Abstract
Patients with nonexudative (“dry”) age-related macular degeneration (AMD) frequently also develop neovascular (“wet”) AMD, suggesting a common pathomechanism. Increased vascular endothelial growth factor A (VEGF-A) has been implicated in the pathogenesis of choroidal neovascularization (CNV) in neovascular AMD, while its role in nonexudative AMD that manifests with progressive retinal pigment epithelium (RPE) and photoreceptor degeneration is not well defined. Mice with overall increased VEGF-A levels develop progressive morphological features of both forms of AMD, suggesting that an increase in VEGF-A has a direct age-dependent adverse effect on RPE and photoreceptor function independently of its CNV-promoting proangiogenic effect. Here we provide evidence for this hypothesis and show that morphological RPE abnormalities and retinal thinning in mice with increased VEGF-A levels correlate with progressive age-dependent attenuation of visual function with abnormal electroretinograms and reduced retinal rhodopsin levels. Retinoid profiling revealed a progressive reduction of 11-cis and all-trans retinal in the retinas of these mice, consistent with an impaired retinoid transport between the RPE and photoreceptors. These findings suggest that increased VEGF-A leads to an age-dependent RPE and retinal dysfunction that occurs also at sites where no CNV lesions form. The data support a central role of increased VEGF-A not only in the pathogenesis of neovascular but also of nonexudative AMD.—Ablonczy, Z., Dahrouj, M., Marneros, A. G. Progressive dysfunction of the retinal pigment epithelium and retina due to increased VEGF-A levels.
Keywords: age-related macular degeneration, visual cycle, retinoids, choroidal neovascularization
Age-related macular degeneration (AMD) is the leading cause of irreversible blindness in the elderly, and the factors that contribute to its development are incompletely understood (1, 2). Clinically, AMD is categorized into the neovascular (“wet”) and the nonexudative (“dry”) forms. Neovascular AMD is characterized by infiltration of proliferating vessels from the underlying choroid into the subretinal space through the retinal pigment epithelium (RPE), which normally separates the highly vascularized choroid from the avascular photoreceptor outer segment layer. This choroidal neovascularization (CNV) impairs vision also through vascular leakage from these pathological vessels. For CNV to occur, the RPE barrier needs to be disrupted, thus linking proper RPE function with pathological neovascularization in neovascular AMD. Vascular endothelial growth factor A (VEGF-A) does not only stimulate endothelial cell proliferation and migration and promotes neovascularization, but it can also impair barrier function of the RPE (3, 4). We have previously shown that treating RPE monolayers in vitro with VEGF-A reduced transepithelial resistance and allowed increased flux through the monolayer (3, 4). Both human and mouse RPE cells express in addition to VEGF-A also the main VEGF-A receptors (Flt1 and Flk1 in mice, termed VEGFR1 and VEGFR2 in humans), and VEGF-A treatment induces activation of pathways downstream of VEGFR2 (4). Thus, VEGF-A can activate VEGFR2-mediated signaling in RPE cells in either an autocrine or a paracrine manner. Consistent with these in vitro observations, mice with increased VEGF-A levels show a progressive age-dependent loss of RPE barrier function with cytoplasmic accumulation of barrier proteins from adherens junctions (e.g., β-catenin) and tight junctions (e.g., ZO-1) (4). Notably, CNV occurs in these mice at sites of RPE barrier breakdown (4). Thus, increased VEGF-A levels link CNV and RPE dysfunction in the development of AMD-like pathologies.
Nonexudative AMD is characterized by age-dependent abnormalities of the RPE and the underlying Bruch's membrane (5, 6). RPE cells undergo atrophic degenerative changes with loss of pigment granules, and distinct deposits in the sub-RPE space accumulate with increased age (5–10). Notably, RPE/Bruch's membrane abnormalities and abnormal sub-RPE deposit formation are associated with progressive photoreceptor degeneration in nonexudative AMD (11). Clinically, nonexudative AMD has been considered as a separate entity from neovascular AMD (5, 6). However, both forms of AMD often cooccur, and patients with nonexudative AMD may progress to develop neovascular AMD in the same eye (12). Genetic association studies have established genetic loci associated with advanced AMD (13, 14). A meta-analysis of genome-wide association studies revealed that the VEGF-A locus is associated with both neovascular and nonexudative AMD (14). Of importance, we found in mice with increased VEGF-A levels (hereafter described as VEGF-Ahyper mice) cardinal features not only of neovascular AMD, but also of nonexudative AMD (4). These mice developed not only VEGF-A-induced RPE barrier breakdown and subsequent CNV lesion formation into the subretinal space, but also age-dependent RPE and retinal atrophy and basal laminar deposit formation (4). These observations suggest that VEGF-A has an important role in the pathogenesis of both forms of AMD, which may form as distinct manifestations of a common underlying process of VEGF-A dysregulation. Thus, increased VEGF-A levels may provide a unifying pathomechanism for both forms of AMD.
Multifactorial insults to the RPE and retina that have been associated with an increased risk of AMD include increased light exposure or smoking (1, 2, 15), which may result in hypoxic and oxidative stress-associated damage to the RPE, both strong inducers of VEGF-A expression in the RPE (16). Thus, risk factors associated with AMD in humans may induce increased VEGF-A expression in the RPE and retina and contribute through this mechanism to AMD development.
The findings in the eyes of mice with increased VEGF-A raise the question whether morphological RPE and retinal abnormalities occur independently of CNV formation, and whether they correlate with functional defects in the RPE and photoreceptors as seen in patients with AMD. Furthermore, it is not known how the observed morphological RPE and photoreceptor abnormalities affect retinoid metabolism and visual function in these mice.
Of importance, the earliest degenerative ocular changes in the eyes of VEGF-Ahyper mice were observed in the RPE of young adult mice with a progressive RPE barrier breakdown, prior to the manifestation of retinal abnormalities with photoreceptor loss (4). Thus, we hypothesized that VEGF-A-induced RPE barrier breakdown affects retinoid transport processes between RPE cells and photoreceptors and impairs the visual cycle, ultimately leading to photoreceptor degeneration and loss of visual function independently of abnormalities caused by VEGF-A-induced CNV.
Here we demonstrate that morphological RPE abnormalities in VEGF-Ahyper mice correlate with a progressive defect in visual function with reduced retinal rhodopsin levels and abnormal electroretinograms (ERGs). These functional defects are associated with abnormal retinoid metabolism and defects in the visual cycle, with decreased 11-cis and all-trans retinal in the retinas of VEGF-Ahyper mice. The observed pathological ERGs, reduced rhodopsin levels, and visual cycle defects are consistent with a progressive age-dependent defect in RPE-photoreceptor interactions, likely due to a VEGF-A-induced RPE barrier breakdown. Our findings provide evidence that increased VEGF-A results in an age-dependent RPE and retinal dysfunction and suggests that increased VEGF-A contributes to the functional and morphological abnormalities observed not only in neovascular AMD but also in nonexudative AMD.
MATERIALS AND METHODS
Animals
The generation of VEGF-Ahyper mice was previously reported (17). Both the rd1 and the rd8 mutations were excluded in the mice analyzed in this study by PCR. The increase of VEGF-A levels occurs in these mice as a consequence of the insertion of an IRES-NLS-lacZ-SV40pA sequence +202 bp 3′ to the STOP codon into the 3′ UTR of the VEGF-A gene locus. In these VEGF-Ahyper mice, nuclear β-galactosidase expression reflects VEGF-A expression at single-cell resolution. These mice were maintained on the original background (CD-1/129 hybrid background; white mice) or on a C57BL/6J (black mice) background. Mice between ages 4 wk and 24 mo were examined, in total >250 mutant and control littermate mice. The described AMD-like pathologies were observed only in mutant mice, while none of the control littermate mice displayed the reported ocular pathologies. Mice in which the IRES-NLS-lacZ-SV40pA sequence was inserted immediately 3′ to the STOP codon into the 3′ UTR of the VEGF-A gene locus are hypomorphic for VEGF-A (VEGF-Ahypo mice) but maintain β-galactosidase expression from the endogenous VEGF-A gene locus (18). While these mice showed β-galacrosidase expression in the eye as seen in VEGF-Ahyper mice, no eye pathologies were observed in these mice, demonstrating that eye pathologies are not caused by insertion of the lacZ sequence. VEGF-A ELISA measurements confirmed increased VEGF-A protein levels in serum and RPE and retinal tissues in VEGF-Ahyper mice (4). VEGF-A levels were confirmed to be increased in the RPE/choroid and retina in the VEGF-Ahyper mice used in this study as well (data not shown). For ERGs, retinal rhodopsin measurements and retinoid profiling experiments 6- and 10-mo-old male VEGF-Ahyper and wild-type (WT) littermate mice were used (n=12–14/group). All animal experiments were performed according to institutional approval by the Medical University of South Carolina and Massachusetts General Hospital.
Immunolabeling
Eyes were fixed in 4% paraformaldehyde. For choroidal flat mounts, eyes were dissected along the ora serrata, and the retina was gently removed from the RPE/choroid tissue. These RPE/choroidal posterior eye cups were permeabilized in 0.5% Triton X and subsequently blocked with serum, in which the secondary antibodies were raised. For frozen sections, eyes were treated with 30% sucrose and subsequently embedded in embedding medium and cut at 7 μm thickness. Tissue permeabilization was performed with 0.5% Triton X, and blocking was performed with blocking serum in which the secondary antibody was raised. Primary antibodies used were rat anti-mouse CD31 (MEC13.3; BD Biosciences, San Jose, CA, USA), rabbit anti-mouse β-galactosidase (A11132; Life Technologies, Grand Island, NY, USA), rabbit anti-mouse NG2 (AB5320; Millipore, Billerica, MA, USA), rat anti-mouse F4/80 (CL8940AP; Cedarlane, Burlington, NC, USA), rat anti-mouse F4/80-Alexa647 conjugate (clone C1:A3-1; Biolegend, San Diego, CA, USA), and rabbit anti-mouse β-catenin (AHO0462; Life Technologies). Cytoskeletal staining was performed with Alexa647-conjugated phalloidin (Life Technologies). Colabeling experiments were combined with single-labeling experiments and experiments omitting both either the primary or the secondary antibodies to distinguish immunolabeling from autofluorescence. DAPI (Life Technologies) was used for staining of nuclei. Choroidal perfusion was assessed by intracardial perfusion with fluorescein-conjugated tomato lectin (Vector Laboratories, Burlingame, CA, USA) at a dilution of 1:100 in PBS.
Morphological examination of eyes and electron microscopy
Electron microscopy was performed as described previously (19). Briefly, eyes were fixed in 1.25% paraformaldehyde and 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4). Eyes were dissected along the ora serrata and cut in half through the optic nerve. After postfixation in 4% osmium tetroxide, and dehydration steps, the eyes were embedded in TAAB epon (Marivac Ltd., Canton de Gore, QC, Canada) and ultrathin sections were used for standard transmission electron microscopy, or 1 μm thin sections were used for toluidine blue staining and light microscopy. Sections were assessed at the level of the optic nerve, and representative retinal images were obtained from areas adjacent to the optic nerve in a paracentral location. Images from similar locations were obtained from each eye, which allowed a direct comparison between VEGF-Ahyper and WT control mouse eyes.
ERGs
ERGs of age-matched 6- and 10-mo-old male VEGF-Ahyper and control littermate mice were recorded (n=12–14/group). Mice were dark-adapted overnight and anesthetized by intraperitoneal injection of 100 mg/kg ketamine and 10 mg/kg xylazine. After pupil dilation (10% phenylephrine hydrochloride and 1% tropicamide), full-field scotopic ERGs were recorded using a UTAS E-2000 system (LKC Technologies, Gaithersburg, MD, USA) using a contact lens electrode brought into electrical contact with the cornea with a drop of methylcellulose (Gonisol; Iolab Pharmaceutical, Claremont, CA, USA). A- and b-wave recordings were averaged in response to 5 flashes (10 ms each) using white light (450–750 nm) at 1.56 cd · s · mm−2 with a 30-s interval between each flash. C waves were averaged in response to five 4-s flashes at 100 cd · s · mm−2 with a 60-s interval between each flash.
Retinoid analyses
All procedures were performed under dim red light on dark-adapted animals (n=12–14 male age-matched mice/group) using methods modified from those previously described (20). Eyecups were dissected, and choroid/RPE tissues were separated from retinal tissues and homogenized in 200 ml of PBS buffer using a microtissue grinder (Fisher Scientific, Pittsburgh, PA, USA). Methanol (300 ml) and hydroxylamine (60 ml, 1 mmol/ml in PBS buffer) were added and samples were vortexed (30 s). After 5 min at 22°C, methylene chloride (300 ml) was added, and the samples were vortexed (30 s) and centrifuged (14,000 g, 1 min). The lower phase was separated, dried under argon, and dissolved in HPLC mobile phase (11.2% ethyl acetate, 2.0% dioxane, and 1.4% octanol in hexane, 90 ml). The syn- and anti-11-cis and all-trans retinal oximes were separated using a Lichrosphere SI-60, 5-mm column (Alltech, Lexington, KY, USA) and quantified by comparison of retention times and absorption properties with pure retinoid isomeric standards.
Rhodopsin measurements
The method for measuring rhodopsin in mouse retinas has been described previously (21). Briefly, retinas were homogenized in 10 mM Tris buffer (pH 7.5) containing 1 mM ethylenediamine tetraacetic acid, 1 mM 4-(2-aminoethyl)-benzenesulfonyl fluoride hydrochloride, protease inhibitor cocktail, and 10 μg DNase I. After centrifugation at 14,200 g for 15 min, pellets were solubilized in 1% dodecylmaltoside (sodium phosphate buffer, pH 7.4) at 4°C for 2 h, centrifuged (88,000 g for 10 min), and the supernatant analyzed in a spectrophotometer. Difference spectra were determined from measurements before and after bleaching with white light in the presence of freshly prepared 20 mM hydroxylamine, pH 6.0 to 7.0. The concentrations of rhodopsin were calculated using the following extinction coefficient: ε(rhodopsin) = 40,000 M/cm.
Fundus imaging
Fundus imaging was performed on a Topcon Imagenet digital angiography system (TRC 50 VT camera and IMAGEnet 1.53 system; Topcon, Paramus, NJ, USA). The photographs were captured with a 20-D lens in contact with the fundus camera lens. Fluorescein angiography, fundus autofluorescence, and optical coherence tomography (OCT) was performed with a Heidelberg Spectralis instrument (Heidelberg Engineering, Heidelberg, Germany) using specific modes of the instrument. To assess autofluorescence of the fundus, images were taken with the Heidelberg Spectralis with the following filter settings: a blue solid state laser for excitation (λ=488 nm) and a 500 nm barrier filter for separating the emission.
Late-phase (5 min postinjection) fluorescein angiography was performed after intraperitoneal injection of 0.1 ml of 1% fluorescein sodium (Akorn, Decatur, IL, USA). OCT data were collected with averaging 100 successive scans in the ART mode. Retinal thickness for WT and VEGF-Ahyper mice (at sites with no lesions) was calculated for each eye by performing 4 similar 15° line scans (in 12, 3, 6, and 9 o'clock positions) around the optic disc in each eye. Independent thickness measurements along these lines were then averaged to obtain the thickness values for each eye. Thickness of lesions in VEGF-Ahyper mice was determined by performing vertical and horizontal scans across the lesions and averaging independent thickness measurements (in the area of lesions) along these lines.
Statistical analyses
A 2-tailed Student's t test was used for statistical analyses. Graphs indicate means ± sd. Values of P < 0.05 were considered to be statistically significant.
RESULTS
Age-dependent progressive ocular abnormalities in mice with increased VEGF-A levels resemble clinical manifestations of both neovascular and nonexudative AMD
VEGF-Ahyper mice, with insertion of an IRES-NLS-lacZ-SV40pA sequence +202 bp 3′ to the STOP codon into the 3′ UTR of the VEGF-A gene locus, which regulates VEGF-A mRNA translation, show an increase of VEGF-A levels in the RPE/choroid, the retina, and the serum (4). Notably, ocular phenotypes in these mice are attributed to increased VEGF-A levels and not to the expression of β-galactosidase, as mice with insertion of the same IRES-NLS-lacZ-SV40pA sequence immediately after the STOP codon into the 3′ UTR of the VEGF-A gene locus are hypomorphic for VEGF-A (VEGF-Ahypo mice), express β-galactosidase also from the endogenous VEGF-A gene locus but do not have these ocular abnormalities.
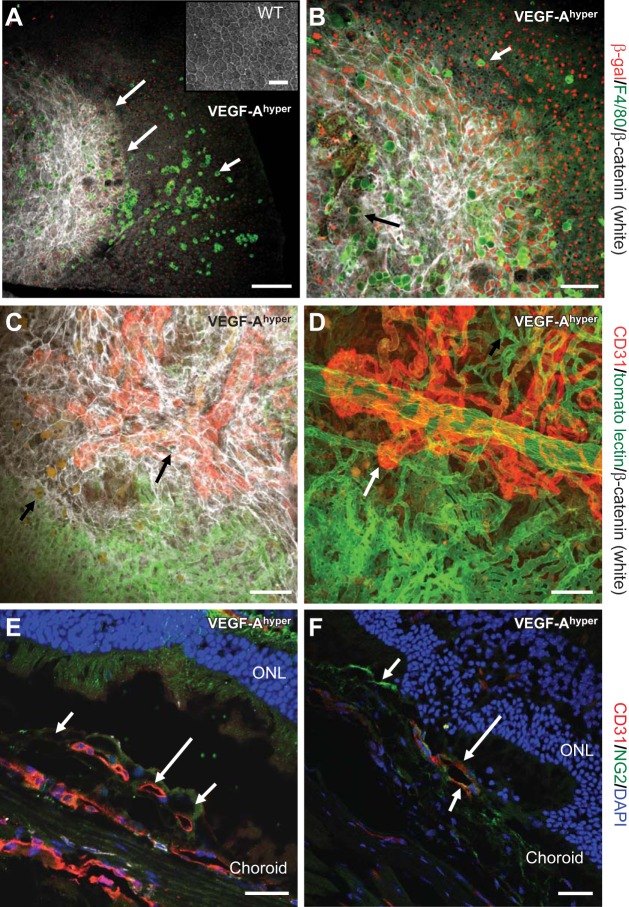
VEGF-Ahyper mice show an age-dependent RPE barrier breakdown with infiltration of activated macrophages (Fig. 1A–B). Immunolabeling shows that RPE cells are the main source of VEGF-A expression in the posterior eye, while activated macrophages do not show immunolabeling for β-galactosidase (and therefore VEGF-A expression; Fig. 1A, B). Subsequently, progressive pathological neovascularization occurs at sites of RPE barrier breakdown that originates from the choroidal vasculature and extends into the sub-RPE and subretinal space (Fig. 1C–F and ref. 4). Choroidal flat-mount staining of VEGF-Ahyper mice that were perfused with fluorescein-labeled tomato lectin and colabeled with CD31 (strongly expressed by neovessels) and β-catenin shows neovascular lesions at sites of RPE barrier breakdown (Fig. 1C, D). These observations strongly suggest that in VEGF-Ahyper mice, the increase of VEGF-A is sufficient to cause RPE barrier breakdown and CNV lesions, resembling neovascular AMD.
Figure 1.
Progressive RPE barrier breakdown and CNV lesion formation in mice with increased VEGF-A levels. A, B) Choroidal flat mount of a 21-mo-old black VEGF-Ahyper mouse. RPE barrier breakdown (indicated as increased β-catenin labeling in white, demarcated by long arrows in A) in VEGF-Ahyper mice is shown with loss of the regular honeycomb RPE cell morphology and by increased cytoplasmic β-catenin labeling (white). In this mouse, almost the entire posterior central fundus shows RPE barrier breakdown with increased β-catenin labeling. F4/80+ macrophages (bright green, short arrow) infiltrate the subretinal space at the site of RPE barrier breakdown. Round autofluorescent sub-RPE deposits (dim green, black arrow in B) are observed at sites of RPE barrier breakdown as well. Red nuclear staining shows labeling for β-galactosidase in these mice, representing VEGF-A expression in pigmented RPE cells (VEGF-Ahyper mice express β-galactosidase from the endogenous VEGF-A locus). Notably, F4/80+ macrophages are β-galactosidase negative. Inset: WT choroidal flat mount with regular honeycomb appearance of RPE cells. C) CNV lesion formation in VEGF-Ahyper mice with neovascularization evolving from choroidal vessels. White VEGF-Ahyper mouse perfused with FITC-tomato lectin and subsequently immunolabeled for CD31 (red) and β-catenin (white). Green vessels represent perfused normal choroidal vasculature, while CD31+ red neovessels originate from the normal choroidal vasculature and form CNV lesions. This CNV lesion formation (red, CD31+vessels, long arrow) occurs at sites of RPE barrier breakdown (white, increased β-catenin). Bottom part of image shows normal choroidal vasculature (green) covered by normal RPE (regular β-catenin labeling); top part shows neovessels (CD31+ in red) covered by irregular RPE cells with increased β-catenin labeling. Autofluorescent sub-RPE deposits appear at sites of RPE barrier breakdown (short arrow). D) Strongly CD31 expressing neovessels (red, long arrow), originating from underlying choroidal vessels (green, FITC-tomato lectin perfusion). White VEGF-Ahyper mouse, age 15 mo, with nonpigmented RPE cells. E) CNV lesions in black VEGF-Ahyper mice protrude into the subretinal space and are CD31+. Frozen section shows CD31+ (red) CNV into the subretinal space (long arrow). Pigmented RPE cells indicated by short arrows. ONL, outer nuclear layer of photoreceptors. F) Vessels in CNV lesions are CD31+ (red, CD31, long arrow) and surrounded in part by NG2+ cells (NG2, green; short arrows). Nuclei labeled blue with DAPI. Black VEGF-Ahyper mouse, age 12 mo. Scale bars = 200 μm (A); 100 μm (B–D); 50 μm (E, F).
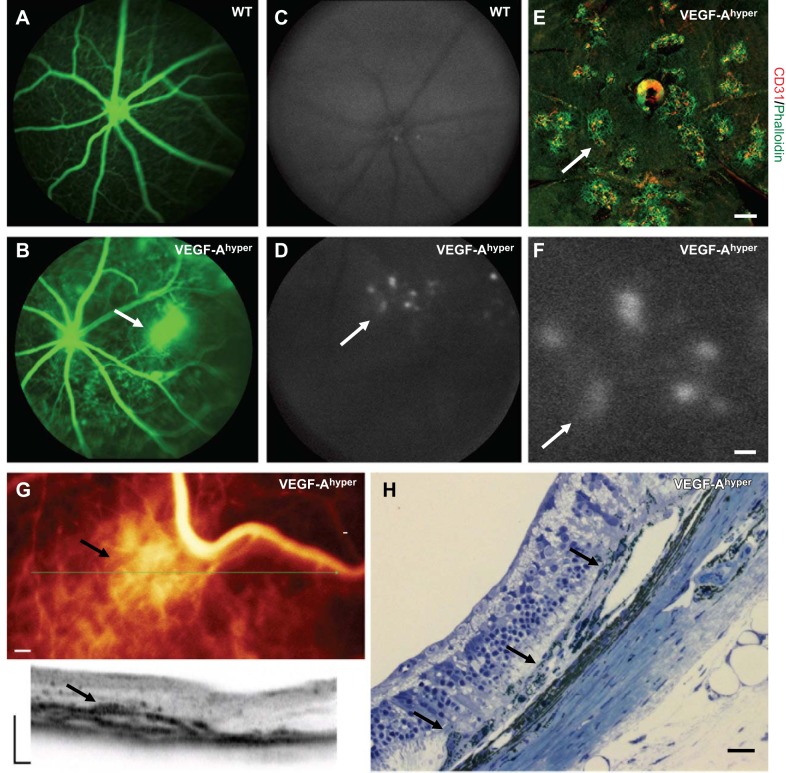
To determine whether morphological findings in VEGF-Ahyper mice result in in vivo abnormalities that correlate with the clinical observations made in eyes of patients with neovascular AMD, we assessed retinal fundus images in groups of 6- and 10-mo-old VEGF-Ahyper mice and control mice by fluorescence angiography (FA). While age- and gender-matched WT control mice showed normal FA images (Fig. 2A), FA in VEGF-Ahyper mice showed neovascular lesions with signs of vascular leakage (Fig. 2B). Fundus imaging prior to fluorescein injection showed areas of increased autofluorescence (Fig. 2D) that were not seen in WT control mice (Fig. 2C), consistent with multifocal RPE barrier breakdown and CNV lesion formation at these sites, as observed in choroidal flat-mount stainings (Fig. 2C–F).
Figure 2.
Fundus imaging in VEGF-Ahyper mice shows CNV resembling findings in neovascular AMD. A, B) Fluorescence angiography shows a neovascular lesion (B, arrow) with fluorescein leakage in a 6-mo-old VEGF-Ahyper mouse, which is not observed in WT control littermate mice (A). C, D) Increased multifocal fundus autofluorescence (arrow) is noticed in VEGF-Ahyper mice (D), but not in WT control mice (C). E) Multifocal CNV lesions (arrow) can be seen in choroidal flat-mount stainings in VEGF-Ahyper mice. CNV lesions show strong staining for neovessels with CD31 (red) at sites of RPE barrier breakdown (phalloidin, green). F) Higher magnification of fundus image shown in D (arrows indicate same lesion with increased fluorescence). Area of increased fluorescence is about the same size as CNV lesions observed by choroidal flat-mount staining (as in E). G) OCT imaging of the fundus of a 10-mo-old VEGF-Ahyper mouse shows a CNV lesion (arrow). Representative image section through this lesion is shown (plane of section through green line indicated, arrow), while adjacent RPE/retina appear normal. H) The in vivo OCT findings reflect the histological findings in these mice that show neovascular CNV membranes with RPE abnormalities (arrows). Scale bars = 200 μm (E–G); 20 μm (H).
Notably, multifocal CNV lesions with CD31+ neovessels at sites of RPE barrier breakdown that were observed in choroidal flat-mount stainings (Fig. 2E), were about the same size as the areas of increased multifocal autofluorescence in the in vivo fundus images (∼200 μm; Fig. 2F). Thus, the in vivo imaging observations correlate well with the choroidal flat-mount stainings and show that the observed areas of increased autofluorescence in the fundus images are multifocal CNV lesions. OCT confirmed areas consistent with CNV lesions and RPE barrier breakdown in VEGF-Ahyper mice (Fig. 2G). The in vivo OCT images mirrored the histological findings of RPE abnormalities and CNV formation at these sites (Fig. 2H).
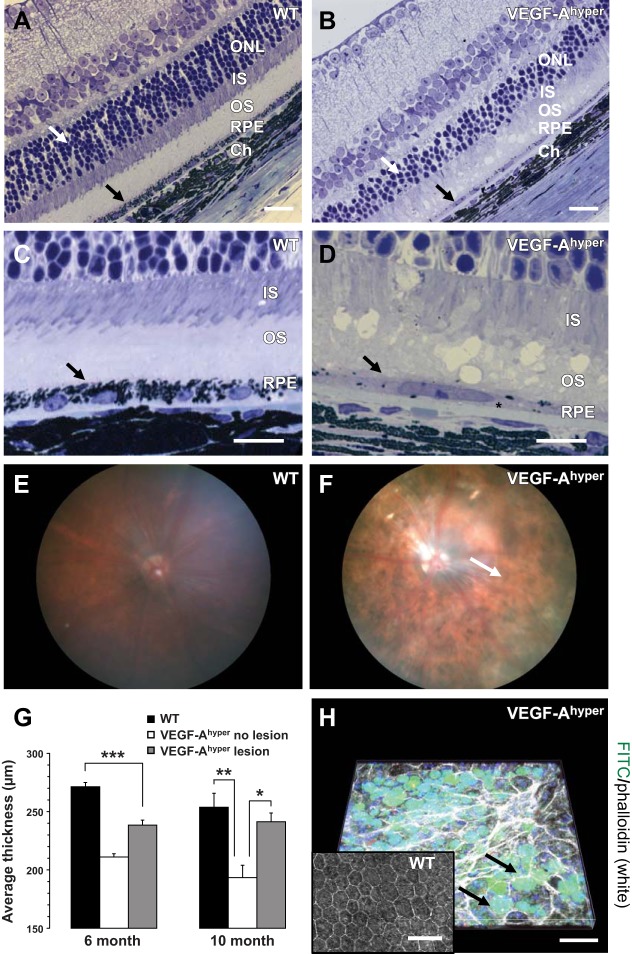
We have recently shown that degenerative changes of photoreceptors occur after morphological abnormalities of RPE cells are observed, suggesting that retinal defects are a consequence of RPE dysfunction (4). In aged VEGF-Ahyper mice, significant degenerative changes in the RPE are noticed with RPE atrophy and loss of pigment granules (Fig. 3B, D). Consistent with these morphological RPE abnormalities, in vivo fundus imaging showed patchy areas of hypopigmentation affecting the entire fundus and not only areas of CNV lesions, resembling pigment abnormalities in the RPE as seen in nonexudative AMD (Fig. 3F).
Figure 3.
Generalized RPE and retinal abnormalities in aged VEGF-Ahyper mice. A–D) Retinal thinning in aged (21-mo-old) VEGF-Ahyper mice is primarily due to loss of photoreceptors with attenuation of the outer nuclear layer (ONL; A, B; white arrows) and the photoreceptor outer segment (OS) and inner segment (IS). Notably, RPE atrophy with loss of pigment granules is noticed in mutant mice (B, black arrow). Sub-RPE deposits in mutant mice resemble basal laminar deposits (D, asterisk). Black arrows indicate RPE. C, D) Higher-magnification images from same eyes as shown in A and B, respectively. Ch, choroid. E, F) Fundus imaging of WT (E) and VEGF-Ahyper (F) mice reveals generalized pigmentary abnormalities of the RPE with patchy areas of hypopigmentation in VEGF-Ahyper mice (F). Areas of hypopigmentation (white arrow) in the fundus images correlate well with the microscopic findings of RPE cell atrophy and focal pigment loss in RPE cells of VEGF-Ahyper mice. G) Measuring retinal thickness in a paracentral location (adjacent to the optic nerve area) by OCT shows a significant thinning of the overall retina in VEGF-Ahyper mice compared to matched control mice, particularly at sites devoid of CNV lesions. Bars represent means ± sd. *P < 0.05, **P < 0.01, ***P < 0.001. H) Confocal microscopy reveals round autofluorescent sub-RPE deposits (appearing as green round deposits when acquired with the fluorescein channel, 488 nm, under the RPE that is apparent by the phalloidin staining in white on top of those deposits) in VEGF-Ahyper mice (arrows). These deposits are not present in WT littermate control mice (inset). Scale bars = 20 μm (A, B); 10 μm (C, D); 50 μm (H).
Of importance, retinal thickness, measured in vivo by OCT, was reduced even at areas devoid of CNV lesions (Fig. 3G), and this retinal atrophy progressed with age and was more pronounced in 10-mo-old VEGF-Ahyper mice when compared to 6-mo-old VEGF-Ahyper mice (Fig. 3G). This observation is consistent with retinal defects in VEGF-Ahyper mice that occur independently of CNV lesions. Retinal thinning observed by OCT in vivo reflects a progressive attenuation of the outer retina in VEGF-Ahyper mice with a reduced photoreceptor outer nuclear layer and photoreceptor inner and outer segments (Fig. 3A–D and ref. 4).
Notably, typical calcified drusen-like deposits were not seen on fundus images (Fig. 3F). Instead, lipid-like autofluorescent round accumulations were noticed by confocal microscopy in the sub-RPE space in VEGF-Ahyper mice (Fig. 3H). These deposits, which we showed previously to be Oil Red O positive (4), were not observed in young VEGF-Ahyper mice or in control littermate mice and appeared concomitant with progressive morphological RPE and retinal abnormalities in aged VEGF-Ahyper mice. Furthermore, significant basal laminar-like sub-RPE deposits were observed in aged VEGF-Ahyper mice (Figs. 3D and 5E), a morphological correlate to findings seen in AMD. Thus, fundus imaging, FA, and OCT reveal striking similarities in the clinical appearance of ocular abnormalities in mice with increased VEGF-A levels with both neovascular and nonexudative AMD, and these in vivo imaging findings correlate well with the observed morphological RPE and retinal abnormalities in these mice.
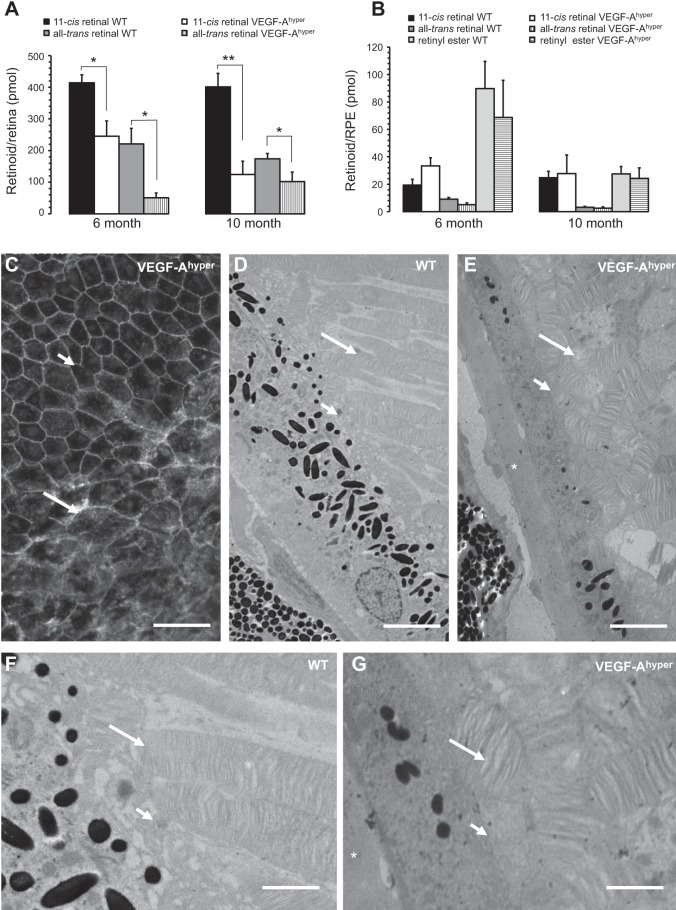
Figure 5.
Retinoid profiling reveals a defect in the visual cycle with reduction of 11-cis and all-trans retinal levels in the retinas of VEGF-Ahyper mice. A) Retinas of VEGF-Ahyper mice show a reduction of 11-cis and all-trans retinal, which is more pronounced in 10-mo-old mutant mice compared to 6-mo-old mice. Bars represent means ± sd. *P < 0.05, **P < 0.01. B) Retinyl esters in the RPE (RPE/choroid) do not show a significant difference between VEGF-Ahyper mice and control mice. Bars represent means ± sd. C) VEGF-Ahyper mice with these visual cycle defects show an RPE barrier breakdown, demonstrated here by cytoplasmic accumulation of β-catenin staining and loss of typical RPE cell honeycomb morphology (long arrow). Adjacent nonlesional RPE cells (short arrow) maintain normal RPE cell morphology in young (2-mo-old) VEGF-Ahyper mice. Adapted from ref. 4. D–G). Defects in visual cycle correlate with abnormalities in the interdigitation of apical RPE cell membranes with photoreceptor outer segments in aged (15-mo-old) VEGF-Ahyper mice, shown by electron microscopy. Photoreceptor outer segments in mutant mice (E, G) are highly disorganized and shortened, compared to WT mice (D, F). Long arrows show photoreceptor outer segments. Short arrows show apical RPE cell membranes. Asterisks indicate electron-dense basal laminar sub-RPE deposits. Panels F and G are magnifications of images in D and E, respectively. Scale bars = 75 μm (C); 3 μm (D, E); 1.5 μm (F, G).
Morphological RPE and retinal abnormalities correlate with abnormal ERGs and reduced retinal rhodopsin levels
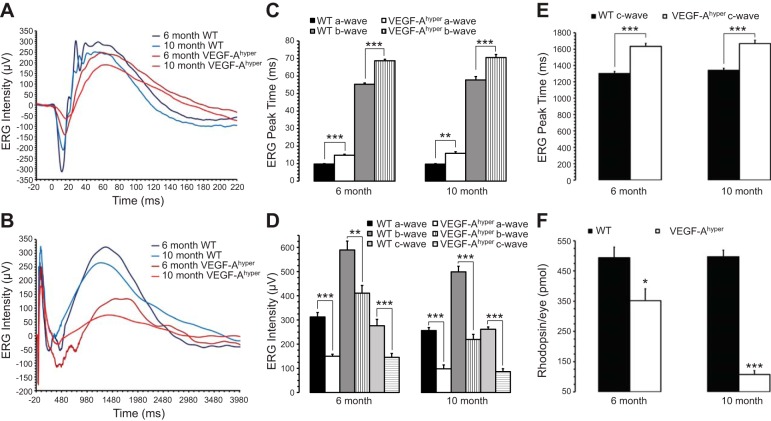
The in vivo imaging experiments in these mice further support the concept that an increase in VEGF-A results not only in CNV, but also in morphological abnormalities of the RPE and retina that resemble nonexudative AMD at sites where no CNV lesions are present. Thus, we aimed to determine whether morphological RPE and retinal abnormalities due to increased VEGF-A levels correlate with progressive age-dependent functional defects in the RPE and retina and loss of vision. First, groups of matched 6- and 10-mo-old VEGF-Ahyper and control mice were used for full-field ERGs. VEGF-Ahyper mice showed a significant decrease of a-, b-, and c-wave amplitudes and delayed peak times (implicit times) for all 3 waves in 6-mo-old mice when compared to WT control littermate mice (Fig. 4A–E). Furthermore, ERG amplitudes further decreased and peak times were further delayed in 10-mo-old VEGF-Ahyper mice (Fig. 4A–E). Thus, the kinetics of all 3 ERG waves were abnormal in VEGF-Ahyper mice, and these abnormalities progressed with increasing age. A waves reflect photoreceptor function, b waves are a measure of inner retinal function (Muller cells and ON bipolar cells), while c waves originate from the RPE. The observation of significantly attenuated a-, b-, and c-wave amplitudes and delayed peak times is consistent with degenerative changes of both the RPE and the retina and correlate with the morphological abnormalities observed in VEGF-Ahyper mice with progressive photoreceptor loss and RPE atrophy (Fig. 3A–D). Furthermore, quantitative retinal rhodopsin measurements showed a significant decrease of rhodopsin levels in 6-mo-old VEGF-Ahyper mice that further decreased by 10 mo of age (Fig. 4F). These rhodopsin quantifications demonstrate a significant progression of the observed RPE and retinal degeneration in VEGF-Ahyper mice between 6 and 10 mo of age (Fig. 4F).
Figure 4.
VEGF-Ahyper mice show abnormal ERGs and reduced retinal rhodopsin levels. A, B) Representative ERG traces of 6- and 10-mo-old VEGF-Ahyper mice and WT control mice show delayed peak times and attenuated a-, b-, and c-wave amplitudes in VEGF-Ahyper mice. These differences increase with progressive age and are more pronounced in groups of 10-mo-old mice compared to groups of 6-mo-old mice. C–E) Quantitative measurements of peak times (C, E) and a-, b-, and c-wave amplitudes (D) in 6- and 10-mo-old VEGF-Ahyper mice and WT control mice show consistent statistically significant differences between VEGF-Ahyper and control mice. F) Rhodopsin quantification shows a progressive age-dependent reduction of rhodopsin levels in the retinas of VEGF-Ahyper mice. Loss of rhodopsin accelerates between 6 and 10 mo of age in VEGF-Ahyper mice. Bars represent means ± sd. *P < 0.05, **P < 0.01, ***P < 0.001.
The fundus imaging data and these functional measurements are consistent with generalized RPE and retinal abnormalities that progress with advanced age in VEGF-Ahyper mice and that are not limited to defects at sites of CNV lesion formation. Thus, correlating the morphological RPE and retinal abnormalities with the findings made by fundus imaging, FA, rhodopsin quantifications, and ERGs demonstrate that VEGF-Ahyper mice have a generalized age-dependent RPE and retinal dysfunction, in support of a central pathogenic role of increased VEGF-A levels for RPE and retinal defects in nonexudative AMD.
Retinoid profiling reveals progressive visual cycle defects in VEGF-Ahyper mice
As morphological RPE abnormalities preceded the manifestation of photoreceptor degeneration in VEGF-Ahyper mice (4), we hypothesized that reduced RPE barrier function leads to a disruption of transport processes between RPE cells and photoreceptor outer segments, resulting in defects in the visual cycle that are associated with photoreceptor degeneration. Thus, we analyzed visual cycle retinoids in the RPE and retina of 6- and 10-mo-old VEGF-Ahyper mice and WT control littermate mice. We found in VEGF-Ahyper mice significantly reduced 11-cis retinal levels in retinas compared to retinas of WT control mice (Fig. 5A). Similarly as with the progressive reduction of rhodopsin levels, 11-cis retinal levels in retinas further decreased in 10-mo-old VEGF-Ahyper mice when compared to 6-mo-old mice, a finding that was not observed in WT control mice (Fig. 5A). All-trans retinal levels in the retinas were also significantly reduced in VEGF-Ahyper mice (Fig. 5A). Notably, retinyl ester levels in the RPE were not significantly different between VEGF-Ahyper mice and control mice (Fig. 5B). These findings suggest a progressive defect in retinoid exchanges between RPE cells and photoreceptors in retinas of aged VEGF-Ahyper mice, likely as a consequence of the age-dependent RPE barrier breakdown that we have observed in VEGF-Ahyper mice (Fig. 5C). This RPE barrier breakdown is demonstrated by a loss of the typical honeycomb pattern morphology of RPE cells in choroidal flat mounts and by cytoplasmic accumulation of β-catenin (Fig. 5C and ref. 4). Electron microscopy demonstrated that RPE abnormalities, such as RPE cell atrophy and pigment loss, are indeed associated with an irregular interdigitation of the apical RPE cell membranes with the photoreceptor outer segments (Fig. 5D–G). Thus, VEGF-A-induced RPE barrier breakdown could explain a disruption of the normal photoreceptor/RPE interactions and the resulting functional visual cycle defects, which result in abnormal ERGs and photoreceptor degeneration with rhodopsin loss.
DISCUSSION
Nonexudative AMD can progress to the neovascular form of AMD, suggesting common pathomechanisms for both forms of AMD (12). Mice with increased VEGF-A levels (VEGF-Ahyper mice) develop features that strongly resemble both forms of AMD, demonstrating that an increase in VEGF-A may provide a unifying pathomechanism for all forms of AMD and that CNV and RPE defects may occur as distinct manifestations of a common underlying process involving VEGF-A dysregulation (4). The AMD-like phenotypes in VEGF-Ahyper mice occur postnatally with progressive age, resembling the age dependency observed in AMD. Consistent with this observation, these phenotypes are not a consequence of developmental ocular defects in VEGF-Ahyper mice, as the RPE, retina, and choroidal vasculature develop normally in these mice (4).
Of importance, no eye pathologies were observed in mice with a hypomorphic VEGF-A allele (VEGF-Ahypo mice; ref. 4). These mice are identical to VEGF-Ahyper mice, except that the IRES-NLS-lacZ-SV40pA sequence was inserted immediately 3′ to the STOP codon into the 3′ UTR of the VEGF-A gene locus in the VEGF-Ahypo mice (resulting in a hypomorphic VEGF-A allele), while this sequence was inserted + 202 bp 3′ to the stop codon in VEGF-Ahyper mice (resulting in a hypermorphic VEGF-A allele and increased VEGF-A levels). Both mouse strains express β-galactosidase from the endogenous VEGF-A gene locus (18), but only VEGF-Ahyper mice develop AMD-like eye pathologies. Thus, the observed phenotypes in VEGF-Ahyper mice correlate with increased VEGF-A levels and are not a consequence of β-galactosidase expression in the RPE. Similarly, RPE-specific overexpression of β-galactosidase did not result in RPE abnormalities (4).
Overexpression of VEGF-A164 in the RPE via adenoviral delivery has previously been reported to result in CNV lesions, which strongly resemble those observed here in the VEGF-Ahyper mice, providing further evidence that the observed abnormalities in VEGF-Ahyper mice are indeed due to increased VEGF-A levels and not due to other strain-specific abnormalities (22). We also excluded the rd1 or rd8 mutation in VEGF-Ahyper mice. Furthermore, VEGF-Ahyper mice (VEGF-AlacZ/WT mice) were obtained through intercrosses with their control littermates (VEGF-AWT/WT mice) for >12 generations, and we examined >250 mice of each genotype. The observed ocular abnormalities always cosegregated with the VEGF-AlacZ/WT allele and were not observed in any of the examined VEGF-AWT/WT mice, which makes it highly unlikely that another mutation may have contributed to the observed ocular abnormalities in VEGF-Ahyper mice.
Our observations in VEGF-Ahyper mice are intriguing, as VEGF-A has largely been viewed as a growth factor that promotes pathological angiogenesis in the eye and in other tissues. However, it is well established that VEGF-A affects epithelial cell interactions, and we have previously shown that RPE cells express VEGF-A receptors (Flt1 and Flk1) and that VEGF-A can potently disrupt epithelial cell junctions and result in a breakdown of RPE barrier function (3, 4). VEGF-A induces VEGFR2-dependent signaling pathway activation in RPE cells and may act either in a paracrine or autocrine manner (4). Notably, VEGF-A is strongly expressed in the RPE cell layer during development and continuously throughout adult life (Fig. 1B and refs. 4, 23). In fact, VEGF-A expression is highest in the RPE compared to other cell types in the posterior eye, and much less VEGF-A is expressed in the inner nuclear layer or ganglion cell layer of the retina (4). In particular, the VEGF-A isoforms VEGF-A120 and VEGF-A164 are expressed in murine RPE cells (VEGF-A121 and VEGF-A165 in human RPE cells; ref. 4). In VEGF-Ahyper mice, about 2-fold higher VEGF-A protein levels are present in the RPE/choroid tissues, and this increase is sufficient to cause a progressive RPE barrier breakdown (4). Macrophages infiltrate the subretinal space concomitant with the VEGF-A-induced RPE barrier breakdown, and this infiltration is accompanied by activation of retinal glia cells that express proangiogenic factors, such as IL-1β and VEGF-A, followed by CNV lesion formation (4). These findings are consistent with a central role of VEGF-A for the development of neovascular AMD. As such, the observed progressive histological CNV formation seen in VEGF-Ahyper mice is reflected in the FA and OCT findings described here that strongly resemble findings in human neovascular AMD (Fig. 2).
However, CNV lesion formation cannot explain the overall RPE and retinal dysfunction and morphological abnormalities that we have observed in VEGF-Ahyper mice at sites where no CNV lesions were present. A generalized RPE atrophy and pigment loss was found by light and electron microscopy, and these findings were consistent with pigment abnormalities in fundus images of VEGF-Ahyper mice (Fig. 3A–F). Furthermore, a significant and progressive attenuation of retinal thickness with photoreceptor loss was found both histologically and in vivo using OCT measurements at areas devoid of CNV lesions (Fig. 3B, G). The age-dependent loss of photoreceptors resulted in a significant reduction of retinal rhodopsin levels (Fig. 4F) and reduced ERG amplitudes and peak times (Fig. 4A–E). These findings led us to hypothesize that the VEGF-A-induced RPE barrier breakdown impairs the visual cycle by disrupting the proper interaction of RPE cells with photoreceptors that is required for retinoid transport processes between them, eventually resulting in photoreceptor degeneration. Electron microscopy showed indeed that RPE defects were associated with abnormal photoreceptors and a diminished interdigitation of apical RPE cell membranes with photoreceptor outer segments (Fig. 5E–G). Retinoid profiling supported this hypothesis and showed that in VEGF-Ahyper mice there is a defect in the visual cycle with a progressive age-dependent reduction of 11-cis and all-trans retinal in the retinas of mutant mice (Fig. 5A). Notably, retinyl ester levels in the RPE, which depend on the function of LRAT and RPE65, were not significantly affected (Fig. 5B). The observed visual cycle abnormalities may explain the progressive photoreceptor degeneration in VEGF-Ahyper mice and are consistent with abnormal ERGs and reduced retinal rhodopsin levels in these mice. Importantly, our findings suggest that an increase in VEGF-A alone is sufficient to cause RPE dysfunction and subsequent retinal degeneration, as is observed in nonexudative AMD. Thus, preventing conditions that promote increased VEGF-A expression, such as oxidative damage or hypoxia (16), is therefore likely to reduce the development not only of neovascular but also of nonexudative AMD pathologies. This hypothesis is consistent with clinical studies in patients with AMD (AREDS1 study; ref. 24), showing that a diet high in antioxidants can slow progression of advanced AMD (25).
Anti-VEGF-A treatments are currently the mainstay of therapy for neovascular AMD (26). However, it has recently been reported that neovascular AMD disease progression often occurs despite long-term treatment with anti-VEGF-A therapies and that long-term anti-VEGF-A treatments can be associated with macular atrophy (27). One possible explanation for the observed macular atrophy may be that chronic inhibition of extracellular VEGF-A at the posterior eye may impair the choriocapillaris, as VEGF-A is continuously expressed by RPE cells and secreted basally toward the chroriocoapillaris and is likely required to maintain a proper choriocapillaris in the adult. Thus, long-term neutralization of VEGF-A may diminish the choriocapillaris and affect RPE function and contribute to macular atrophy.
This possible adverse effect of anti-VEGF-A treatments on RPE function and the macula is not contradictory to the hypothesis that increased VEGF-A levels can cause RPE dysfunction and progressive retinal atrophy, as seen in VEGF-Ahyper mice. While chronic anti-VEGF-A antibody treatments may cause macular atrophy in humans, possibly by disrupting the choriocapillaris-maintaining function of VEGF-A, increased VEGF-A levels in the RPE of VEGF-Ahyper mice are likely causing RPE defects and disrupting the visual cycle through direct effects on the RPE and not the choriocapillaris (which appeared normal in VEGF-Ahyper mice by electron microscopic examination). This hypothesis is based on the observation that VEGF-A can disrupt RPE barrier function directly, either through paracrine or autocrine effects (4). Our data suggest that inhibiting increased VEGF-A signaling specifically in the RPE would likely reduce AMD pathologies, including RPE and photoreceptor atrophy and CNV lesion formation, while neutralizing anti-VEGF-A antibodies may have adverse effects on the choriocapillaris and could cause RPE and macular atrophy. Thus, our findings may instruct novel therapeutic approaches for both forms of AMD in targeting VEGF-A signaling specifically in the RPE or preventing the accumulation of factors that induce increased VEGF-A expression in the RPE, such as oxidative damage or hypoxia.
Acknowledgments
The authors thank Drs. Andras Nagy, Lucile Miquerol, and Annette Damert (University of Toronto, Toronto, ON, Canada) for providing VEGF-Ahyper mice and VEGF-Ahypo mice, Patrice Goletz for technical help, and Drs. Rosalie Crouch and Jie Fan for helpful discussions.
This work was supported by a U.S. National Institutes of Health (NIH)/National Eye Institute (NEI) grant to A.G.M. (NEI R01-EY019297), an NIH/NEI grant to Z.A. (NEI R01-EY019065), funding from the Medical Scientist Training Program at the Medical University of South Carolina (MUSC; NIH/National Institute of General Medical Sciences grant T32 GM008716) to M.D., an unrestricted grant of Research to Prevent Blindness to the Department of Ophthalmology at MUSC, and the South Carolina Lions Association.
Footnotes
- AMD
- age-related macular degeneration
- CNV
- choroidal neovascularization
- ERG
- electroretinogram
- FA
- fluorescence angiography
- RPE
- retinal pigment epithelium
- OCT
- optical coherence tomography
- VEGF-A
- vascular endothelial growth factor A
- WT
- wild type
REFERENCES
- 1. Friedman D. S., O'Colmain B. J., Munoz B., Tomany S. C., McCarty C., de Jong P. T., Nemesure B., Mitchell P., Kempen J. (2004) Prevalence of age-related macular degeneration in the United States. Arch. Ophthalmol. 122, 564–572 [DOI] [PubMed] [Google Scholar]
- 2. Van Leeuwen R., Klaver C. C., Vingerling J. R., Hofman A., de Jong P. T. (2003) The risk and natural course of age-related maculopathy: follow-up at 6 1/2 years in the Rotterdam study. Arch. Ophthalmol. 121, 519–526 [DOI] [PubMed] [Google Scholar]
- 3. Ablonczy Z., Dahrouj M., Tang P. H., Liu Y., Sambamurti K., Marmorstein A. D., Crosson C. E. (2011) Human retinal pigment epithelium cells as functional models for the RPE in vivo. Invest. Ophthalmol. Vis. Sci. 52, 8614–8620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marneros A. G. (2013) NLRP3 inflammasome blockade inhibits VEGF-A-induced age-related macular degeneration. Cell Rep. 4, 945–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bird A. C., Bressler N. M., Bressler S. B., Chisholm I. H., Coscas G., Davis M. D., de Jong P. T., Klaver C. C., Klein B. E., Klein R., et al. (1995) An international classification and grading system for age-related maculopathy and age-related macular degeneration. The International ARM Epidemiological Study Group. Surv. Ophthalmol. 39, 367–374 [DOI] [PubMed] [Google Scholar]
- 6. Kliffen M., van der Schaft T. L., Mooy C. M., de Jong P. T. (1997) Morphologic changes in age-related maculopathy. Microsc. Res. Tech. 36, 106–122 [DOI] [PubMed] [Google Scholar]
- 7. Curcio C. A., Presley J. B., Millican C. L., Medeiros N. E. (2005) Basal deposits and drusen in eyes with age-related maculopathy: evidence for solid lipid particles. Exp. Eye Res. 80, 761–775 [DOI] [PubMed] [Google Scholar]
- 8. Curcio C. A., Millican C. L. (1999) Basal linear deposit and large drusen are specific for early age-related maculopathy. Arch. Ophthalmol. 117, 329–339 [DOI] [PubMed] [Google Scholar]
- 9. Rudolf M., Malek G., Messinger J. D., Clark M. E., Wang L., Curcio C. A. (2008) Sub-retinal drusenoid deposits in human retina: organization and composition. Exp. Eye Res. 87, 402–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Curcio C. A., Messinger J. D., Sloan K. R., McGwin G., Medeiros N. E., Spaide R. F. (2013) Subretinal drusenoid deposits in non-neovascular age-related macular degeneration: morphology, prevalence, topography, and biogenesis model. Retina 33, 265–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Curcio C. A., Medeiros N. E., Millican C. L. (1996) Photoreceptor loss in age-related macular degeneration. Invest. Ophthalmol. Visual Sci. 37, 1236–1249 [PubMed] [Google Scholar]
- 12. Sunness J. S., Gonzalez-Baron J., Bressler N. M., Hawkins B., Applegate C. A. (1999) The development of choroidal neovascularization in eyes with the geographic atrophy form of age-related macular degeneration. Ophthalmology 106, 910–919 [DOI] [PubMed] [Google Scholar]
- 13. Fritsche L. G., Chen W., Schu M., Yaspan B. L., Yu Y., Thorleifsson G., Zack D. J., Arakawa S., Cipriani V., Ripke S., Igo R. P., Jr., Buitendijk G. H., Sim X., Weeks D. E., Guymer R. H., Merriam J. E., Francis P. J., Hannum G., Agarwal A., Armbrecht A. M., Audo I., Aung T., Barile G. R., Benchaboune M., Bird A. C., Bishop P. N., Branham K. E., Brooks M., Brucker A. J., Cade W. H., Cain M. S., Campochiaro P. A., Chan C. C., Cheng C. Y., Chew E. Y., Chin K. A., Chowers I., Clayton D. G., Cojocaru R., Conley Y. P., Cornes B. K., Daly M. J., Dhillon B., Edwards A. O., Evangelou E., Fagerness J., Ferreyra H. A., Friedman J. S., Geirsdottir A., George R. J., Gieger C., Gupta N., Hagstrom S. A., Harding S. P., Haritoglou C., Heckenlively J. R., Holz F. G., Hughes G., Ioannidis J. P., Ishibashi T., Joseph P., Jun G., Kamatani Y., Katsanis N., C N. K., Khan J. C., Kim I. K., Kiyohara Y., Klein B. E., Klein R., Kovach J. L., Kozak I., Lee C. J., Lee K. E., Lichtner P., Lotery A. J., Meitinger T., Mitchell P., Mohand-Said S., Moore A. T., Morgan D. J., Morrison M. A., Myers C. E., Naj A. C., Nakamura Y., Okada Y., Orlin A., Ortube M. C., Othman M. I., Pappas C., Park K. H., Pauer G. J., Peachey N. S., Poch O., Priya R. R., Reynolds R., Richardson A. J., Ripp R., Rudolph G., Ryu E., Sahel J. A., Schaumberg D. A., Scholl H. P., Schwartz S. G., Scott W. K., Shahid H., Sigurdsson H., Silvestri G., Sivakumaran T. A., Smith R. T., Sobrin L., Souied E. H., Stambolian D. E., Stefansson H., Sturgill-Short G. M., Takahashi A., Tosakulwong N., Truitt B. J., Tsironi E. E., Uitterlinden A. G., van Duijn C. M., Vijaya L., Vingerling J. R., Vithana E. N., Webster A. R., Wichmann H. E., Winkler T. W., Wong T. Y., Wright A. F., Zelenika D., Zhang M., Zhao L., Zhang K., Klein M. L., Hageman G. S., Lathrop G. M., Stefansson K., Allikmets R., Baird P. N., Gorin M. B., Wang J. J., Klaver C. C., Seddon J. M., Pericak-Vance M. A., Iyengar S. K., Yates J. R., Swaroop A., Weber B. H., Kubo M., Deangelis M. M., Leveillard T., Thorsteinsdottir U., Haines J. L., Farrer L. A., Heid I. M., Abecasis G. R. (2013) Seven new loci associated with age-related macular degeneration. Nat. Genet. 45, 433–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yu Y., Bhangale T. R., Fagerness J., Ripke S., Thorleifsson G., Tan P. L., Souied E. H., Richardson A. J., Merriam J. E., Buitendijk G. H., Reynolds R., Raychaudhuri S., Chin K. A., Sobrin L., Evangelou E., Lee P. H., Lee A. Y., Leveziel N., Zack D. J., Campochiaro B., Campochiaro P., Smith R. T., Barile G. R., Guymer R. H., Hogg R., Chakravarthy U., Robman L. D., Gustafsson O., Sigurdsson H., Ortmann W., Behrens T. W., Stefansson K., Uitterlinden A. G., van Duijn C. M., Vingerling J. R., Klaver C. C., Allikmets R., Brantley M. A., Jr., Baird P. N., Katsanis N., Thorsteinsdottir U., Ioannidis J. P., Daly M. J., Graham R. R., Seddon J. M. (2011) Common variants near FRK/COL10A1 and VEGFA are associated with advanced age-related macular degeneration. Hum. Mol. Genet. 20, 3699–3709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Klein R., Peto T., Bird A., Vannewkirk M. R. (2004) The epidemiology of age-related macular degeneration. Am. J. Ophthalmol. 137, 486–495 [DOI] [PubMed] [Google Scholar]
- 16. Klettner A., Roider J. (2009) Constitutive and oxidative-stress-induced expression of VEGF in the RPE are differently regulated by different Mitogen-activated protein kinases. Graefes Arch. Clin. Exp. Ophthalmol. 247, 1487–1492 [DOI] [PubMed] [Google Scholar]
- 17. Miquerol L., Gertsenstein M., Harpal K., Rossant J., Nagy A. (1999) Multiple developmental roles of VEGF suggested by a LacZ-tagged allele. Dev. Biol. 212, 307–322 [DOI] [PubMed] [Google Scholar]
- 18. Damert A., Miquerol L., Gertsenstein M., Risau W., Nagy A. (2002) Insufficient VEGFA activity in yolk sac endoderm compromises haematopoietic and endothelial differentiation. Development 129, 1881–1892 [DOI] [PubMed] [Google Scholar]
- 19. Marneros A. G., Keene D. R., Hansen U., Fukai N., Moulton K., Goletz P. L., Moiseyev G., Pawlyk B. S., Halfter W., Dong S., Shibata M., Li T., Crouch R. K., Bruckner P., Olsen B. R. (2004) Collagen XVIII/endostatin is essential for vision and retinal pigment epithelial function. EMBO. J. 23, 89–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim J. Y., Zhao H., Martinez J., Doggett T. A., Kolesnikov A. V., Tang P. H., Ablonczy Z., Chan C. C., Zhou Z., Green D. R., Ferguson T. A. (2013) Noncanonical autophagy promotes the visual cycle. Cell 154, 365–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fan J., Rohrer B., Moiseyev G., Ma J. X., Crouch R. K. (2003) Isorhodopsin rather than rhodopsin mediates rod function in RPE65 knock-out mice. Proc. Natl. Acad. Sci. U. S. A. 100, 13662–13667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Spilsbury K., Garrett K. L., Shen W. Y., Constable I. J., Rakoczy P. E. (2000) Overexpression of vascular endothelial growth factor (VEGF) in the retinal pigment epithelium leads to the development of choroidal neovascularization. Am. J. Pathol. 157, 135–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marneros A. G., Fan J., Yokoyama Y., Gerber H. P., Ferrara N., Crouch R. K., Olsen B. R. (2005) Vascular endothelial growth factor expression in the retinal pigment epithelium is essential for choriocapillaris development and visual function. Am. J. Pathol. 167, 1451–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Age-Related Eye Disease Study Research Group (2001) A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E and beta carotene for age-related cataract and vision loss: AREDS report no. 9. Arch. Ophthalmol. 119, 1439–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chew E. Y., Clemons T. E., Agron E., Sperduto R. D., Sangiovanni J. P., Kurinij N., Davis M. D. (2013) Long-term effects of vitamins C and E, beta-carotene, and zinc on age-related macular degeneration: AREDS report no. 35. Ophthalmology 120, 1604–1611, e1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Martin D. F., Maguire M. G., Ying G. S., Grunwald J. E., Fine S. L., Jaffe G. J. (2011) Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 364, 1897–1908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rofagha S., Bhisitkul R. B., Boyer D. S., Sadda S. R., Zhang K. (2013) Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP). Ophthalmology 120, 2292–2299 [DOI] [PubMed] [Google Scholar]