Abstract
Background
Zinc (Zn) supplementation has been shown to reduce the incidence of diarrhea and to protect animals from intestinal diseases, but the mechanisms of this protective effect against virus infection in vivo have not yet been elucidated. Transmissible gastroenteritis virus (TGEV) causes diarrhea in piglets with an age-dependent decrease of severity.
Results
We used 60 weaned piglets that were divided into three groups to evaluate the effect of different Zn levels added to a conventional diet (50 mg Zn/kg diet, Znlow, control group). The other groups received the diet supplemented with ZnO at final concentrations of 150 mg Zn/kg diet (Znmed), or 2,500 mg/kg diet (Znhigh). Oral challenge infection with TGEV was performed when the pigs had been fed for 1 week with the respective diet. Half of the piglets of each group were sacrificed at day 1 and 18 after challenge infection. Fecal consistency was improved and body weights increased in the Znhigh group when compared to the other groups, but no direct effect of Zn concentrations in the diet on fecal TGEV shedding and mucosal immune responses was detectable. However, in the Znhigh group, we found a prevention of villus atrophy and decreased caspase-3-mediated apoptosis of jejunal epithelium. Furthermore, pigs receiving high Zn diet showed a down-regulation of interferon (IFN)-α, oligoadenylate synthetase (OAS), Zn transporter SLC39A4 (ZIP4), but up-regulation of metallothionein-1 (MT1), as well as the Zn transporters SLC30A1 (ZnT1) and SLC30A5 (ZnT5). In addition, forskolin-induced chloride secretion and epithelial resistance were controlled at a physiological level in the Znhigh but not the other groups. Finally, in the Znhigh group, we documented an earlier and higher systemic TGEV-specific serum antibody response.
Conclusions
These results suggest that high dietary Zn could provide enhanced protection in the intestinal tract and stimulate the systemic humoral immune response against TGEV infection.
Keywords: Zinc oxide, Coronavirus, Transmissible gastroenteritis virus, Cytokine, Morphometry, Electrophysiology, Zinc transporters
Background
Several in vitro studies have shown that zinc (Zn) has broad-spectrum antiviral activity against a variety of viruses, such as human immunodeficiency virus, transmissible gastroenteritis virus (TGEV), equine arteritis virus, and severe acute respiratory syndrome coronavirus [1-6]. Many potential mechanisms have been suggested to explain the potential beneficial effect of Zn against virus infections. For example, Zn mediates antiviral effects through the inhibition of nidovirus RNA-dependent RNA polymerases or other proteins essential for the different phases of the viral life cycle [5,6]. In addition, Zn participates in initiating and maintaining robust immune responses, in particular cytokine production and modulation of the activity of immune cells [7]. Zn induces the production of innate interferon (IFN)-α and also immune IFN-γ, and can potentiate the antiviral action of IFN-α, but not of IFN-γ [8]. Clearance of viral infections requires cytotoxic T lymphocytes, which are also highly dependent on the presence of Zn [7]. Antibody production during both the first and an immunological memory response is disturbed by Zn deficiency [9,10], indicating that Zn is necessary for optimal results following vaccination.
In swine nutrition, especially in the North American swine industry, high levels of Zn oxide (ZnO, 2,000-3,000 ppm) are often added to the diet of weaned pigs, since such addition was shown to reduce non-specific post-weaning diarrhea and improve performance in this critical period of dietary change [11-13]. Diarrhea is caused by impaired intestinal epithelial barrier function, which most likely leads to malnutrition and decreased uptake of micronutrients, including Zn. It was shown that oral Zn supplementation with high doses was able to counteract this loss, improve intestinal mucosal integrity as well as absorption of water and electrolytes [12,14]. Furthermore, it leads to a faster regeneration of the gut epithelium [15]. However, because of environmental concerns, the maximum level of Zn allowed in pig diets was set up to 150 ppm in the European Union, irrespective of the Zn formulation [16].
Zn homeostasis is maintained in the body through a variety of transporters and Zn binding proteins [17]. High levels of dietary Zn provided as ZnO have been recently shown to outbalance Zn homeostasis with increased accumulation of Zn in various organs including the small intestine of piglets [18,19]. Since intestinal Zn uptake can also take place through passive diffusion, it is likely that very high dietary Zn levels would indirectly increase the intestinal barrier function as a protection mechanism of the epithelium. In addition, metallothionein that is induced by Zn accumulation in intestinal tissue may also protect the tissue from oxidative damage.
Due to suboptimal immune functions, newborn as well as weaned piglets are particularly susceptible to infection by various pathogens, among them TGEV, which causes severe to mild gastroenteritis in piglets, depending on the age [20,21]. Our previous study [5] showed that high Zn levels markedly reduced TGEV titers as well as viral RNA and protein synthesis in vitro, but there is no report about antiviral effects of Zn supplementation in pigs. The aim of this study was to close the knowledge gap and evaluate the antiviral potential and possible protection mechanisms of increased dietary Zn supplementation against TGEV infection in weaned piglets.
Results
High-dose Zn prevents diarrhea in piglets but does not affect other trace elements and virus shedding
TGEV infection caused only mild symptoms and there was no difference in dehydration, anorexia, lethargy and body temperature when the different Zn treatment groups were compared. However, the body weight in Znhigh was higher at 7, 14 and 18 dpi in comparison to both other groups (Figure 1A). Furthermore, the fecal score from 2 to 7 dpi was also higher in Znhigh compared to the Znlow control group (Figure 1B).
Figure 1.
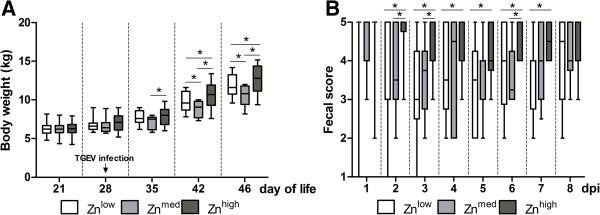
Body weight and fecal score of TGEV-infected piglets. Piglets fed with Znlow, Znmed and Znhigh were orally infected with TGEV. (A) Body weight was recorded at given time points and (B) fecal scores (from 1 to 5, where 1 means watery and 5 hard and dry stool) were recorded daily after infection. Boxes indicate medians (n = 10) (horizontal lines) and the lower and upper quartiles (bottoms and tops of the boxes). The vertical bars in the box plots indicate the minimal and maximal values recorded. Asterisk indicates statistically significant difference (p ≤ 0.05) between the groups.
Serum and liver Zn concentrations were higher in the Znhigh group as compared to the Znmed and Znlow groups, but other trace elements were not affected. In addition, there was an increased Zn, manganese and iron concentration, but decreased copper concentration, in both liver and serum from 1 to 18 dpi (Additional file 1: Table S1).
Low amounts of shedding TGEV could be detected by qPCR from 1 to 6 dpi, irrespective of Zn feeding group. The highest incidence was observed at 4 dpi with 4 out of 10 positive TGEV shedding piglets in each group.
Systemic and mucosal immune responses
Figure 2 shows the systemic and mucosal immune responses of piglets as measured by ELISA. At the time of challenge, all piglets fed with different concentrations of Zn were negative for TGEV-specific serum IgG antibodies. The serum antibody response after infection occurred earlier in Znhigh piglets and was measurable already at 7 dpi, when all piglets from this group tended to develop higher (P = 0.06) TGEV-specific serum antibody titers than piglets in the Znlow group. At 11 dpi, seroconversion was clearly detectable in almost all animals. Greater antibody levels were detected in Znmed (P = 0.009) and Znhigh (P = 0.03) groups compared to the Znlow group at 14 dpi (Figure 2A).
Figure 2.
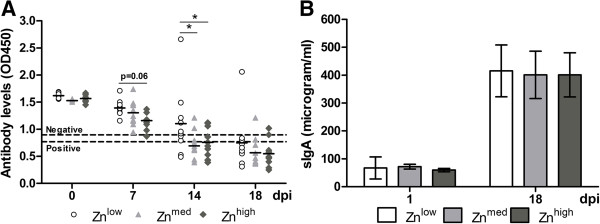
Development of systemic and mucosal immune responses after TGEV infection. (A) TGEV-specific antibodies measured by competitive ELISA are shown as optical densities (OD), measured at 450 nm, from 0 to 18 dpi. An OD450 value higher than 0.9 was considered negative (upper dashed line), an OD450 value lower than 0.77 was considered positive (lower dashed line), and an OD450 value between 0.9 and 0.77 was considered questionable. (B) The concentration of sIgA in intestinal wash fluid was measured by a direct ELISA. Results are shown as the mean value ± standard deviation. Asterisk indicates statistically significant difference (P ≤ 0.05) between the groups.
The levels of sIgA antibodies in intestinal wash fluids were increased 6 to 7-fold from 1 to 18 dpi, showing that mucosal adaptive humoral immune response had also been induced after infection, but the differences between the dietary groups were only marginal (Figure 2B).
Gene expression profiles
To further elucidate the potential effect of Zn supplementation on the immune response and on Zn transport, we examined the expression of genes for cytokines and metal binding/transport proteins in intestinal tissues in the three Zn treatment groups. There was a statistically significant increase of IFN-α expression in the Znlow group compared to Znhigh group (P = 0.009). 2′, 5′-oligoadenylate synthetase (OAS) is one of IFN-stimulated gene products (ISGs), and there was a decreased expression of this enzyme in the Znlow group (P = 0.01) (Figure 3). Expression of ZIP4 was higher and ZnT1, ZnT5 and MT1 were lower in Znhigh group compared with two other groups (Table 1). Expression of IL-6, TNF-α and ZnT2 did not differ between treatments.
Figure 3.
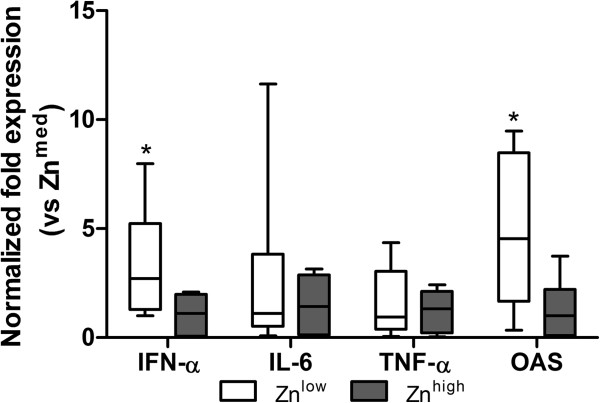
Cytokine expression in intestinal tissues of Zn-treated piglets at 1 dpi. The expression of selected cytokines was assessed by quantitative RT-PCR. The expression of IFN-α and OAS was significantly increased in the Znlow compared to the Znhigh group. Boxes indicate medians (n = 10) (horizontal lines) and the lower and upper quartiles (bottoms and tops of boxes). The vertical bars in the box plots indicate the minimal and maximal values recorded. Asterisk indicates statistically significant differences (P ≤ 0.05) between Zn treatment groups.
Table 1.
Mean relative gene expression of zinc transporters and metallothionein in jejunal tissue of piglets at 1 and 18 dpi 1
| Gene |
1 dpi |
18 dpi |
Significance |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Zn low | Zn med | Zn high | Zn low | Zn med | Zn high | Diet | Time | Diet × Time | |
|
ZIP4 |
1,22 ± 0,16 |
1,46 ± 0,16 |
0,44 ± 0,08 |
3,91 ± 0,44 |
1,68 ± 0,295 |
0,39 ± 0,18 |
*** |
** |
** |
|
ZnT1 |
0,94 ± 0,11 |
0,81 ± 0,08 |
1,47 ± 0,35 |
0,63 ± 0,06 |
0,77 ± 0,075 |
1,38 ± 0,23 |
** |
ns |
ns |
|
ZnT2 |
1,39 ± 0,44 |
0,50 ± 0,14 |
1,82 ± 0,54 |
1,10 ± 0,58 |
1,09 ± 0,634 |
0,85 ± 0,40 |
ns |
ns |
ns |
|
ZnT5 |
0,68 ± 0,06 |
0,61 ± 0,10 |
0,86 ± 0,13 |
0,72 ± 0,10 |
0,50 ± 0,07 |
1,04 ± 0,14 |
** |
ns |
ns |
| MT1 | 0,12 ± 0,02 | 0,12 ± 0,01 | 2,59 ± 4,23 | 0,10 ± 0,01 | 0,15 ± 0,031 | 11,71 ± 4,28 | *** | ns | ns |
Data are means ± SEs, n = 10/group. Significances are depicted as: **, P < 0.01; ***, P < 0.001.
1Abbreviations: MT1 metallothionein-1, ZIP4 Zn transporter SLC39A4, ZnT1 Zn transporter SLC30A1, ZnT2 Zn transporter SLC30A2, ZnT5 Zn transporter SLC30A5.
Histology and immunohistochemistry
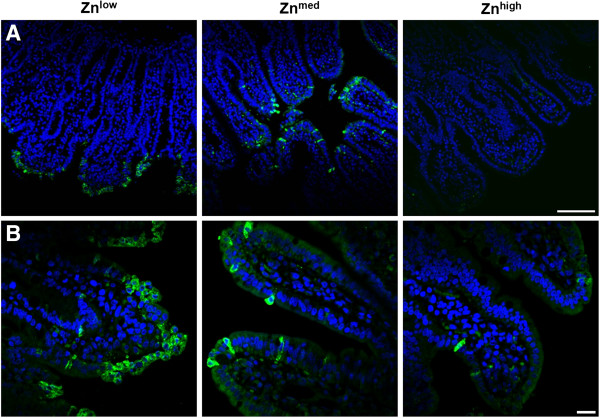
To further investigate the effect of Zn supplementation on TGEV infection in piglets, histological changes in intestinal tissues of piglets were examined. Piglets from the Znlow group showed a destruction of the architecture of intestinal tissue with villous atrophy, which resulted in a significant reduction of the intestinal surface area by a factor of 1.38 (n = 6, P = 0.009) compared to the Znmed group where no villus atrophy was observed (n = 6) (Figure 4A and B). No further changes in villus morphology in the Znhigh group (n = 6) could be detected (Figure 4A and B). In piglets from the Znlow group, the majority of jejunal enterocytes was caspase-3-positive while being morphologically altered, whereas in Znmed jejunal tissue the number of caspase-3-positive cells were drastically reduced and there was a further reduction of apoptotic cells in Znhigh animals (Figure 5A and B). The epithelial architecture of Znmed and Znhigh jejunum was apparently not impaired. The crypt epithelium was not affected by TGEV infection at any Zn concentration.
Figure 4.
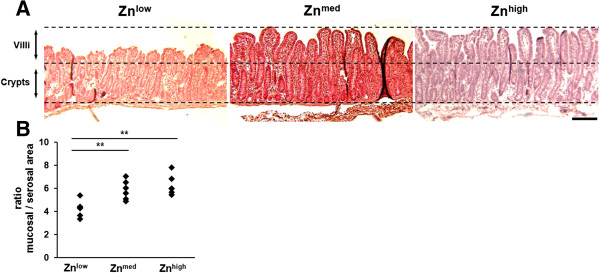
Morphometrical analysis of jejunal tissue at 1 dpi. (A) Jejunal tissue from piglets fed with Znlow, Znmed, and Znhigh diets, respectively, were sliced and H&E stained. The bottom and upper dashed lines indicate the basal and apical borders of the epithelium. The middle dashed line represents the transition zone from where crypts go down and villi go up. TGEV infection in Znlow piglets resulted in villus atrophy, which could be prevented by Znmed and Znhigh diets. Scale bar = 200 μm. (B) H&E-stained jejunal specimens were morphometrically analyzed by measuring the lengths of apical epithelial and the mucosa’s muscular linings. The ratio of mucosal-to-serosal surface area represents a measurement for the effective epithelial area, which was significantly decreased under Znlow (P ≤ 0.01, n =6) compared to Znmed and Znhigh (n = 6 each). The latter conditions were not significantly different.
Figure 5.
Immunofluorescence staining of caspase-3 in jejunal epithelium at 1 dpi. Apoptotic cells are presented by cells being positive for cleaved caspase-3 (as depicted in green). Nuclei (DAPI staining) are presented in blue. (A) When the jejunal epithelium was affected by TGEV infection, only the villus lining was apoptotic, while the crypt epithelium stayed intact. Scale bar = 100 μm. (B) Under Znlow diet, most of jejunal enterocytes were cleaved caspase-3-positive and their shape was distorted, whereas under Znmed treatment, the amount of cleaved caspase-3 signals was reduced and the cell shape was not affected. Under Znhigh, apoptotic cells were extremely rare and cell morphology was not impaired. Scale bar = 20 μm.
Intestinal epithelial resistance, paracellular permeability, and active transport
Taking into account the changes in the surface area (corr), Repi, corr, as a measure of epithelial integrity, was lower in tissues from the Znlow group (20.8 ± 2.2 Ω•cm2, n = 8), compared to Znmed (29.8 ± 2.8 Ω•cm2, n = 10; Znlow vs. Znmed, P = 0.08) and Znhigh (31.4 ± 2.7 Ω•cm2, n = 10; Znlow vs. Znhigh, P = 0.01) (Figure 6A). Surprisingly, permeability to the paracellular marker fluorescein did not significantly differ in TGEV-challenged jejunal epithelia in any of the animals (Figure 6B). As an ex vivo measure for in vivo diarrhea susceptibility, forskolin (FSK)-induced chloride secretion was quantified as increase in short-circuit current, ΔISCFSK,corr. It was found to be increased in jejunal tissue from TGEV-infected Znlow piglets (ΔISCFSK, corr, 110 ± 11 μA/cm2, n = 8) compared to Znmed (54 ± 5 μA/cm2, Znlow vs. Znmed, P = 0.0002, n = 9) and Znhigh (50 ± 7 μA/cm2, Znlow vs. Znhigh, P < 0.0001, n = 10) (Figure 7A), whereas ΔISCFSK, corr of Znmed and Znhigh did not significantly differ. In contrast, average glucose-induced short-circuit currents (ΔISCGlc, corr) were not different in jejunal tissues from piglets fed the tested Zn concentrations, however, against expectations, the range of ΔISCGlc, corr values was greatly increased in Znlow compared to the other two conditions (Figure 7B).
Figure 6.
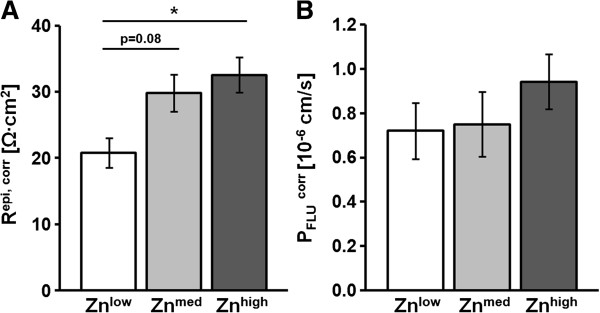
Epithelial resistance and paracellular permeability at 1 dpi. All values were corrected for the epithelial surface area (corr). (A) Epithelia from the Znlow group exhibited significantly decreased Repi, corr values (P = 0.01, n = 8) compared to the Znhigh group (n = 10) and a trend to values lower than those of the Znmed group (P = 0.08, n = 10). (B) Fluorescein permeability (PFLUcorr) did not significantly differ between Zn groups.
Figure 7.
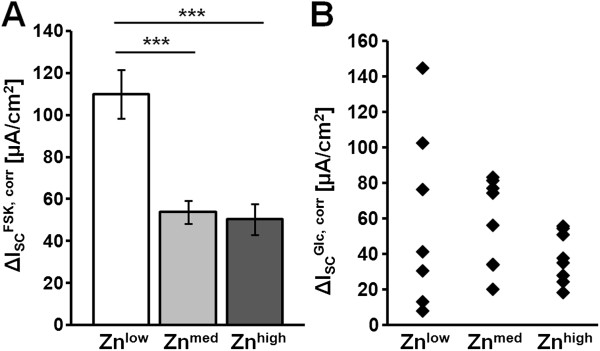
Active transport of jejunal epithelium at 1 dpi. All values were corrected for the epithelial surface area (corr). (A) Stimulation by forskolin (FSK) resulted in a significantly increased chloride secretory response (∆ISCFSK, corr) in the Znlow group (P ≤ 0.001, n = 8) when compared to the Znmed (n = 9) and Znhigh groups (n = 10). (B) Glucose-induced short-circuit currents (∆ISCGlc, corr) of Znlow (n = 7), Znmed (n = 8), and Znhigh (n = 8) were not significantly different. Note the big spread of individual values in the Znlow group.
Discussion
High doses of ZnO (2,000 – 3,000 mg ZnO/kg diet) added to the diets of newly-weaned piglets were shown to improve performance and to reduce the occurrence of unspecific diarrhea [13]. TGEV infection causes villus atrophy with severe and frequently fatal diarrhea in newborn pigs, while the clinical signs in older piglets or in adult pigs are milder or inapparent because of a higher replacement rate of enterocytes compared to newborn piglet [22-24]. However, asymptomatic older piglets may serve as carriers, and the high mutation rates of coronavirus genomes may lead to the generation of more virulent genotypes. For this study we infected weaned piglets with a cell-culture adapted TGEV strain, for which direct antiviral effects of Zn were proven in vitro[5], and tested if direct antiviral and/or systemic effects of different Zn levels could be observed in vivo. Critical factors for the reduction in the mortality and morbidity of piglets from TGE include a reduction in the infectious agent, and oral rehydration therapy for the treatment of dehydration and metabolic acidosis associated with acute diarrhea [12].
In this study, TGEV infection caused only mild clinical symptoms, which can be explained by the piglets’ age and by the use of a tissue-culture adapted virus strain, which was chosen on purpose in order to directly compare results from this study with previous in vitro results [25]. Furthermore, in our study the piglets were provided with a relatively comfortable environment to minimize stress other than that induced by infection. It should be stressed that our experimental conditions vary substantially from those in commercial farming conditions. This could also contribute to the absence of severe clinical signs after infection. However, this study demonstrated that feeding the Znhigh diet improved the fecal score and led to higher body weights after infection in comparison to the Znmed and Znlow groups. Several studies demonstrated that feeding high levels of Zn reduces the incidence and severity of diarrhea and improved fecal consistency [26,27]. Our results are consistent with these findings showing that the Znhigh group had higher fecal scores compared to the Znmed and Znlow groups after infection. There was also a direct correlation between Zn levels and histological changes. TGEV infection in the Znlow group led to destruction of the enterocytes of jejunal villi as reflected by marked villus atrophy. It has been reported that Zn plays a fundamental role in maintaining epithelial barrier integrity and function. For example, supplementation of Zn reduced methotrexate-induced intestinal damage and resulted in faster recovery [28], while it reduced intestinal permeability in Bangladeshi children with acute diarrhea and persistent diarrhea syndrome [29]. Furthermore, feeding supplemental Zn to rats with experimental colitis improved mucosal repair by regulating tight junction permeability [30]. In agreement with these data, higher Repi, corr values in the Znmed and Znhigh group indicate that Zn may prevent epithelial barrier loss induced by TGEV. The dramatic increase in ∆ISCFSK, corr observed in the Znlow group despite the reduced surface area may be interpreted as a protective mechanism. Chloride secretion is the basis of secretory diarrhea and might be regarded as a mechanism to rapidly extrude pathogens. The increase in ∆ISCGlc, corr observed in some of the animals from the Znlow group may be a compensatory effect and is in agreement with the observation that, even in the Znlow group, animals gained weight at a normal rate. This considerably larger variance in the Znlow group indicates that some animals were able to compensate reduced glucose uptake due to the loss of villi by increasing glucose transport capacity.
Zn is also important for the production of antibodies against intestinal pathogens [31]. In agreement with such finding, TGEV-specific serum antibody titers were detected earlier and at higher levels in Znhigh when compared to Znlow piglets. This finding may be the result of multiple effects of Zn on antigen-presenting cells, T-cells and antibody-producing B-lymphocytes. IgA is the primary immunoglobulin isotype induced at the mucosal surface. Secretory IgA (sIgA) in mucosal secretions provides protection against bacterial and viral pathogens and neutralizes microbial toxins [32]. Zn can influence sIgA levels by altering the cytokine profile of stimulated immune cells residing in the gut-associated lymphatic tissue (GALT) [33]. Furthermore, sIgA responses are mediated through activated Th2 cells producing, among other, abundant amount of IL-6 [34]. In this study, there was an increased sIgA levels from 1 to 18 dpi, indicating that adaptive mucosal immune responses were induced by TGEV infection but not influenced by the diet. This finding is in accordance with results from Broom and colleagues [33], who also showed only slight differences of intestinal IgA concentrations between animals either receiving low or high levels of dietary ZnO. In accordance with these findings, the expression of IL-6 in the intestinal tissues also showed no difference between the dietary groups at 1 dpi. Therefore, it is questionable if Zn plays a role in enhancing intestinal IgA concentration.
TGEV infection in piglets is characterized by a robust and early IFN-α production in intestinal secretions and in other organs [35,36]. At 1 dpi, higher IFN-α gene expression levels in the Znlow group may be consistent with a more severe TGEV infection or higher virus loads in this group compared to the Znhigh group. The activation of OAS, one of the IFN-stimulated gene products (ISGs), can lead to apoptosis [37], potentially by indirectly triggering cleavage of caspase-3 [38]. The increased OAS level in the Znlow group may reflect TGEV-induced apoptosis in intestinal epithelial, as it was shown, that the amount of caspase-3-positive cells was markedly increased in the Znlow compared with the Znmed and Znhigh group. This finding is in agreement with the histological observations: the high-grade villus atrophy in the Znlow and normal jejunal mucosal morphology in the Znhigh group.
Metallothionein is known to be induced by exposure to heavy metal cations [39], and in line with a former study [18], the expression level of MT1 was higher with concomitant high levels of Zn in piglets (Additional file 2: Table S2). As reported previously, this indicates an outbalanced Zn homeostasis although the expression of Zn transporters in the jejunum was regulated to reduce Zn uptake (ZIP4) from the gut lumen and increase the export of Zn from epithelial cells (ZnT1, ZnT5) in the Znhigh group. However, an increased level of Zn in intestinal tissue and the induction of metallothionein may explain our observations of improved intestinal mucosal integrity and histological changes, which are in line with other studies [12,14]. Thus, changes in absorption of water and electrolytes may counteract TGEV infection, which causes impaired intestinal epithelial barrier function and decreased uptake of micronutrients.
The paracellular epithelial barrier is highly regulated both under normal conditions and in disease. During infection, TNF-α is a mediator between the immune system and the intestinal epithelial barrier by altering tight junction proteins and their cellular localization. More specifically, the cytokine up-regulates the pore-forming protein claudin-2 [40] and redistributes the tightening protein claudin-1 [41]. This leads to an impaired barrier function which is associated with increased paracellular permeability. However, our in vitro work [25] and the in vivo study presented here did not find any up- or down-regulation of TNF-α after TGEV infection in any of the experimental groups. The outcome is, however, in accordance with the data set of permeability to fluorescein (PFLUcorr), as paracellular permeability was not affected. The data could, therefore, be interpreted as a result of a different pathological mechanism, possibly mediated by IFN-α, rather than by TNF-α, as up-regulation of the former in Znlow piglets was clearly evident.
Conclusions
This study provides data that supplementation of the post-weaning diet with high levels of ZnO resulted in earlier and higher TGEV-specific antibody response, modulation of cytokine expression, and prevention of disruption of the intestinal barrier integrity. Our findings might also be interesting for infections with other coronaviruses like SARS-CoV, which could cause gastrointestinal symptoms and diarrhea as well.
Methods
Virus and cells
The TGEV strain Purdue 46-MAD (kindly provided by Dr. C. Schwegmann-Wessels, Institut für Virologie, Tierärztliche Hochschule Hannover) was used in this study. To prepare TGEV stocks, a stable mycoplasma-free swine testicle (ST) cell line supporting the growth of TGEV was used. Cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM; PAN-Biotech, Aidenbach, Germany) supplemented with 10% heat-inactivated fetal calf serum (Hyclone), and 1% penicillin/streptomycin (Biochrom, Berlin, Germany) at 37°C with 5% CO2. Stock virus was propagated in ST cells to a titer of 1.00E + 07 plaque-forming unit (PFU)/mL.
Animals and experimental design
German landrace piglets (n = 60) of both sexes from a TGEV-free herd (Leibniz Institut für Nutztierbiologie, Dummerstorf, Germany) were weaned at the age of 21 days and were randomly assigned to three different dietary groups (20 animals each). Diets either contained no additional ZnO (Znlow: 50 mg Zn/kg diet), or were supplemented with analytical grade ZnO (>98% purity) to contain 150 mg Zn/kg diet (Znmed) or 2,500 mg Zn/kg diet (Znhigh). The Znlow diet represents the regular feed of the animals and the Znlow group, therefore, represented the control group for this experiment. At 26 days of age, animals were moved to a containment facility (Bundesinstitut für Risikobewertung, Berlin, Germany) where they were randomly allocated to 6 pens per group. The piglets were fed the respective diet ad libitum in a pelleted form, water was also provided ad libitum by nipple drinkers. The pens were of equal size (2.8 m2), equipped with a feeding automate with 5 feeding places and a nipple drinker. The floors were covered with rubber mate and a red light lamp was placed above to provide additional heat. Room temperature was kept at 25 ± 1°C with humidity of 50 - 60% and constant air volume exchange. The pens were thoroughly cleaned by brushing the floors and walls with consecutive flushing with lukewarm water in the mornings. Superficial flushing of the floors was additionally performed in the late afternoon. At day 28 of age, all piglets were challenged orally with 2 mL TGEV with a titer of 1.0E+07 PFU/mL. In each group, half of the piglets (n = 10 per group) were sacrificed 1 day post infection (dpi) in order to examine acute infection, since piglets reportedly display strong symptoms of gastroenteritis within 20 h post-infection [42], and the other half (n = 10 per group) were sacrificed 18 dpi to see the effect of Zn on adaptive immune response.
Clinical follow-up and sampling
The study was approved by the local animal welfare authority (Landesamt für Gesundheit und Soziales, Berlin, Germany) under the registration number G 0116/12. Animals were clinically examined on arrival. Blood samples were collected at 0, 4, 7, 11, 14 and 18 dpi. Sera were used to determine TGEV-specific antibodies. Piglets were monitored daily for rectal temperature and body weight was recorded once weekly. Fecal scores (from 1 to 5, where 1 means watery and 5 dry and hard stool) were also recorded daily up to 12 dpi. Fecal swabs were taken before and then daily after infection for the detection of virus shedding. At necropsy (both 1 and 18 dpi), 10 – 15 cm long samples from the descending duodenum, jejunum (approximately 100 cm distal to the duodeno-jejunal flexure), ileum (distal 15 cm), spleen and jejunal mesenteric lymph nodes were taken to determine gene expression profiles. Furthermore, defined pieces of the jejunum of the same length were washed with 25 mL PBS and intestinal fluid was collected to detect adaptive immune response (sIgA). Additionally, 15 cm of mid jejunum (1 dpi) were removed, cut open, and rinsed with and transported in cooled saline solution (0.9% NaCl, 1 mM CaCl2). Jejunal tissue was stripped off the muscle layer and explants were mounted in Ussing chambers for electrophysiological analysis as described in detail below. Part of each intestinal tissue sample was additionally fixed in buffered 4% formalin.
Confirmation of Zn status
Trace element status of the pigs was determined in serum and liver tissue as described previously [18]. Briefly, organs were freeze dried, incinerated and hydrolysed in concentrated hydrochloric acid. Serum samples were hydrolysed directly. Trace element concentration was determined by atomic absorption spectrometry in an AAS vario 6 spectrometer (Analytik Jena, Jena, Germany).
Real-time quantitative RT-PCR (qRT-PCR) analysis
Total RNA was extracted from 20 mg of jejunum sample or 200 μL of serum using a Nucleo-Spin® RNA II Kit for Tissue (Macherey & Nagel, Düren, Germany) following the manufacturer’s instructions. Reverse transcription (RT) was performed using the RevertAidTM First Strand cDNA Synthesis Kit (Fermentas, St. Leon-Rot, Germany) according to the manufacturer’s instructions. PCR reactions were performed in a total volume of 25 μL in an iCycler iQ5® detection system (Bio-Rad Laboratories, München, Germany). Melt curve analysis and agarose gel electrophoresis were performed after completion of each assay to confirm specificity of the amplification. For the following target genes: Zn transporters SLC30A1 (ZnT1), SLC30A2 (ZnT2), SLC30A5 (ZnT5), SLC39A4 (ZIP4), and metallothionein (MT1), quantitative real-time RT-PCR was performed using the one-step QRT-PCR master mix kit (Brilliant®II SYBR®Green, Agilent Technologies, Santa Clara, USA) as described previously [18].
TGEV genome copy numbers were quantified using a TaqMan fluorescent quantitative (q) PCR assay as previously shown [25]. Expression levels of IFN-α, IL-6, OAS, and TNF-α were calculated using the Delta-Delta-Ct-Method calculation [43]. Four commonly used reference genes (β-2 microglobulin, RPL13, RPL19 and SDHA) were selected for normalization of gene expression. A mean expression value (normalization factor) for the four reference genes was calculated to enable normalization of gene expression data for all the genes of interest. Samples from Znmed group were used the references for the calculations. For the expression of Zn transporters ZnT1, ZnT2, ZnT5, ZIP4 and MT1, standard curves were generated using serial dilutions of pooled RNA (within a range from 5–200 ng/μL) from 20 samples to convert Ct values into arbitrary values. These values were then normalized using the mean values of the house-keeping genes and then used for statistical comparisons. The names of genes, primer sequences, annealing temperature, and references are listed in Additional file 1: Table S1.
Enzyme-linked immunosorbent assay (ELISA)
Blood samples were collected on 0, 4, 7, 11, 14 and 18 dpi, and TGEV-specific IgG antibodies were determined using a commercial ELISA (INGEZIM TGEV 2.0, Ingenasa). Intestinal wash fluid was collected on 1 and 18 dpi and mucosal sIgA antibodies in the supernatants were also measured using a commercial ELISA (Pig IgA ELISA Kit, Bethyl Laboratories, Montgomery, USA) following the manufacturer’s instructions.
Electrophysiology
Jejunal specimens were mounted in modified Ussing chambers to carry out impedance measurements as described previously [44]. In brief, one-path impedance spectroscopy was performed to determine epithelial (Repi) and subepithelial (Rsub) contributions to the transepithelial resistance (TER). Resistances of bath solution without tissue as well as electrode offsets were recorded prior to each experiment and subtracted from experimental data. Preparations were allowed to equilibrate for 45 min and Repi was measured as previously described [45].
As pathogen-induced secretory diarrhea is caused by excessive chloride secretion with accompanied osmotically driven water flux, forskolin (Calbiochem®, Merck, Darmstadt, Germany; final concentration 10 μM), a secretagogue agent, was added basolaterally in supplemented Ringer’s solution (113.6 mM NaCl, 5.4 mM KCl, 1.2 mM MgCl2, 1.2 mM CaCl2, 21 mM NaHCO3, 0.6 mM NaH2PO4, 2.4 mM Na2HPO4, 10 mM D(+)-glucose, 2.5 mM glutamine, 10 mM D(+)-mannose, 0.5 mM β-OH-butyrate, 50 mg/l piperacillin, 4 mg/l imipenem; pH 7.4 when equilibrated with carbogen) in order to trigger a chloride secretory response. For testing the sodium-coupled and therefore electrogenic glucose absorption via SGLT1, glucose (Roth, Karlsruhe, Germany; final concentration 10 mM) was added apically in glucose-free Ringer’s solution (113.6 mM NaCl, 5.4 mM KCl, 1.2 mM MgCl2, 1.2 mM CaCl2, 21 mM NaHCO3, 0.6 mM NaH2PO4, 2.4 mM Na2HPO4; pH 7.4 when equilibrated with carbogen). After administration of effectors, changes in short-circuit current (∆ISC) were recorded.
Permeability to fluorescein (332 Da) represents a measure for the paracellular leakiness/tightness of the epithelial layer and was ascertained in Ussing chamber experiments under voltage clamp conditions. After equilibrating jejunal specimens in supplemented Ringer’s solution, fluorescein (Sigma-Aldrich, St. Louis, USA) was added apically (final concentration, 50 μM). At 30 and 90 min post administration, basolateral samples were replaced with Ringer’s solution. Fluorescein concentrations were determined with a fluorometer at 525 nm (Infinite M200, Tecan, Crailsheim, Germany) and permeabilities were calculated.
Morphometry
Formalin-fixed tissue sections were stained with hematoxylin and eosin (H&E) using standard staining protocols and analyzed using the freehand line selection tool of Image J (Rasband, ImageJ, NIH, Bethesda, Maryland; http://rsb.info.nih.gov/ij/).
The surface area of the jejunal mucosa was assessed as the ratio of mucosal-to-serosal surface area from the lengths of apical epithelial as well as muscular mucosa linings in equivalent fields of view of adjacent sections. Crypt/villus height and density were determined from five sections per piglet, each ~750 μm in width.
Immunofluorescence staining
In order to highlight caspase-3-mediated apoptotic events in jejunal tissue, formalin-fixed tissue slices were rehydrated (xylene, increasing ethanol series), heated at 95 °C in citrate buffer (10 mM citric acid, pH 6.0) for 15 min, and then washed in phosphate-buffered saline (PBS). Tissue slices were then incubated in blocking solution (6% goat serum + 1% BSA in PBS) for 30 min at room temperature (RT) before incubation with rabbit anti-cleaved caspase-3 (Cell Signaling Technology, Cambridge, UK) at a 1:250 dilution in blocking solution for 60 min at RT. After several washings, tissue slices were incubated with goat anti-rabbit F(ab')2 conjugated with DyLight™488 (Jackson ImmunoResearch, Newmarket, UK) at a 1:500 dilution and 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI, 1 μg/mL; Roche, Mannheim, Germany) for 60 min at RT in the dark. After washing, sections were embedded using ProTaqs Mount Fluor (Biocyc, Luckenwalde, Germany).
Images were taken with an inverted Zeiss LSM 510 META confocal laser-scanning microscope (Carl Zeiss, Jena, Germany). Digital images were processed using Fiji imaging software [46] and Zeiss LSM 510 META software.
Statistical analyses
Calculations were performed with SPSS® Version 21 (IBM, Armonk, USA) and GraphPad Prism 5 (GraphPad Software Inc, La Jolla, USA). Two-factorial mixed models were applied to calculate residuals for all variables (fixed factor: diet, time, diet*time) and a random effect (animal). Data from gene expression were used for the one-factorial model (fixed factor: diet). F-Test was applied for fixed effects and interaction effects with subsequent LSD post hoc test. Electrophysiology and morphometric data are expressed as means ± standard error of the mean (SEM), and statistical analyses were carried out using a one-way ANOVA with Tukey HSD post hoc test. Significances are depicted as: *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; tendencies are given as 0.05 ≤ P ≤ 0.1.
Abbreviations
dpi: Day post infection; H&E: Hematoxylin and eosin; IFN-α: Interferon alpha; MT1: Metallothionein-1; IL-6: Interleukin-6; OAS: Oligoadenylate synthetase; RPL13: 60S ribosomal protein L13; SDHA: Succinate dehydrogenase subunit A; ST cells: Swine testicle cells; TER: Transepithelial resistance; TGEV: Transmissible gastroenteritis virus; TNF-α: Tumor necrosis factor-alpha; ZIP4: Zn transporter SLC39A4; ZnT1: Zn transporter SLC30A1; ZnT2: Zn transporter SLC30A2; ZnT5: Zn transporter SLC30A5.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
MB, WC and NO conceived and designed experiments. WC, SSZ, DG, RP, PJ, and ZW performed the experiments; WC, MB SSZ, RP, and ST performed statistical analyses of experimental data. WC, MB, and NO prepared the draft of the manuscript; and MB, and NO had primary responsibility for the final content. All authors critically revised the manuscript and approved the final version.
Supplementary Material
Detailed qRT-PCR primers and conditions used in this study.
Mean concentrations of trace elements (mg/kg fresh matter1) in liver and serum at 1 and 18 dpi.
Contributor Information
Weidong Chai, Email: weidong.chai@fu-berlin.de.
Silke S Zakrzewski, Email: silke.zakrzewski@charite.de.
Dorothee Günzel, Email: dorothee.guenzel@charite.de.
Robert Pieper, Email: robert.pieper@fu-berlin.de.
Zhenya Wang, Email: zhenya.wang0371@gmail.com.
Sven Twardziok, Email: sven.twardziok@charite.de.
Pawel Janczyk, Email: pawel.janczyk@bfr.bund.de.
Nikolaus Osterrieder, Email: no34@cornell.edu.
Michael Burwinkel, Email: burwinkelm@rki.de.
Acknowledgements
The authors would like to acknowledge the animal welfare officer Mechthild Ladwig, Bundesinstitut für Risikobewertung, for fruitful discussions and input considering the animal care and welfare. All animal technicians supervised by Dr. Stefanie Banneke receive our great appreciation for their engagement. We further acknowledge Enno Luge for his excellent technical assistance. We thank Prof. Michael Schmidt, Institut für Immunologie, Freie Universität Berlin, for critical reading of the manuscript. The superb technical assistance of Bettina Esch, Institut für Virologie, Christiane Palissa, Institut für Immunologie, and Anja Fromm, Institut für Klinische Physiologie, is gratefully acknowledged.
References
- Arens M, Travis S. Zinc salts inactivate clinical isolates of herpes simplex virus in vitro. J Clin Microbiol. 2000;38:1758–1762. doi: 10.1128/jcm.38.5.1758-1762.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne N, Stegall R, Montano R, Meador M, Stanberry LR, Milligan GN. Efficacy and toxicity of zinc salts as candidate topical microbicides against vaginal herpes simplex virus type 2 infection. Antimicrob Agents Ch. 2005;49:1181–1183. doi: 10.1128/AAC.49.3.1181-1183.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraguchi Y, Sakurai H, Hussain S, Anner BM, Hoshino H. Inhibition of HIV-1 infection by zinc group metal compounds. Antiviral Res. 1999;43:123–133. doi: 10.1016/S0166-3542(99)00040-6. [DOI] [PubMed] [Google Scholar]
- Suara RO, Crowe JE Jr. Effect of zinc salts on respiratory syncytial virus replication. Antimicrob Agents Ch. 2004;48:783–790. doi: 10.1128/AAC.48.3.783-790.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z, Burwinkel M, Palissa C, Ephraim E, Schmidt MF. Antiviral activity of zinc salts against transmissible gastroenteritis virus in vitro. Vet Microbiol. 2012;160:468–472. doi: 10.1016/j.vetmic.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Velthuis AJ, van den Worm SH, Sims AC, Baric RS, Snijder EJ, van Hemert MJ. Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6:e1001176. doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbeck S, Rink L, Haase H. Modulating the immune response by oral zinc supplementation: a single approach for multiple diseases. Archivum Immunologiae et Therapiae Experimentalis. 2008;56:15–30. doi: 10.1007/s00005-008-0003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg K, Bolt G, Andersen H, Owen TC. Zinc potentiates the antiviral action of human IFN-alpha tenfold. J Interferon Cytokine Res. 2001;21:471–474. doi: 10.1089/10799900152434330. [DOI] [PubMed] [Google Scholar]
- Fraker PJ, Jardieu P, Cook J. Zinc deficiency and immune function. Archives Dermatol. 1987;123:1699–1701. doi: 10.1001/archderm.1987.01660360152028. [DOI] [PubMed] [Google Scholar]
- Fraker PJ, Gershwin ME, Good RA, Prasad A. Interrelationships between zinc and immune function. Fed Proc. 1986;45:1474–1479. [PubMed] [Google Scholar]
- Fischer Walker C, Black RE. Zinc and the risk for infectious disease. Annu Rev Nutr. 2004;24:255–275. doi: 10.1146/annurev.nutr.23.011702.073054. [DOI] [PubMed] [Google Scholar]
- Hoque KM, Binder HJ. Zinc in the treatment of acute diarrhea: current status and assessment. Gastroenterology. 2006;130:2201–2205. doi: 10.1053/j.gastro.2006.02.062. [DOI] [PubMed] [Google Scholar]
- Poulsen HD. Zinc-Oxide for Weanling Piglets. Acta Agr Scand a-An. 1995;45:159–167. [Google Scholar]
- Ghishan FK. Transport of electrolytes, water, and glucose in zinc deficiency. J Pediatr Gastroenterol Nutr. 1984;3:608–612. doi: 10.1097/00005176-198409000-00022. [DOI] [PubMed] [Google Scholar]
- Bettger WJ, O'Dell BL. A critical physiological role of zinc in the structure and function of biomembranes. Life sciences. 1981;28:1425–1438. doi: 10.1016/0024-3205(81)90374-X. [DOI] [PubMed] [Google Scholar]
- Commission Regulation (EC) No 1334/2003. Conditions for authorisation of a number of additives in feedingstuffs belonging to the group of trace elements. Official J European Union. 2003;187:11. [Google Scholar]
- Lichten LA, Cousins RJ. Mammalian zinc transporters: nutritional and physiologic regulation. Ann Rev Nutr. 2009;29:153–176. doi: 10.1146/annurev-nutr-033009-083312. [DOI] [PubMed] [Google Scholar]
- Martin L, Lodemann U, Bondzio A, Gefeller EM, Vahjen W, Aschenbach JR, Zentek J, Pieper R. A high amount of dietary zinc changes the expression of zinc transporters and metallothionein in jejunal epithelial cells in vitro and in vivo but does not prevent zinc accumulation in jejunal tissue of piglets. J Nutr. 2013;143:1205–1210. doi: 10.3945/jn.113.177881. [DOI] [PubMed] [Google Scholar]
- Martin L, Pieper R, Schunter N, Vahjen W, Zentek J. Performance, organ zinc concentration, jejunal brush border membrane enzyme activities and mRNA expression in piglets fed with different levels of dietary zinc. Arch Anim Nutr. 2013;67:248–261. doi: 10.1080/1745039X.2013.801138. [DOI] [PubMed] [Google Scholar]
- Cavanagh D. Nidovirales: a new order comprising Coronaviridae and Arteriviridae. Arch Virol. 1997;142:629–633. [PubMed] [Google Scholar]
- Sola I, Castilla J, Pintado B, Sanchez-Morgado JM, Whitelaw CB, Clark AJ, Enjuanes L. Transgenic mice secreting coronavirus neutralizing antibodies into the milk. J Virol. 1998;72:3762–3772. doi: 10.1128/jvi.72.5.3762-3772.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingartl HM, Derbyshire JB. Evidence for a putative second receptor for porcine transmissible gastroenteritis virus on the villous enterocytes of newborn pigs. J Virol. 1994;68:7253–7259. doi: 10.1128/jvi.68.11.7253-7259.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BM, Han YW, Kim SB, Rahman MM, Uyangaa E, Kim JH, Roh YS, Kim B, Han SB, Hong JT, Kim K, Eo SK. Enhanced protection against infection with transmissible gastroenteritis virus in piglets by oral co-administration of live attenuated Salmonella enterica serovar Typhimurium expressing swine interferon-alpha and interleukin-18. Comp Immunol Microbiol Infect Dis. 2011;34:369–380. doi: 10.1016/j.cimid.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Moon HW. Epithelial cell migration in the alimentary mucosa of the suckling pig. Proc Soc Exp Biol Med Soc Exp Biol Med. 1971;137:151–154. doi: 10.3181/00379727-137-35533. [DOI] [PubMed] [Google Scholar]
- Chai W, Burwinkel M, Wang Z, Palissa C, Esch B, Twardziok S, Rieger J, Wrede P, Schmidt MF. Antiviral effects of a probiotic Enterococcus faecium strain against transmissible gastroenteritis coronavirus. Archives of virology. 2013;158:799–807. doi: 10.1007/s00705-012-1543-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Guo Y. Supplemental zinc reduced intestinal permeability by enhancing occludin and zonula occludens protein-1 (ZO-1) expression in weaning piglets. Br J Nutr. 2009;102:687–693. doi: 10.1017/S0007114509289033. [DOI] [PubMed] [Google Scholar]
- Mavromichalis I, Webel DM, Parr EN, Baker DH. Growth-promoting efficacy of pharmacological doses of tetrabasic zinc chloride in diets for nursery pigs. Can J Anim Sci. 2001;81:387–391. doi: 10.4141/A01-005. [DOI] [Google Scholar]
- Tran CD, Howarth GS, Coyle P, Philcox JC, Rofe AM, Butler RN. Dietary supplementation with zinc and a growth factor extract derived from bovine cheese whey improves methotrexate-damaged rat intestine. Am J Clin Nutr. 2003;77:1296–1303. doi: 10.1093/ajcn/77.5.1296. [DOI] [PubMed] [Google Scholar]
- Roy SK, Behrens RH, Haider R, Akramuzzaman SM, Mahalanabis D, Wahed MA, Tomkins AM. Impact of zinc supplementation on intestinal permeability in Bangladeshi children with acute diarrhoea and persistent diarrhoea syndrome. J Pediatr Gastroenterol Nutr. 1992;15:289–296. doi: 10.1097/00005176-199210000-00010. [DOI] [PubMed] [Google Scholar]
- Sturniolo GC, Fries W, Mazzon E, Di Leo V, Barollo M, D'Inca R. Effect of zinc supplementation on intestinal permeability in experimental colitis. J Lab Clin Med. 2002;139:311–315. doi: 10.1067/mlc.2002.123624. [DOI] [PubMed] [Google Scholar]
- Sargeant HR, Miller HM, Shaw MA. Inflammatory response of porcine epithelial IPEC J2 cells to enterotoxigenic E. coli infection is modulated by zinc supplementation. Mol Immunol. 2011;48:2113–2121. doi: 10.1016/j.molimm.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Pacheco S, Acuna CL, Switzer KC, Wang Y, Gilmore X, Harriman GR, Mbawuike IN. Immunoglobulin A-deficient mice exhibit altered T helper 1-type immune responses but retain mucosal immunity to influenza virus. Immunology. 2002;105:286–294. doi: 10.1046/j.0019-2805.2001.01368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broom LJ, Miller HM, Kerr KG, Knapp JS. Effects of zinc oxide and Enterococcus faecium SF68 dietary supplementation on the performance, intestinal microbiota and immune status of weaned piglets. Res Vet Sci. 2006;80:45–54. doi: 10.1016/j.rvsc.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Simecka JW. Mucosal immunity of the gastrointestinal tract and oral tolerance. Adv Drug Deliv Rev. 1998;34:235–259. doi: 10.1016/S0169-409X(98)00042-8. [DOI] [PubMed] [Google Scholar]
- Charley B, Riffault S, Van Reeth K. Porcine innate and adaptative immune responses to influenza and coronavirus infections. Ann N Y Acad Sci. 2006;1081:130–136. doi: 10.1196/annals.1373.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riffault S, Carrat C, van Reeth K, Pensaert M, Charley B. Interferon-alpha-producing cells are localized in gut-associated lymphoid tissues in transmissible gastroenteritis virus (TGEV) infected piglets. Vet Res. 2001;32:71–79. doi: 10.1051/vetres:2001111. [DOI] [PubMed] [Google Scholar]
- Player MR, Torrence PF. The 2-5A system: modulation of viral and cellular processes through acceleration of RNA degradation. Pharmacol Ther. 1998;78:55–113. doi: 10.1016/S0163-7258(97)00167-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusch L, Zhou A, Silverman RH. Caspase-dependent apoptosis by 2',5'-oligoadenylate activation of RNase L is enhanced by IFN-beta. J Interferon Cytokine Res. 2000;20:1091–1100. doi: 10.1089/107999000750053762. [DOI] [PubMed] [Google Scholar]
- Ishii K, Usui S, Yamamoto H, Sugimura Y, Tatematsu M, Hirano K. Decreases of metallothionein and aminopeptidase N in renal cancer tissues. J Biochem. 2001;129:253–258. doi: 10.1093/oxfordjournals.jbchem.a002852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankertz J, Amasheh M, Krug SM, Fromm A, Amasheh S, Hillenbrand B, Tavalali S, Fromm M, Schulzke JD. TNFalpha up-regulates claudin-2 expression in epithelial HT-29/B6 cells via phosphatidylinositol-3-kinase signaling. Cell Tissue Res. 2009;336:67–77. doi: 10.1007/s00441-009-0751-8. [DOI] [PubMed] [Google Scholar]
- Amasheh M, Fromm A, Krug SM, Amasheh S, Andres S, Zeitz M, Fromm M, Schulzke JD. TNFalpha-induced and berberine-antagonized tight junction barrier impairment via tyrosine kinase, Akt and NFkappaB signaling. J Cell Sci. 2010;123:4145–4155. doi: 10.1242/jcs.070896. [DOI] [PubMed] [Google Scholar]
- Schwegmann-Wessels C, Herrler G. Transmissible gastroenteritis virus infection: a vanishing specter. DTW Deutsche tierarztliche Wochenschrift. 2006;113:157–159. [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Gitter AH, Schulzke JD, Sorgenfrei D, Fromm M. Ussing chamber for high-frequency transmural impedance analysis of epithelial tissues. J Biochem Bioph Methods. 1997;35:81–88. doi: 10.1016/S0165-022X(97)00028-6. [DOI] [PubMed] [Google Scholar]
- Fromm M, Palant CE, Bentzel CJ, Hegel U. Protamine reversibly decreases paracellular cation permeability in Necturus gallbladder. J Membr Biol. 1985;87:141–150. doi: 10.1007/BF01870660. [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detailed qRT-PCR primers and conditions used in this study.
Mean concentrations of trace elements (mg/kg fresh matter1) in liver and serum at 1 and 18 dpi.