Abstract
Temporal order memory, or remembering the order of events, is critical for everyday functioning and is difficult for patients with mild cognitive impairment (MCI). It is currently unclear whether these patients have difficulty acquiring and/or retaining such information and whether deficits in these patients are in excess of “normal” age-related declines. Therefore, the current study examined age and disease-related changes in temporal order memory as well as whether memory load played a role in such changes. Young controls (n=25), older controls (n=34), and MCI patients (n=32) completed an experimental task that required the reconstruction of sequences that were 3, 4, or 5-items in length both immediately after presentation (i.e., immediate recall) and again following a 10-minute delay (i.e., delayed recall). During the immediate recall phase, there was an effect of age largely due to reduced performance at the two longest span lengths. Older controls and MCI patients only differed during the 5 span (controls > MCI). During the delayed recall, however, there were significant effects of both age and MCI regardless of span length. In MCI patients, immediate recall was significantly correlated with measures of executive functioning while delayed recall performance was only related to other memory tests. These findings suggest that MCI patients experience initial temporal order memory deficits at the point when information begins to exceed working memory capacity and become dependent on medial temporal lobe functioning. Longer-term deficits are due to an inability to retain information, consistent with the characteristic medial temporal lobe dysfunction in MCI.
1. Introduction
Remembering the order in which events occur is critical in our everyday lives, whether for traveling from one location to another, baking cookies, or relaying our medical history. We refer to this process as temporal order memory hereafter. Neuroanatomically, both the prefrontal and medial temporal cortices have been implicated in temporal order memory processing (see review by Dickerson and Eichenbaum, 2010). Although the exact contributions of each region and how they interact are still under investigation, some trends are emerging. Learning and recalling the order of events requires the online maintenance and comparison of both the items and the time at which they occurred. These cognitive processes would presumably be mediated by working memory (see Baddeley, 2003 for a comprehensive review). The prefrontal cortex plays a critical role in both the mental holding (ventrolateral) and manipulation (dorsolateral) of information within working memory (D'Esposito et al., 1998; Wager & Smith, 2003). Not surprisingly then, the prefrontal cortex has been implicated in perceiving the passage of time (Wittmann et al., 2010), judging the order and frequency of recent events (Cabeza et al., 1997; Cabeza et al., 2000; Shimamura, Janowsky, and Squire, 1990), and in the planning and self-monitoring of response sequences (Milner et al., 1985; Stuss, Binns, Murphy, and Alexander, 2002). Such findings have been supported by temporal order learning deficits in patients with frontal lobe lesions (Shimamura et al., 1990; Kesner, Hopkins, and Fineman, 1994) and through functional neuroimaging studies that revealed prefrontal involvement during the encoding of temporally relevant information (Amiez and Petrides, 2007). A recent neuroimaging study suggests that the prefrontal cortex plays a greater role during encoding than subsequent retrieval of temporally-based information (Duarte et al., 2010); findings that suggest other brain structures are important for the “long-term” retention of this information. The medial temporal lobes are critical for forming new memories (Squire and Zola, 1996) and are believed to bind the various aspects of memories together (i.e., the “what” and the “when”) (see reviews by Dickerson and Eichenbaum, 2010 & Mayes et al., 2004). Earlier studies have shown medial temporal involvement in sequence memory (Ross, Brown, and Stern, 2009). Together, these findings suggest that prefrontally-mediated working memory may be especially critical during the initial acquisition and encoding of temporal information. This process would seemingly depend on Baddeley's (2003) episodic buffer, which is conceptualized as a “…limited capacity store that binds together information to form integrated episodes” (p. 836). Such episodes would then be transferred into “long-term” memory via the medial temporal (hippocampal) memory system.
A considerable body of evidence suggests that cognitive abilities that are mediated by the prefrontal cortex (i.e., executive abilities) show decline in healthy aging (Braver and West, 2008), including those related to temporal processing (Salthouse and Miles, 2002). Additionally, hippocampal subregions, namely the dentate gyrus, also appear to show age-related decline (Brickman et al., 2011; for a review see Small et al., 2011). It is perhaps not surprising, then, that temporal order memory generally declines with age (Cabeza et al., 2000; Spencer and Raz, 1995). However, these brain regions show further decline in patients with mild cognitive impairment (MCI), which is generally viewed as a clinical precursor of Alzheimer's disease (AD) and primarily characterized by learning and memory deficits (Albert et al., 2011; Petersen, 2004). The medial temporal lobes have long been known to be a site of early AD-related pathology (see Braak, Griffing, and Arai, 1999 for a review) and additional hippocampal subregions (entorhinal cortex) are disproportionately affected by MCI and AD (see review by Small et al., 2011). Many patients with MCI also demonstrate disease pathology within the prefrontal cortex (Ewers et al., 2011). Consistent with the pathological processes in these key brain regions, temporal order memory deficits have been reported in patients with AD (Hampstead et al., 2010; Hanseeuw et al., 2011, Johnson and Kesner, 1997; Madsen and Kesner, 1995) and MCI (Bellassen et al., 2012). In fact, this latter study suggested that temporal order memory is a sensitive marker for subsequent conversion to AD (Bellassen et al., 2012). It is unclear, however, whether these deficits arise from an inability to acquire / encode or to retain temporally based information.
Therefore, the current study used a novel Temporal Sequencing Task (TST) to address two key questions (i.e., goals). First, are the presumed temporal order memory deficits in MCI in excess of “normal” age-related changes? To answer this question, we adopted a cross-sectional design that compared performances of healthy young adults, healthy older adults, and patients with MCI. Second, at what stage of memory processing do the deficits (if present) emerge as a result of age and MCI? Specifically, do the deficits arise because of a failure to acquire/encode the temporal order or because of an inability to retain this information? The former would suggest prefrontally-mediated working memory impairment (e.g., within the episodic buffer) leads to these deficits whereas the latter would support a primary role of the medial temporal memory system. To answer this question, the TST required participants to recall sequences of items both immediately after presentation (i.e., within the confines of working memory) and again after a 10-minute delay (i.e., within the confines of the medial temporal lobe memory system). Based on the literature reviewed above, we predicted a primary effect of age during both the immediate and delayed recall portions of the TST. Because MCI patients typically demonstrate greater memory (i.e., medial temporal lobe dysfunction) than working memory (i.e., prefrontally-mediated) impairment, we predicted that the effect of MCI would be superimposed on these age-effects during the delayed recall phase.
We previously reported a linear relationship between span length and temporal order memory in patients with AD (Hampstead et al., 2010) while another study revealed a significant relationship between span and medial temporal lobe volume in MCI patients (Wenger et al., 2010). Therefore, we performed exploratory analyses to examine whether span length affected performance on the TST as a function of age and MCI.
2. Methods
2.1. Participants
A total of 91 individuals participated in this study. Thirty-two patients had been diagnosed with MCI according to Petersen's criteria (Petersen, 2004), of these, 6 were single-domain (amnestic) and the remaining 26 were multi-domain (amnestic + at least one other cognitive domain). Specifically, there was a subjective report of cognitive decline (provided by the patient or an informant) and objective evidence of impairment on standardized neuropsychological testing that was below expectations relative to their peers. As can be seen in Table 1, the MCI group averaged about 1.6 standard deviations below the mean on the RBANS Delayed Memory Index. This index provided an independent measure of memory functioning that had not been used in the diagnostic process. Additionally, it is based on performances on three separate memory tests, which is important because previous research has shown a lower risk of reversion from MCI to normal when multiple memory test performances are used (Chang et al., 2010; Loewenstein et al., 2009). Critical for the diagnosis of MCI, instrumental activities of daily living (IADLs) were relatively preserved as measured by the Functional Activities Questionnaire (Pfeffer et al., 1982). Healthy older controls (n=34) and healthy young controls (n=25) were free of subjective complaints or objective evidence of cognitive impairment (all performances were required to be within normal limits on the measures listed below) and were fully independent in all IADLs. All participants were also required to score within normal limits on the Beck Depression Inventory – II (Beck et al., 1996) (young controls) or Geriatric Depression Scale (Yesavage et al., 1983) (older controls and MCI). General exclusion criteria included a history of neurologic injury or disease (e.g., stroke, moderate or severe traumatic brain injury, and epilepsy), psychiatric disorders (e.g., severe depression, bipolar disorder, and schizophrenia), current or past alcohol or drug abuse, and a history of learning or attentional disorders. The Institutional Review Board of Emory University and the Research and Development Committee of the Atlanta VAMC approved the study. All participants provided written informed consent.
Table 1.
Demographic and baseline neuropsychological test performances and between-group differences.
| Young Controls (n=25) | Older Controls (n=34) | MCI (n=32) | One-way ANOVAs | Post-hoc Bonferroni p-values | ||||
|---|---|---|---|---|---|---|---|---|
| M(SD) | M(SD) | M(SD) | F 2,88 | p-value | Young vs. Older | Young vs. MCI | Older vs. aMCI | |
| Age (years) | 20.52 (2.40) | 68.12 (8.19) | 71.41 (9.55) | 374.25 | <0.001 | <0.001 | <0.001 | 0.256 |
| Education (years) | 14.40 (1.53) | 16.31 (2.44) | 16.84 (2.69) | 8.29 | 0.001 | 0.01 | 0.001 | 1.0 |
| WTAR (Standard score) | ||||||||
| Predicted VIQ | 113.12 (8.34) | 111.41 (8.65) | 111.94 (8.89) | 0.29 | 0.752 | |||
| Predicted PIQ | 109.56 (6.54) | 108.88 (6.99) | 108.97 (6.64) | 0.09 | 0.918 | |||
| Predicted FSIQ | 112.36 (8.78) | 111.06 (9.12) | 111.78 (8.90) | 0.16 | 0.856 | |||
| MMSE (raw score) | 29.36 (1.25) | 29.56 (0.66) | 27.09 (2.37) | 22.92 | <0.001 | 1.0 | <0.001 | <0.001 |
| RBANS Indices (Std. score) | ||||||||
| Total | 105.36 (8.49) | 104.56 (11.71) | 83.66 (13.83) | 33.85 | <0.001 | 1.0 | <0.001 | <0.001 |
| Immediate Memory | 104.64 (13.19) | 103.32 (12.69) | 82.5 (16.94) | 22.75 | <0.001 | 1.0 | <0.001 | <0.001 |
| Visuospatial/Constructional | 104.68 (10.28) | 103.59 (13.29) | 93.09 (16.65) | 6.5 | 0.002 | 1.0 | 0.007 | 0.01 |
| Language | 99.36 (11.73) | 102.74 (11.71) | 91.69 (9.53) | 8.61 | <0.001 | 0.741 | 0.032 | <0.001 |
| Attention | 112.12 (14.37) | 103.47 (15.68) | 94.41 (17.27) | 8.76 | <0.001 | 0.127 | <0.001 | 0.069 |
| Delayed Memory | 100.88 (10.20) | 103.82 (10.17) | 75.41 (20.30) | 36.38 | <0.001 | 1.0 | <0.001 | <0.001 |
| Trails A (T-score) | 54.16 (10.52) | 50.35 (8.92) | 43.34 (11.16) | 8.43 | <0.001 | 0.479 | <0.001 | 0.019 |
| Trails B (T-score) | 53.56 (10.24) | 52.44 (8.37) | 43.88 (11.90) | 8.18 | 0.001 | 1.0 | 0.002 | 0.003 |
| Trails B/A ratio (raw scores) | 2.54 (0.78) | 2.45 (0.85) | 2.66 (1.07) | 0.44 | 0.643 | |||
| WMS/KBNA (% Accuracy) | F2,86 | |||||||
| Automatized Accuracy | 97.92 (6.26) | 98.50 (3.58) | 95.70 (18.14) | 0.53 | 0.590 | |||
| Nonautomatized Accuracy | 94.92 (7.74) | 88.31 (10.79) | 84.22 (21.04) | 3.73 | 0.028 | 0.263 | 0.024 | 0.792 |
| EWCST (raw score) | F2,85 | |||||||
| # Sorts | 5.96 (0.54) | 5.00 (0.95) | 3.47 (1.93) | 26.24 | <0.001 | 0.021 | <0.001 | <0.001 |
| % Perseverative Errors | 6.16 (11.31) | 12.61 (11.26) | 23.71 (19.11) | 10.56 | <0.001 | 0.287 | <0.001 | 0.009 |
| # Set Loss Errors | 0.32 (0.56) | 1.00 (1.15) | 1.53 (1.72) | 6.33 | 0.003 | 0.135 | 0.002 | 0.291 |
MMSE = mini-mental state exam; RBANS = Repeatable Battery for the Assessment of Neuropsycho logical Status (Standard Scores provided); WMS/KBNA = Boston Revision of the Wechsler Memory Scale Mental Control subtest; EWCST = Emory Short form of the Wisconsin Card Sorting Test.
2.2. Materials
2.2.1. Neuropsychological Tests
Each participant completed a brief neuropsychological screening protocol to ensure that 1) participants with MCI had not progressed to AD or reverted to normal and 2) young and older control participants were, in fact, cognitively intact. The protocol included the Mini Mental Status Exam (MMSE) (Folstein et al., 1975), the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) (Randolph et al., 1998), Trail Making Test A & B, the Emory Short form of the Wisconsin Card Sorting Test (Heaton et al., 1993; but see Stringer, 1996), the Wechsler Test of Adult Reading (Wechsler, 2001), the Boston Revision of the Wechsler Memory Scale Mental Control subtest (WMS/KBNA) (Lamar et al., 2002).
2.2.2 The Temporal Sequencing Task (TST)
The stimuli used for the TST were taken from the classic line drawings of Snodgrass and Vanderwort (1980). We selected a total of 55 images through an iterative process and according to the following caveats. First, there were a comparable number of living (n=28) and non-living (n=27) drawings, which was deemed important since there is debate about whether patients with AD demonstrate greater deficits for living than non-living items (Laws et al., 2007). “Living” stimuli consisted of humans, animals, and plants. “Non-living” stimuli consisted of household items, methods of transportation, and manmade tools. The number of available stimuli differed within each of these 6 categories, so we matched stimuli across living and non-living domains such that human = methods of transportation, animals = household items, and plants = tools.
Using the selected stimuli, we created 12 target sequences that were three, four, and five images in length (four sequences per span length). Two additional sequences served as practice items. Within each sequence, each category was represented a maximum of one time (the specific item from that category was pseudo-randomly selected). Across sequences, the ordinal position of the items was pseudo-randomized so that living and non-living items appeared in each ordinal position of the sequence an equal number of times.
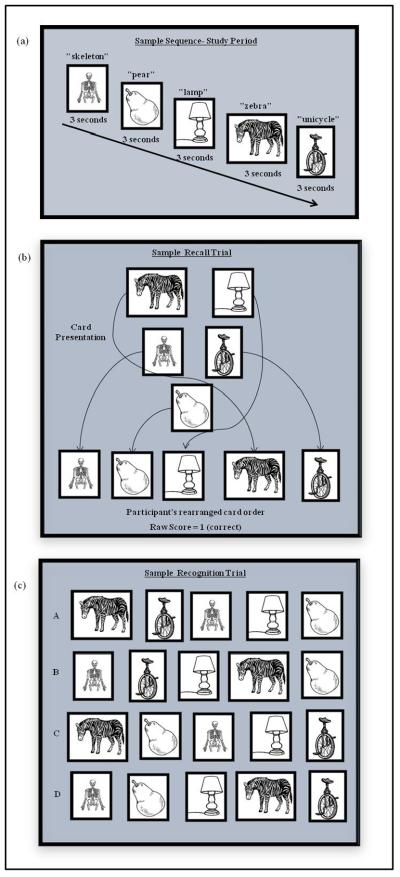
The TST consisted of 3 phases: 1) study & immediate recall, 2) delayed recall, and 3) recognition. The order in which the 12 target sequences were presented was initially randomized and then held constant across all participants. During the study & immediate recall phase, participants first viewed each image of a sequence for three seconds (presented on a laptop screen via a Microsoft Office PowerPoint® presentation). The experimenter said the name of each image as it appeared and the subject was instructed to repeat it. This process ensured participants were attending to, and accurately perceiving, each stimulus. After each entire sequence was presented (Figure 1a), the participant reconstructed it using individual paper cards that contained the images (Figure 1b). Participants were given as much time as needed to re-create the sequence, after which the cards were removed and the next sequence was presented. The dependent variable was the number of sequences correctly reproduced (score of 0–12). Participants were not informed that there would be a delayed recall phase until after the last immediate recall trial had been completed.
Figure 1.
Sample TST stimulus sequence. First, participants saw an entire sequence, one stimulus at a time on a computer screen (a). During the recall trials (both immediate and delayed), stimuli were presented in a non-linear grouping on individual paper cards and participants recreated the sequence (b). During recognition, participants selected the correct sequence from among four options that were presented on a piece of paper (c).
During the 10-minute delay, participants completed study questionnaires (e.g., Geriatric Depression Scale), but not any other perceptual or memory test that could mimic or otherwise interfere with TST performance. The delayed recall phase began after this delay. Participants were given the individual image cards comprising a given sequence and instructed to put them in the correct order (Figure 1b). Each of the 12 sequences was presented individually and participants were given as much time as necessary. The dependent variable was again the number of sequences correctly reproduced (score of 0–12).
The recognition phase began immediately after the last delayed recall sequence had been recreated. There were 12 trials in the recognition phase, one for each sequence. During each trial, participants chose the correct sequence from among four possible choices: the correct sequence, a sequence where only the last image was incorrect, a sequence where only the first image was incorrect, or one where both the first and last images were incorrect (Figure 1c). These foils were meant to control for possible primacy and recency effects. The four choices were presented on a sheet of paper. The dependent variable was the number of target sequences selected (score of 0–12).
2.3. Statistical analyses
All analyses were performed using SPSS 18.0. Between-group differences on demographic variables and baseline neuropsychological data were assessed with multiple one-way ANOVAs. Our primary research goals were to examine the effects of age and MCI on temporal order memory as a function of time; therefore, we used a analysis of variance (ANOVA) tests were considered significant at p < 0.05. Unless otherwise noted, we used the false discovery rate (FDR) to correct for multiple comparisons. The FDR minimizes both type I and type II error (Storey, 2003), wherein the corrected p = (computed p (#tests+1))/(2*(#tests)). This resulted in an FDR corrected p ≤ 0.0291.
With regard to addressing the study primary hypotheses, we first examined the effects of age and MCI on overall performance (total number correct) during the three phases of the TST using a 3 (between subjects factors: young controls, older controls, MCI) × 3 (within subjects factors: immediate recall, delayed recall, recognition) repeated measures ANOVA. We used the false discovery rate (FDR) to correct for multiple comparisons across all analyses related to the TST since the FDR minimizes both type I and type II error relative to other correction methods (e.g., Sidak, Bonferroni) (Storey, 2003). The significance threshold is determined using the following formula: corrected p = (computed p (#tests+1))/(2*(#tests)). To minimize the number of post-hoc analyses performed, we (1) examined age effects by contrasting the young vs. older control groups and (2) examined the effects of MCI by contrasting the older controls vs. MCI group. This resulted in an FDR corrected p ≤ 0.0291 for our primary hypotheses (i.e., 2 contrasts during the immediate, delayed, and recognition phases = 6 total contrasts).
A secondary aim was to examine whether TST recall performance was affected by span length in the context of age- and disease status. We used independent t-tests to examine age (i.e., young vs. older controls) and “disease” (i.e., older controls vs. MCI) effects. In all, 12 comparisons were performed (2 time points, 6 contrasts/phase) for an FDR corrected p ≤ 0.027.
We then performed correlational analyses (Pearson's r) to examine the relationship between TST performance (total immediate recall and delayed recall) and relevant standardized neuropsychological measures that were significantly different between the groups. Given the study goals, we included neuropsychological measures of memory (the RBANS Delayed Memory Index) and executive functioning, which included the RBANS Attention Index, WMS/KBNA non-automatized index, and the EWCST. For the EWCST, we chose to use the number of correct sorts because it provides the best overall indication of performance. Correlation results were assessed at an FDR corrected p ≤ 0.026 to account for all 24 comparisons.
3. Results
3.1. Group characteristics
Demographic and neuropsychological data are provided in Table 1. As expected, the young control group was significantly younger and had fewer years of education (largely because many were undergraduates) than the other two groups. The two older groups were comparable in terms of age and education. The MCI group demonstrated significant deficits in learning and memory, with relatively more mild impairments in visuocontstruction, language (due to reduced semantic fluency), and some measures of attention and executive functioning. Thus, the pattern of neuropsychological impairment in our MCI group is consistent with individuals who are at increased risk of converting to dementia, especially due to AD (Petersen, 2004).
3.2. TST performance
3.2.1. Overall TST performances
TST performances can be seen in Figure 2. There was a significant main effect of time because scores declined after the delay (F2,88 = 137.93, p < 0.001, pη2 = 0.76), a significant main effect of group (F2,88 = 47.80, p < 0.001., pη2 = 0.52), and a significant group x time interaction (F2,88 = 9.65, p < 0.001, pη2= 0.18). Post hoc analyses revealed that during TST immediate recall, young controls performed significantly better than the older control (t(57) = 4.72, p < 0.001; Cohen's d = 1.32). Although there was a treand for the older controls to outperform the MCI patients (t(64) = 2.02, p = 0.048, Cohen's d = .50), this did not survive the FDR correction threshold of p ≤ 0.0291 (though see span length analyses for additional comparisons). During the delayed recall phase, significant age (young vs. older controls: t(57) = 4.73, p < 0.001, Cohen's d = 1.26) and MCI effects were observed (older controls vs. MCI: t(64) = 4.66, p < 0.001, Cohen's d = 1.15. These differences were also evident during the recognition phases of the TST (young vs. older controls: t(57) = 3.62, p = 0.001, Cohen's d = 1.0; older controls vs. MCI: t(64) = 5.92, p < 0.001, Cohen's d = 1.3).
Figure 2.
Overall accuracy (raw scores) by group for each phase of the TST.
3.2.2. Correlations between the TST and neuropsychological data
Results of correlation analyses can be seen in Table 2. In the young control group, delayed recall TST performance was associated with the WMS/KBNA non-automatized accuracy, which is generally viewed as a measure of working memory. However, the relationship between this measure and immediate TST recall fell just short of FDR-based correction (observed p = 0.029 vs. threshold of p = 0.026). Within the older control group, TST performance was unrelated to any variable at either time point. In the MCI group, TST immediate recall was positively correlated with all selected measures of executive functioning whereas delayed recall was correlated with only the RBANS Delayed Memory Index.
Table 2.
Correlation results (Pearson's r, with p-values in parentheses) of TST Immediate Recall (IR) and Delayed Recall (DR) number correct with neuropsychological variables.
| Young Controls (n=25) | Older Controls (n=34) | MCI (n=32) | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| IR | DR | IR | DR | IR | DR | |
| RBANS DMI | −0.20 (0.351) | −0.02 (0.915) | 0.08 (0.672) | 0.26 (0.135) | 0.37 (0.040) | 0.49 (0.004)* |
| RBANS Att. Index | 0.06 (0.791) | −0.01 (0.979) | −0.06 (0.749) | 0.14 (0.442) | 0.56 (0.001)* | −0.14 (0.451) |
| WMS/KBNA NA | 0.44 (0.029) | 0.47 (0.018)* | 0.12 (0.502) | 0.30 (0.089) | 0.81 (O.001)* | 0.30 (0.113) |
| EWCST #Sorts | −0.05 (0.822) | 0.05 (0.813) | 0.31 (0.071) | 0.23 (0.200) | 0.58 (0.001)* | 0.16 (0.389) |
p ≤ 0.026, cutoff for significance after correction for multiple comparisons using the false discovery rate (FDR).
RBANS = Repeatable Battery for the Assessment of Neuropsychological Status; DMI = Delayed Memory Index; WMS/KBNA NA = Boston Revision of the Wechsler Memory Scale Mental Control subtest, non-automatized accuracy; EWCST = Emory Short form of the Wisconsin Card Sorting Test.
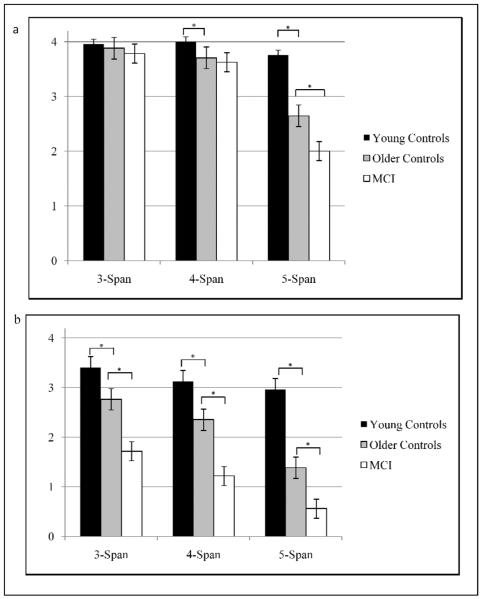
3.2.3. Span length comparisons
During immediate recall, the two control groups demonstrated comparable performances for 3-span items (t(57) = 1.05, p = 0.265, d = 0.29); however, the younger controls performed significantly better than the older controls for 4-span (t(57) = 2.53, p = 0.014, d = 0.72) and 5-span (t(57) = 4.59, p < 0.001, d = 1.28) items. In regards to “disease” effects: MCI and older controls were comparable at the 3-span (t(64) = 0.99, p = 0.332, d = 0.24) and 4-span (t(64) = 0.51, p = 0.612, d = 0.13), but the older controls performed significantly better during the 5-span items (t(64) = 2.45, p = 0.017, d =0.60).
During the delayed recall, the young controls performed significantly better than the older controls across all span lengths: 3-span (t(57) = 2.81, p = 0.007, d = 0.75), 4-span (t(57) = 2.42, p = 0.019, d = 0.64) and 5-span (t(57) = 5.15, p < 0.001, d = 1.39). Similarly, older controls performed significantly better than MCI patients regardless of span length: 3-span (t(64) = 4.23, p < 0.001, d = 1.04), 4-span (t(64) = 3.86, p < 0.001, d = 0.95), and 5-span (t(64) = 2.84, p = 0.006, d = 0.70).
4. Discussion
The first goal of the current study was to examine whether temporal order memory deficits in MCI are in excess of “normal” age-related declines. The second goal was to identify whether MCI patients are impaired due to an inability to initially acquire / encode or whether it is a failure to retain the temporal information. The TST was designed to specifically answer this second question. Episodic memories generally combine information about the content (i.e., “who” or “what”) and the context (i.e., “where” or “when”). The TST provided participants with all of the content so that memory for “what” was not confounding performance and, as a result, participants were forced to learn and retain the context, especially the “when.” As discussed below, the results revealed that immediate recall deficits within the MCI group only exceed age-related changes at the most taxing span length (5-span) whereas delayed recall deficits were superimposed on age effects regardless of span length.
The TST immediate recall trials were posited to depend on prefrontally mediated working memory because participants had to mentally hold and then compare the temporal information while reconstructing the sequences. We found a general effect of age that supports previous findings of reduced temporal order memory during aging (Parkin, Walter, and Hunkin, 1995). It is relevant to note that older adults demonstrate greater reductions in learning than retention (Petersen et al., 1992) as well as in memory for context rather than content (Spencer and Raz, 1995); changes that also have been associated with prefrontal functioning (Cabeza et al., 2000). However, the age-related effects on immediate TST recall only emerged as the memory load increased, which is consistent with earlier work showing that older adults have more difficulty with span tasks compared with younger adults (Iachini et al., 2008; Kessels et al., 2010). The effect of span is variable in MCI since some studies have reported only age-related declines (Guarch et al., 2008; Kramer et al., 2006) whereas others revealed additional disease-related impairments (Gagnon and Belleville, 2011; Kessels et al., 2010; Saunders and Summers, 2010). There was only a trend toward significance in the total number of sequences correctly recalled between the older control and MCI groups during this stage of the TST, but subsequent analyses revealed that MCI patients were disproportionately affected by the highest memory load condition (i.e., the 5-span). Together, these findings raise the possibility that temporal order deficits arise either directly as the result of reduced working memory or through the inefficient transfer of information from working memory into “long-term” memory. This latter possibility may represent a deficient episodic buffer within Baddeley's (2003) working memory model and is further supported by findings of other groups (Kessels et al., 2010), including findings that span length is associated with medial temporal lobe integrity in MCi patients (Wenger et al., 2010). What was clear, however, is that the greatest between-group differences were observed during the delayed recall phase when demands on the medial temporal memory system were greatest (see delayed recall discussion below).
The general pattern of correlation results also supported the role of prefrontally mediated processes during the immediate recall phase of the TST, with working memory abilities appearing especially relevant. However, this relationship appeared to change with age and again with disease status. Our inclusion criteria required healthy participants to be fully cognitively intact, which limited the variability in scores that is necessary for correlational analyses. This may explain why no significant correlations were found for the control groups. However, a strong trend (0.003 short the FDR correction threshold) was observed in which performance of the young controls was related to a measure of working memory (WMS/KBNA non-automatized index) and raises the possibility that those with stronger working memory abilities were better able to perform the TST. In the MCI group, immediate TST recall was significantly related to all selected measures of executive functioning and likely suggests that the acquisition / encoding of temporal information becomes more difficult as patients progress toward AD (i.e., as disease pathology affects the structure and function of the prefrontal cortex and its associated networks).
We expected the TST delayed recall phase to be dependent on the medial temporal lobes because it required the retention of information over time and, because associative memory (e.g., binding the “what” and “when” of the TST stimuli) is known to be hippocampally mediated (see review by Mayes et al., 2004). The results demonstrated an effect of age with the younger outperforming the older controls and an additional effect of MCI, where older controls outperformed those with MCI. This effect was observed regardless of span length. Many standardized memory tests include measures of both recall and recognition in order to more clearly delineate whether memory deficits are due to deficient retrieval (i.e., recall performance > recognition) versus retention (i.e., recall performance = recognition) of previously learned information. We included the recognition phase of the TST for precisely this purpose. MCI patients continued to show significant deficits relative to the other groups during the recognition phase, suggesting that their primary failure was in the retention of information rather than its retrieval. It is relevant that delayed recall performance was significantly related to the RBANS Delayed Memory Index in MCI patients, providing further support for role of the “long-term” medial temporal memory system during this phase of the TST. Interestingly, a growing body of evidence suggests that age and Alzheimer's disease have differential effects on hippocampal subregions, specifically the dentate gyrus and entorhinal cortex, respectively (Brickman et al., 2011; see Small et al., 2011 for a review). In addition, a recent study demonstrated that those MCI patients who demonstrate impaired temporal order memory are more likely to convert to AD (Bellassen et al., 2012). Our findings provide evidence for both an age- and an additional disease-related decline in performance following the passage of time, which is when medial temporal lobe demands are presumably highest. Future studies should investigate whether this impairment is linked to the integrity of the dentate gyrus versus entorhinal cortex, which could provide further support for the use of temporal order memory tasks in the detection of MCI and conversion to AD.
4.1. Limitations
As with any study involving MCI, there is some degree of etiological uncertainty since 14–30% of those diagnosed subsequently revert to normal (Boyle et al., 2006; Manly et al., 2008). Two factors increase our confidence in our diagnostic procedures. First, all patients demonstrated objective memory deficits during a clinical evaluation and, as a result, had been diagnosed with MCI during a consensus conference prior to their referral to our study. Second, the patients demonstrated persistent memory test impairment on independent measures (average memory test performance of −1.64 SD on the RBANS Delayed Memory Index) at the time the current study was performed. These factors suggest that many of our patients will progress to AD. Another potential concern may be that our patients demonstrated impairment that was not restricted to memory but, rather, encompassed other cognitive domains. Although this increased the variance within our MCI sample (presumably reflecting the spectrum from near “normal” to AD/dementia), the results of the TST reinforce that prefrontal and medial temporal lobe abilities contribute somewhat differently to temporal order memory as a function of the cognitive processes being utilized. In many ways, the cognitive heterogeneity of the sample may have increased our ability to detect these relationships. Future studies could use structural (e.g., volumetrics or cortical thickness) and functional neuroimaging to more definitively examine the brain regions associated with performance. Effective connectivity analyses might be especially informative in regards to the interactions between these regions. We cannot rule out the possibility that our inclusion criteria for the older controls (i.e., that all performances were average or better) inadvertently restricted the “normal” variability that is found in this population and limited the correlation analyses as a result. Similarly, ceiling effects may be of concern in the younger control group given their near perfect performances on some portions of the TST (e.g., recognition phase). In this respect, our relatively short span lengths may actually have led to an underestimate of age-related decline. Despite such limitations, the results clearly indicate that both age and disease (i.e., MCI) have significant effects on temporal order memory.
5. Conclusion
Our findings have several potential implications. First, it is possible that temporal order memory deficits emerge as the direct result of working memory impairments because patients would be unable to mentally hold and manipulate the temporal information for long enough to engage the medial temporal memory system. Our findings of both age- and disease-related impairment with increased span length support this possibility. However, this is also the point at which the medial temporal lobe memory system is engaged and information is “transferred” into long-term memory; akin to Baddeley's (2003) episodic buffer. Our findings of marked disease-related decline in delayed recall performance suggest that temporal order memory deficits are primarily attributed to an inability to retain information in MCI patients, presumably due to the primary disease pathology within the medial temporal lobes. Any executive dysfunction appears to play a secondary role, at least based on our available data. There is widespread acceptance that early identification of those at risk for AD is critical for modifying the disease course and prolonging quality of life. Future studies could examine the sensitivity and specificity of the TST or other similar measures as they relate to the early detection of MCI and subsequent conversion to AD. Consideration of specific hippocampal subregions (e.g., entorhinal cortex) may be especially helpful in this regard. Our finding that prefrontally mediated temporal abilities are relatively preserved in MCI is important from a rehabilitative standpoint. For example, we recently reported that mnemonic strategy training improves associative memory and is related to the integrity of prefrontally mediated abilities (Hampstead et al., 2012a). Further, training partially restored hippocampal functioning in patients with MCI (Hampstead et al., 2012b). Thus, the TST could also serve as an independent measure through which the efficacy of cognitive rehabilitation is assessed. In sum, measures of temporal order memory may hold promise for further examining some of the most critical research questions within the field of aging and dementia.
Figure 3.
Accuracy by group and span length during the immediate recall trial (a), and delayed recall trial (b). * p ≤ 0.027, the FDR corrected p value for independent samples t-test comparisons to determine differences due to age (young controls vs. older controls) and disease status (older controls vs. MCI).
Acknowledgements
This study was supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, and Rehabilitation Research and Development Service through grant B6366W. The Emory Alzheimer's Disease Research Center (NIA: 2P50AG025688) also supported this study.
Footnotes
The contents of this manuscript do not represent the views of the Department of Veterans Affairs or the United States Government. Portions of this work were presented at the 2011 and 2012 annual meetings of the International Neuropsychological Society.
REFERENCES
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendation from the National Institute on Aging- Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiez C, Petrides M. Selective involvement of the mid-dorsolateral prefrontal cortex in the coding of the serial order of visual stimuli in working memory. Proceedings of the National Academy of Sciences, U.S.A. 2007;104(34):13786–13791. doi: 10.1073/pnas.0706220104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A. Working memory: Looking back and looking forward. Nature Reviews: Neuroscience. 2003;4:829–839. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory manual. 2nd ed. Psychological Corporation; San Antonio. TX: 1996. [Google Scholar]
- Bellassen V, Igloi K, de Souza LC, Dubois B, Rondi-Reig L. Temporal order memory assessed during spatiotemporal navigation as a behavioral cognitive marker for differential Alzheimer's disease diagnosis. The Journal of Neuroscience. 2012;32(6):1942–1952. doi: 10.1523/JNEUROSCI.4556-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle PA, Wilson RS, Aggarwal NT, Tang Y, Bennett DA. Mild cognitive impairment: risk of Alzheimer disease and rate of cognitive decline. Neurology. 2006;67:441–445. doi: 10.1212/01.wnl.0000228244.10416.20. [DOI] [PubMed] [Google Scholar]
- Braak E, Griffing K, Arai K. Neuropathology of Alzheimer's disease: What is new since A. Alzheimer? European Archives of Psychiatry and Clinical Neuroscience. 1999;249:14–22. doi: 10.1007/pl00014168. [DOI] [PubMed] [Google Scholar]
- Braver TS, West R. Working memory, executive control, and aging. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. 3rd ed. Psychology Press; New York: 2008. pp. 311–372. [Google Scholar]
- Brickman AM, Stern Y, Small SA. Hippocampal subregions differentially associate with standardized memory tests. Hippocampus. 2011;21(9):923–928. doi: 10.1002/hipo.20840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Mangels J, Nyberg L, Habib R, Houle S, McIntosh AR, Tulving E. Brain regions differentially involved in remembering what and when: A PET study. Neuron. 1997;19:863–870. doi: 10.1016/s0896-6273(00)80967-8. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Houle S, Mangels JA, Nyberg L. Age-related differences in neural activity during item and temporal-order memory retrieval: a positron emission tomography study. Journal of Cognitive Neuroscience. 2000;12(1):197–206. doi: 10.1162/089892900561832. [DOI] [PubMed] [Google Scholar]
- Chang YL, Bondi MW, Fennema-Notestine C, McEvoy LK, Hagler DJ, Jacobson MW, Dale AM. Brain substrates of learning and retention in mild cognitive impairment diagnosis and progression to Alzheimer's disease. Neuropsychologia. 2010;48:1237–1247. doi: 10.1016/j.neuropsychologia.2009.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YL, Jacobson MW, Fennema-Notestine C, Hagler DJ, Jr., Jennings RG, Dale AM, McEvoy LK. Level of executive function influences verbal memory in amnestic mild cognitive impairment and predicts prefrontal and posterior cingulate thickness. Cerebral Cortex. 2010;20(6):1305–1313. doi: 10.1093/cercor/bhp192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que PASA? The posterior anterior shift in aging. Cerebral Cortex. 2008;18(5):1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Esposito M, Aquirre GK, Zarahn E, Ballard D, Shin RK, Lease J. Functional MRI studies of spatial and nonspatial working memory. Cognitive Brain Research. 1998;7:1–13. doi: 10.1016/s0926-6410(98)00004-4. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Eichenbaum H. The episodic memory system: neurocircuitry and disorders. Neuropsychopharmacology. 2010;35(1):86–104. doi: 10.1038/npp.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte A, Henson RN, Knight RT, Emery T, Graham KS. The orbitofrontal cortex is necessary for temporal context memory. Journal of Cognitive Neuroscience. 2010;22(8):1819–1831. doi: 10.1162/jocn.2009.21316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewers M, Sperling RA, Klunk WE, Weiner MW, Hampel H. Neuroimaging markers for the prediction and early diagnosis of Alzheimer's disease dementia. Trends in Neurosciences. 2011;34(8):430–442. doi: 10.1016/j.tins.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gagnon LG, Belleville S. Working memory in mild cognitive impairment and Alzheimer's disease: contribution of forgetting and predictive value of complex span tasks. Neuropsychology. 2011;25(2):226–236. doi: 10.1037/a0020919. [DOI] [PubMed] [Google Scholar]
- Ganguli M, Snitz BE, Saxton JA, Chang CC, Lee CW, Vander Bilt J, Petersen RC. Outcomes of mild cognitive impairment by definition: a population study. Archives of Neurology. 2011;68(6):761–767. doi: 10.1001/archneurol.2011.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarch J, Marcos T, Salamero M, Gastó C, Blesa R. Mild cognitive impairment: A risk indicator of later dementia, or a preclinical phase of the disease? International Journal of Geriatric Psychiatry. 2008;23:257–265. doi: 10.1002/gps.1871. [DOI] [PubMed] [Google Scholar]
- Hampstead BM, Libon DJ, Moelter ST, Swirsky-Sacchetti T, Scheffer L, Platek SM. Temporal order memory difference in Alzheimer's disease and vascular dementia. Journal of Clinical and Experimental Neuropsychology. 2010;32(6):645–654. doi: 10.1080/13803390903418918. [DOI] [PubMed] [Google Scholar]
- Hampstead BM, Sathian K, Phillips PA, Amaraneni A, Delaune WR, Stringer AY. Mnemonic strategy training improves memory for object location associations in both healthy elderly and patients with amnestic mild cognitive impairment: A randomized, single-blind study. Neuropsychology. 2012a;26(3):385–399. doi: 10.1037/a0027545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampstead BM, Stringer AY, Stilla RF, Giddens M, Sathian K. Mnemonic strategy training partially restores hippocampal activity in patients with mild cognitive impairment. Hippocampus. 2012b;22(8):1652–1658. doi: 10.1002/hipo.22006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanseeuw B, Dricot L, Kavec M, Grandin C, Seron X, Ivanoiu A. Associative encoding deficits in amnestic mild cognitive impairment: A volumetric and functional MRI study. NeuroImage. 2011;56(3):1743–1748. doi: 10.1016/j.neuroimage.2011.03.034. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtis G. Wisconsin Card Sorting Test (WCST) manual revised and expanded. Psychological Assessment Resources Inc; Odessa: 1993. [Google Scholar]
- Iachini T, Ruggiero G, Ruotolo F, Pizza R. Age and gender differences in some components of spatial cognition. In: Benninghouse HT, Rosset AG, editors. Women and Aging: New Research. Nova Science Publishers; New York: 2008. [Google Scholar]
- Johnson DL, Kesner RP. Comparison of temporal order memory in early and middle stage Alzheimer's disease. Journal of Clinical and Experimental Neuropsychology. 1997;19(1):83–100. doi: 10.1080/01688639708403839. [DOI] [PubMed] [Google Scholar]
- Kessels RPC, Meulenbroek O, Fernandez G, Olde Rikkert MGM. Spatial working memory in aging and mild cognitive impairment: Effects of task load and contextual cueing. Aging, Neuropsychology, and Cognition. 2010;17:556–574. doi: 10.1080/13825585.2010.481354. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Hopkins RO, Fineman B. Item and order dissociation in humans with prefrontal cortex damage. Neuropsychologia. 1994;32(8):881–891. doi: 10.1016/0028-3932(94)90040-x. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Nelson A, Johnson JK, Yaffe K, Glenn S, Rosen HJ, Miller BL. Multiple cognitive deficits in amnestic mild cognitive impairment. Dementia and Geriatric Cognitive Disorders. 2006;22(4):306–311. doi: 10.1159/000095303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamar M, Price CC, Davis KL, Kaplan E, Libon DJ. Capacity to maintain mental set in dementia. Neuropsychologia. 2002;40:435–445. doi: 10.1016/s0028-3932(01)00125-7. [DOI] [PubMed] [Google Scholar]
- Laws KR, Adlington RL, Gale TM, Moreno-Martinez FJ, Sartori G. A meta analytic review of category naming in Alzheimer's disease. Neuropsychologia. 2007;45:2674–2682. doi: 10.1016/j.neuropsychologia.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Loewenstein DA, Acevedo A, Small BJ, Agron J, Crocco E, Duara R. Stability of different subtypes of mild cognitive impairment among the elderly over a 2-to 3- year follow-up period. Dementia and Geriatric Cognitive Disorders. 2009;27:418–423. doi: 10.1159/000211803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen J, Kesner RP. The temporal distance effect in subjects with dementia of the Alzheimer type. Alzheimer Disease & Associated Disorders. 1995;9(2):94–100. doi: 10.1097/00002093-199509020-00006. [DOI] [PubMed] [Google Scholar]
- Manly JJ, Tang MX, Schupf N, Stern Y, Vonsattel JPG, Mayeux R. Frequency and course of mild cognitive impairment in a multiethnic community. Annals of Neurology. 2008;63(4):494–506. doi: 10.1002/ana.21326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes AR, Holdstock JS, Isaac CL, Montaldi D, Grigor A, Gummer A, Norman KA. Associative recognition in a patient with selective hippocampal lesions and relatively normal item recognition. Hippocampus. 2004;14(6):763–84. doi: 10.1002/hipo.10211. [DOI] [PubMed] [Google Scholar]
- Milner B, Petrides M, Smith ML. Frontal lobes and the temporal organization of memory. Human Neurobiology. 1985;4:137–142. [PubMed] [Google Scholar]
- Moscovitch M, Winocur G. Frontal lobes, memory, and aging. Annals of the New York Academy of Sciences. 1995;769:119–150. doi: 10.1111/j.1749-6632.1995.tb38135.x. [DOI] [PubMed] [Google Scholar]
- Parkin AJ, Walter BM, Hunkin M. Relationships between normal aging, frontal lobe function, and memory for temporal and spatial information. Neuropsychology. 1995;9:304–312. [Google Scholar]
- Petersen RC. Mild cognitive impairment as a diagnostic entity. Journal of International Medicine. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith G, Kokmen E, Ivnik RJ, Tangalos EG. Memory function in normal aging. Neurology. 1992;42(2):396–401. doi: 10.1212/wnl.42.2.396. [DOI] [PubMed] [Google Scholar]
- Pfeffer RI, Kurosaki TT, Harrah CH, Chance JM, Filos S. Measurement of functional activities in older adults in the community. The Journals of Gerontology. 1982;37(3):323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- Rajah MN, Languay R, Valiquette L. Age-related changes in prefrontal cortex activity are associated with behavioral deficits in both temporal and spatial context memory retrieval in older adults. Cortex. 2010;46:535–549. doi: 10.1016/j.cortex.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Rami L, Sole-Padulles C, Fortea J, Bosch B, Llado A, Antonell A, Molinuevo JL. Applying the new research diagnostic criteria: MRI findings and neuropsychological correlations of prodromal AD. International Journal of Geriatric Psychiatry. 2012;27(2):127–134. doi: 10.1002/gps.2696. [DOI] [PubMed] [Google Scholar]
- Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. Journal of Clinical and Experimental Neuropsychology. 1998;20(3):310–319. doi: 10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]
- Ross RS, Brown TI, Stern CE. The retrieval of learned sequences engages the hippocampus: Evidence from fMRI. Hippocampus. 2009;19(9):790–799. doi: 10.1002/hipo.20558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA, Miles JD. Aging and time-sharing aspects of executive control. Memory & Cognition. 2002;30(4):572–582. doi: 10.3758/bf03194958. [DOI] [PubMed] [Google Scholar]
- Saunders NLJ, Summers MJ. Attention and working memory deficits in mild cognitive impairment. Journal of clinical and experimental Neuropsychology. 2010;32(4):350–357. doi: 10.1080/13803390903042379. [DOI] [PubMed] [Google Scholar]
- Shimamura AP, Janowsky JS, Squire LR. Memory for the temporal order of events in patients with frontal lobe lesions and amnesic patients. Neuropsychologia. 1990;28(8):803–813. doi: 10.1016/0028-3932(90)90004-8. [DOI] [PubMed] [Google Scholar]
- Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nature Reviews Neuroscience. 2011;12:585–601. doi: 10.1038/nrn3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: Norms for name agreement, image agreement, familiarity and visual complexity. Journal of Experimental Psychology: Human Learning and Memory. 1980;6:174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- Spencer WD, Raz N. Differential effects of aging on memory for content and context: a meta-analysis. Psychology and Aging. 1995;10(4):527–539. doi: 10.1037//0882-7974.10.4.527. [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola SM. Structure and function of declarative and nondeclarative memory systems. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:13515–13522. doi: 10.1073/pnas.93.24.13515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey JD. The positive false discovery rate: A Bayesian interpretation and the q-value. Annals of Statistics. 2003;31(6):2013–2035. [Google Scholar]
- Stringer AY. A guide to adult neuropsychological diagnosis. F.A. Davis; Philadelphia, PA: 1996. [Google Scholar]
- Stuss DT, Binns MA, Murphy KJ, Alexander MP. Dissociations within the anterior attentional system: effects of task complexity and irrelevant information on time speed and accuracy. Neuropsychology. 2002;16(4):500–13. doi: 10.1037//0894-4105.16.4.500. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Test of Adult Reading (WTAR) The Psychological Corporation; San Antonio, TX: 2001. [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: A meta-analysis. Cognitive, Affective, & Behavioral Neuroscience. 2003;3(4):255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Wenger MK, Negash S, Petersen RC, Petersen L. Modeling and estimating recall processing capacity: sensitivity and diagnostic utility in application to mild cognitive impairment. Journal of Mathematical Psychology. 2010;54(1):73–89. doi: 10.1016/j.jmp.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann M, Simmons AN, Aron JL, Paulus MP. Accumulation of neural activity in the posterior insula encodes the passage of time. Neuropsychologia. 2010;48(10):3110–3120. doi: 10.1016/j.neuropsychologia.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leier VO. Development and validation of a geriatric depression screening scale: a preliminary report. Journal of Psychiatric Research. 1983;17(1):37–4. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]