Abstract
Secretion of proteins and neurotransmitters from large dense core vesicles (LDCVs) is a highly regulated process. Adrenal LDCV formation involves the granin proteins chromogranin A (CgA) and chromogranin B (CgB); CgA- and CgB-derived peptides regulate catecholamine levels and blood pressure. We investigated function of the granin VGF (nonacronymic) in LDCV formation and the regulation of catecholamine levels and blood pressure. Expression of exogenous VGF in nonendocrine NIH 3T3 fibroblasts resulted in the formation of LDCV-like structures and depolarization-induced VGF secretion. Analysis of germline VGF-knockout mouse adrenal medulla revealed decreased LDCV size in noradrenergic chromaffin cells, increased adrenal norepinephrine and epinephrine content and circulating plasma epinephrine, and decreased adrenal CgB. These neurochemical changes in VGF-knockout mice were associated with hypertension. Germline knock-in of human VGF1–615 into the mouse Vgf locus rescued the hypertensive knockout phenotype, while knock-in of a truncated human VGF1–524 that lacks several C-terminal peptides, including TLQP-21, resulted in a small but significant increase in systolic blood pressure compared to hVGF1–615 mice. Finally, acute and chronic administration of the VGF-derived peptide TLQP-21 to rodents decreased blood pressure. Our studies establish a role for VGF in adrenal LDCV formation and the regulation of catecholamine levels and blood pressure.—Fargali, S., Garcia, A. L., Sadahiro, M., Jiang, C., Janssen, W. G., Lin, W.-J., Cogliani, V., Elste, A., Mortillo, S., Cero, C., Veitenheimer, B., Graiani, G., Pasinetti, G. M., Mahata, S. K., Osborn, J. W., Huntley, G. W., Phillips, G. R., Benson, D. L., Bartolomucci, A., Salton, S. R. The granin VGF promotes genesis of secretory vesicles, and regulates circulating catecholamine levels and blood pressure.
Keywords: adrenal, chromaffin granule, CG, large dense core vesicle, LDCV, norepinephrine
Chromogranin and secretogranin proteins are synthesized in neuroendocrine and neuronal cells, where they colocalize with peptide hormones, neurotrophins, and neurotransmitters within the matrix of the chromaffin granule (CG) in the adrenal medulla or large dense core vesicle (LDCV) in endocrine glands and neurons (1, 2). These proteins are rich in acidic residues and condense to form aggregates in the acidic and calcium ion rich milieu of the trans-Golgi-network and subsequent secretory granule compartments (1–3), trapping neuropeptides and hormones within the nascent LDCV core, thereby segregating them from constitutively secreted proteins. Among the chromogranin proteins, chromogranin A (CgA), chromogranin B (CgB), and secretogranin II (SgII) contribute to the formation of normal dense core granules (DCGs) and are expressed abundantly in adrenal medullary chromaffin cell catecholaminergic LDCVs (4–9). Both CgA and CgB play a significant role in cardiac function and blood pressure (BP) regulation (10, 11).
VGF (nonacronymic), a member of the extended granin family (2, 12), is stored in secretory granules and is expressed widely in neuronal, neuroendocrine, and endocrine cells (13, 14). The mature VGF protein contains 10 highly conserved dibasic sites for proteolytic processing by proprotein convertases (14). Similar to other granins, processing of VGF generates a number of bioactive peptides (13, 15), which exert effects on energy and water balance, memory, and depressive behavior (16–25). Like CgA and CgB, VGF aggregates in the calcium ion-rich and low-pH conditions that are found in secretory vesicles (26, 27), and VGF along with its processed peptides are released from the regulated secretory pathway (26). Based on its tissue, cellular, and subcellular distribution, VGF is therefore well positioned to modulate the regulated secretion of hormones and growth factors, potentially insulin (28) and brain-derived neurotrophic factor (BDNF; ref. 22).
Many studies of CG/LDCV biogenesis have been carried out in adrenal medullary chromaffin cells that store and release bioactive molecules, including catecholamines, neuropeptides, and granins. These cells serve as an important cog in the autonomic control of the circulatory and cardiovascular systems. VGF was originally cloned from PC12 cells, an adrenal medullary pheochromocytoma-derived cell line (29), as a nerve growth factor (NGF)-inducible gene product (30–32). Western blot analysis indicated that VGF was expressed in rat adrenal medulla (13), and recent immunohistochemical studies demonstrated widespread expression of VGF in bovine, swine, and rat adrenal medulla, with different distributions of VGF-derived peptides noted in epinephrine (adrenalin) and norepinephrine (noradrenalin) cells (33). Given these findings, the well-defined cellular makeup of the adrenal medulla, and the characteristic appearances of epinephrine- and norepinephrine-containing CGs/LDCVs in mouse chromaffin cells (34), we investigated whether VGF plays a role in regulating the structure and/or content of LDCVs. Our studies indicate that VGF is granulogenic, and that germline VGF-knockout mice have abnormal chromaffin cell CGs/LDCVs and increased adrenal catecholamine content and are hypertensive. Germline knock-in of full-length human VGF1–615 coding sequence into the mouse Vgf locus normalizes BP, while knock-in of a truncated human VGF1–524 that lacks several VGF-derived C-terminal peptides, including TLQP-21, results in a small but significant increase in systolic BP (SBP) compared to hVGF1–615-knock-in mice. Finally, infusion of TLQP-21 lowers BP and normalizes obesity-associated hypertension. Our studies suggest that VGF and/or specific VGF-derived peptides play a nonredundant role in the regulated secretory pathway, and in the regulation of catecholamine levels and BP.
MATERIALS AND METHODS
Mouse strains
The VGF-knockout mouse line was generated as described previously (ref. 35; Regeneron Pharmaceuticals, Inc., Tarrytown, NY, USA) using F1H4 ES cells (a 129B6/F1-derived cell line) and a bacterial artificial chromosome (BAC)-based targeting vector, deleting the entire Vgf coding sequence and inserting an in-frame lacZ reporter gene and neomycin (neo)-selection cassette. Male chimeras were mated with C57BL/6J females to produce F1 breeders, and experiments were performed on N2F1 mice (>83% C57BL/6J background). This line of VGF-knockout mice (36) is extremely similar in phenotype to an independent line of VGF-knockout mice that was extensively characterized on mixed 129Sv/C57BL/6J and homogeneous C57BL/6J backgrounds (16, 37, 38).
Humanized VGF-knock-in mouse lines were generated by modification of a previously described targeting construct, utilizing mouse Vgf genomic sequences (16), and human BAC (ImaGenes GmbH clone RZPDB737B0725D; B-Bridge International, Mountain View, CA, USA) and VGF genomic clones (gift of A. Levi, University of Rome, Rome, Italy; ref. 39). Human VGF coding sequence was contained on a single exon flanked by loxP sites, and a selectable phosphoglycerate kinase (PGK)-neo cassette [flanked by flippase recombinase target (FRT) and loxP sites], derived from plasmid PGKneoF2L2DTA (Dr. P. Soriano, Icahn School of Medicine at Mount Sinai; Addgene, Cambridge, MA, USA), was inserted 3′ to the Vgf polyadenylation signal (see Fig. 6). A 2.2-kb Sfi 1-SphI fragment that contained human VGF coding, 5′-UTR, and 3′-UTR sequences, replaced the 2.3-kb KpnI-XbaI fragment that included mouse Vgf coding, 5′-UTR, and 3′-UTR sequences. Inserted human sequences encoded either full-length human VGF (aa 1–615), or a truncated human VGF protein (aa 1–524), the latter created by introducing a single-nucleotide polymorphism (SNP; rs35400704), by PCR, which created a stop codon. Human VGF1–615 (hVGF) and VGF1–524 (SNP) targeting constructs were electroporated into 129Sv/J-derived R1 ES cells by the Mouse Genetics and Gene Targeting Core Facility (Icahn School of Medicine at Mount Sinai) as described previously (16). G418-resistant clones were selected, and 3 correctly targeted clones for each construct were identified, expanded, and injected into C57BL/6 blastocysts to generate chimeras. Germline transmission was obtained in 2 founders, each derived from an independent targeted clone, for each line (hVGF and SNP). Male chimeras were mated with C57BL/6J females to produce F1 breeders and experiments were performed on N2F1 mice. Mice were provided food and water ad libitum. All animal studies were conducted in accordance with the U.S. National Institutes of Health Guidelines for the Care and Use of Experimental Animals, using protocols approved by the Institutional Animal Care and Use Committee of the Icahn School of Medicine at Mount Sinai.
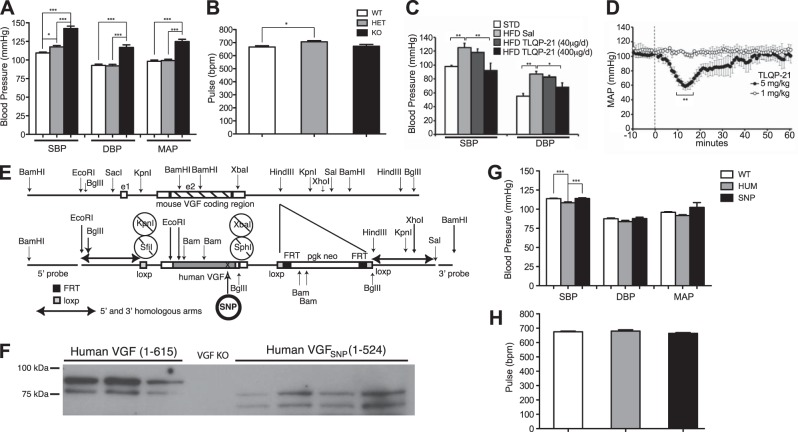
Figure 6.
Germline VGF ablation increases BP that is rescued by germline knock-in of human VGF1–615 into the mouse Vgf locus, consistent with reduced BP in VGF peptide TLQP-21-treated mice and rats. A, B) BP (A) and pulse (B) were recorded utilizing tail plethysmography in awake wild-type Vgf+/+, heterozygous-knockout Vgf+/−, and homozygous-knockout Vgf−/− mice, as described in Materials and Methods. SBP, DBP, MAP, and pulse (bpm) are shown, and SBP, DBP, and MAP were significantly increased in Vgf−/− (KO) compared to Vgf+/+ (WT) and Vgf+/− (HET) mice (means±sem; n=9–12 mice/group). *P < 0.05, ***P < 0.0001; 1-way ANOVA. C) Chronic 14-d treatment of obese, hypertensive mice with TLQP-21 (400 μg/d) decreased SBP and DBP, recorded by intracarotid catheterization under anesthesia, while a lower dose of TLQP-21 (40 μg/d) was ineffective. STD, standard diet; sal, saline. *P < 0.05, **P < 0.01 vs. HFD-sal group. D) Acute intravenous infusion of TLQP-21 (5 mg/kg) rapidly and transiently decreased MAP in freely moving Sprague-Dawley rats (F(61,122)=4.8, P<0.0001; n=4) measured by telemetry, while infusion of a lower dose of TLQP-21 (1 mg/kg) was ineffective (n=2). Dashed line marks peptide infusion. **P < 0.01 vs. baseline; ANOVA with Tukey's post hoc comparison. E–H) Germline knock-in of human VGF1–615 or an SNP-encoded, truncated human VGF1–524 into the mouse Vgf locus rescued the hypertensive phenotype resulting from germline VGF ablation. E) Gene-targeting strategy used to generate hVGF and SNP lines. F) Western blotting with anti-VGF antisera was used to identify full-length human VGF1–615 and truncated human VGF1–524 in total brain lysates from homozygous hVGF and SNP mice, respectively. G, H) SBP, DBP, and MAP (G) and pulse (bpm; H) were measured by tail plethysmography in awake wild-type (WT), and homozygous hVGF1–615 (HUM) and hVGF1–524 (SNP) mice (means±sem; n=9–12 mice/group). Note that SBP in homozygous hVGF1–615 is slightly but significantly lower than homozygous hVGF1–524 mice that lack TLQP-21 (G). ***P < 0.0001; 1-way ANOVA.
BP measurement
For studies in VGF-knockout and VGF-knock-in lines, BP was measured in awake mice using noninvasive, single-animal, tail-cuff plethysmography (Hatteras Instruments, Cary, NC, USA). Mice were immobilized in a restraining chamber, and the tail was inserted through a cuff and secured in a slot with tape. Mice were acclimated to handling and BP measurement daily for 10–14 d and, prior to each BP recording session, underwent 5 preliminary cycles for which data were not recorded. The following 10 measurements of pulse rate, SBP, diastolic BP (DBP), and mean arterial pressure (MAP) were recorded; measurements were taken on 3–5 successive days, and mean values were calculated.
To analyze BP modulation by TLQP-21 peptide in obese hypertensive mice compared to controls, male CD1 mice (Charles River, Calco, Italy) were fed for 12 wk on either a standard diet (4RF21, 10.6% kcal from fat and 3.9 kcal/g; Mucedola, Milan, Italy) or a high-fat diet (HFD; modified 4RF21, 45% kcal from fat and 5.2 kcal/g; Mucedola). Control mice were treated with sterile saline, and HFD-fed mice were treated with sterile saline or TLQP-21 (40 or 400 μg/d, suspended in sterile saline) for 14 d, all delivered subcutaneously via osmotic minipump (Alzet 1002; Durect Corp., Cupertino, CA, USA; n=6/group), as described previously (25). Prefilled osmotic minipumps were sterilely implanted subcutaneously, in the dorsum of mice anesthetized with ketamine (100 mg/kg) and xylazine (5 mg/kg). Hemodynamic measurements were performed under ketamine (50 mg/kg) and xylazine (2.5 mg/kg) anesthesia, 2 wk postimplantation. To determine heart function, a 1.4-F Millar-Micro Tip catheter (Millar Instruments, Houston, TX, USA) was inserted into the right carotid artery to record systolic and diastolic arterial BP. The transducer was then advanced into the left ventricle (LV) to measure LV systolic pressure (LVP; mmHg), LV end-diastolic pressure (LVEDP; mmHg), and heart rate (HR; bpm). Values were recorded digitally and analyzed using the Power Lab ML 845/4 channels (2Biological Instruments, Besozzo-Varese, Italy). BP and HR were analyzed with a 1-way ANOVA followed by Neumans-Keuls post hoc tests.
To measure acute effects of TLQP-21 peptide on BP, male Sprague-Dawley rats (Charles River Laboratory, Wilmington, MA, USA) were implanted with a radiotelemetry transmitter (model TA11PA-C40; Data Sciences International, St. Paul, MN, USA) for continuous monitoring of MAP and HR and an intravenous (i.v.) catheter for systemic drug delivery. The transmitter catheter was implanted into the descending aorta via the femoral artery. The i.v. catheter was advanced to the abdominal vena cava via the femoral vein, tunneled through a spring that was attached to the skin between the scapulae, and attached to a swivel above the cage. Rats were conscious and freely moving in their home cages for the duration of the study. Experiments were initiated ≥2 wk after surgery. Each cage was placed on a receiver (model RPC1; DSI, St. Paul, MN, USA) that was connected to a computer via a data exchange matrix (DSI). TLQP-21 peptide infusion (1 or 5 mg/kg dissolved in 100 ml saline/1 min; n=2 and 3, respectively) was performed between 10:00 and 10:30 AM during the resting phase of the animal. MAP and HR data were collected continuously at 500 Hz for 10 min at 10-s intervals (averaged over 1 min) before (baseline) and for 60 min after the peptide or saline bolus infusion. These procedures were described previously in greater detail (40). MAP and HR were analyzed with a 1-way ANOVA followed by Neumans-Keuls post hoc tests.
Western blot analysis
Western blot analysis was performed essentially as described previously (26). Briefly, young adult mice (6–8 wk old) were anesthetized, and tissues were isolated, rinsed in ice-cold Ringers solution (125 mM NaCl, 2.5 mM KCl, 1.3 mM MgSO4, 1 mM NaH2PO4, 26.2 mM NaHCO3, 2.5 mM CaCl2, and 11 mM glucose, pH 7.3), and homogenized in lysis buffer [50 mM Tris-HCl, 150 mM NaCl, 0.1% sodium dodecyl sulfate (SDS), 0.5% deoxycholate, 1% nonidet P-40, and 0.02% sodium azide] containing protease inhibitor cocktail (Complete; Roche Diagnostics, Indianapolis, IN, USA) at 4°C. Homogenates were cleared by centrifugation, and samples containing equal protein amounts were electrophoresed on 10% SDS-polyacrylamide gel electrophoresis (PAGE) gels and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA, USA). Membranes were blocked overnight at 4°C in PBS containing 5% nonfat dried milk (Carnation; Nestlé, Vevey, Switzerland) and 5% newborn calf serum (NCS; Gemini Bioproducts, West Sacramento, CA, USA), then were incubated with primary antibodies in blocking buffer overnight at 4°C, washed at 25°C, probed with horseradish peroxidase (HRP)-conjugated secondary antibody for 60 min at 25°C, and washed, and signals were detected using chemiluminescent substrate (Pierce Fisher Thermo Scientific, Rockford IL, USA). Primary antibody dilutions were rabbit polyclonal anti-VGF (41), 1:1000; goat anti-CgA (Santa Cruz Biotechnology, Dallas, TX, USA), 1:2000; rabbit polyclonal anti-CgA (Drs. Y. Peng Loh, U.S. National Institutes of Health, Bethesda, MD, USA, and Sucheta Vaingankar, University of California, San Diego), 1:1000; rabbit polyclonal anti-CgB (Dr. Reiner Fischer-Colbrie, Innsbruck Medical University, Innsbruck, Austria), 1:2000; rabbit polyclonal anti-SgII (Dr. Reiner Fischer-Colbrie), 1:2000; rabbit polyclonal anti-SgIII (Santa Cruz Biotechnology), 1: 2000; mouse anti-green fluorescent protein (GFP; Clontech, Mountain View, CA, USA), 1:2000; and actin (MAB1501; Chemicon International, Temecula, CA, USA), 1:5000. Secondary anti-rabbit-HRP and anti-mouse-HRP (GE Health Care Biosciences, Pittsburgh, PA, USA) were diluted 1:2000. Films were scanned, digital images were analyzed by ImageJ (U.S. National Institutes of Health, Bethesda, MD, USA), and band densities were compared by ANOVA and Tukey's post hoc test.
Immunofluorescence analysis
Rat PC12 pheochromocytoma cells were cultured in RPMI 1640 containing 15% (v/v) fetal bovine serum at 37°C with 5% CO2, were replated onto collagen I-coated 18-mm glass coverslips at a density of 40,000 cells/coverslip, and were incubated for 3 d, then processed for immunofluorescence. To examine the cellular localization of VGF and CgB, PC12 cells were rinsed with ice-cold PBS, fixed with 4% paraformaldehyde for 30 min, and washed 5 min with PBS, 3 times. The cells were then blocked with 2% normal donkey serum and 2% normal goat serum (Jackson ImmunoResearch Laboratories, West Baltimore, PA, USA), 0.2% triton-X 100 in PBS for 1h. Double immunofluorescence was performed, incubating the cells with a primary rabbit polyclonal anti-VGF antibody [ref. 41; purified IgG prepared using Melon Gel Resin (Thermo Scientific, Rockford, IL, USA), as described by the manufacturer], diluted at 1:50 in the blocking buffer as described above, and a primary mouse monoclonal anti-CgB (BD Transduction Laboratories, San Jose, CA, USA) antibody, diluted 1:200. Cells were incubated overnight at 4°C in a moist chamber, washed 3 times in PBS, and incubated for 1 h at room temperature in darkness with Alexa Fluor 568 conjugated goat anti-rabbit IgG (Invitrogen, Grand Island, NY, USA) for detection of VGF (1:400) and dye 488-conjugated donkey anti-mouse IgG for detection of CgB (1:200) in PBS, followed by extensive PBS washing. The slides were mounted using mounting medium (Vectashield; Vector Laboratories, Burlingame, CA, USA) and analyzed at higher magnification (×63) using a Zeiss LSM 510 confocal microscope (Carl Zeiss, Oberkochen, Germany). Final images from 20 PC12 cells were magnified (×4 zoom), and dense core vesicle (DCV)-like particles expressing immunoreactive VGF and CgB were quantified (n=10–14 DCVs/cell). Images of 20 cells were analyzed using ImageJ to determine colocalization (means±sem). Analysis of VGF and CgB coexpression in adrenal medullary chromaffin cells was similarly performed.
Coimmunoprecipitation analysis
After reaching 80% confluence, PC12 cells were rinsed twice with ice-cold PBS and lysed in ice-cold lysis buffer (150 mM NaCl, 1% nonidet P40, 50 mM Tris, and 0.5% deoxycholate, pH 7.4). After 45 min on ice, cells were scraped off, and lysates were centrifuged at 14,000 rpm for 10 min at 4°C. Supernatants were incubated with anti-VGF (rabbit polyclonal anti-VGF; ref. 41), normal rabbit serum (negative control), normal goat serum (negative control), or goat anti-CgB (Santa Cruz Biotechnology), and protein A/G PLUS (Santa Cruz Biotechnology), overnight at 4°C with rotary agitation. The precipitates were collected by centrifugation, washed 4 times with lysis buffer, boiled in 2× sample buffer (42) for 5 min, and analyzed by SDS-PAGE and immunoblotting as described previously (36).
Electron microscopy (EM)
Mice (6–8 wk old) were anesthetized by intraperitoneal injection with 2.5% tribromoethanol in isotonic saline and were perfused with 2.5% glutaraldehyde and 5% paraformaldehyde in 0.1 M sodium phosphate buffer (pH 7.4) at 25°C. Adrenals were removed, immersed in the same fixative buffer at 4°C overnight, and then were incubated for 1 h in 1% osmium tetroxide in 0.1 M sodium phosphate buffer (pH 7.4), washed in PBS, and dehydrated by successive incubation in progressively increasing concentrations of ethanol from 50 to 100%, as described previously (43). Tissues were then incubated 3 times for 10 min in acetone, then overnight in equal parts acetone and epoxy resin. Tissues were set in epoxy resin the following day. Thin sections (90 nm) were obtained using a Diatome diamond knife (Diatome AG, Biel, Switzerland) on a Reichert-Jung ultramicrotome (Leica Microsystems, Wetzlar, Germany) and mounted on nickel grids. Sections were stained with uranyl acetate prior to EM imaging. Digital EM images were obtained at final magnifications of 10,000 and 30,000 using a Hitachi H-7000 microscope (Hitachi, Tokyo, Japan) and Megaplus ES 4.0 CCD camera (Kodak, Rochester, NY, USA) with the aid of Advanced Microscopy Techniques V542 2.0 imaging software (Advanced Microscopy Techniques Corp., Woburn, MA, USA).
Morphometric analysis
LDCV core area, cytoplasmic area, area of the volume around the core, LDCVs with the membrane around the core, and LDCV number were obtained by manual tracing and computation using digital EM images and MetaMorph 6.0 (Molecular Devices LLC, Sunnyvale, CA, USA). Image thresholding was utilized to define and select secretory granule core areas; then core perimeters were defined with the boundary function, and thresholding was removed. Nonsecretory granule areas captured by this method were identified by manual inspection and were deleted, and secretory granule areas not captured were traced manually. LDCVs quantified had electron-dense cores, continuous vesicle membrane surrounding the dense core, and an approximately circular geometry. From the various areas that were randomly sampled, norepinephrine cells represented the most abundant source of quantifiable electron-dense LDCVs, as noted previously with EM analysis of murine adrenal chromaffin cells (34). So, to obtain comparisons with the most statistical power across genotype, we focused our EM quantification on this class of LDCVs. EM micrographs (×30,000) were captured randomly from the adrenal medullas of 6- to 8-wk-old Vgf+/+ and Vgf−/− mice (4 adrenal medullas per genotype; 19 Vgf+/+ and 18 Vgf−/− EM micrographs; n=3228 Vgf+/+ LDCVs; n=2551 Vgf−/− LDCVs). Photomicrographs were obtained by systematic uniform random sampling of the ∼10 sections that were prepared from each adrenal medulla. A grid was superimposed on each section, and random images were collected at regular intervals. Images from multiple sections were obtained, and those of sufficient image contrast, restricted to NE-containing LDCVs, were selected for final image analysis. All image capture and subsequent analysis were performed blind to specimen genotype; only images containing norepinephrine LDCVs were analyzed, and the mean DCG number per square micrometer of cytoplasm was determined. Data were analyzed by unpaired, 2-tailed Student's t test, and LDCV core area distributions were determined using Prism (GraphPad, San Diego, CA, USA).
Examination of LDCV-like structures in transfected NIH 3T3 cells by correlative light and electron microscopy (CLEM)
NIH 3T3 cells were cotransfected with plasmids encoding VGF (full-length rat VGF cDNA in pcDNA3) and GFP (pEGFPN1; Clontech) in a 10:1 ratio, respectively, or with pEGFPN1 alone, using Lipofectamine (Life Technologies, Grand Island, NY, USA), and cells were plated onto gridded glass-bottom imaging dishes (MaTek Corp., Ashland, MA, USA) at a density of 20,000 cells/well. In separate experiments, NIH 3T3 cells were transfected with previously described plasmids (26) encoding either GFP or VGF1–65:GFP:VGF452–617, a VGF:GFP fusion protein that is sorted into LDCVs and the regulated secretory pathway in PC12 cells. After 72 h, transfected cells were fixed in gluteraldehyde and processed for CLEM as previously detailed (44). CLEM allows individual GFP-positive cells to be visualized by confocal microscopy and their locations identified, and after embedding and sectioning, these identified cells are then subsequently analyzed by transmission EM.
Analysis of regulated secretion in transfected NIH 3T3 cells
Non-neuroendocrine NIH 3T3 fibroblasts were transfected with pcDNA3 plasmids encoding either the full-length rat VGF1–617 protein, GFP, VGF1–65:GFP:VGF452–617, a VGF:GFP fusion protein that is sorted into LDCVs and the regulated secretory pathway in PC12 cells, or Igκ:GFP, a constitutively secreted fusion protein that contains the Igκ chain leader sequence (pSecTag2/Hygro; Life Technologies; ref. 26). To measure regulated secretion of VGF or VGF1–65:GFP:VGF452–617 from transfected 3T3 cells, sister cultures were rinsed, incubated in serum-free medium for 30 min (basal secretion), rinsed, and incubated in medium supplemented with 2 mM BaCl2 for 30 min (regulated secretion) (7). In some cases, cultures were pretreated with 1 μg/ml cycloheximide (CHX) for 2 h prior to release experiments, which were carried out as described above, but also in the presence of 1 μg/ml CHX or vehicle (0.01% ethanol) (26). Medium samples were precipitated with 0.1 volume 100% trichloroacetic acid/0.2% deoxycholic acid for 24 h at 4°C. After centrifugation, pellets were washed with acetone, dried, resuspended in 2× Laemmli buffer, and analyzed by SDS-PAGE and Western blotting (26).
Adrenal, heart, and plasma catecholamine analysis
Mice (8–10 wk old) were anesthetized with isoflurane. Blood (0.5–0.8 ml) was withdrawn by cardiac puncture of the LV using a 25-gauge needle (Becton Dickinson, Franklin Lakes, NJ, USA). Blood samples were transferred into K3EDTA 5-ml glass tubes (Becton Dickinson) and were immediately centrifuged at 14,000 rpm for 5 min at 4°C, and plasma was transferred into a fresh tube and frozen at −80°C. Adrenal, cardiac, and plasma samples were stored at −80°C, and catecholamine levels were quantified by HPLC as previously detailed (45) and are expressed as nanograms per milliliter per microgram protein.
RESULTS
Expression of exogenous VGF in nonendocrine NIH 3T3 fibroblasts stimulates granulogenesis and regulated secretion
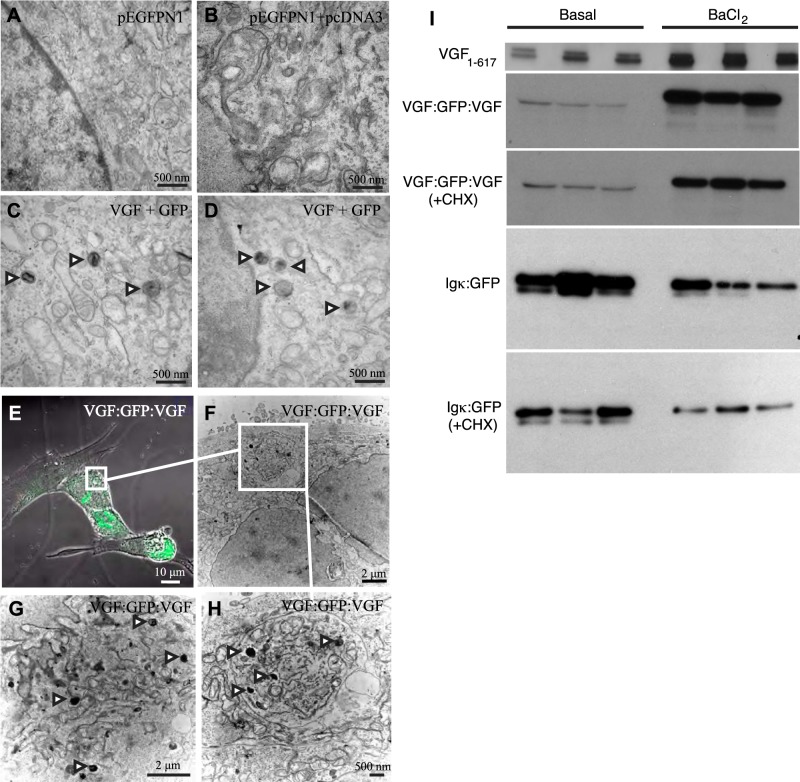
Previous studies have demonstrated that expression of exogenous granin proteins CgA, CgB, and SgII in cells that lack a regulated secretory pathway induces LDCV-like structures and regulated secretion (6, 7, 46–49). To determine whether expression of exogenous VGF stimulates the neogenesis of granule-like structures in nonendocrine cells, plasmids encoding full-length rat VGF1–617 and GFP were transfected into NIH 3T3 cells. We then utilized CLEM (44) to visualize individual GFP-positive cells by confocal microscopy, and after embedding and sectioning, to analyze these identified cells by transmission EM. Expression of cotransfected plasmids encoding full-length rat or mouse VGF1–617 and GFP (molar ratio 10:1, respectively) in 3T3 cells resulted in the formation of LDCV-like structures in GFP-positive cells but not in GFP-negative or empty vector-transfected cells (Fig. 1A–D). Moreover, transfection of 3T3 cells with a construct encoding the fusion protein VGF1–65:GFP:VGF452–617, which contains the required sorting signals for targeting into the regulated secretory pathway in PC12 cells (26), also resulted in the formation of LDCV-like structures (Fig. 1E–H), while transfection with plasmid encoding GFP did not (not shown). In addition, Western blotting of culture medium from transfected NIH 3T3 fibroblasts, collected after 30 min of basal followed by 30 min of stimulated (2mM BaCl2) release, demonstrated depolarization-induced, regulated secretion of both VGF1–617 and VGF1–65:GFP:VGF452–617 (Fig. 1I). On the other hand, secretion of Igk:GFP, previously shown to be constitutively secreted from PC12 cells (26), was not stimulated by BaCl2 in transfected 3T3 cells, and was actually significantly reduced (P<0.05; Fig. 1I). Treatment with protein synthesis inhibitors blocks constitutive protein secretion (50) but has less effect on regulated secretion, presumably a result of the larger pool of proteins stored in LDCVs in the regulated secretory pathway. Robust, regulated secretion of VGF1–65:GFP:VGF452–617 was still observed in transfected 3T3 cells that were both CHX treated and BaCl2 stimulated, while Igk-GFP secretion decreased in CHX-treated, BaCl2-stimulated cells (Fig. 1I). Thus, expression of exogenous VGF is sufficient to stimulate formation of LDCV-like structures, allowing regulated secretion of VGF, and a VGF:GFP fusion protein that contains previously defined VGF sorting signals (26), from non-neuroendocrine NIH 3T3 fibroblasts, in vitro.
Figure 1.
Expression of exogenous VGF in nonendocrine NIH 3T3 cells results in the formation of LDCV-like vesicles, visualized by CLEM, and regulated secretion of VGF. A–D) NIH 3T3 cells were cotransfected with plasmids encoding GFP and either full-length rat VGF or empty vector, and identified GFP-positive cells were processed for transmission EM as detailed in Materials and Methods. Representative transmission electron micrographs of 3T3 cells transfected with pEGFPN1 alone (A), and those cotransfected with pEGFPN1 and empty pcDNA3 vector (B), or pEGFPN1 and rat VGF1–617 expression plasmids (C, D), are shown. Arrowheads indicate LDCV-like structures. E–H) NIH 3T3 cells were similarly transfected with plasmid encoding VGF1–65:GFP:VGF452–617 (VGF:GFP:VGF), and LDCV-like structures were visualized in GFP-positive, green cells (E) by CLEM (F–H). Arrowheads indicate LDCV-like structures. I) To measure secretion, NIH 3T3 cells were transfected with expression plasmids encoding rat VGF1–617, VGF1–65:GFP:VGF452–617 (VGF:GFP:VGF), or constitutively secreted Igκ:GFP (26). After 48–72 h, cells were rinsed, incubated for 30 min in serum-free medium (basal release), rinsed, and incubated for 30 min in serum-free medim supplemented with 2 mM BaCl2 (stimulated release). In a subset of experiments, cells were pretreated for 2 h with CHX (1 μg/ml), which blocks constitutive but has less effect on regulated secretion, or with vehicle, and basal or stimulated release was assayed as described above, in the presence of CHX or vehicle. Secreted VGF, VGF:GFP:VGF, or Igκ:GFP proteins were quantified by Western blot analysis and ImageJ, as described in Materials and Methods. Normalized to basal release (1±0.2), VGF secretion was stimulated 2.8 ± 0.1-fold by BaCl2 (P=0.004, 2-tailed Student's t test). VGF:GFP:VGF secretion under basal conditions (1±0.3) was stimulated 18.6 ± 1.3-fold by BaCl2 (P<0.001, ANOVA with Tukey's post hoc comparison, n=3), while basal + CHX release (2±0.4) was stimulated 12.2 ± 0.7-fold in BaCl2 + CHX-treated cells (P<0.001, ANOVA with Tukey's post hoc comparison, n=3) (all values normalized to VGF:GFP:VGF secretion under basal conditions). Igκ:GFP secretion under basal conditions (1±0.12) was not stimulated by BaCl2 treatment but rather was significantly reduced (0.4±0.14; P<0.05, ANOVA with Tukey's post hoc comparison, n=3). Secretion of Igκ:GFP in the presence of CHX (0.5±0.07) was not stimulated by BaCl2 + CHX (0.2±0.03; nonsignificant compared to CHX). Igκ:GFP secretion in BaCl2 + CHX-treated cells was significantly reduced compared to basal secretion (P=0.0025) (all values normalized to Igκ:GFP secretion under basal conditions). Results are representative of ≥3 experiments.
Number of LDCVs per square micrometer of cytoplasm is unchanged in Vgf−/− in comparison to Vgf+/+ adrenal chromaffin cells
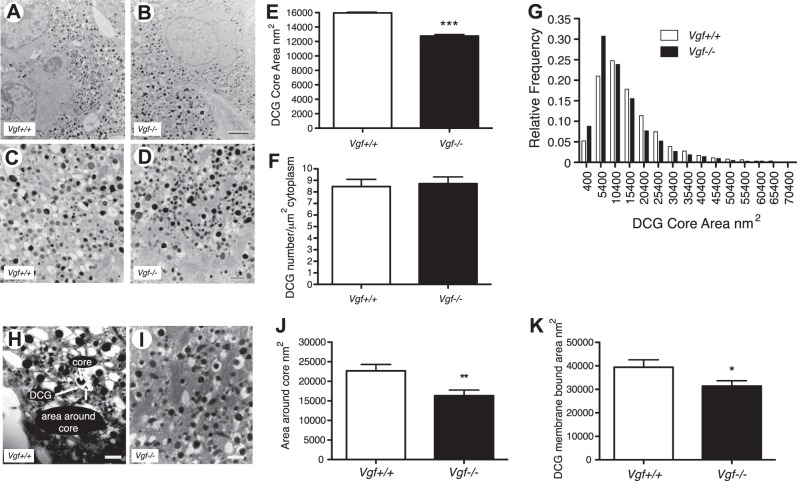
To determine whether VGF is required for LDCV biogenesis in vivo, we took advantage of the characteristic appearances of epinephrine- and norepinephrine-containing LDCVs in mouse adrenal medullary chromaffin cells (34) to investigate whether germline Vgf gene ablation is associated with alterations in the structure, number, and/or content of LDCVs. Norepinephrine cells represented the most abundant source of quantifiable electron-dense LDCVs, as noted previously (34), so we focused our EM quantification on this class of LDCVs. The mean ± sem numbers of norepinephrine-containing LDCVs per square micrometer of cytoplasm in adrenal chromaffin cells from 6- to 8-wk old Vgf+/+ and Vgf−/− mice, quantified as detailed in Materials and Methods, were not significantly different (Vgf+/+: 8.5±0.6, n=3228; Vgf−/−: 8.7 ± 0.6, n=2551; P = 0.773, 2-tailed Student's t test; Fig. 2A–F).
Figure 2.
DCG (LDCV) area but not number is decreased in adrenal medullary chromaffin cells from VGF-knockout (Vgf−/−) compared to wild-type (Vgf+/+) mice. A–D) Representative EM micrographs of Vgf+/+ (A, C) and Vgf−/− (B, D) adrenal medullary chromaffin cells, taken at ×10,000 (A, B) and at ×30,000 (C, D). Scale bars = 2 μm (A, B); 500 nm (C, D). E–G) Tissues were prepared for EM, and ×30,000 images were analyzed using MetaMorph to determine mean LDCV core area in square nanometers (E), LDCV number per square micrometer of cytoplasm (F), and the distribution of LDCVs having specified square nanometer areas (G). Values are means ± sem. LDCV core area in Vgf+/+ chromaffin cells was significantly different from that in Vgf−/− cells (n=3228 Vgf+/+ DCVs; n=2551 Vgf−/− DCVs). ***P < 0.0001; 2-tailed Student's t test. H–K) As shown (H), the area around the dense core (J) and the area of the membrane-bound LDCV (K) were both significantly decreased in Vgf−/− chromaffin cells (I). *P < 0.05, **P < 0.005; unpaired 2-tailed Student's t test.
Proportion of smaller LDCVs is increased in adrenal chromaffin cells from Vgf−/− in comparison to Vgf+/+ mice
To determine whether the size of LDCVs might be affected by VGF ablation, areas of LDCV cores were measured from EM micrographs of wild-type and VGF-knockout adrenal medullary chromaffin cells, and mean LDCV area and the distribution of granules, sorted into bins on the basis of area, were determined. Norepinephrine-containing chromaffin cells in Vgf−/− mice were found to have a mean LDCV area that was 20% less than in Vgf+/+ mice (P<0.0001; 2-tailed Student's t test; Fig. 2A–E), reflective of a 44% increase in the fraction of dense core, electron-opaque granules within the 6000- to 11,000-nm2 range, and a 30% decrease in the fraction of DCGs in the 26,000- to 36,000-nm2 range, in chromaffin cells of the Vgf−/− group compared to the Vgf+/+ group (Fig. 2G). Two-tailed Student's t test of the two distributions showed that the difference between the means was significant, with P < 0.0001. LDCVs visualized by EM are often heterogeneous in size, and in many cases, the electron-dense core of the LDCV is not surrounded by directly contiguous, tightly apposed membrane (see Fig. 2H). In comparison to LDCVs from wild-type adrenal chromaffin cells, those from Vgf−/− adrenal chromaffin cells were generally found to have reduced area surrounding the dense core (Fig. 2J). The mean area around the core of Vgf−/− LDCVs (16,344 nm2) was 28% less than that of Vgf+/+ LDCVs (22,676 nm2) (P<0.001; 2-tailed Student's t test), which correlated with reduced LDCV core area and reduced area of the membrane-bound granule (Fig. 2E, K). Thus, in the absence of VGF protein expression, the mean size of norepinephrine-containing LDCVs is reduced in Vgf−/− compared to Vgf+/+ adrenal chromaffin cells, and the relative number of smaller LDCVs is increased.
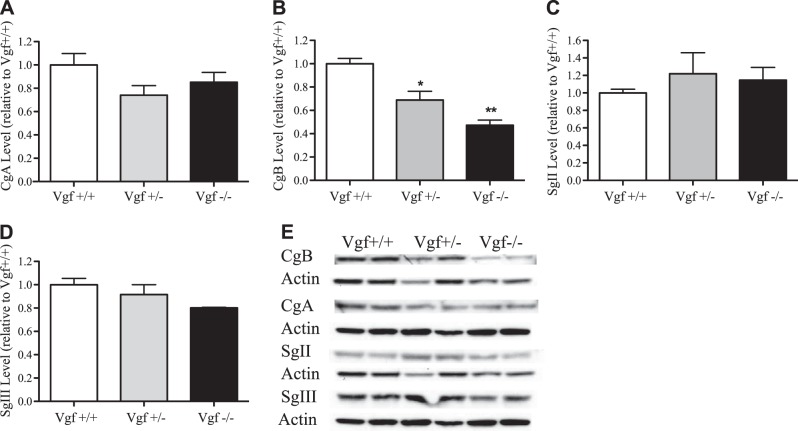
Adrenal CgB protein levels are significantly decreased in Vgf+/− and Vgf−/− mice compared to wild-type mice
Since LDCV size in adrenal medullary chromaffin cells was affected by VGF ablation, we examined whether the levels of chromogranins or secretogranins, the most abundant proteins in LDCVs, were affected. Relative levels of CgA, CgB, SgII, and SgIII in adrenal glands from age-matched, 6- to 8-wk-old VGF homozygous-knockout Vgf−/− (n=4), heterozygous-knockout Vgf+/− (n=5), and wild-type Vgf+/+ (n=5) mice, were determined by Western blot analysis. CgB levels were significantly decreased in heterozygous VGF-knockout mice to 68.7% of wild-type levels, and in homozygous VGF-knockout mice to 47.3% of wild-type animals (ANOVA; Tukey's post hoc test; P<0.05, P<0.005; Fig. 3B, E). Adrenal CgA, SgII, and SgIII levels did not significantly differ among the VGF genotypes (ANOVA; Tukey's post hoc test; Fig. 3).
Figure 3.
Adrenal CgB protein levels are reduced in Vgf+/− and Vgf−/− mice in comparison to wild-type Vgf+/+ mice. Adrenal glands were isolated from wild-type Vgf+/+, heterozygous-knockout Vgf+/−, and homozygous-knockout Vgf−/− mice and extracted in RIPA buffer, and Western blot analysis for CgA, CgB, SgII, and SgIII was carried out. Levels of CgA (A), CgB (B), SgII (C), and SgIII (D) were determined by densitometry of scanned films (E) and normalized to their respective actin loading controls, and are expressed relative to the level of the respective wild-type granin (means±sem; n=2–5 mice/group). *P < 0.05, **P < 0.005; ANOVA with Tukey's post hoc comparison.
Coimmunoprecipitation of a complex containing VGF and CgB, and coexpression of VGF and CgB in PC12 and adrenal medullary chromaffin cells
As shown in Fig. 3, VGF ablation in heterozygous and homozygous germline knockout mice was associated with a selective reduction in the levels of the LDCV protein CgB in the adrenal medulla, suggesting that lack of VGF could potentially have an effect on CgB sorting into the regulated pathway, stability, and/or processing, perhaps through a protein:protein interaction. We hypothesized that VGF and CgB are colocalized in LDCVs, potentially forming a complex, and investigated this using indirect immunofluorescence and coimmunoprecipitation analysis. As shown in Fig. 4, immunoprecipitation of proteins from PC12 cell extracts with polyclonal VGF antibody and immunoblot analysis with anti-CgB (Fig. 4A), or immunoprecipitation with anti-CgB and immunoblot analysis using anti-VGF (Fig. 4B), indicated that native CgB and VGF proteins form a complex. In contrast, immunoprecipitation with anti-CgA, anti-SgII, or anti-SgIII failed to pull down VGF (Fig. 4C).
Figure 4.
Coexpression of CgB and VGF in adrenal chromaffin and PC12 pheochromocytoma cells. A, B) Coimmunoprecipitation analysis was used to determine whether CgB and VGF associate in PC12 pheochromocytoma cells. PC12 cell lysates were immunoprecipitated with rabbit anti-VGF or normal rabbit IgG (A), and with goat anti-CgB or normal goat IgG (B), and complexes were collected and analyzed by SDS-PAGE and Western blotting with anti-CgB (A) and anti-VGF (B; asterisks indicate nonspecific bands). PC12 cell lysate (25 μg) was coanalyzed as a positive control and represented 5% of the input lysate immunoprecipitated. C) PC12 cell lysates were immunoprecipitated with anti-SgII, anti-CgA, or anti-SgIII, and analyzed by SDS-PAGE and Western blotting with anti-VGF; no coimmunoprecipitated VGF was identified (asterisks indicate nonspecific bands). D) Western blot analysis revealed VGF protein (arrows ∼85 and ∼90 kDa) in adrenal gland lysates from male and female wild-type mice and rat PC12 cells. E–J) VGF and CgB distributions in PC12 cells were analyzed by indirect immunofluorescence and confocal microscopy. Coexpression of VGF (red; E, H) and CgB (green; F, I) is shown in merge panels (yellow; G, J). K–Q) VGF (red; K, O) and CgB (green; L, P) were similarly coexpressed (yellow; M, N, Q) in sections of mouse adrenal gland (K–N) and, shown at lower magnification, rat adrenal gland (O–Q).
To determine whether VGF and CgB were coexpressed in mouse adrenal medullary chromaffin cells, we first confirmed expression of VGF in wild-type male and female mouse adrenal glands (Fig. 4D) by Western blot analysis. We then sought to determine the degree of coexpression of these proteins in LDCVs by immunofluorescence analysis. VGF (red) and CgB (green) were first visualized in rat PC12 pheochromocytoma cells by indirect immunofluorescence and confocal microscopy (Fig. 4E–J), as detailed in Materials and Methods. Merged images (Fig. 4G, J; yellow) indicate a high degree of colocalization (93±1.7%) of CgB and VGF immunoreactivity in LDCV-like structures, in PC12 cells. We determined that VGF and CgB were also highly coexpressed (92±0.02%) in mouse adrenal medullary chromaffin cells (Fig. 4K–N; yellow), and similarly distributed in rat adrenal medullary chromaffin cells shown at lower magnification (Fig. 4O–Q; yellow).
Increased adrenal catecholamine content and increased plasma epinephrine levels are associated with hypertension in VGF-knockout mice
To determine whether abnormalities in the structure of chromaffin cell LDCVs and/or adrenal chromogranin and secretogranin protein content could have potential physiological consequences in VGF-knockout mice, we measured adrenal, plasma, and cardiac catecholamine levels. Both norepinephrine and epinephrine content were significantly elevated in Vgf−/− compared to Vgf+/+ adrenal glands (Fig. 5A, B), which was associated with significantly increased plasma epinephrine levels (Fig. 5D), while cardiac muscle epinephrine and norepinephrine levels were not significantly elevated in Vgf−/− mice compared to wild-type mice (Fig. 5E–F). These alterations in adrenal catecholamine and granin content and circulating catecholamine levels in Vgf−/− mice were associated with a significant increase in SBP, DBP, and MAP, but not HR (Fig. 6A, B).
Figure 5.
Adrenal catecholamine content and plasma epinephrine levels are increased in VGF homozygous-knockout compared to wild-type mice. Norepinephrine (A, C, E) and epinephrine (B, D, F) levels were quantified in adrenal gland (A, B), plasma (C, D), and heart (E, F), from wild-type Vgf+/+ (WT), heterozygous-knockout Vgf+/− (HET), and homozygous-knockout Vgf−/− (KO) mice, as described in Materials and Methods (means±sem; n=4–8 mice/group). *P < 0.05, **P < 0.005, ***P < 0.0001; ANOVA with Tukey's post hoc comparison.
Administration of the VGF-derived peptide TLQP-21 decreases BP
Consistent with increased BP in mice lacking VGF, chronic or acute treatment of wild-type mice or rats with the C-terminal internal VGF-derived peptide TLQP-21 (17) decreased BP. Mice were fed an HFD for 12 wk to establish obesity-induced hypertension (51) and were subsequently treated for 14 d with saline or TLQP-21 (40 or 400 μg/d delivered subcutaneously via osmotic minipump) and BP and HR, determined via intracarotid catheterization under anesthesia on d 14, were compared to control mice fed a standard diet (Fig. 6C). Chronic high-dose TLQP-21 treatment for 2 wk normalized obesity-induced hypertension (Fig. 6C) and, in agreement with our previous work, did not change body weight (25). In addition, acute i.v. infusion of high-dose (5 mg/kg) but not low dose (1 mg/kg) TLQP-21 peptide into Sprague-Dawley rats transiently and significantly decreased MAP, with the maximum effect observed between 9 and 16 min postinfusion (Fig. 6D). Neither chronic nor acute peptide treatment had a significant effect on HR (data not shown).
Germline knock-in of human VGF1–615 or a truncated human VGF1–524 coding sequence into the mouse Vgf locus normalizes BP
To determine whether human VGF was functional in mice and could rescue the hypertensive phenotype resulting from germline VGF ablation, we knocked full-length human VGF1–615 into the mouse Vgf locus. Human VGF-coding 5′- and 3′-UTR sequences replaced equivalent mouse sequences downstream of the mouse Vgf promoter (Fig. 6E). In addition, we also used site-directed mutagenesis to introduce a known SNP (rs35400704), creating a stop codon, which resulted in the translation of a truncated human VGF1–524 protein that lacked several bioactive C-terminal peptides (Fig. 6E). Southern and PCR analysis confirmed correctly targeted integration, and Western blot analysis of brain extracts using anti-VGF antisera confirmed that hVGF1–615 and hVGF1–524 were expressed from the targeted alleles (Fig. 6F). Homozygous expression of either hVGF1–615- or hVGF1–524-knock-in alleles essentially normalized BP (Fig. 6G) compared to homozygous germline-knockout Vgf−/− mice (Fig. 6A). SBP was slightly but significantly increased in hVGF1–524 mice compared to hVGF1–615 mice (P<0.0001). These data suggest that human full-length VGF1–615 or truncated VGF1–524 protein, and/or processed peptides derived from either of these human VGF proteins, can functionally compensate for germline Vgf ablation with respect to the regulation of BP, and that lack of VGF C-terminal peptides, including TLQP-21, in homozygous hVGF1–524-knock-in mice, results in a small but significant increase in SBP compared to full-length hVGF1-615-knock-in mice.
DISCUSSION
Granin proteins, including CgA, CgB, and SgII, contribute to LDCV formation, as determined in vitro, primarily through the analysis of granin-transfected non-neuroendocrine cell lines or through RNAi- or antisense-mediated down-regulation of specific granins in neural and endocrine secretory cell lines (4–9, 46–49, 52, 53), as well as in knockout and transgenic mouse lines, in vivo (45, 54–56). Expression of exogenous chromogranins and secretogranins, including CgA, CgB, and SgII, and also a number of proteins secreted via the regulated pathway, including provasopressin, prooxytocin, and proopiomelanocortin, stimulate the formation of LDCV-like vesicles in nonendocrine cells (6, 7, 46–49, 52, 53). VGF shares structural properties and distribution with CgA, CgB, and SgII (2, 12), and based on findings presented here, stimulates neogenesis of LDCV-like structures in nonendocrine cells, with similar efficiency to CgA and CgB (2–4 LDCVs/transfected cell; ref. 7), and these VGF-containing vesicles also undergo regulated secretion.
Analyses of two different mouse knockout lines and a transgenic CgA-deficient line suggest that CgA is critically involved in but not required for granule biogenesis and catecholamine secretion (56), although significant variability in phenotype occurs among the lines (45, 54, 55). Changes in CgB and/or SgII protein levels in adrenal tissue were noted in these CgA-deficient lines, which suggests some functional redundancy among the granin proteins, but incomplete compensation, as decreased catecholamine storage was noted. Recently, CgA/CgB double-knockout mice were generated, and these are fertile but have atypical, large LDCVs and reduced adrenal catecholamine content (56). In addition, both CgA- and CgB-knockout mice were hypertensive, consistent with genetic association studies in hypertensive patients (45, 57).
We found that expression in nonendocrine NIH 3T3 cells of exogenous full-length VGF or a VGF:GFP fusion protein that contains the required sorting signals for targeting or retention into the regulated secretory pathway resulted in the formation of LDCV-like structures, visualized by CLEM, as well as regulated VGF secretion. Although LDCV number was preserved in Vgf−/− adrenal glands, suggesting largely intact biogenesis, mean LDCV area in norepinephrine chromaffin cells was significantly reduced, adrenal CgB levels were decreased, adrenal catecholamine content was increased, and Vgf−/− mice were hypertensive. Interestingly, common genetic variation at the human CHGB locus, particularly in the proximal promoter, is associated with decreased CgB expression, increased catecholamine secretion, and hypertension (57). We noted that VGF and CgB were coexpressed in adrenal medulla and were colocalized and could be coimmunoprecipitated in PC12 pheochromocytoma cells; further studies would be needed to determine whether decreased adrenal CgB levels in germline VGF-knockout mice are responsible for their increased BP and catecholamine levels.
The VGF:CgB interaction we detected suggests potential coaggregation of these proteins as part of a larger complex in the dense core lumen, either by direct association, or possibly indirect association via binding to a shared sorting receptor, membrane anchor, or matrix protein. No interaction was detected with CgA, SgII, or SgIII, suggesting that nonspecific coaggregation of VGF with other granins in the LDCV lumen is less likely. Based on similarities in the hypertensive phenotypes of CgA-, CgB-, and VGF-knockout mice, and the VGF:CgB interaction detected here, it would be interesting to examine adrenal and other tissues from CgB- and CgA-knockout mice for alterations in VGF expression. Increased circulating catecholamine levels in VGF-knockout mice could consequently be an indirect result of lower LDCV CgB content, and reported effects that this has of increasing circulating catecholamine levels and blood pressure in CgB-knockout mice (58), although it is important to note that the capacity to concentrate and secrete catecholamines, and adrenal epinephrine content, are all reduced in an independent line of CgB-knockout mice (59), so the observed increase in adrenal catecholamine content in VGF-knockout mice is unlikely to result from decreased CgB levels per se. Additional studies will be necessary to determine whether VGF modulation of the levels of CgB protein, catestatin, or perhaps a newly described CgB-derived peptide, hCHGB60–67 (58), that inhibits catecholamine release in response to acetylcholine, are involved in BP regulation by VGF.
We propose that VGF, like CgA, CgB, and SgII, promotes granule neogenesis and is also involved in the regulation of LDCV protein and neurotransmitter content. The significant neuronal, endocrine, and neuroendocrine phenotypic abnormalities that result from Vgf gene ablation in mice, including elevated BP noted here, and altered fertility and energy and glucose homeostasis, noted previously (16, 37, 38), could result from the loss of the VGF precursor and/or VGF-derived peptides or, alternatively, secondary alterations in secretory pathway function and LDCV content.
Future studies could assess regulated secretory pathway function and LDCV size and content, in other tissues of VGF-knockout mice including hypothalamus, where abnormalities in anorexigenic and orexigenic neuropeptide secretion could contribute to their lean, hypermetabolic phenotype (16, 37), and pancreas, where VGF and insulin are coexpressed and cosecreted from beta cells (28, 60, 61). Recent studies further suggest increased sympathetic nervous system activity as a critical underlying mechanism for increased lipolysis and decreased white adipose stores in VGF-knockout mice (62) and mice treated with the VGF-derived peptide TLQP-21 (25). This finding raises the possibility that hypertension in Vgf−/− mice may be alternatively or in part driven by increased vascular or cardiac sympathetic nervous system activity rather than by high circulating plasma epinephrine levels. At least with respect to BP regulation, we further found that expression of either human VGF1–615 or truncated VGF1–524, lacking several bioactive C-terminal peptides, encoded by genes knocked into the mouse Vgf locus, is sufficient to rescue the hypertensive phenotype of mice lacking VGF1–617. Notably, mice expressing truncated human VGF1–524 had slightly but significantly higher SBP than those expressing human VGF1–615. In addition, small differences in SBP and DBP levels were noted when comparing VGF-knockout mice on a mixed >83% C57Bl/6:<17% 129/Sv background with humanized VGF lines on a mixed ∼50% C57Bl/6:∼50% 129/Sv background (Fig. 6), perhaps secondary to differences in strain background. Consequently, BP was always measured in experimental and wild-type mice from the same line within each individual monitoring session. Small but significant differences in SBP between hVGF1-615 and hVGF1–524 mice were consistent with lower SBP and DBP and normalization of obesity-associated hypertension, measured following administration of the C-terminal internal peptide TLQP-21 (rat/mouse VGF556–576; human VGF554–574) to rats and mice. Notably, TLQP-21 is not synthesized in homozygous germline VGF-knockout and hVGF1–524 mice. Finally, in addition to TLQP-21, the VGF-derived neuroendocrine regulatory peptides (NERPs), expressed in hVGF1–524 mice, including NERP-1 and NERP-2, which block (21), and NERP-3, which stimulates (24), arginine-vasopressin (AVP) release from the posterior pituitary, could via this mechanism potentially modulate salt retention, fluid balance, and BP, although our preliminary studies did not detect differences in plasma AVP levels between Vgf−/− and Vgf+/+ mice (unpublished results). Taken together, these findings are consistent with a novel role for VGF in autonomic circulatory control, much as this protein and the VGF-derived peptide TLQP-21 have been suggested to function in autonomic outflow pathways to regulate peripheral adiposity (25, 62), but not cardiovascular function or anatomy, including HR, mass to chamber volume ratio, and myocardial fibrosis (studies presented here and ref. 25).
In summary, our studies have identified a broader role for the secreted neuronal and endocrine protein and peptide precursor, VGF, in LDCV formation and the regulation of vesicle content, which suggests further that regulated secretion of other growth factors, hormones, neurotransmitters, and/or neuropeptides may be compromised in neuronal, neuroendocrine, and endocrine cells lacking VGF, much as the effects of CgB ablation on hormone secretion extend from the adrenal to pancreatic islets (63), where defects in insulin, glucagon, and somatostatin secretion have been reported.
Acknowledgments
The authors thank director Kevin Kelley and staff of the Mouse Genetics Core Facility (Icahn School of Medicine at Mount Sinai) for their assistance with Vgf gene targeting in ES cells and the generation of humanized VGF-knock-in mouse lines.
This work was supported by an American Psychological Association Diversity Program in Neuroscience predoctoral fellowship (A.L.G.), U.S. National Institutes of Health (NIH) Endocrine Training grant 5T32DK07645 (S.F.), NIH grant RO1 DK071308 (S.R.S.), NIH grant RO1 MH086499 (S.R.S.), NIH grant R21/R33 MH083496 (S.R.S.), the Diabetes Action Research and Education Foundation (S.R.S.), a Minnesota Partnership for Biotechnology and Medical Genomics, Decade of Discovery in Diabetes grant (A.B.), NIH grant R01 HL076312 (J.W.O.), and a U.S. Department of Veterans Affairs Research Career Scientist Award (S.K.M.).
Footnotes
- AVP
- arginine-vasopressin
- BAC
- bacterial artificial chromosome
- BP
- blood pressure
- BDNF
- brain-derived neurotrophic factor
- CG
- chromaffin granule
- CgA
- chromogranin A
- CgB
- chromogranin B
- CHX
- cycloheximide
- CLEM
- correlative light and electron microscopy
- DBP
- diastolic blood pressure
- DCG
- dense core granule
- DCV
- dense core vesicle
- EM
- electron microscopy
- FRT
- flippase recombinase target
- GFP
- green fluorescent protein
- HFD
- high-fat diet
- HR
- heart rate
- HRP
- horseradish peroxidase
- i.v.
- intravenous
- LDCV
- large dense core vesicle
- LV
- left ventricle
- MAP
- mean arterial pressure
- neo
- neomycin
- NERP
- neuroendocrine regulatory peptide
- PGK
- phosphoglycerate kinase
- PAGE
- polyacrylamide gel electrophoresis
- PVDF
- polyvinylidene difluoride
- SDS
- sodium dodecyl sulfate
- SgII
- secretogranin II
- SgIII
- secretogranin III
- SNP
- single-nucleotide polymorphism
- SBP
- systolic blood pressure
REFERENCES
- 1. Huttner W. B., Gerdes H. H., Rosa P. (1991) The granin (chromogranin/secretogranin) family. Trends Biochem. Sci. 16, 27–30 [DOI] [PubMed] [Google Scholar]
- 2. Bartolomucci A., Possenti R., Mahata S. K., Fischer-Colbrie R., Loh Y. P., Salton S. R. (2011) The extended granin family: structure, function, and biomedical implications. Endocr. Rev. 32, 755–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Loh Y. P., Kim T., Rodriguez Y. M., Cawley N. X. (2004) Secretory granule biogenesis and neuropeptide sorting to the regulated secretory pathway in neuroendocrine cells. J. Mol. Neurosci. 22, 63–71 [DOI] [PubMed] [Google Scholar]
- 4. Ozawa H., Takata K. (1995) The granin family–its role in sorting and secretory granule formation. Cell Struct. Funct. 20, 415–420 [DOI] [PubMed] [Google Scholar]
- 5. Natori S., Huttner W. B. (1996) Chromogranin B (secretogranin I) promotes sorting to the regulated secretory pathway of processing intermediates derived from a peptide hormone precursor. Proc. Natl. Acad. Sci. U. S. A. 93, 4431–4436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim T., Tao-Cheng J. H., Eiden L. E., Loh Y. P. (2001) Chromogranin A, an “on/off” switch controlling dense-core secretory granule biogenesis. Cell 106, 499–509 [DOI] [PubMed] [Google Scholar]
- 7. Huh Y. H., Jeon S. H., Yoo S. H. (2003) Chromogranin B-induced secretory granule biogenesis: comparison with the similar role of chromogranin A. J. Biol. Chem. 278, 40581–40589 [DOI] [PubMed] [Google Scholar]
- 8. Malosio M. L., Giordano T., Laslop A., Meldolesi J. (2004) Dense-core granules: a specific hallmark of the neuronal/neurosecretory cell phenotype. J. Cell Sci. 117, 743–749 [DOI] [PubMed] [Google Scholar]
- 9. Hotta K., Hosaka M., Tanabe A., Takeuchi T. (2009) Secretogranin II binds to secretogranin III and forms secretory granules with orexin, neuropeptide Y, and POMC. J. Endocrinol. 202, 111–121 [DOI] [PubMed] [Google Scholar]
- 10. Loh Y. P., Cheng Y., Mahata S. K., Corti A., Tota B. (2012) Chromogranin A and derived peptides in health and disease. J. Mol. Neurosci. 48, 347–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang K., Chen Y., Wen G., Mahata M., Rao F., Fung M. M., Vaingankar S., Biswas N., Gayen J. R., Friese R. S., Mahata S. K., Hamilton B. A., O'Connor D. T. (2011) Catecholamine storage vesicles: role of core protein genetic polymorphisms in hypertension. Curr. Hypertens. Rep. 13, 36–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Helle K. B. (2004) The granin family of uniquely acidic proteins of the diffuse neuroendocrine system: comparative and functional aspects. Biol. Rev. Camb. Philos. Soc. 79, 769–794 [DOI] [PubMed] [Google Scholar]
- 13. Trani E., Ciotti T., Rinaldi A. M., Canu N., Ferri G. L., Levi A., Possenti R. (1995) Tissue-specific processing of the neuroendocrine protein VGF. J. Neurochem. 65, 2441–2449 [DOI] [PubMed] [Google Scholar]
- 14. Salton S. R., Ferri G. L., Hahm S., Snyder S. E., Wilson A. J., Possenti R., Levi A. (2000) VGF: a novel role for this neuronal and neuroendocrine polypeptide in the regulation of energy balance. Front. Neuroendocrinol. 21, 199–219 [DOI] [PubMed] [Google Scholar]
- 15. Trani E., Giorgi A., Canu N., Amadoro G., Rinaldi A. M., Halban P. A., Ferri G. L., Possenti R., Schinina M. E., Levi A. (2002) Isolation and characterization of VGF peptides in rat brain. Role of PC1/3 and PC2 in the maturation of VGF precursor. J. Neurochem. 81, 565–574 [DOI] [PubMed] [Google Scholar]
- 16. Hahm S., Mizuno T. M., Wu T. J., Wisor J. P., Priest C. A., Kozak C. A., Boozer C. N., Peng B., McEvoy R. C., Good P., Kelley K. A., Takahashi J. S., Pintar J. E., Roberts J. L., Mobbs C. V., Salton S. R. (1999) Targeted deletion of the Vgf gene indicates that the encoded secretory peptide precursor plays a novel role in the regulation of energy balance. Neuron 23, 537–548 [DOI] [PubMed] [Google Scholar]
- 17. Bartolomucci A., La Corte G., Possenti R., Locatelli V., Rigamonti A. E., Torsello A., Bresciani E., Bulgarelli I., Rizzi R., Pavone F., D'Amato F. R., Severini C., Mignogna G., Giorgi A., Schinina M. E., Elia G., Brancia C., Ferri G. L., Conti R., Ciani B., Pascucci T., Dell'omo G., Muller E. E., Levi A., Moles A. (2006) TLQP-21, a VGF-derived peptide, increases energy expenditure and prevents the early phase of diet-induced obesity. Proc. Natl. Acad. Sci. U. S. A. 103, 14584–14589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hunsberger J. G., Newton S. S., Bennett A. H., Duman C. H., Russell D. S., Salton S. R., Duman R. S. (2007) Antidepressant actions of the exercise-regulated gene VGF. Nat. Med. 13, 1476–1482 [DOI] [PubMed] [Google Scholar]
- 19. Jethwa P. H., Warner A., Nilaweera K. N., Brameld J. M., Keyte J. W., Carter W. G., Bolton N., Bruggraber M., Morgan P. J., Barrett P., Ebling F. J. (2007) VGF-derived peptide, TLQP-21, regulates food intake and body weight in Siberian hamsters. Endocrinology 148, 4044–4055 [DOI] [PubMed] [Google Scholar]
- 20. Thakker-Varia S., Krol J. J., Nettleton J., Bilimoria P. M., Bangasser D. A., Shors T. J., Black I. B., Alder J. (2007) The neuropeptide VGF produces antidepressant-like behavioral effects and enhances proliferation in the hippocampus. J. Neurosci. 27, 12156–12167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yamaguchi H., Sasaki K., Satomi Y., Shimbara T., Kageyama H., Mondal M. S., Toshinai K., Date Y., Gonzalez L. J., Shioda S., Takao T., Nakazato M., Minamino N. (2007) Peptidomic identification and biological validation of neuroendocrine regulatory peptide-1 and -2. J. Biol. Chem. 282, 26354–26360 [DOI] [PubMed] [Google Scholar]
- 22. Bozdagi O., Rich E., Tronel S., Sadahiro M., Patterson K., Shapiro M. L., Alberini C. M., Huntley G. W., Salton S. R. (2008) The neurotrophin-inducible gene Vgf regulates hippocampal function and behavior through a brain-derived neurotrophic factor-dependent mechanism. J. Neurosci. 28, 9857–9869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Toshinai K., Yamaguchi H., Kageyama H., Matsuo T., Koshinaka K., Sasaki K., Shioda S., Minamino N., Nakazato M. (2010) Neuroendocrine regulatory peptide-2 regulates feeding behavior via the orexin system in the hypothalamus. Am. J. Physiol. Endocrinol. Metab. 299, E394–E401 [DOI] [PubMed] [Google Scholar]
- 24. Fujihara H., Sasaki K., Mishiro-Sato E., Ohbuchi T., Dayanithi G., Yamasaki M., Ueta Y., Minamino N. (2012) Molecular characterization and biological function of neuroendocrine regulatory peptide-3 in the rat. Endocrinology 153, 1377–1386 [DOI] [PubMed] [Google Scholar]
- 25. Possenti R., Muccioli G., Petrocchi P., Cero C., Cabassi A., Vulchanova L., Riedl M. S., Manieri M., Frontini A., Giordano A., Cinti S., Govoni P., Graiani G., Quaini F., Ghe C., Bresciani E., Bulgarelli I., Torsello A., Locatelli V., Sanghez V., Larsen B. D., Petersen J. S., Palanza P., Parmigiani S., Moles A., Levi A., Bartolomucci A. (2012) Characterization of a novel peripheral pro-lipolytic mechanism in mice: role of VGF-derived peptide TLQP-21. Biochem. J. 441, 511–522 [DOI] [PubMed] [Google Scholar]
- 26. Garcia A. L., Han S. K., Janssen W. G., Khaing Z. Z., Ito T., Glucksman M. J., Benson D. L., Salton S. R. (2005) A prohormone convertase cleavage site within a predicted alpha -helix mediates sorting of the neuronal and endocrine polypeptide VGF into the regulated secretory pathway. J. Biol. Chem. 280, 41595–41608 [DOI] [PubMed] [Google Scholar]
- 27. Gentile F., Cali G., Zurzolo C., Corteggio A., Rosa P., Calegari F., Levi A., Possenti R., Puri C., Tacchetti C., Nitsch L. (2004) The neuroendocrine protein VGF is sorted into dense-core granules and is secreted apically by polarized rat thyroid epithelial cells. Exp. Cell Res. 295, 269–280 [DOI] [PubMed] [Google Scholar]
- 28. Stephens S. B., Schisler J. C., Hohmeier H. E., An J., Sun A. Y., Pitt G. S., Newgard C. B. (2012) A VGF-derived peptide attenuates development of type 2 diabetes via enhancement of islet beta-cell survival and function. Cell Metab. 16, 33–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Greene L. A., Tischler A. S. (1976) Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc. Natl. Acad. Sci. U. S. A. 73, 2424–2428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Levi A., Eldridge J. D., Paterson B. M. (1985) Molecular cloning of a gene sequence regulated by nerve growth factor. Science 229, 393–395 [DOI] [PubMed] [Google Scholar]
- 31. Cho K. O., Skarnes W. C., Minsk B., Palmieri S., Jackson-Grusby L., Wagner J. A. (1989) Nerve growth factor regulates gene expression by several distinct mechanisms. Mol. Cell. Biol. 9, 135–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Salton S. R., Fischberg D. J., Dong K. W. (1991) Structure of the gene encoding VGF, a nervous system-specific mRNA that is rapidly and selectively induced by nerve growth factor in PC12 cells. Mol. Cell. Biol. 11, 2335–2349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. D'Amato F., Noli B., Brancia C., Cocco C., Flore G., Collu M., Nicolussi P., Ferri G. L. (2008) Differential distribution of VGF-derived peptides in the adrenal medulla and evidence for their selective modulation. J. Endocrinol. 197, 359–369 [DOI] [PubMed] [Google Scholar]
- 34. Grabner C. P., Price S. D., Lysakowski A., Fox A. P. (2005) Mouse chromaffin cells have two populations of dense core vesicles. J. Neurophysiol. 94, 2093–2104 [DOI] [PubMed] [Google Scholar]
- 35. Valenzuela D. M., Murphy A. J., Frendewey D., Gale N. W., Economides A. N., Auerbach W., Poueymirou W. T., Adams N. C., Rojas J., Yasenchak J., Chernomorsky R., Boucher M., Elsasser A. L., Esau L., Zheng J., Griffiths J. A., Wang X., Su H., Xue Y., Dominguez M. G., Noguera I., Torres R., Macdonald L. E., Stewart A. F., DeChiara T. M., Yancopoulos G. D. (2003) High-throughput engineering of the mouse genome coupled with high-resolution expression analysis. Nat. Biotechnol. 21, 652–659 [DOI] [PubMed] [Google Scholar]
- 36. Watson E., Fargali S., Okamoto H., Sadahiro M., Gordon R. E., Chakraborty T., Sleeman M. W., Salton S. R. (2009) Analysis of knockout mice suggests a role for VGF in the control of fat storage and energy expenditure. BMC Physiol. 9, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hahm S., Fekete C., Mizuno T. M., Windsor J., Yan H., Boozer C. N., Lee C., Elmquist J. K., Lechan R. M., Mobbs C. V., Salton S. R. (2002) VGF is required for obesity induced by diet, gold thioglucose treatment and agouti, and is differentially regulated in POMC- and NPY-containing arcuate neurons in response to fasting. J. Neurosci. 22, 6929–6938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Watson E., Hahm S., Mizuno T. M., Windsor J., Montgomery C., Scherer P. E., Mobbs C. V., Salton S. R. (2005) VGF ablation blocks the development of hyperinsulinemia and hyperglycemia in several mouse models of obesity. Endocrinology 146, 5151–5163 [DOI] [PubMed] [Google Scholar]
- 39. Canu N., Possenti R., Ricco A. S., Rocchi M., Levi A. (1997) Cloning, structural organization analysis, and chromosomal assignment of the human gene for the neurosecretory protein VGF. Genomics 45, 443–446 [DOI] [PubMed] [Google Scholar]
- 40. Veitenheimer B., Osborn J. W. (2011) Role of spinal V1a receptors in regulation of arterial pressure during acute and chronic osmotic stress. Am. J. Physiol. 300, R460–R469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chakraborty T. R., Tkalych O., Nanno D., Garcia A. L., Devi L. A., Salton S. R. (2006) Quantification of VGF- and pro-SAAS-derived peptides in endocrine tissues and the brain, and their regulation by diet and cold stress. Brain Res. 1089, 21–32 [DOI] [PubMed] [Google Scholar]
- 42. Laemmli U. K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 43. Elste A. M., Benson D. L. (2006) Structural basis for developmentally regulated changes in cadherin function at synapses. J. Comp. Neurol. 495, 324–335 [DOI] [PubMed] [Google Scholar]
- 44. Hanson H. H., Reilly J. E., Lee R., Janssen W. G., Phillips G. R. (2010) Streamlined embedding of cell monolayers on gridded glass-bottom imaging dishes for correlative light and electron microscopy. Microsc. Microanal. 16, 747–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mahapatra N. R., O'Connor D. T., Vaingankar S. M., Hikim A. P., Mahata M., Ray S., Staite E., Wu H., Gu Y., Dalton N., Kennedy B. P., Ziegler M. G., Ross J., Mahata S. K. (2005) Hypertension from targeted ablation of chromogranin A can be rescued by the human ortholog. J. Clin. Invest. 115, 1942–1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Beuret N., Stettler H., Renold A., Rutishauser J., Spiess M. (2004) Expression of regulated secretory proteins is sufficient to generate granule-like structures in constitutively secreting cells. J. Biol. Chem. 279, 20242–20249 [DOI] [PubMed] [Google Scholar]
- 47. Courel M., Rodemer C., Nguyen S. T., Pance A., Jackson A. P., O'Connor D T., Taupenot L. (2006) Secretory granule biogenesis in sympathoadrenal cells: identification of a granulogenic determinant in the secretory prohormone chromogranin A. J. Biol. Chem. 281, 38038–38051 [DOI] [PubMed] [Google Scholar]
- 48. Stettler H., Beuret N., Prescianotto-Baschong C., Fayard B., Taupenot L., Spiess M. (2009) Determinants for chromogranin A sorting into the regulated secretory pathway are also sufficient to generate granule-like structures in non-endocrine cells. Biochem. J. 418, 81–91 [DOI] [PubMed] [Google Scholar]
- 49. Courel M., Soler-Jover A., Rodriguez-Flores J. L., Mahata S. K., Elias S., Montero-Hadjadje M., Anouar Y., Giuly R. J., O'Connor D. T., Taupenot L. (2010) Pro-hormone secretogranin II regulates dense core secretory granule biogenesis in catecholaminergic cells. J. Biol. Chem. 285, 10030–10043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cowley D. J., Moore Y. R., Darling D. S., Joyce P. B., Gorr S. U. (2000) N- and C-terminal domains direct cell type-specific sorting of chromogranin A to secretory granules. J. Biol. Chem. 275, 7743–7748 [DOI] [PubMed] [Google Scholar]
- 51. Esler M., Straznicky N., Eikelis N., Masuo K., Lambert G., Lambert E. (2006) Mechanisms of sympathetic activation in obesity-related hypertension. Hypertension 48, 787–796 [DOI] [PubMed] [Google Scholar]
- 52. Inomoto C., Umemura S., Egashira N., Minematsu T., Takekoshi S., Itoh Y., Itoh J., Taupenot L., O'Connor D. T., Osamura R. Y. (2007) Granulogenesis in non-neuroendocrine COS-7 cells induced by EGFP-tagged chromogranin A gene transfection: identical and distinct distribution of CgA and EGFP. J. Histochem. Cytochem. 55, 487–493 [DOI] [PubMed] [Google Scholar]
- 53. Montero-Hadjadje M., Elias S., Chevalier L., Benard M., Tanguy Y., Turquier V., Galas L., Yon L., Malagon M. M., Driouich A., Gasman S., Anouar Y. (2009) Chromogranin A promotes peptide hormone sorting to mobile granules in constitutively and regulated secreting cells: role of conserved N- and C-terminal peptides. J. Biol. Chem. 284, 12420–12431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kim T., Zhang C. F., Sun Z., Wu H., Loh Y. P. (2005) Chromogranin A deficiency in transgenic mice leads to aberrant chromaffin granule biogenesis. J. Neurosci. 25, 6958–6961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hendy G. N., Li T., Girard M., Feldstein R. C., Mulay S., Desjardins R., Day R., Karaplis A. C., Tremblay M. L., Canaff L. (2006) Targeted ablation of the chromogranin a (Chga) gene: normal neuroendocrine dense-core secretory granules and increased expression of other granins. Mol. Endocrinol. 20, 1935–1947 [DOI] [PubMed] [Google Scholar]
- 56. Diaz-Vera J., Camacho M., Machado J. D., Dominguez N., Montesinos M. S., Hernandez-Fernaud J. R., Lujan R., Borges R. (2012) Chromogranins A and B are key proteins in amine accumulation, but the catecholamine secretory pathway is conserved without them. FASEB J. 26, 430–438 [DOI] [PubMed] [Google Scholar]
- 57. Zhang K., Rao F., Rana B. K., Gayen J. R., Calegari F., King A., Rosa P., Huttner W. B., Stridsberg M., Mahata M., Vaingankar S., Mahboubi V., Salem R. M., Rodriguez-Flores J. L., Fung M. M., Smith D. W., Schork N. J., Ziegler M. G., Taupenot L., Mahata S. K., O'Connor D. T. (2009) Autonomic function in hypertension; role of genetic variation at the catecholamine storage vesicle protein chromogranin B. Circ. Cardiovasc. Genet. 2, 46–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhang K., Biswas N., Gayen J. R., Miramontes-Gonzalez J. P., Hightower C. M., Mustapic M., Mahata M., Huang C. T., Hook V. Y., Mahata S. K., Vaingankar S., O'Connor D. T. (2013) Chromogranin B: intra- and extra-cellular mechanisms to regulate catecholamine storage and release, in catecholaminergic cells and organisms. [E-pub ahead of print] J. Neurochem. 10.1111/jnc.12527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Diaz-Vera J., Morales Y. G., Hernandez-Fernaud J. R., Camacho M., Montesinos M. S., Calegari F., Huttner W. B., Borges R., Machado J. D. (2010) Chromogranin B gene ablation reduces the catecholamine cargo and decelerates exocytosis in chromaffin secretory vesicles. J. Neurosci. 30, 950–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Possenti R., Rinaldi A. M., Ferri G. L., Borboni P., Trani E., Levi A. (1999) Expression, processing, and secretion of the neuroendocrine VGF peptides by INS-1 cells. Endocrinology 140, 3727–3735 [DOI] [PubMed] [Google Scholar]
- 61. Snyder S. E., Peng B., Pintar J. E., Salton S. R. (2003) Expression of VGF mRNA in developing neuroendocrine and endocrine tissues. J. Endocrinol. 179, 227–235 [DOI] [PubMed] [Google Scholar]
- 62. Fargali S., Scherer T., Shin A. C., Sadahiro M., Buettner C., Salton S. R. (2012) Germline ablation of VGF increases lipolysis in white adipose tissue. J. Endocrinol. 215, 313–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Obermuller S., Calegari F., King A., Lindqvist A., Lundquist I., Salehi A., Francolini M., Rosa P., Rorsman P., Huttner W. B., Barg S. (2010) Defective secretion of islet hormones in chromogranin-B deficient mice. PLoS One 5, e8936. [DOI] [PMC free article] [PubMed] [Google Scholar]