5.91 ± 2.19 | 5.82 ± 1.88 | 0.51 | 0.88 |
| Static compliance of the respiratory system (ml/cmH2O) | 50 ± 15 | 56 ± 38 | 0.018 | 48 ± 14 | 52 ± 14 | <0.001 | 0.14 |
| Total chest radiographic score | 4.6 ± 3.2 | 4.4 ± 2.8 | 0.19 | 4.7 ± 3.3 | 5.0 ± 3.2 | 0.39 | 0.035 |
Data as mean ± SD,
Paired t-test,
Analysis of covariance (ANCOVA)
Donor lung utilization did not differ significantly between treatment groups (Table 4). Donor lung utilization also did not differ significantly between treatment groups after excluding donors who were deemed unlikely to be lung donors at enrollment. Excluding unlikely lung donors, donor lung utilization was 37% (61/167) in the albuterol group and 37% (67/183) in the placebo group. Donor lung utilization was also not improved by albuterol in the subgroup with initial PaO2/FiO2 ratio < 300. In fact, lung utilization was higher in the placebo treated group (22/108, 20%) compared to the albuterol treated group (11/120, 9%, p = 0.023) when donors with PaO2/FiO2 ratio > 300 were excluded.
Table 4.
Organ utilization in the placebo versus albuterol groups
| Placebo | Albuterol | P value | |
|---|---|---|---|
| Lung | 78/246 (32%) | 74/260 (29%) | 0.44 |
| Lung (excluding donors less likely to donate)1 | 67/183 (37%) | 61/167 (37%) | 1.0 |
| Heart | 99/246 (40%) | 95/260 (37%) | 0.41 |
| Liver | 198/246 (81%) | 213/260 (82%) | 0.73 |
| Pancreas | 68/246 (28%) | 53/260 (20%) | 0.061 |
| Kidney | 216/246 (88%) | 199/260 (77%) | 0.001 |
| Kidney (excluding donors less likely to donate)2 | 138/143 (97%) | 121/130 (93%) | 0.27 |
Donors less likely to donate included those > 65 years old or > 50 pack years smoking or history of chronic lung disease or positive serologies
Donors less likely to donate included those with diabetes, chronic kidney disease, hypertension or positive serologies
There were no differences in liver, heart or pancreas utilization (Table 4). However, kidney utilization was lower in the albuterol treated group compared to the placebo group (77% vs. 88%, p = 0.001). Kidney function did not appear to drive this disparity. Indeed, serum creatinine was similar at enrollment between the two groups (Table 1) and changes in blood urea nitrogen and creatinine between enrollment and procurement were also not different (data not shown). To further investigate potential reasons for the disparity in kidney utilization, we considered underlying comorbidities that could plausibly affect potential for kidney donation. We compared the incidence of comorbidities including diabetes, hypertension, chronic kidney disease and positive serologies (hepatitis B, C and human immunodeficiency virus) between placebo treated and albuterol treated donors. There was a trend toward more comorbidities in the albuterol group (47% vs. 40% with any comorbidity, p = 0.10). When potential donors with any comorbidity were excluded, the kidney utilization rate was similar between the albuterol and placebo groups (Table 4). We also compared the use of vasoactive drugs between the two treatment groups. There were no differences in the number of donors receiving vasoactive drugs (need for any norepinephrine, epinephrine, dopamine, or phenylephrine during donor management period) between donors receiving albuterol and those receiving placebo and there were no differences in total doses received of norepinephrine, epinephrine or dopamine. Donors in the albuterol arm received a slightly higher total dose of phenylephrine during the donor management period [93.8 mg (44.0 – 162.1) vs. 76.1 mg (25.5 – 139.3), p = 0.035].
One or both lungs that were not utilized for transplantation were recovered for physiologic evaluation from 213 donors. Physiologic indices are summarized in Table 5. There was no difference in lung weight, a measure of the degree of pulmonary edema, or bronchoalveolar lavage cell count in albuterol versus placebo-treated lungs. Donor lungs from the albuterol group were more likely to have a measurable rate of alveolar fluid clearance (83% vs. 70%, p = 0.042). However, the median rate of alveolar fluid clearance was not different in donor lungs that had been treated with albuterol compared to placebo [9.5 (3.8 – 15.4) %/h vs. 7.0 (0 – 14.3), p = 0.11].
Table 5.
Physiologic, cell and protein data in recovered lungs that were not utilized for transplantation
| N | Placebo | N | Albuterol | P Value | |
|---|---|---|---|---|---|
| Left lung weight (g) | 103 | 427 ± 131 | 106 | 445 ± 142 | 0.31 |
| Right lung weight (g) | 100 | 402 ± 112 | 103 | 423 ± 123 | 0.20 |
| Alveolar fluid clearance (%/h) | 89 | 7.0 (0 – 14.3) | 87 | 9.5 (3.8 – 15.4) | 0.11 |
| BAL cell count (× 103 × ml) | 101 | 797 (521 – 1492) | 104 | 740 (469 – 1539) | 0.94 |
| BAL protein (mg/dL) | 76 | 58 (15– 108) | 70 | 42 (26 – 88) | 0.96 |
Data as Mean ± SD or Median (IQR).
Importantly, albuterol administration did not impact transplant recipient survival. Indeed, across all solid organ transplant groups, there were no significant differences in either thirty day (not shown) or one-year survival (Table 6).
Table 6.
One-year survival and hazard ratio for survival in organ recipients randomized to placebo versus albuterol
| Placebo | Albuterol | Comparison | ||||
|---|---|---|---|---|---|---|
| N | 1-year Survival1 (%) [95% CI] |
N | 1-year Survival1 (%) [95% CI] |
Hazard Ratio1,2 95% CI] |
P-value1 | |
| Lung recipients | 80 | 0.89 [0.84 – 0.95] | 79 | 0.83 [0.76 – 0.90] | 1.46 [0.86 – 2.48] | 0.16 |
| Liver recipients | 203 | 0.88 [0.85 – 0.93] | 221 | 0.90 [0.86 – 0.93] | 0.89 [0.56 – 1.42] | 0.63 |
| Kidney recipients | 416 | 0.97 [0.95 – 0.98] | 371 | 0.97 [0.96 – 0.99] | 0.74 [0.42 – 1.3] | 0.29 |
| Heart recipients | 97 | 0.85 [0.79 – 0.92] | 92 | 0.92 [0.87 – 0.97] | 0.53 [0.26 – 1.07] | 0.08 |
: Survival probabilities, hazard ratios, and confidence intervals were estimated with univariate Cox proportional-hazards models. To account for repeated measures due to shared donors (except for heart recipients), a GEE approach was used in the Cox proportional hazard model. The lung transplantation model was further adjusted for transplantation type (single, double, heart/lung).
: Albuterol vs. Placebo
DISCUSSION
In this large, randomized, blinded, placebo-controlled clinical trial, administration of 5.0 mg of aerosolized albuterol sulfate every 4 hours during the donor management period did not improve donor oxygenation, static compliance of the respiratory system, donor radiographic findings, lung utilization, or the degree of pulmonary edema in the recovered lung. Donors who received albuterol were more likely to have tachycardia, and less likely to be kidney donors.
The lack of benefit of aerosolized albuterol in the critically ill donor population is consistent with the outcome of two recent large randomized clinical trials of beta-2 adrenergic agonists in acute lung injury. In a National Heart Blood and Lung Institute Acute Respiratory Distress Syndrome Clinical Trials Network study of a similar dose of aerosolized albuterol, there was no benefit in 282 patients with acute lung injury and the study was stopped early for futility (24). In a United Kingdom multicenter study of intravenous salbutamol in 326 patients with acute lung injury, there was also no benefit of intravenous salbutamol (25). Furthermore, that study was stopped early because of increased mortality in the salbutamol treated arm. Taken together, these three studies suggest that the use of either inhaled or intravenous beta-adrenergic agonists to enhance the resolution of pulmonary edema is not beneficial across a variety of clinical settings.
While this study did not demonstrate a clinical benefit of albuterol therapy, it provides important insights for future trials aimed at improving donor management and utilization. This is especially relevant for lung transplantation given the extremely low donor utilization rate of ~20%. Indeed, this study is not only one of the first, large scale, randomized trials of an intervention during the donor management period but also one of the first to study a pharmacologic agent. While randomized studies of preservation solutions at the time of organ recovery are numerous, large randomized trials of interventions in donor management are scant. Consequently, donor management continues to be driven by physiologic rationale and uncontrolled studies rather than by evidence based on clinical trials. This study, taken together with a recent study of two different ventilator strategies during the donor management period (26), suggests donor management trials are feasible, can be performed ethically (13), and can address important questions in donor management.
There are several potential explanations for the lack of efficacy of albuterol. First, pulmonary edema may be less of a clinical problem in organ donors than we expected based on our prior studies of ex vivo human lungs. In our prior study of lungs recovered but not utilized for transplantation, we found that the majority had pulmonary edema as measured by the lung wet-to-dry weight ratio (8). However, the sample size was modest (n = 29) and the majority of the lung wet-to-dry weight ratio measurements were consistent with only mild pulmonary edema. Similarly in this study, the mean lung weights were elevated compared to the normal lung weight by approximately 250 g, suggestive of mild pulmonary edema (20). By radiograph, the average total chest radiographic score, a quantitative index of pulmonary edema (16) was in the 4–5 range, consistent with mild but not severe pulmonary edema. It is also possible that the aerosolized albuterol could not effectively reach flooded or atelectatic airspaces. We have previously reported that aerosolization of 3–4mg of albuterol in mechanically ventilated patients with hydrostatic pulmonary edema or acute lung injury led to concentrations in the airspace of 10−6M (14). This represents a concentration that is on the plateau of the dose response curve for enhanced alveolar fluid clearance in the ex vivo human lung (15). While our dosing strategy of 5mg every four hours would presumably lead to equivalent, if not greater, airspace concentrations, it was not possible to measure air space concentrations of albuterol in the current study. In addition, atelectasis was common on donor chest radiographs (16) and aerosolized albuterol would likely not be delivered to atelectatic regions. Indeed, it is possible that some of the total chest radiographic density score was due to atelectasis rather than pulmonary edema and would not be impacted by albuterol. Whether albuterol might have a beneficial effect on lung fluid balance if delivery issues were bypassed by adding it to the perfusate during ex vivo lung perfusion is a question that will need to be addressed in future prospective studies.
Although albuterol did not show a therapeutic benefit, both the placebo and the albuterol treated groups had a significant improvement in oxygenation as measured by the change in PaO2/FiO2 ratio from study enrollment to organ procurement. It is not possible to conclude from the study whether the aerosolization procedure itself (of saline or albuterol) was responsible for changes in donor oxygenation over time or whether this was a function of other aspects of routine donor management. The only other deviation from routine CTDN donor management that was used in the study protocol was a suggested tidal volume of 10 cc/kg predicted body weight which was lower than tidal volumes routinely used by the CTDN at the time this study was initiated. All other aspects of ventilator management and donor management were done by standard CTDN protocol and were not altered for this study. Given the report by Mascia and colleagues (26) suggesting that a short course of lower tidal volume is associated with better oxygenation and higher rates of lung utilization among potential lung donors, it is possible that lower tidal volume was beneficial in the current study. Future prospective trials are needed to test the value of protective ventilatory strategies in donors managed over longer periods of time than in the study by Mascia and colleagues.
The overall donor lung utilization among donors enrolled in this study was approximately ~30%, higher than reported national donor lung utilization rates of approximately 20% during the study period (1). Current and historical donor lung utilization rates for the California Transplant Donor Network are consistently higher than national averages. One reason for the high donor lung utilization rate is donor mix, which may differ from other OPOs. Another possible explanation is the active involvement of a small group of experienced Advanced Practice Coordinators in all aspects of donor management and organ allocation at the CTDN, a practice model that differs from most other OPOs.
An unexpected finding was that kidney utilization was significantly lower in the albuterol treated group. This difference appeared to be explained by an imbalance in comorbiditities that might impact kidney utilization, rather than differences in kidney function. After excluding potential donors with comorbidities that might preclude kidney donation such as diabetes, hypertension or positive viral serologies, kidney utilization rates were not different between albuterol treated and placebo treated donors. Also reassuring is the finding that survival was similar in recipients of either albuterol or placebo treated donor kidneys. Although this analysis suggests that the difference in kidney utilization was driven primarily by imbalances in comorbidities at randomization, our findings underscore the importance of prospectively assessing outcomes in other organs in future donor management trials aimed at improving lung function.
Another unexpected finding was a non-significant trend towards better long term outcomes in recipients of hearts from albuterol treated donors compared to placebo. Since the number of heart recipients was low, the study may have been underpowered to demonstrate any effect of albuterol on long term outcomes in heart recipients. A potential explanation for trends towards different outcomes of heart transplantation could be that albuterol treatment altered endogenous catecholamine production or need for exogenous catecholamines. However, plasma norepinephrine and epinephrine levels were not different between albuterol and placebo treated donors (data not shown) and there were no differences in the need for or total doses of epinephrine, norepinephrine or dopamine. The higher total dose of phenylephrine in the albuterol-treated donors may reflect beta-2 adrenergic receptor mediated peripheral vasodilation. Another potential explanation for the trend towards better outcomes of heart recipients in the albuterol group is that high dose albuterol caused coronary vasodilation which may have been protective at the time of myocardial ischemia reperfusion. Albuterol has been reported to cause coronary vasodilation in healthy humans (27, 28).
In summary, in a large randomized blinded clinical trial in 506 brain-dead organ donors, high dose aerosolized albuterol was not beneficial compared to placebo and cannot be recommended as routine donor management. This study establishes the feasibility of large scale randomized trials during the period of active donor management and lays the groundwork to carry out additional rigorous evaluations of donor management practices in the future.
Acknowledgments
We thank Dr. Ellen Wright Clayton and Dr. Art Caplan for ethical consultation during the design of this clinical trial. We also are grateful to the members of the data safety and monitoring committee, Dr. Roy Brower, Dr. Jason Christie, Dr. John Newman and Dr. Chang Yu. Finally, this study would not have been possible without the tremendous dedication and hard work of all the transplant coordinators, advanced practice coordinators, surgical coordinators and support staff at the California Transplant Donor Network as well as members of Dr. Matthay’s laboratory including Jason Abbott. This study was supported by NIH HL088263 and NIH HL103836. The funding source had no role in the study design or interpretation of findings.
Abbreviations
- PaO2
arterial partial pressure of oxygen
- FiO2
fraction of inspired oxygen
- CTDN
California Transplant Donor Network
- bpm
beats per minute
- UNOS
United Network for Organ Sharing
- OPTN
Organ Procurement and Transplantation Network
- HRSA
Health Resources and Services Administration
Footnotes
Publisher's Disclaimer: UNOS/OPTN Disclaimer: Some of the data reported here have been supplied by the United Network for Organ Sharing (UNOS) as the contractor for the Organ Procurement and Transplantation Network (OPTN). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the OPTN or the U.S. Government.”
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Valapour M, Paulson K, Smith JM, Hertz MI, Skeans MA, Heubner BM, et al. OPTN/SRTR 2011 Annual Data Report: lung. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013;13(Suppl 1):149–177. doi: 10.1111/ajt.12024. [DOI] [PubMed] [Google Scholar]
- 2.Edwards LB, Keck BM. Thoracic organ transplantation in the US. Clin Transpl. 2002:29–40. [PubMed] [Google Scholar]
- 3.Klein AS, Messersmith EE, Ratner LE, Kochik R, Baliga PK, Ojo AO. Organ donation and utilization in the United States, 1999–2008. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010;10(4 Pt 2):973–986. doi: 10.1111/j.1600-6143.2009.03008.x. [DOI] [PubMed] [Google Scholar]
- 4.Ojo AO, Heinrichs D, Emond JC, McGowan JJ, Guidinger MK, Delmonico FL, et al. Organ donation and utilization in the USA. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2004;4(Suppl 9):27–37. doi: 10.1111/j.1600-6135.2004.00396.x. [DOI] [PubMed] [Google Scholar]
- 5.Rogers FB, Shackford SR, Trevisani GT, et al. Neurogenic pulmonary edema in fatal and nonfatal head injuries. J Trauma. 1995;39:860–868. doi: 10.1097/00005373-199511000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Matthay MA, Folkesson HG, Clerici C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiol Rev. 2002;82:569–600. doi: 10.1152/physrev.00003.2002. [DOI] [PubMed] [Google Scholar]
- 7.Ware LB, Fang X, Wang Y, Sakuma T, Hall TS, Matthay MA. Selected contribution: mechanisms that may stimulate the resolution of alveolar edema in the transplanted human lung. J Appl Physiol. 2002;93:1869–1874. doi: 10.1152/japplphysiol.00252.2002. [DOI] [PubMed] [Google Scholar]
- 8.Ware LB, Wang Y, Fang X, Warnock M, Sakuma T, Hall TS, et al. Assessment of lungs rejected for transplantation and implications for donor selection. Lancet. 2002;360:619–620. doi: 10.1016/s0140-6736(02)09774-x. [DOI] [PubMed] [Google Scholar]
- 9.Perkins GD, McAuley DF, Thickett DR, Gao F. The beta-agonist lung injury trial (BALTI): a randomized placebo-controlled clinical trial. Am J Respir Crit Care Med. 2006;173(3):281–287. doi: 10.1164/rccm.200508-1302OC. [DOI] [PubMed] [Google Scholar]
- 10.Perkins GD, Nathani N, McAuley DF, Gao F, Thickett DR. In vitro and in vivo effects of salbutamol on neutrophil function in acute lung injury. Thorax. 2007;62(1):36–42. doi: 10.1136/thx.2006.059410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bosmann M, Grailer JJ, Zhu K, Matthay MA, Sarma JV, Zetoune FS, et al. Anti-inflammatory effects of beta2 adrenergic receptor agonists in experimental acute lung injury. FASEB J. 2012;26(5):2137–2144. doi: 10.1096/fj.11-201640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ware LB, Koyama T, Billheimer D, Landeck M, Johnson E, Brady S, et al. Advancing donor management research: design and implementation of a large, randomized, placebo-controlled trial. Ann Intensive Care. 2011;1(1):20. doi: 10.1186/2110-5820-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rey MM, Ware LB, Matthay MA, Bernard GR, McGuire AL, Caplan AL, et al. Informed consent in research to improve the number and quality of deceased donor organs. Crit Care Med. 2011;39(2):280–283. doi: 10.1097/CCM.0b013e3181feeb04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atabai K, Ware LB, Snider ME, Koch P, Daniel B, Nuckton TJ, et al. Aerosolized beta2-adrenergic agonists achieve therapeutic levels in the pulmonary edema fluid of ventilated patients with acute respiratory failure. Intensive Care Med. 2002;28:705–711. doi: 10.1007/s00134-002-1282-x. [DOI] [PubMed] [Google Scholar]
- 15.Sakuma T, Folkesson HG, Suzuki S, Okaniwa G, Fujimura S, Matthay MA. Beta-adrenergic agonist stimulated alveolar fluid clearance in ex vivo human and rat lungs. Am J Respir Crit Care Med. 1997;155:506–512. doi: 10.1164/ajrccm.155.2.9032186. [DOI] [PubMed] [Google Scholar]
- 16.Ware LB, Neyrinck A, O'Neal HR, Lee JW, Landeck M, Johnson E, et al. Comparison of chest radiograph scoring to lung weight as a quantitative index of pulmonary edema in organ donors. Clin Transplant. 2012:65–71. doi: 10.1111/j.1399-0012.2011.01591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.2004 Annual Report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant Data 1994–2003. Rockville, MD: Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation; Richmond, VA: United Network for Organ Sharing; Ann Arbor, MI: University Renal Research and Education Association; 2004. [Google Scholar]
- 18.Sekine Y, Waddell TK, Matte-Martyn A, Pierre AF, de Perrot M, Fischer S, et al. Risk quantification of early outcome after lung transplantation: donor, recipient, operative, and post-transplant parameters. J Heart Lung Transplant. 2004;23(1):96–104. doi: 10.1016/s1053-2498(03)00034-2. [DOI] [PubMed] [Google Scholar]
- 19.Knoben J, Anderson P. Handbook of clinical drug data. 7th edition ed. Hamilton: Drug Intelligence Publications Inc.; 1993. [Google Scholar]
- 20.O'Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35(3):549–556. [PubMed] [Google Scholar]
- 21.Lan KKG, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika. 1983;70:659–663. [Google Scholar]
- 22.Hintze J. PASS. 2008 Available from: [Google Scholar]
- 23.Liang KY, Zeger SL. Regression analysis for correlated data. Annu Rev Public Health. 1993;14:43–68. doi: 10.1146/annurev.pu.14.050193.000355. [DOI] [PubMed] [Google Scholar]
- 24.Matthay MA, Brower RG, Carson S, Douglas IS, Eisner M, Hite D, et al. Randomized, placebo-controlled clinical trial of an aerosolized beta(2)-agonist for treatment of acute lung injury. Am J Respir Crit Care Med. 2011;184(5):561–568. doi: 10.1164/rccm.201012-2090OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao Smith F, Perkins GD, Gates S, Young D, McAuley DF, Tunnicliffe W, et al. Effect of intravenous beta-2 agonist treatment on clinical outcomes in acute respiratory distress syndrome (BALTI-2): a multicentre, randomised controlled trial. Lancet. 2012;379(9812):229–235. doi: 10.1016/S0140-6736(11)61623-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mascia L, Pasero D, Slutsky AS, Arguis MJ, Berardino M, Grasso S, et al. Effect of a lung protective strategy for organ donors on eligibility and availability of lungs for transplantation: a randomized controlled trial. JAMA. 2010;304(23):2620–2627. doi: 10.1001/jama.2010.1796. [DOI] [PubMed] [Google Scholar]
- 27.Perry R, Joseph MX, De Pasquale CG, Chew DP, Yiu D, Aylward PE, et al. High-resolution transthoracic echocardiography of the left anterior descending coronary artery: a novel noninvasive assessment of coronary vasoreactivity. J Am Soc Echocardiogr. 2008;21(2):134–138. doi: 10.1016/j.echo.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 28.Barbato E, Piscione F, Bartunek J, Galasso G, Cirillo P, De Luca G, et al. Role of beta2 adrenergic receptors in human atherosclerotic coronary arteries. Circulation. 2005;111(3):288–294. doi: 10.1161/01.CIR.0000153270.25541.72. [DOI] [PubMed] [Google Scholar]