Abstract
Polycystin 2 (PC2) is a calcium-dependent calcium channel, and mutations to human PC2 (hPC2) are associated with polycystic kidney disease. The C-terminal tail of hPC2 contains 2 EF hand motifs, but only the second binds calcium. Here, we investigate whether these EF hand motifs serve as a calcium sensor responsible for the calcium dependence of PC2 function. Using NMR and bioinformatics, we show that the overall fold is highly conserved, but in evolutionarily earlier species, both EF hands bind calcium. To test whether the EF hand motif is truly a calcium sensor controlling PC2 channel function, we altered the number of calcium binding sites in hPC2. NMR studies confirmed that modified hPC2 binds an additional calcium ion. Single-channel recordings demonstrated a leftward shift in the calcium dependence, and imaging studies in cells showed that calcium transients were enhanced compared with wild-type hPC2. However, biophysics and functional studies showed that the first EF hand can only bind calcium and be functionally active if the second (native) calcium-binding EF hand is intact. These results suggest that the number and location of calcium-binding sites in the EF hand senses the concentration of calcium required for PC2 channel activity and cellular function.—Kuo, I. Y., Keeler, C., Corbin, R., Ćelić, A., Petri, E. T., Hodsdon, M. E., Ehrlich, B. E. The number and location of EF hand motifs dictates the calcium dependence of polycystin-2 function.
Keywords: TRP channels, kidney, ion channels, intracellular release, signaling
Polycystin 2 (PC2) is a calcium (Ca2+)-permeable transient receptor potential (TRP) channel whose activity depends on cytoplasmic Ca2+. Inheritable mutations in PC2, together with mutations in polycystin 1 (PC1), account for >90% of autosomal dominant polycystic kidney disease (ADPKD), a systemic genetic disorder affecting 1:500 to 1:1000 births. Over 99% of PC2 resides on the endoplasmic reticulum (ER), where it acts as an intracellular Ca2+ release channel. In addition, important functions of PC2 have also been associated with localization to primary cilia (1–4). However, how PC1 and PC2 function under normal conditions, and how ADPKD-associated mutations in these proteins result in systemic cyst formation remain poorly understood. One possible molecular pathway that can contribute to cyst formation is via altered Ca2+ signaling. Altered Ca2+ signaling is already known to be correlated with a number of human disease states (5), and PC2 is a prime target, not only because it is a Ca2+-conducting channel, but also because its channel activity is regulated by cytoplasmic Ca2+ concentration in a bell-shaped manner: PC2 channels are activated at high nanomoloar concentrations of Ca2+, reach maximal activity with increasing concentrations, and are inactivated at low micromolar levels of Ca2+ (6).
TRP channels are conserved from yeast to humans and serve as cellular sensors in a wide range of physiological processes. However, little is known about how TRP channels transduce stimuli into channel activity, and the structural changes governing TRP channel gating remain unclear. PC2, a member of the TRPP family, has the same overall architecture as other TRP family channels, with 6 transmembrane domains and cytoplasmic N- and C-terminal tails (7). Four PC2 units are believed to oligomerize as a tetramer to form a functional channel. Several groups, including ours, have applied biochemical and biophysical methods to investigate the structural and functional basis of PC2 channel activity. Based on these studies, the C-terminal cytoplasmic region of human PC2 (hPC2) contains several functional domains: an EF hand, a flexible acidic linker, and a coiled-coil domain (8–11). Moreover, all PC2 orthologs appear to contain the same C-terminal cytoplasmic domain architecture as found in hPC2, namely a Ca2+-binding EF hand domain connected by an acidic linker to a coiled coil domain; this condition suggests functional conservation of these domains. The functional significance of these C-terminal domains is highlighted by the fact that many ADPKD-associated mutations in PC2 result in truncated protein products, several of which lack the Ca2+-binding EF hand (e.g., N720X, ΔL736-N737, R742X, and Y762X; see http://pkdb.mayo.edu) (12, 13).
Of the discrete functional domains in PC2, the EF hand is of particular interest, as EF hands can serve as Ca2+ buffers (e.g., parvalbumin) and/or molecular switches, where Ca2+ binding induces conformational changes that lead to changes in the activity of a protein; for example, calmodulin, voltage-dependent sodium channels, voltage-dependent Ca2+ channels, and 2-pore channels (14–17). EF hands are well-characterized Ca2+-binding motifs with a helix-loop-helix fold (18, 19). In canonical EF hands, the loop contains 12 aa, 6 of which coordinate Ca2+. EF hand motifs often appear in pairs, with closely apposed EF hand motifs frequently displaying cooperative Ca2+ binding (19–21). Herein, we refer to a pair of EF hands as a domain, and a single helix-loop-helix EF hand as a motif.
Like the other proteins described above, hPC2 contains a pair of EF hand motifs, which on Ca2+ binding, facilitate conformational changes hypothesized to be involved in channel gating (11, 22, 23). However, for hPC2, only the second motif can functionally bind Ca2+, due to deletion of 4 aa in the first EF hand motif that are required for Ca2+ binding. Interestingly, sequence analysis suggests that these 4 aa are absent in PC2 in vertebrates but are present in PC2 orthologs in evolutionarily earlier invertebrate organisms. For example, sea urchin PC2 (suPC2) contains two complete EF hand motifs, where both are predicted to bind Ca2+. The suPC2 ortholog is believed to play physiologically different roles (e.g., spermatogenesis) than hPC2 (22, 24), which suggests the intriguing possibility that evolutionary changes to the EF hand may have adapted PC2 channels for new functions in vertebrates.
Previously, we have shown that the EF hand in hPC2 undergoes a folding transition on binding Ca2+ and that the conformation and oligomerization state of the C-terminal region is sensitive to Ca2+, with increasing Ca2+ concentrations causing the C-terminal cytoplasmic tail to transition from a mixture of extended oligomers to a compact dimer (11, 23). Moreover, point mutations disabling the Ca2+-binding site in the EF hand result in loss of PC2 channel activity (23). Based on these and other studies, it is tempting to speculate that the EF hand could directly sense intracellular Ca2+ and delineate the Ca2+ dependence of PC2 channel activity. To address this possibility, in the present study we set out to determine the functional significance of the two EF hand motifs in hPC2 by using comparative structural biology and protein spectroscopy. Our research here will aid in our understanding of how the EF hand senses Ca2+ within PC2 and how this action transmits to channel gating. To test how the EF hand modulates the activity profile of PC2 channels, we use these structural results to guide creation of a series of modified PC2 constructs with different Ca2+-binding potentials. In this work, PC2 channels with altered EF hand motifs are functionally compared to hPC2 channels using single-channel recordings and live cell Ca2+ imaging. Our studies provide the first direct evidence that the EF hand domain in hPC2 determines the threshold of Ca2+ necessary for PC2 channel activation and suggests that the number and location of Ca2+ binding sites in the EF hand are directly responsible for sensing the concentration of Ca2+ required for PC2 channel activity and cellular function.
MATERIALS AND METHODS
Plasmid constructs
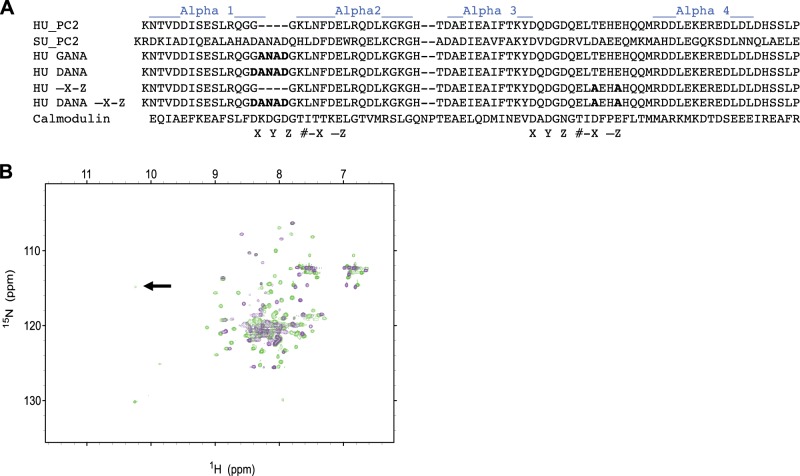
For the NMR studies, suPC2-EF (K655-E738) was amplified from a sea urchin (Strongylocentrotus purpuratus) cDNA library by PCR and cloned into a pET21 bacterial expression vector with an N-terminal His tag. For functional studies in mammalian cells, full-length suPC2 and the C-terminal tail of suPC2 (K655–V907) were PCR amplified from a sea urchin cDNA library. suPC2 was cloned upstream of mCherry in a pCDNA3.1 vector. Site-directed mutagenesis (QuikChange kit; Aligent, Santa Clara, CA, USA) was used to generate the hPC2-GANA (insertion of the sequence ANAD between G732 and G733), hPC2-DANA (mutation of G732 to D732 and insertion of the sequence ANAD between D732 and G733) and –X-Z mutations of full-length hPC2 (T771A and E774A). The mutations and PC2 constructs used in this study can be found in Figs. 4A and 5A.
Figure 4.
Insertion of DANAD into hPC2-EF results in two Ca2+ binding sites. A) Constructs and the mutants created for this study. B) Overlaid 1H-15N HSQC NMR spectra of hPC2-EF (violet contours) and DANA hPC2-EF (green contours) at 600-MHz proton frequency. Proteins were in 25 mM TRIS and 150 mM KCl buffer (pH 7.4). Arrow indicates amide protons involved in hydrogen bonding with the side-chain carboxyl oxygen atom of the corresponding aspartate residue in the DANA construct, indicating a second Ca2+ binding site.
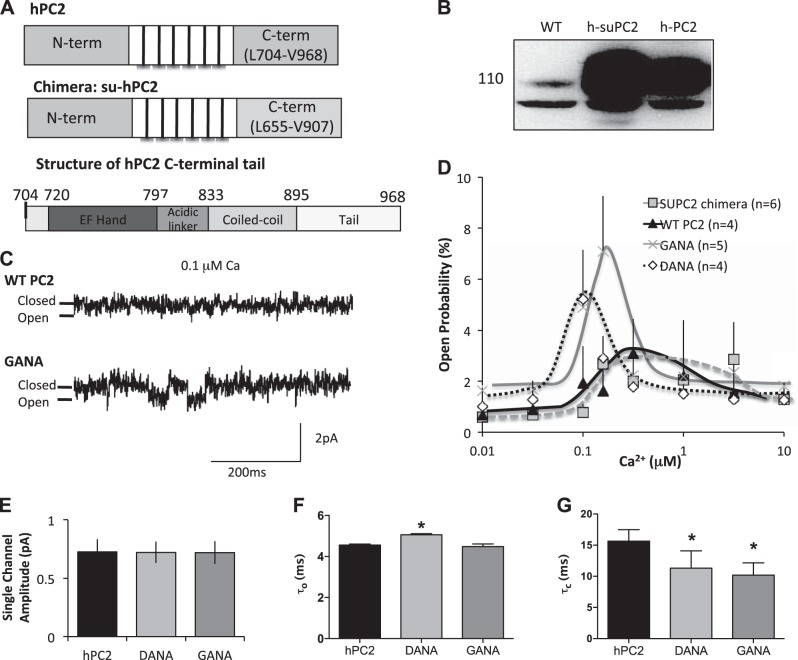
Figure 5.
Mutation of the EF hands alters the Ca2+ dependence of PC2. A) Creation of the h-suPC2 chimera (top), and overall structure of the C-terminus hand of hPC2 (bottom). Numbers refer to amino acid residues that are at the start of each domain within the C terminus. B) Expression of constructs. Lanes from left to right: endogenous expression of PC2, expression of the h-suPC2 chimera, and expression of hPC2 in LLC-PK1 cells. C). Example traces of DANA and hPC2 with 0.1 μM Ca2+. Downward deflections represent channel openings. D) Single-channel open probability curves as a function of cis Ca2+ concentrations. E) Single-channel amplitude is not altered. Results are presented as means ± sem; n = 4–6. F) Channel open dwell time (τo) with 0.1 μM Ca2+. G) Interevent interval (i.e., closed time, τc) with 0.1 μM Ca2+. Results in F and G are means ± sem of ≥300 events from n = 3 experiments. *P < 0.05 vs. hPC2.
Sequence alignment and analysis
Multalign (http://www.multalin.toulouse.inra.fr), Clustal Omega (http://www.clustal.org/omega), and Phylogeny.fr (http://www.phylogeny.fr) were used to align sequences, determine sequence similarity and identity, and construct the phylogenetic tree. The program JalView (http://www.jalview.org) was used to view and edit aligned sequences.
Protein expression and purification
suPC2-EF (K655-E738 of suPC2 cloned into a pET21 vector) was expressed in BL21-RIPL (DE3) Escherichia coli cells. Transformed BL21 cells were grown at 37°C to an OD600nm of 0.5, induced with 1 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) in minimal medium (supplemented with 15N and 13C carbon and nitrogen sources when necessary), and purified by nickel-affinity chromatography as described previously (11, 23). As preliminary NMR spectra indicated aggregation, presumably due to disulfide-linked dimerization, the reducing agent 5 mM tris-(2-carboxyethyl) phosphine:DCl (TCEP) was used in all subsequent experiments; this was found to greatly improve protein stability and NMR spectral quality. Size-exclusion chromatography in conjunction with size-exclusion column multiple-angle laser light scattering (SEC-MALS) was used to confirm a monomeric state.
NMR structural studies
NMR structural studies were conducted as described previously (22, 23). NMR samples contained ∼700 μM suPC2-EF in 25 mM Tris pH 7.38; 150 mM NaCl; 20 mM CaCl2; 5 mM TCEP; 5% D2O; 0.05% NaN3; and a cocktail of protease inhibitors. NMR spectra were collected at 25°C (Varian INOVA, 600 MHz; Varian Medical Systems Inc., Palo Alto, CA, USA) and processed in NMRPipe (U.S. National Institute of Diabetes and Kidney Diseases, Bethesda, MD, USA; http://spin.niddk.nih.gov/NMRPipe). Resonance-specific chemical shifts were assigned using 2-dimensional (2D)1H-15N heteronuclear single quantum coherence (HSQC), 1H-13C HSQC, (3D)HNCO, HN(CA)CO, HNCACB, HN(CO)CA, HCACO, HCC(CO)NH, 15N total correlation spectroscopy (TOCSY)-HSQC (total correlation spectroscopy), HCCH-TOCSY, (2D)1H-13C HSQC, and (3D)13C nuclear Overhauser enhancement spectroscopy (NOESY)-HSQC spectra. Spectra have been submitted to BioMagResBank (University of Wisconsin, Madison, WI, USA; http://www.bmrb.wisc.edu), accession number 19633.
Steady-state 1H and 15N NOE measurements, NMR relaxation times, and dipolar couplings
Steady-state 1H-15N NOE experiments included sensitivity enhancement, water flip-back, and coherence selection via pulse field gradients with a 9 s saturation and 6 s recycle delay; 128 transients were collected at 9 kHz (f2) and 2.1 kHz (f1). NMR relaxation times (T1 and T2) were extracted from 2 series each of 1H-15N HSQC spectra with delays of 100, 300, 500, 700, and 1000 ms for T1 and 10, 30, 70, 150, 190, and 250 ms for T2, with a 1 s recycle delay. NMR peak heights were determined by the “rh” command in Sparky [University of California–San Francisco (UCSF), San Francisco, CA, USA; http://www.cgl.ucsf.edu/home/sparky], and the program CurveFit (25) was used for exponential fitting of dihedral angle and NOE distance restraints. Backbone ψ and μ torsion (dihedral) restraints were calculated using TALOS (NMRPipe). NOESY correlations were identified in 3D 15N-NOESY-HSQC and (aromatic) 13C-NOESY-HSQC in Sparky and interpreted in CYANA (http://www.cyana.org). Several iterations of automated NOESY interpretation, structure calculation, and restraint analysis were used to calculate the final distance restraints. Dihedral angle restraints were included to improve convergence. To measure residual dipolar couplings, anisotropic orientational restraints were obtained from Ca2+-bound 15N-labeled suPC2-EF with 10 mg/mL Pf1 phage, or 7% strained polyacrylamide gel, and were compared to 15N suPC2-EF lacking phage. 1 JNH coupling constants were measured using a spin-state-selective 1H, 15N-HSQC pulse sequence. Structure analysis was performed with the UCSF Chimera package (http://www.cgl.ucsf.edu/chimera) (26). Coordinates for suPC2-EF and structural constraints were evaluated using PSVS 1.4 (http://psvs-1_4-dev.nesg.org), with structure quality evaluators ProCheck, Prosall, and MolProbity (27). The program PALES (28) was used to determine the correlation between experimentally determined RDC values and RDC values back-calculated from structure. Correlation is expressed as both the Q factor (RMS(Dcalc − Dobs)/RMS(Dobs), where Dcalc and Dobs are calculated and observed RDC values) and Pearson's R coefficient. The Dali server (DaliLite 3; http://ekhidna.biocenter.helsinki.fi/dali_server/) was used to assess the similarity of suPC2-EF to existing structures (29).
Single-channel recordings in planar lipid bilayers
Microsomes were isolated from pig kidney epithelial cells (LLC-PK1) overexpressing the various PC2 constructs and incorporated into planar lipid bilayers as described previously (6). To monitor the Ca2+ dependence of PC2 channel activity, channels were recorded with a holding potential of 0 mV and with 50 mM Ba2+ dissolved in 250 mM HEPES, pH 7.35, in the trans side as a charge carrier. The cis side contained 110 mM Tris dissolved in 250 mM HEPES, pH 7.35. The Ca2+ concentration was calculated and is presented as the log [Ca2+]. Channel data were acquired at 10 kHz, and filtered at 2 kHz (Warner, Hartford, CT, USA). Open probability was determined by using current recordings from at least 90 s of continuously recorded data, and analyzed using pClamp 8 (Molecular Devices, Sunnyvale, CA, USA).
Cell culture
LLC-PK1 cells were maintained in Dulbecco's minimal essential medium supplemented with 10% fetal bovine serum and 5% CO2. Cells were transiently transfected (Lipofectamine 2000, Life Technologies, Grand Island, NY, USA) with the appropriate PC2 construct and cotransfected with dsRed.
Immunoblotting and immunofluorescence
PC2 overexpression was confirmed by immunoblotting and immunofluorescent staining. For immunoblotting, cell lysates were separated by SDS-PAGE and transferred to PVDF membranes. After incubation in primary antibody overnight, membranes were washed and incubated in secondary antibodies conjugated to horseradish peroxidase (HRP). The HRP signal was detected using an enhanced chemical luminescent kit (WestDura; ThermoScientific, Waltham, MA, USA). For immunofluorescent studies, cells were fixed with 2% paraformaldehyde 24–48 h post-transfection. PC2 was detected using either N-terminal or C-terminal directed primary antibodies against hPC2. Images were captured using a Zeiss LSM710 Duo confocal microscope (Carl Zeiss, Heidelberg, Germany).
Ca2+ imaging
Experiments to monitor changes in intracellular Ca2+ were performed by optically imaging Fluo-4-AM loaded cells (5 μM) using a Zeiss LSM710Duo confocal microscope. Studies were carried out in imaging buffer (130 mM NaCl, 1 mM MgSO4, 1.2 mM KH2PO4, 4.7 mM KCl, 19.7 mM HEPES, and 5 mM dextrose, pH 7.3) supplemented with 1.25 mM Ca2+ (Ca2+-containing buffer) or 0.1 mM EGTA and 1.25 mM MgCl2 (Ca2+-free buffer). Ca2+ transients were induced by applying 10 or 100 nM vasopressin or 1 μM ATP to activate the G-protein-coupled PLC pathway. Fluorescent intensity ratios were normalized to the first 10 s of the baseline recording.
Isothermal titration calorimetry (ITC)
ITC experiments were conducted with ∼100 μM protein in the chamber (25 mM TRIS, 150 mM KCl, and 1 mM TCEP, pH 7.4) and 4.7 mM Ca2+ as the injectant. Calorimetry experiments were conducted on a MicroCal VP-ITC isothermal titration calorimeter (GE Healthcare Life Sciences, Pittsburgh, PA, USA). Samples were degassed prior to each experiment. The first injection volume for each calorimetry experiment was 2 μl and was discarded from the data series. Typical injection volumes were 5 or 8 μl. All baseline corrections were conducted using Origin 7.0 (OriginLab Corp., Northampton, MA, USA) software with the MicroCal ITC add-on program provided by the manufacturer. Baseline corrected data were exported as ascii text from the MicroCal program before being further analyzed in custom Mathematica scripts (30).
Statistical analyses
Data were entered into Prism (GraphPad, San Diego, CA, USA) for 1-way ANOVA, followed by Bonferroni's multiple comparison tests to determine significance, where P < 0.05 is considered significant.
Unless stated otherwise, chemicals were obtained from Sigma (St. Louis, MO, USA).
RESULTS
Phylogenetic analyses and sequence alignments predict that invertebrate PC2 orthologs contain two putative Ca2+-binding EF hand motifs, whereas vertebrate PC2 proteins contain only one Ca2+-binding EF hand motif
In the present study, we examined the structural and functional roles of the EF hand domain in the Ca2+-dependent activity of hPC2 channels. Comparative structural biology and bioinformatics have the potential to provide functional insight into human proteins through analysis of evolutionary changes in protein structural domains.
To provide a framework for analysis of the functional significance of the two EF hand motifs in hPC2 channels, we conducted structure-based sequence alignments of EF hand domains from PC2 orthologs using the NMR structure of hPC2-EF [Protein Data Bank (PDB) ID 2KQ6] as a reference and compared our results with the canonical EF hand found in calmodulin (22). Sequence alignments were calculated in Clustal Omega using BLAST search results from the cytoplasmic tail of hPC2 and analyzed in JalView. For clarity, sequence alignments have been truncated to show only the EF hand domains (Fig. 1A). The NMR structure of hPC2-EF was used to estimate the secondary structure of PC2-EF hand orthologs. As can be seen, all PC2 orthologs appear to contain two helix-loop-helix EF hand motifs based on similarity to human calmodulin and comparison with an EF hand test sequence. The second EF hand motif is highly conserved across all PC2 orthologs and appears to be functional, with all residues necessary for Ca2+ binding. However, the first EF hand motif appears to be nonfunctional in vertebrate PC2 orthologs due to the loss of 4 residues necessary for Ca2+ coordination. These results are in agreement with structural and Ca2+-binding studies conducted on the hPC2-EF hand domain (22).
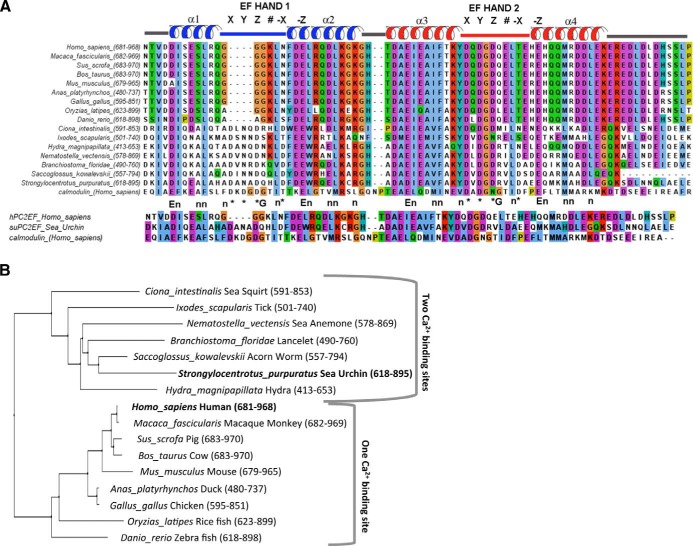
Figure 1.
Alignment and phylogenic tree of the C-terminus of PC2. A) Alignment of EF hands from PC2 orthologs, including hPC2 and suPC2. Sequence alignments were calculated in Clustal Omega using BLAST search results from the cytoplasmic tail of hPC2 and analyzed in JalView. B) Phylogenic tree of the C-terminal tail of PC2. Note that PC2 orthologs from chordates and nonchordates fall into either groups containing 2 Ca2+ sites or 1 Ca2+ site. The C terminus of suPC2 was used an input sequence under a BLAST-explorer search to find similar proteins using Phylogeny.fr (36). Example sequences across different species were then selected and aligned using Clustal Omega. The phylogeny tree was then constructed using Pylogeny.fr (37). Numbers refer to the amino acids chosen for analysis.
To determine the evolutionary relationship of PC2 orthologs to hPC2, multiple sequence alignments comparing full-length PC2 proteins was conducted. The degree of sequence similarity across the C-terminal tail of PC2 orthologs is significant, with the PC2-EF hand domain sharing ∼43% identity between suPC2 and hPC2 orthologs. To analyze the extent of evolutionary conservation of the functional domains within the C-terminal PC2, phylogenetic analysis was conducted on this region alone (Fig. 1B). As can be seen, PC2 orthologs are clearly divided into two broad groups: nonchordates, which are considered to be evolutionarily earlier, have two Ca2+-binding EF hand motifs; and chordates, which have one Ca2+-binding EF hand motif.
These results are consistent with our sequence alignment results and strongly suggest that invertebrate PC2 orthologs contain two functional Ca2+-binding EF hand motifs, whereas in vertebrate PC2 orthologs the first Ca2+ binding site (corresponding to EF hand 1) has been lost.
NMR spectral analysis shows that the suPC2-EF ortholog contains two functional Ca2+-binding EF hand motifs
Based on the above findings, the Ca2+-binding properties of the PC2-EF hand domain appear to have changed during evolution from invertebrates to vertebrates. Because sea urchin is a well-studied model organism, we focused on the suPC2-EF hand domain. We first examined whether suPC2EF was monomeric. In the presence of 1 mM TCEP, suPC2-EF was determined to be monomeric by SEC-MALS analysis, like hPC2-EF (Fig. 2A). To experimentally determine whether both predicted EF hand motifs in suPC2 bind Ca2+, 1H-15N HSQC NMR spectra were recorded for the suPC2-EF hand domain in saturating (20 mM) Ca2+ conditions (Fig. 2B). This concentration was chosen to enable a direct comparison with previous NMR structures of PC2 (11, 23). 1H-15N HSQC NMR spectra show that suPC2-EF forms a well-ordered, α-helical structure typical of EF hand domains. In addition, under saturating Ca2+ concentrations, proton chemical shifts for Q675 and R709 were significantly shifted to ∼10 ppm (Fig. 2A and Supplemental Fig. S1). These shifts are a diagnostic signature of Ca2+-bound EF hands (31), where the amide proton of each of these residues is involved in hydrogen bonding with the side-chain carboxyl oxygen atom (C′O) of the corresponding aspartate residue at the +X position of the individual EF hand motifs. In contrast, this shift was not observed for loop resides in the first EF hand motif of hPC2-EF (22). These NMR results are in agreement with the above sequence alignment and phylogenetic analysis.
Figure 2.
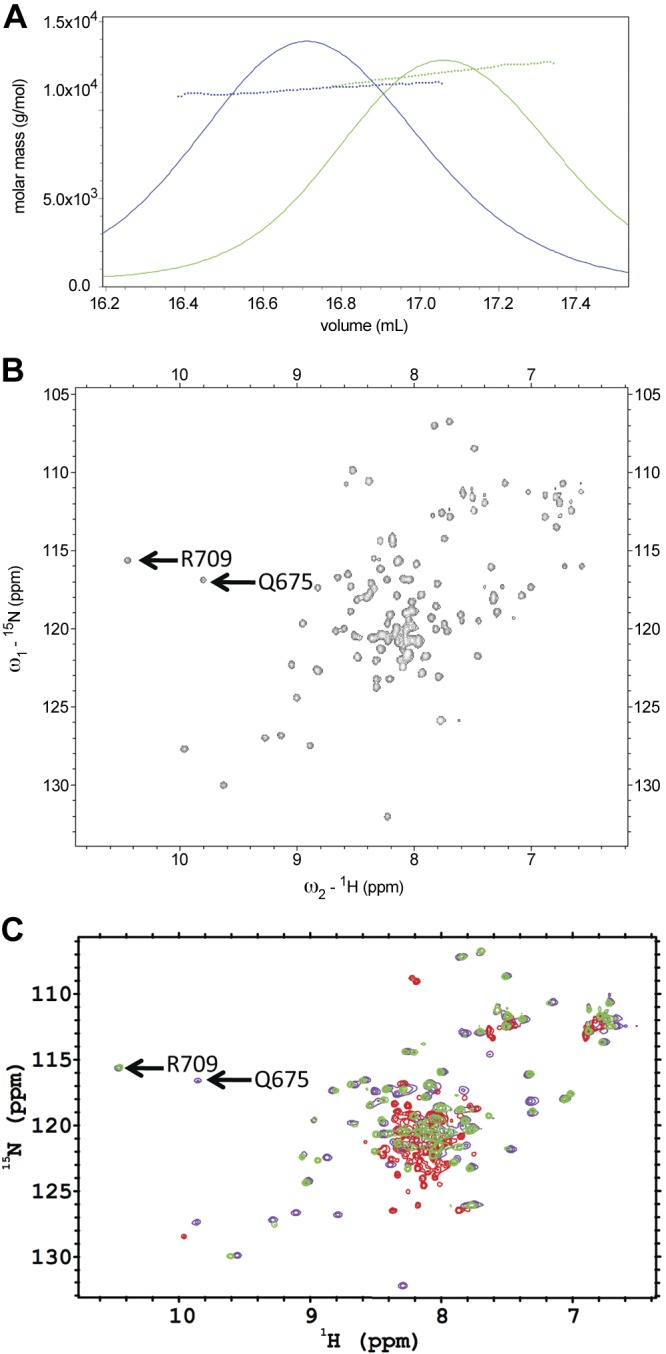
SEC-MALS and NMR spectra of PC2 proteins. A) SEC-MALS analysis of suPC2-EF (12.7 kDa, green trace) and hPC2EF (11.4 kDa, blue trace) indicate that both proteins are monomeric in 25 mM TRIS (pH 7.4), 150 mM KCl, 1 mM Ca2+, and 1 mM TCEP buffer. B) 1H-15N HSQC spectra of 15N suPC2-EF at 600-MHz proton frequency. Arrows indicate Q675 and R709 residues that show shifts due to the amide protons involved in hydrogen bonding with the side-chain carboxyl oxygen atom of the corresponding aspartate residue at the +X position of the individual EF hand motifs. C) Overlaid 1H-15N HSQC NMR spectra of 15N suPC2EF-1 protein under various solvent conditions collected at 25°C. Violet contours depict data from a sample with a protein concentration of 450 μM with 2 mM TRIS (pH 7.4), 150 mM NaCl, 1 mM TCEP, and 20 mM CaCl2. Green contours depict data collected on a sample with 87 μM protein under similar solvent conditions, but without added CaCl2; whereas red contours depict data collected on a sample with 70 μM protein under similar solvent conditions without added CaCl2 and with 200 μM 5, 5′-dimethyl BAPTA to bind residual calcium found in the buffer.
Under nominal Ca2+ (estimated to be 10 μM Ca2+), only the proton chemical shift in the 10 ppm region associated with R709 shift was observed, indicating that the binding of Ca2+ to the second EF hand had a higher affinity than the first site (Fig. 2B). The peak associated with Q675 was no longer observed after the addition of the calcium chelator BAPTA (200 μM) to bind residual Ca2+, confirming that the first EF hand also bound Ca2+ (Fig. 2B, C). With BAPTA present, a loss of several chemical shifts occurred, which would suggest that those residues are involved in the binding of Ca2+. These data indicate that the two EF hands of suPC2EF both bind Ca2+ and that the two sites have different affinities, with the first site (associated with Q675) having a higher affinity than the second site.
NMR structural studies reveal that hPC2-EF and suPC2-EF have similar folds with key structural differences affecting Ca2+ binding in the first EF hand motif
We hypothesize that the Ca2+-binding properties of the EF hand domain in PC2 are directly responsible for conferring Ca2+ dependence to PC2 channels. Because hPC2 and suPC2 channels contain different numbers of functional Ca2+ binding sites and are expected to play different physiological roles, we speculate that the PC2-EF domain evolved to respond to different levels of cytoplasmic Ca2+. To provide a structural framework for this hypothesis, we wanted to compare the structures of hPC2-EF and suPC2-EF (as a representative of invertebrate PC2 orthologs). NMR structural studies were conducted on the suPC2-EF in saturating (20 mM) Ca2+ conditions. The 3-dimensional structure of suPC2-EF was determined using CYANA (32), and checked for structural restraints (Table 1). NMR chemical shift assignments were established for nearly all backbone atoms from residues S11 to E107. According to CYANA, 99.2% of the typically assignable hydrogens were assigned for this residue range (4 more 1H chemical shifts). Representative 3D NOSEY strips are provided in Supplemental Fig. S3.
Table 1.
Constraints and structural statistics summary
| Parameter |
Value |
|---|---|
| Conformational constraints | |
| NOE-based distance constraints | |
| Total | 1363 |
| Intraresidue [i = j] | 265 |
| Sequential [|i – j| = 1] | 354 |
| Medium range [1 < |i – j| < 5] | 339 |
| Long range [|i – j| ≥ 5] | 405 |
| NOE constraints per residue | 14.3 |
| Dihedral angle constraints | 156 |
| RDC constraints | |
| Bacteriophage Pf1 | 78 |
| Strained acrylamide gel | 47 |
| Residual constraint violations | |
| Distance violations > 0.5 Å per structure | 0.3 |
| RMS of distance violation per constraint | 0.02 |
| Maximum distance violation (Å) | 0.62 |
| Dihedral angle violations > 5° per structure | 0.15 |
| RMS of dihedral angle violation per constraint (deg) | 0.67 |
| Maximum dihedral angle violation (deg) | 5.78 |
| Structural analysis statisticsa | |
| RMSD | |
| All backbone atoms | |
| All residues (Å) | 2.4 |
| Ordered residues (Å)b | 0.9 |
| All heavy atoms | |
| All residues (Å) | 2.7 |
| Ordered residues (Å)b | 1.5 |
| Ramachandran plot summary from Procheck | |
| Most favored regions (%) | 86.3 |
| Additionally allowed regions (%) | 12.2 |
| Generously allowed regions (%) | 0.3 |
| Disallowed regions (%) | 1.1 |
| Global structural quality Z score | |
| Verify3D | −2.09 |
| Prosall [−ve] | 0.33 |
| Procheck [ϕ-ψ] | −0.12 |
| Procheck [all] | −0.89 |
| MolProbity Clashscore | −0.52 |
Generated by PSVS 1.4 (http://psvs-1_4-dev.nesg.org).
Residues 21–102.
We validated the NMR refinement solution by comparing the correlation between experimentally measured RDCs (Table 1) and our structure of PC2-EF using the program PALES (28). Good agreement (calculated Q factor of 0.055 and a Pearson's R coefficient of 0.996) was found between the RDCs predicted by the structure of PC2-EF and experimentally measured Pf1 phage NH (backbone amide) RDCs (Supplemental Fig. S2). In addition, NH RDCs were measured in an alternate alignment medium, 7% strained polyacrylamide gels, to obtain independent alignment tensors for additional cross-validation (Supplemental Fig. S3).
The structure of suPC2-EF has a PDB accession code of 2MHH. The NMR data have been deposited into the BioMagResBank, accession number 19633. The overall 3D fold of suPC2-EF is similar to canonical EF hand domains, with 2 helix-loop-helix EF hand motifs (residues K655 to C688, and residues D693 to L734), connected by a short linker of 4 aa (R689 to A692) (Fig. 3A). However, although the overall 3D fold is conserved between hPC2-EF and suPC2-EF, key differences indicate possible important implications for the Ca2+ dependence of PC2 channel activity (Fig. 3B).
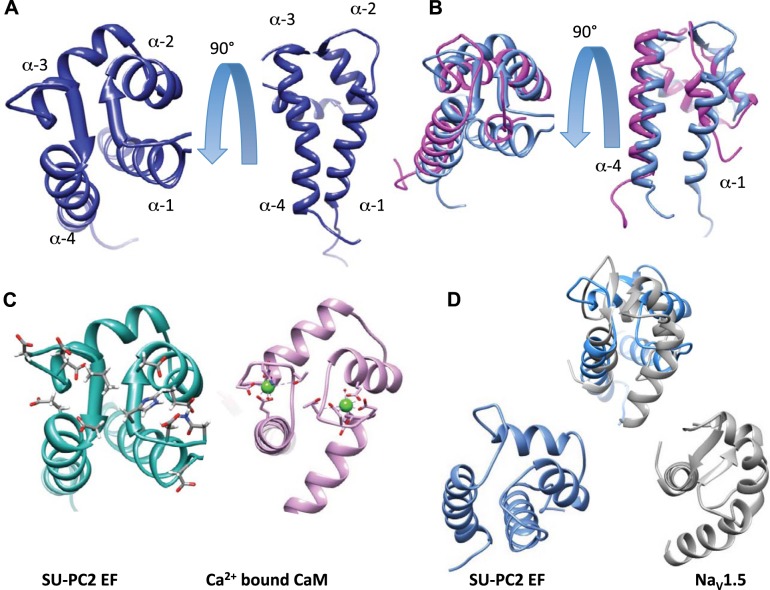
Figure 3.
A) Structure of Ca2+-bound suPC2-EF and comparison to other EF structures. Backbone ribbon for the top model (taken from the top 20 conformers). B) Comparison of suPC2-EF (blue) and hPC2-EF (magenta; PDB ID: 2KQ6). C) Side-by-side comparison of suPC2 and CaM (PDB ID: 1CLL) with the residues that coordinate Ca2+ represented with Corey-Pauling-Koltun (CPK) stick models. CaM shows the coordinating residues, with green Ca2+ spheres. D) Overlap comparison and side-by-side comparison of the ribbon structures of suPC2-EF (blue) and NaV1.2 EF (gray; PDB ID: 4DCK).
To further analyze the structural evolution of the PC2-EF hand domain, we compared the structure of suPC2-EF with known protein structures using the Dali server, which ranks proteins according to structural similarity (defined by a Z score) via a structure superposition algorithm (29). Superposition of the structure of hPC2-EF (PDB ID: 2KQ6) with suPC2-EF resulted in a very high Z score of 6.3, indicating a similar overall fold. The superimposed structures were visualized using the program Chimera (26). Overall both structures are similar, with the parallel β strands forming the Ca2+ coordination loops overlaying at similar angles (Fig. 3B). However, 3 striking differences are apparent. First, the effect of the 4 missing residues in the helix-loop-helix of hPC2-EF can be clearly seen: The “Ca2+ binding pocket” and the requisite amino acids necessary to coordinate Ca2+ binding are absent (Fig. 3B). Second, helix 1 in suPC2-EF is longer than in hPC2-EF. As the region immediately N-terminal to helix 1 in hPC2-EF is susceptible to proteolytic cleavage and presumed to be unstructured (11), it is likely that the presence of two Ca2+ binding sites in suPC2-EF helps stabilize helix 1. Alternatively, because the first helical turn in suPC2-EF contains 3 non-native amino acids (a cloning artifact), it is possible, but unlikely, that these non-native residues affect the structure of suPC2-EF. However, NMR spectra for native suPC2-EF and suPC2-EF containing the introduced cloning artifact were virtually identical (data not shown). Third, helices α1 and α2 of the first EF hand motif in suPC2-EF are roughly perpendicular, enabling the α1-α2 loop to assume the proper geometry for Ca2+ coordination, as expected for canonical Ca2+-binding EF hand motifs. In contrast, α1 and α2 are parallel in hPC2-EF, consistent with loss of the Ca2+ binding site. Moreover, interhelical angles between helices α3 and α4, which comprise the second EF hand motif, are practically identical between hPC2-EF and suPC2-EF. This is consistent with the finding that the second EF hand motif contains a functional Ca2+ binding site, which is necessary in both vertebrate and invertebrate PC2 orthologs.
In support of the assumption that suPC2-EF contains two functional EF hands, superposition of the solution structure of suPC2-EF with the well-known EF hand protein calmodulin (PDB ID: 2W73) yielded a higher Dali server Z score of 6.4. Closer inspection revealed many structural similarities, particularly in the regions responsible for coordinating Ca2+ (Fig. 3C). This finding strongly suggests that suPC2 has the ability to bind Ca2+ at two sites, again in contrast with hPC2-EF. One difference was noted in the first and fourth α-helices; in calmodulin the helices are perpendicular to each other, whereas the same α-helices in suPC2-EF are more parallel in orientation. However, these helices participate in opposing EF hand motifs and interhelical angles often vary among opposed canonical EF hand pairs.
To determine whether the PC2-EF hand domain contains structural similarities to EF hands in other ion channel proteins, we also superimposed the structure of suPC2-EF with the structure of an EF hand domain from the sodium channel NaV1.5 (PDB ID:4DCK) (Fig. 3D). Like PC2, NaV1.5 has an EF hand domain located in the C-terminus that is involved in channel gating (16); thus, we compared the Ca2+-binding motifs of NaV1.5 and PC2. The EF hand of NaV1.5, like hPC2-EF, binds Ca2+, which in turn induces a conformational change in its C-terminal tail; however, in the case of Nav1.5, this conformation change reveals a calmodulin binding site (16). Although some overall similarities between suPC2-EF and NaV1.5 are visible, clear differences are apparent in the orientation of the first α-helix and the length of the linker joining the two EF hand motifs. However, these differences do not preclude either NaV1.5 or PC2-EF from binding Ca2+. Not surprisingly, the Dali Z score between suPC2-EF and NaV1.5 was lower (2.6) than that measured for calmodulin (6.3). Taken together, comparative structural analysis shows that suPC2-EF has two functional Ca2+-binding regions, unlike hPC2-EF, and that the general features of the suPC2-EF are similar to the structures of other canonical EF hands.
Creation of modified hPC2 channels with different Ca2+-binding potentials via structure-guided mutagenesis of the EF hand domain
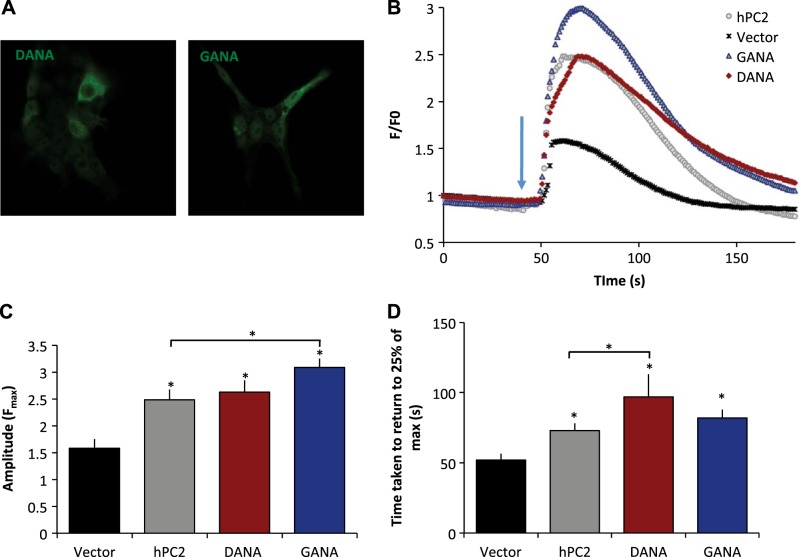
We propose that the EF hand motifs in hPC2 function as a Ca2+ sensor responsible for the Ca2+-dependence of PC2 channel activity. Using NMR and bioinformatics, we have shown that the overall fold of this Ca2+ sensor is highly conserved but that the first EF hand motif in hPC2 has lost the ability to bind Ca2+. Using the NMR structure of suPC2-EF as a guide, we created two modified hPC2 constructs designed to reintroduce the lost residues necessary for Ca2+ binding into the first helix-loop-helix EF hand motif. The first hPC2 construct, hPC2-GANA (corresponding to insertion of the sequence ANAD between G732 and G733), introduced 4 residues selected because they correspond to residues responsible for Ca2+ coordination in the same region of the first EF hand motif of suPC2-EF (Figs. 1A and 4). The reintroduction of the ANAD sequence in EF hand 1 of hPC2 provides 2 residues at the +Y and +Z position in the first EF hand, enabling the site to bind Ca2+. The second hPC2 construct, hPC2-DANA (mutation of G732D and insertion of the sequence ANAD between G732D and G733), was constructed by mutating Gly732 to Asp, to better reflect the preference of an aspartate residue at the +X position of EF hand motifs (19). To the best of our knowledge, EF hand structures where the residue at position +X is a glycine have been reported, and almost all known structures contain an aspartate residue at this position (19). The Gly-to-Asp substitution in hPC2-GANA-EF would thus be expected to alter the Ca2+-binding potential of the PC2-EF hand. To ensure that these two constructs had an additional Ca2+ binding site, 1H-15N HSQC NMR spectra were recorded under saturating (20 mM) Ca2+ conditions (see Fig. 4B for an overlay of hPC2-DANA-EF and hPC2-EF 1H-15N HSQC NMR spectra). 1H-15N HSQC NMR spectra show that the addition of the 4 aa did not disrupt the well-ordered, α-helical structure. More importantly, under saturating Ca2+ concentrations, we observed a resonance at 10 ppm (Fig. 4B, arrow), similar to those observed for suPC2-EF. Thus, these data suggest that the addition of a second functional Ca2+ binding site between the α1-α2 loops is well-tolerated, and both sites can bind Ca2+.
The addition of a second Ca2+ binding site to the EF hand domain of hPC2 alters the Ca2+ dependence of PC2 channel activity
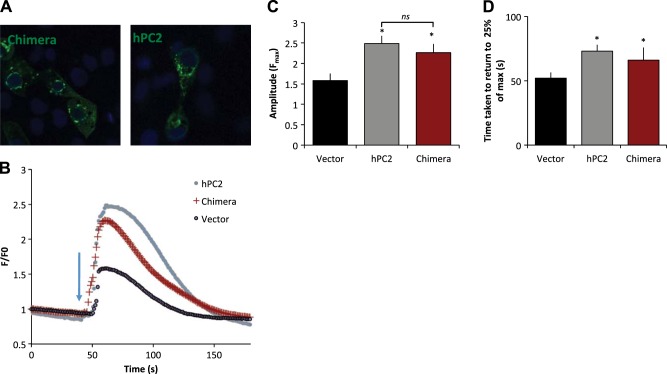
If the EF hand domain of hPC2 is truly a Ca2+ sensor, changing its Ca2+-binding properties (e.g., number of binding sites and/or location) should alter the Ca2+ dependence of PC2 channels. To investigate the functional implications of two Ca2+ binding sites in the EF hand of hPC2, we used single-channel experiments in planar lipid bilayers. We first established that our modified hPC2 variants could be overexpressed in LLC-PK1 cells (Figs. 5B, 6A, and 7A). We initially started with a human suPC2 (h-suPC2) chimera construct (Fig. 5B), because we reasoned that the C terminus could be considered as a modular functional domain and could tolerate substitution. Although we found that substitution of the C terminus of hPC2 for that of suPC2 did not statistically change the activity or the Ca2+ sensitivity compared to hPC2, this may be due to the multiple amino acid differences within the C-terminal tail introduced by the h-suPC2 chimera. Thus, we then compared our modified PC2 channels (hPC2-DANA and hPC2-GANA) with hPC2. Over the Ca2+ range tested, we found that both GANA and DANA hPC2 channels displayed higher open channel probabilities at the optimal Ca2+ concentration (∼6.5% compared to ∼4%) than hPC2 (Fig. 5C, D). In addition, the concentration range over which Ca2+ modulates channel activity was narrower, with a shift to lower Ca2+ concentrations (Fig. 5D) for both activation and inhibition. The Ca2+ dependence curves describing of the activity of the GANA and the DANA constructs were not significantly different from each other. At the 0.1 μM Ca2+ concentration, the open probability was significantly different among wild type (WT) and the two constructs with the additional calcium binding site (DANA and GANA). At 0.3 μM Ca2+, the open probability of the GANA construct was significantly higher than the WT construct. As the PC2 currents are small at 0 mV, we also conducted experiments with −10 mV holding potential to increase the driving force for calcium flux. Our results with the GANA construct indicate that currents are larger, as expected, but the same Ca2+ dependence is observed at both −10 mV and 0 mV (Supplemental Fig. S4).
Figure 6.
Expression and effect of chimeric and full-length PC2. A) Distribution of chimeric and full-length hPC2 in LLC-PK1cells. B) LLC-PK1 cells were transfected with empty vector (gray), or PC2 (black) or h-suPC2 chimera (red). Ca2+ transients elicited after the addition of 100 nM vasopressin (blue arrow). Traces represent mean data, n = 8–28, where n represents number of cells from at least two independent experiments. C) Average amplitude responses. D) Average time taken for the transient to return to 25% of the maximum amplitude. Results are presented as mean ±sem; n = 8–28, where n represents number of cells from at least two independent experiments. Asterisks represent P < 0.05 compared to Vector.
Figure 7.
Effect of two PC2-EF hands on Ca2+ transients. A) hPC2-GANA and hPC2-DANA localized intracellularly. B) LLC-PK1 cells were transfected with empty vector (gray), PC2 (black), DANA (red), or GANA (blue). Traces represent mean data, n = 8–28, where n represents number of cells from ≥2 independent experiments. C) Average amplitude responses. D) Average time taken for the transient to return to 25% of the maximum amplitude. Results are presented as means± sem; n = 8–28, where n represents number of cells from ≥2 independent experiments. *P < 0.05 vs. vector.
To better characterize how the mutations were altering the channel activity, the event dwell time (τo) and interevent intervals (τc) were also analyzed (Fig. 5E, F). At 0.1 μM Ca2+, the channel open dwell time was significantly longer for the DANA construct compared with WT, although the open time was still relatively short (∼5 ms). The closed time for both the GANA and the DANA constructs were significantly lower for both constructs compared with the WT, which is consistent with an increase in channel openings as observed in 0.1 μM Ca2+. The current amplitude for all the constructs was unchanged (∼0.7 pA; Fig. 5E). Taken together, the data suggest that the addition of the Ca2+ binding site alters the sensitivity of opening and changes the probability that the channel will open, but does not alter the mean open time, once the channel has opened. These single-channel experiments provide the first evidence that alterations to the Ca2+-binding properties of the PC2-EF hand motif directly alter the Ca2+ dependence of PC2 channel activity, as well as channel open probability.
h-suPC2 chimeras with two Ca2+-binding EF hand motifs enhance Ca2+ signaling compared with control cells
To further investigate the h-suPC2 chimera construct, which in the bilayer assay did not show a functional difference in Ca2+ sensitivity vs. hPC2, we conducted live cell Ca2+ imaging experiments. Cells overexpressing the h-suPC2 chimera showed a large-amplitude Ca2+ signaling response to 100 nM vasopressin, comparable in amplitude and duration to cells transfected with full-length hPC2 (Fig. 6B, C). This concentration of vasopressin was used because it was more comparable to the agonist concentration used in other studies (6) and induced a more reproducible response than lower concentrations (Supplemental Fig. S4). Similar responses were observed with the addition of 1 μM ATP (Supplemental Fig. S4), indicating that the change in the intracellular Ca2+ is due to activation of the PLC pathway. In both hPC2- and h-suPC2-transfected cells, this amplitude was significantly higher compared with vector only control (F/F0=1.58 for vector, 2.48 for hPC2 and 2.26 for h-suPC2 chimera). In addition, the duration of the Ca2+ transient was increased for both hPC2 and h-suPC2 compared with empty vector controls (Fig. 6D). Similar experiments were conducted in extracellular Ca2+-containing buffer, as well as a Ca2+-free buffer. Under both conditions, the results were similar, indicating that the Ca2+ responses examined in these experiments were due to changes in intracellular Ca2+ signaling, rather than influx from the extracellular environment. In these experiments monitoring Ca2+ transients, we interpret the amplitude to be a reflection of the effect of Ca2+ release from the ER as a consequence of PC2 activation, as well as release via the InsP3R (vector only). The duration is a measure of the uptake back into the ER stores, as well as sustained release through open InsP3R or PC2. The data from the chimeric h-suPC2 construct suggest an enhancement of the Ca2+ transients as compared to vector-only-transfected cells; however, no significant change was found in the Ca2+ transient in h-suPC2 cells compared with hPC2-transfected cells. This result additionally suggests no additive effect of h-suPC2 on the InsP3R-dependent Ca2+ release. Overall, these data are consistent with the single-channel results, as we did not observe a shift in the Ca2+-dependent response.
Modified hPC2 channels engineered to contain two Ca2+-binding EF hand motifs display enhanced Ca2+ signaling compared to hPC2
Because our single-channel data suggested that a Ca2+ sensitivity shift in PC2 channel activity on addition of a second functional EF hand motif, we then examined the effects of these specific variants on Ca2+ signaling in live cells. We used an N-terminal anti-PC2 antibody to ensure that both the hPC2-DANA and hPC2-GANA are expressed in LLC-PK1 cells and have similar levels of expression (Fig. 7A). Strikingly, on addition of vasopressin, the amplitude of Ca2+-response transients from hPC2-GANA was significantly higher than hPC2, (Fig. 7B, C). The duration of the response, measured as the time taken to return to 25% of the maximal response for both hPC2-GANA and hPC2-DANA, was significantly longer compared to vector control, and that of hPC2-DANA but not hPC2-GANA was significantly longer than hPC2 (Fig. 7D). The duration of the response, measured as the time taken to return to 25% of the maximal response for both hPC2-GANA and hPC2-DANA, was significantly longer compared to vector control (Fig. 7D). The duration of the response of hPC2-DANA was significantly longer than that of hPC2. Similar results were also observed when using Ca2+-free medium conditions, indicating that the Ca2+ responses were due to Ca2+ release from intracellular stores (data not shown). These results suggest that inclusion of a second Ca2+ binding site in the EF hand domain enhances Ca2+ signaling and that the enhanced Ca2+ transients observed can be directly attributed to modifications to the Ca2+-binding properties of the PC2-EF hand domain. As only a modest change was found in the amplitude, it is likely that the effect of adding the second EF hand has a larger effect on the sustained Ca2+-induced Ca2+ response, via PC2 and/or InsP3R. These results are in agreement with our single-channel studies and provide additional evidence supporting the hypothesis that PC2-EF directly confers Ca2+ dependence to PC2 channels.
Location of the Ca2+ binding site in the hPC2-EF hand domain is not interchangeable: modified hPC2 channels with a Ca2+ binding site only in the first EF hand motif are not functional
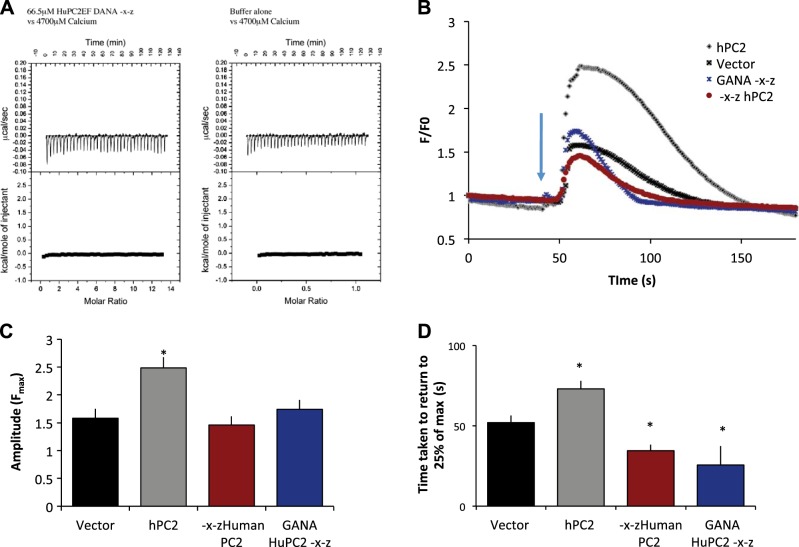
Our NMR data show that the suPC2 construct, as well as modified hPC2 with two sites, can bind two Ca2+ ions (Fig. 4B). In many EF hand proteins, these two sites are not equivalent but act in concert. To address this, we asked whether a single Ca2+ binding site in the first EF hand motif alone is sufficient for PC2 channel function, without the presence of the second (native) Ca2+ binding site. In other words, are the two EF hand motifs in hPC2 interchangeable? To test whether the location of the Ca2+ binding site in PC2 channels is interchangeable, we mutated the second, native Ca2+ binding site in both hPC2-GANA-EF and hPC2-DANA-EF to make it unable to bind Ca2+. This modified PC2 variant was named hPC2 GANA–X-Z and contains only one (non-native) Ca2+ binding site, located in the first EF hand motif between α-helices 1 and 2. Our previous NMR data indicate that mutation of the first site from suPC2 still enabled Ca2+ binding, as measured under saturating (20 mM) Ca2+conditions (30), suggesting that the first site is not necessary for maintaining the overall EF hand domain fold. However, to our surprise, we found that introducing the second mutation abolished all binding of Ca2+, as measured by ITC for both the hPC2 GANA-X-Z and the hPC2 DANA-X-Z (see Fig. 8A for DANA-X-Z). We have previously determined that hPC2-EF binds Ca2+, both under the exact same conditions (30), and without a competing chelator. To confirm these results, we monitored the function of these versions of PC2 by recording Ca2+ transients. We have shown previously that hPC2-EF-X-Z with no functional Ca2+ binding sites does not bind Ca2+ (9) and that hPC2-X-Z channels are not functional (23). In cell-based experiments, we found that the amplitude of Ca2+ transients in cells expressing hPC2-GANA-X-Z were not significantly different than vector-only controls or to the hPC2-X-Z construct previously studied (10). However, Ca2+ transients were significantly less than hPC2 (Fig. 8B), and the transients were of shorter duration than both the vector control and hPC2 (Fig. 8C). The difference in duration between hPC2–X-Z and huPC2-GANA-X-Z was not significant. Taken together, our ITC results suggest that Ca2+ binding to the first EF hand motif can be achieved only if the second EF hand is intact and binds Ca2+, which suggests cooperativity or sequential binding between the two sites. This result is further confirmed by the Ca2+ transient measurements, which suggests that the hPC2-X-Z and the hPC2-GANA-X-Z have similar functional characteristics. Finally, these results provide a functional explanation for the evolutionary conservation of the second Ca2+ binding site over the first site.
Figure 8.
The first EF hand cannot bind Ca2+ in the absence of the second EF hand. A) Titration of 66.5 μM huPC2-EF DANA-X-Z or buffer alone (25 mM TRIS, 150 mM KCl, and 1 mM TCEP, pH 7.4) against 4.7 mM Ca2+. B) Ca2+ transients elicited after the addition of 100 nM vasopressin (blue arrow). LLC-PK1 cells were transfected with empty vector (gray) PC2 (black), hPC2-X-Z (red), or hPC2-GANA-X-Z (blue). Traces represent mean data, n = 8–22, where n represents number of cells from ≥2 independent experiments. C) Average amplitude responses. D) Average time taken for the transient to return to 25% of the maximum amplitude; n = 8–22, where n represents number of cells from ≥2 independent experiments. *P < 0.05 vs. vector.
DISCUSSION
Ca2+ is an important signaling ion, and intracellular Ca2+ concentrations within human cells are tightly regulated. One way for Ca2+ signals to be transmitted is through binding to EF hand-containing proteins. In most instances, EF hands consist of a pair of Ca2+-binding motifs, yet hPC2 contains only one functional Ca2+ binding site. Here, we demonstrate by NMR spectroscopy that PC2 from an invertebrate PC2 ortholog, sea urchin, has two Ca2+-binding EF hands. We used results from comparative structural studies of hPC2-EF (1 Ca2+ site) and suPC2-EF (2 Ca2+ binding sites) to guide functional investigations into how Ca2+ binding to the EF hand in hPC2 modulates the Ca2+ dependence of PC2 channel activity. To demonstrate directly that the PC2-EF hand is truly a Ca2+ sensor, changing its Ca2+-binding properties must alter the Ca2+ dependence of PC2 channels. We show that by reintroducing the second Ca2+ binding site into hPC2, we can alter the Ca2+-binding and functional response of hPC2. We also show that the two sites in PC2-EF are not equivalent in mediating Ca2+ signaling in mammalian cells, with the number and location of the EF hands dictating channel function. Taken together, these results strongly suggest that the EF hand domain in PC2 is directly responsible for sensing cytoplasmic levels of Ca2+, and translating this signal into PC2 channel activity.
In several ion channel proteins, including the TRP channels, the binding of Ca2+ to an EF hand motif is believed to mediate channel gating. This is achieved through Ca2+ dependent conformational changes (10). Unlike the more “traditional” EF hand motifs associated with Ca2+ buffering proteins, the EF hand motifs associated with ion channels are somewhat unusual. For example, in NaV1.5, the canonical 12 residues in the EF hand are shifted, and in the absence of an IQ motif (which binds calmodulin), form a relatively weak Ca2+ binding site (16). In hPC2, the first EF hand is missing critical residues required to coordinate Ca2+, although the helix-loop-helix structure of this nonfunctional EF hand motif still provides stability to the second Ca2+-binding EF hand motif (22). We were intrigued that, while the basic helix-loop-helix in the first EF hand of vertebrate PC2 orthologs is retained, the loop appears to have lost the ability to bind Ca2+. Here, we show that modifications to the Ca2+-binding properties of the PC2-EF hand domain alter Ca2+ signaling. Specifically, addition of a second Ca2+ binding site to the PC2-EF hand domain shifts the Ca2+ dependence and Ca2+ gating properties of hPC2 channels. Indeed, the sensitivity of these modified PC2 channels to the concentration of free Ca2+ is enhanced, as indicated in our bilayer studies, strongly suggesting that the EF hand domain has sensitivity in the physiological range of Ca2+ and plays a direct role in Ca2+-dependent PC2 channel regulation. Moreover, we have observed an enhancement of Ca2+ signaling responses, indicating that PC2-EF acts as a Ca2+ sensing functional domain rather than as a Ca2+ buffering protein.
If changes in intracellular Ca2+ levels regulate PC2 channel openings and closings, as would be predicted by the bell-shaped Ca2+ response curve of hPC2, and if the EF hand domain is involved in sensing those changes, the presence of an EF hand with altered Ca2+ affinity would be expected to alter the Ca2+ signaling properties and sensitivity of PC2 channels, as we have observed here. Our results indicate that the two EF hand motifs are not interchangeable and are not equivalent in their ability to mediate Ca2+ signaling in mammalian cells. In addition, our results suggest that regardless of introduction of a functional Ca2+ binding site at site 1, it cannot act to bind Ca2+ in the absence of Ca2+ binding at site 2. These results reinforce the idea of cooperativity, or sequential binding between the two motifs. Nonmutually exclusive Ca2+ binding is present in many other proteins with canonical EF hands. Moreover, these results may also suggest that a conformational change occurs when Ca2+ binds to the second EF hand in hPC2, and this binding is required before Ca2+ can bind to the reintroduced first site in hPC2. This interpretation of our results are consistent with our previous findings, whereby binding of Ca2+ to the C-terminus tail of hPC2 resulted in a change of the oligomerization state of PC2 (23).
Mammalian PC2 acts as a Ca2+ dependent Ca2+ channel, where it responds by opening and closing within the physiological range of ∼100 nM to 10 μM Ca2+. In contrast, suPC2-EF has two binding sites that appear to be saturated in this range of Ca2+. Although we have not determined the absolute affinity of suPC2-EF hands for Ca2+, our NMR experiments carried out in nominal Ca2+, which is estimated to be ∼10 μM, demonstrate that only the second EF hand in suPC2-EF was fully bound by Ca2+, whereas the addition of 200 μM BAPTA successfully chelated both Ca2+ binding sites. This observation indicates that the two EF hands in suPC2 have differing affinities for Ca2+ and that Ca2+ constitutively binds to the first site under physiological cytoplasmic conditions. Our NMR and functional data suggests that the second site has a Kd in the higher micromolar range. These data are consistent with our ITC measurements of suPC2-EF, where a mutation that prevents Ca2+ binding to the first site resulted in a measured Ca2+-binding Kd of 1.95 μM (30). It is possible that hPC2 may have lost the second functional site in the EF hand in order to better respond to local cytoplasmic Ca2+ levels present in vertebrate cells (i.e., in the high nanomolar-to-low micromolar range). In the case of hPC2, cooperativity may also arise from the interactions established by the C-terminal coiled-coil, which has already been shown to oligomerize (8, 9). Such an interaction may enable EF hand domains from neighboring PC2 molecules to interact. Indeed, our previous studies have demonstrated that the binding of Ca2+ causes the C-terminal tail of PC2 to move from an extended oligomer state to a compact dimer (23).
In addition to direct effects on the EF hand, it is possible that the mutations introduced to hPC2 also change the interaction of PC2 with other Ca2+ release channels, namely the InsP3R and RyR, which interact with PC2, or PC1 (10, 33–35). The RyR is functionally responsive to the C terminus of PC2 and can bind the N terminus of PC2 without overt functional effect (34). However, the interaction region for the InsP3R has been shown to be the acidic linker that lies downstream of the EF hand, which suggests that the EF hand does not have a direct effect but rather has an indirect effect on PC2 binding to protein partners (10). In addition, our single-channel data suggest effects of Ca2+ independent of the effects on InsP3R or RyR. In contrast, the dominant-negative effects observed for the PC2-X-Z and the PC2-GANA-X-Z variants suggests that the position where the Ca2+ ion binds within the EF hand is a critical factor for both PC2 activity and its effects on amplifying intracellular Ca2+ release, and/or on its interaction with the InsP3R or RyR. Our results with the PC2-GANA-X-Z construct are broadly consistent with previous findings, where overexpression of the C terminus of PC2 significantly reduced the amplitude of InsP3-mediated Ca2+ responses but also prolonged the duration of transients (35).
In summary, our studies reveal that modifications to the EF hand domain designed to enhance Ca2+ binding alter the Ca2+ sensitivity of PC2 channels. Thus, these studies provide the first direct evidence that the EF hand is an important Ca2+ sensor, which functions in the gating mechanisms involved in the activity of PC2 ion channels. Study of PC2 using the approach described gives us insight into how PC2 and other Ca2+-dependent channels are regulated, which may help in the understanding Ca2+ -dependent channel gating and, more specifically, the Ca2+-dependent functions of PC2. Insight into the control of the PC2 channel may lead to the design of agents, for example, peptides that can target and modulate the activity of PC2. Such agents may be useful therapies in the treatment of ADPKD, where the activity of PC2 is compromised.
Supplementary Material
Acknowledgments
The authors thank Victor Vacquier (Scripps Institution of Oceanography, University of California–San Diego, La Jolla, CA, USA) for providing the sea urchin cDNA library. The authors thank Yifei Yang for helpful discussions. The authors thank Yiqiang Cai and Stephan Somlo (Yale University) for providing the PC2 construct and PC2 antibodies. Salim Acimi assisted with early Ca2+ imaging experiments. E.T.P. and A.C. thank the Ministry of Education and Science, Republic of Serbia, for support (projects 173014 and 172021) during preparation of this article.
The SEC-LS/UV/RI instrumentation was supported by U.S. National Institutes of Health (NIH) award 1S10RR023748-01 (Keck Facility, Yale University). This work was funded by NIH grants P30 DK090744I, R01 DK61747, and R01 DK087844 (B.E.E. and M.E.H), and by American Heart Association postdoctoral fellowship R10682 (I.Y.K).
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- 2D
- 2-dimensional
- 3D
- 3-dimensional
- ADPKD
- autosomal dominant polycystic kidney disease
- ER
- endoplasmic reticulum
- hPC2
- human polycystin 2
- h-suPC2
- human sea urchin polycystin 2
- HSQC
- heteronuclear single quantum coherence
- IPTG
- isopropyl β-d-1-thiogalactopyranoside
- ITC
- isothermal titration calorimetry
- NOE
- nuclear Overhauser enhancement spectroscopy
- NOESY
- nuclear Overhauser enhancement spectroscopy
- PC1
- polycystin 1
- PC2
- polycystin 2
- SEC-MALLS
- size -exclusion column multiple-angle laser light scattering
- suPC2
- sea urchin polycystin 2
- TCEP
- tris-(2-carboxyethyl) phosphine:DCl
- TOCSY
- total correlation spectroscopy
- TRP
- transient receptor potential
- WT
- wild type
REFERENCES
- 1. Yoshiba S., Shiratori H., Kuo I. Y., Kawasumi A., Shinohara K., Nonaka S., Asai Y., Sasaki G., Belo J. A., Sasaki H., Nakai J., Dworniczak B., Ehrlich B. E., Pennekamp P., Hamada H. (2012) Cilia at the node of mouse embryos sense fluid flow for left-right determination via Pkd2. Science 338, 226–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nauli S. M., Alenghat F. J., Luo Y., Williams E., Vassilev P., Li X., Elia A. E., Lu W., Brown E. M., Quinn S. J., Ingber D. E., Zhou J. (2003) Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat. Genet. 33, 129–137 [DOI] [PubMed] [Google Scholar]
- 3. Sharif-Naeini R., Folgering J. H., Bichet D., Duprat F., Lauritzen I., Arhatte M., Jodar M., Dedman A., Chatelain F. C., Schulte U., Retailleau K., Loufrani L., Patel A., Sachs F., Delmas P., Peters D. J., Honore E. (2009) Polycystin-1 and -2 dosage regulates pressure sensing. Cell 139, 587–596 [DOI] [PubMed] [Google Scholar]
- 4. Koulen P., Cai Y., Geng L., Maeda Y., Nishimura S., Witzgall R., Ehrlich B. E., Somlo S. (2002) Polycystin-2 is an intracellular calcium release channel. Nat. Cell. Biol. 4, 191–197 [DOI] [PubMed] [Google Scholar]
- 5. Berridge M. J., Bootman M. D., Roderick H. L. (2003) Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 4, 517–529 [DOI] [PubMed] [Google Scholar]
- 6. Cai Y., Anyatonwu G., Okuhara D., Lee K. B., Yu Z., Onoe T., Mei C. L., Qian Q., Geng L., Wiztgall R., Ehrlich B. E., Somlo S. (2004) Calcium dependence of polycystin-2 channel activity is modulated by phosphorylation at Ser812. J. Biol. Chem. 279, 19987–19995 [DOI] [PubMed] [Google Scholar]
- 7. Kuo I. Y., Ehrlich B. E. (2012) Ion channels in renal disease. Chem. Rev. 112, 6353–6372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yu Y., Ulbrich M. H., Li M. H., Buraei Z., Chen X. Z., Ong A. C., Tong L., Isacoff E. Y., Yang J. (2009) Structural and molecular basis of the assembly of the TRPP2/PKD1 complex. Proc. Natl. Acad. Sci. U. S. A. 106, 11558–11563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Giamarchi A., Feng S., Rodat-Despoix L., Xu Y., Bubenshchikova E., Newby L. J., Hao J., Gaudioso C., Crest M., Lupas A. N., Honore E., Williamson M. P., Obara T., Ong A. C., Delmas P. (2010) A polycystin-2 (TRPP2) dimerization domain essential for the function of heteromeric polycystin complexes. EMBO J. 29, 1176–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sammels E., Devogelaere B., Mekahli D., Bultynck G., Missiaen L., Parys J. B., Cai Y., Somlo S., De Smedt H. (2010) Polycystin-2 activation by inositol 1,4,5-trisphosphate-induced Ca2+ release requires its direct association with the inositol 1,4,5-trisphosphate receptor in a signaling microdomain. J. Biol. Chem. 285, 18794–18805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Celic A., Petri E. T., Demeler B., Ehrlich B. E., Boggon T. J. (2008) Domain mapping of the polycystin-2 C-terminal tail using de novo molecular modeling and biophysical analysis. J. Biol. Chem. 283, 28305–28312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hateboer N., Veldhuisen B., Peters D., Breuning M. H., San-Millan J. L., Bogdanova N., Coto E., van Dijk M. A., Afzal A. R., Jeffery S., Saggar-Malik A. K., Torra R., Dimitrakov D., Martinez I., de Castro S. S., Krawczak M., Ravine D. (2000) Location of mutations within the PKD2 gene influences clinical outcome. Kidney Int. 57, 1444–1451 [DOI] [PubMed] [Google Scholar]
- 13. Harris P. C., Torres V. E. (2009) Polycystic kidney disease. Annu. Rev. Med. 60, 321–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schulze C., Sticht H., Meyerhoff P., Dietrich P. (2011) Differential contribution of EF-hands to the Ca(2)(+)-dependent activation in the plant two-pore channel TPC1. Plant. J. 68, 424–432 [DOI] [PubMed] [Google Scholar]
- 15. Brunet S., Scheuer T., Klevit R., Catterall W. A. (2005) Modulation of CaV1.2 channels by Mg2+ acting at an EF-hand motif in the COOH-terminal domain. J. Gen. Physiol. 126, 311–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chagot B., Potet F., Balser J. R., Chazin W. J. (2009) Solution NMR structure of the C-terminal EF-hand domain of human cardiac sodium channel NaV1.5. J. Biol. Chem. 284, 6436–6445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Miloushev V. Z., Levine J. A., Arbing M. A., Hunt J. F., Pitt G. S., Palmer A. G., 3rd (2009) Solution structure of the NaV1.2 C-terminal EF-hand domain. J. Biol. Chem. 284, 6446–6454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Capozzi F., Casadei F., Luchinat C. (2006) EF-hand protein dynamics and evolution of calcium signal transduction: an NMR view. J. Biol. Inorg. Chem. 11, 949–962 [DOI] [PubMed] [Google Scholar]
- 19. Gifford J. L., Walsh M. P., Vogel H. J. (2007) Structures and metal-ion-binding properties of the Ca2+-binding helix-loop-helix EF-hand motifs. Biochem. J. 405, 199–221 [DOI] [PubMed] [Google Scholar]
- 20. Chazin W. J. (2011) Relating form and function of EF-hand calcium binding proteins. Acc. Chem. Res. 44, 171–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grabarek Z. (2006) Structural basis for diversity of the EF-hand calcium-binding proteins. J. Mol. Biol. 359, 509–525 [DOI] [PubMed] [Google Scholar]
- 22. Petri E. T., Celic A., Kennedy S. D., Ehrlich B. E., Boggon T. J., Hodsdon M. E. (2010) Structure of the EF-hand domain of polycystin-2 suggests a mechanism for Ca2+-dependent regulation of polycystin-2 channel activity. Proc. Natl. Acad. Sci. U. S. A. 107, 9176–9181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ćelić A. S., Petri E. T., Benbow J., Hodsdon M. E., Ehrlich B. E., Boggon T. J. (2012) Calcium-induced conformational changes in the C-terminal tail of polycystin-2 are necessary for channel gating. J. Biol. Chem. 287, 17232–17240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Neill A. T., Moy G. W., Vacquier V. D. (2004) Polycystin-2 associates with the polycystin-1 homolog, suREJ3, and localizes to the acrosomal region of sea urchin spermatozoa. Mol. Reprod. Dev. 67, 472–477 [DOI] [PubMed] [Google Scholar]
- 25. Palmer A. G., 3rd. (1997) Probing molecular motion by NMR. Curr. Opin. Struct. Biol. 7, 732–737 [DOI] [PubMed] [Google Scholar]
- 26. Pettersen E. F., Goddard T. D., Huang C. C., Couch G. S., Greenblatt D. M., Meng E. C., Ferrin T. E. (2004) UCSF Chimera–a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 [DOI] [PubMed] [Google Scholar]
- 27. Bhattacharya A., Tejero R., Montelione G. T. (2007) Evaluating protein structures determined by structural genomics consortia. Proteins 66, 778–795 [DOI] [PubMed] [Google Scholar]
- 28. Zweckstetter M., Bax A. (2000) Prediction of sterically induced alignment in a dilute liquid crystalline phase: Aid to protein structure determination by NMR. J. Am. Chem. Soc. 122, 3791–3792 [Google Scholar]
- 29. Holm L., Rosenstrom P. (2010) Dali server: conservation mapping in 3D. Nucleic Acids Res. 38, W545–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Keeler C., Poon G., Kuo I. Y., Ehrlich B. E., Hodsdon M. E. (2013) An explicit formulation approach for the analysis of calcium binding to EF-hand proteins using isothermal titration calorimetry. Biophys. J. 105, 2843–2853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Biekofsky R. R., Martin S. R., Browne J. P., Bayley P. M., Feeney J. (1998) Ca2+ coordination to backbone carbonyl oxygen atoms in calmodulin and other EF-hand proteins: 15N chemical shifts as probes for monitoring individual-site Ca2+ coordination. Biochemistry (Mosc.) 37, 7617–7629 [DOI] [PubMed] [Google Scholar]
- 32. Herrmann T., Guntert P., Wuthrich K. (2002) Protein NMR structure determination with automated NOE assignment using the new software CANDID and the torsion angle dynamics algorithm DYANA. J. Mol. Biol. 319, 209–227 [DOI] [PubMed] [Google Scholar]
- 33. Mekahli D., Sammels E., Luyten T., Welkenhuyzen K., van den Heuvel L. P., Levtchenko E. N., Gijsbers R., Bultynck G., Parys J. B., De Smedt H., Missiaen L. (2012) Polycystin-1 and polycystin-2 are both required to amplify inositol-trisphosphate-induced Ca2+ release. Cell Calcium 51, 452–458 [DOI] [PubMed] [Google Scholar]
- 34. Anyatonwu G. I., Estrada M., Tian X., Somlo S., Ehrlich B. E. (2007) Regulation of ryanodine receptor-dependent calcium signaling by polycystin-2. Proc. Natl. Acad. Sci. U. S. A. 104, 6454–6459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li Y., Wright J. M., Qian F., Germino G. G., Guggino W. B. (2005) Polycystin 2 interacts with type I inositol 1,4,5-trisphosphate receptor to modulate intracellular Ca2+ signaling. J. Biol. Chem. 280, 41298–41306 [DOI] [PubMed] [Google Scholar]
- 36. Dereeper A., Audic S., Claverie J. M., Blanc G. (2010) BLAST-EXPLORER helps you building datasets for phylogenetic analysis. BMC 10, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dereeper A., Guignon V., Blanc G., Audic S., Buffet S., Chevenet F., Dufayard J. F., Guindon S., Lefort V., Lescot M., Claverie J. M., Gascuel O. (2008) Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 36, W465–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.