Abstract
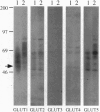
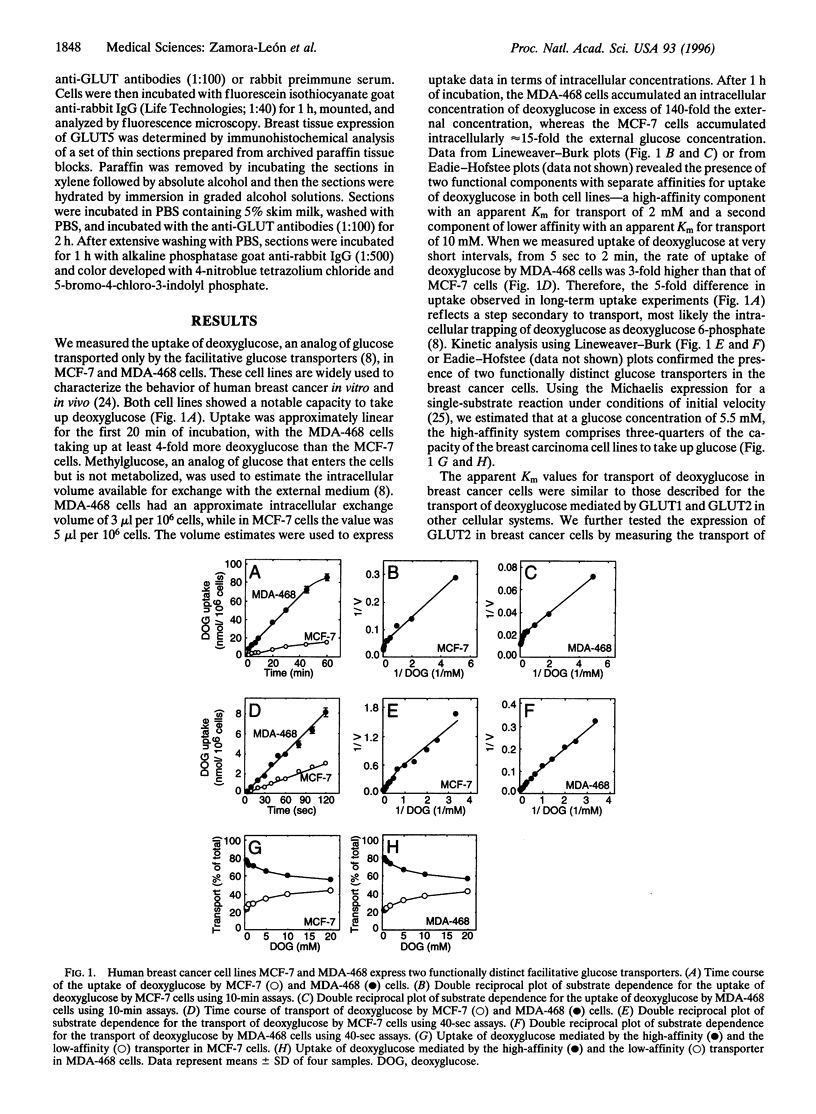
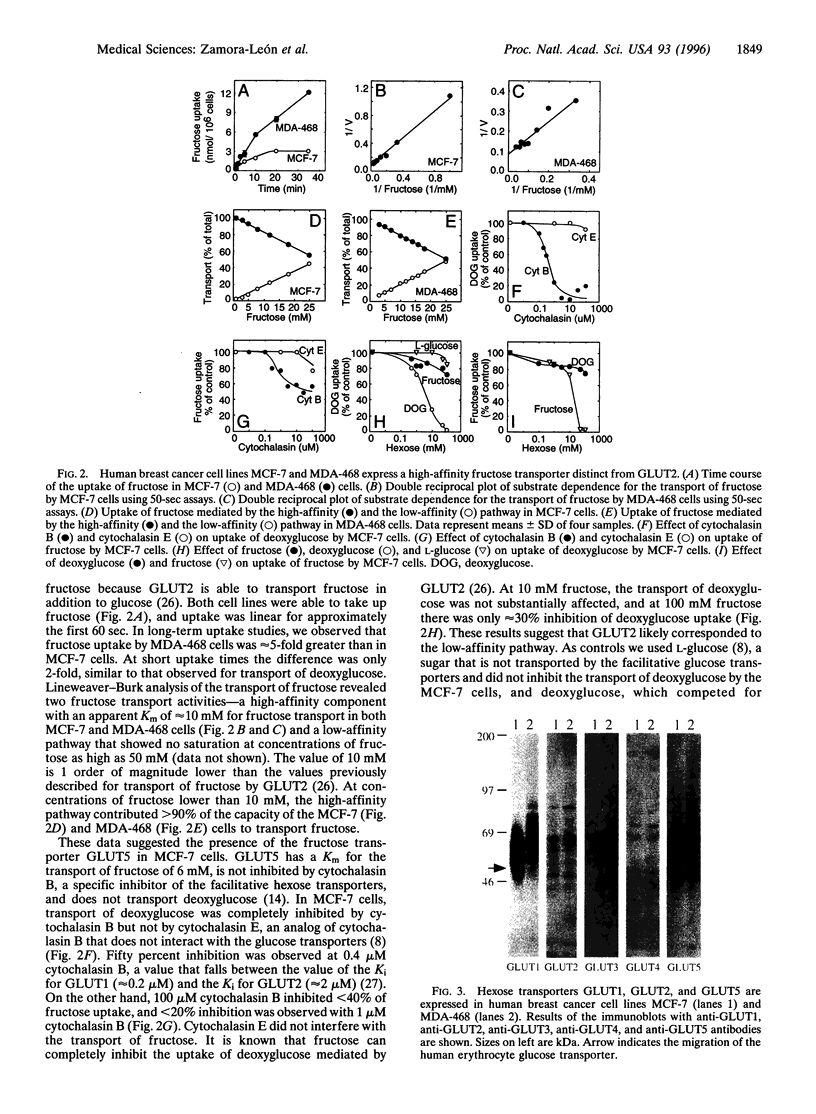
The primary metabolic characteristic of malignant cells is an increased uptake of glucose and its anaerobic metabolism. We studied the expression and function of the glucose transporters in human breast cancer cell lines and analyzed their expression in normal and neoplastic primary human breast tissue. Hexose uptake assays and immunoblotting experiments revealed that the breast carcinoma cell lines MCF-7 and MDA-468 express the glucose transporters GLUT1 and GLUT2, isoforms expressed in both normal and neoplastic breast tissue. We also found that the breast cancer cell lines transport fructose and express the fructose transporter GLUT5. Immunolocalization studies revealed that GLUT5 is highly expressed in vivo in human breast cancer but is absent in normal human breast tissue. These findings indicate that human breast cancer cells have a specialized capacity to transport fructose, a metabolic substrate believed to be used by few human tissues. Identification of a high-affinity fructose transporter on human breast cancer cells opens opportunities to develop novel strategies for early diagnosis and treatment of breast cancer.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod J. D., Pilch P. F. Unique cytochalasin B binding characteristics of the hepatic glucose carrier. Biochemistry. 1983 Apr 26;22(9):2222–2227. doi: 10.1021/bi00278a025. [DOI] [PubMed] [Google Scholar]
- Baselga J., Norton L., Masui H., Pandiella A., Coplan K., Miller W. H., Jr, Mendelsohn J. Antitumor effects of doxorubicin in combination with anti-epidermal growth factor receptor monoclonal antibodies. J Natl Cancer Inst. 1993 Aug 18;85(16):1327–1333. doi: 10.1093/jnci/85.16.1327. [DOI] [PubMed] [Google Scholar]
- Birnbaum M. J., Haspel H. C., Rosen O. M. Transformation of rat fibroblasts by FSV rapidly increases glucose transporter gene transcription. Science. 1987 Mar 20;235(4795):1495–1498. doi: 10.1126/science.3029870. [DOI] [PubMed] [Google Scholar]
- Birnbaum M. J. Identification of a novel gene encoding an insulin-responsive glucose transporter protein. Cell. 1989 Apr 21;57(2):305–315. doi: 10.1016/0092-8674(89)90968-9. [DOI] [PubMed] [Google Scholar]
- Brown R. S., Wahl R. L. Overexpression of Glut-1 glucose transporter in human breast cancer. An immunohistochemical study. Cancer. 1993 Nov 15;72(10):2979–2985. doi: 10.1002/1097-0142(19931115)72:10<2979::aid-cncr2820721020>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Burant C. F., Takeda J., Brot-Laroche E., Bell G. I., Davidson N. O. Fructose transporter in human spermatozoa and small intestine is GLUT5. J Biol Chem. 1992 Jul 25;267(21):14523–14526. [PubMed] [Google Scholar]
- Burnol A. F., Leturque A., Loizeau M., Postic C., Girard J. Glucose transporter expression in rat mammary gland. Biochem J. 1990 Aug 15;270(1):277–279. doi: 10.1042/bj2700277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carruthers A. Facilitated diffusion of glucose. Physiol Rev. 1990 Oct;70(4):1135–1176. doi: 10.1152/physrev.1990.70.4.1135. [DOI] [PubMed] [Google Scholar]
- Colville C. A., Seatter M. J., Jess T. J., Gould G. W., Thomas H. M. Kinetic analysis of the liver-type (GLUT2) and brain-type (GLUT3) glucose transporters in Xenopus oocytes: substrate specificities and effects of transport inhibitors. Biochem J. 1993 Mar 15;290(Pt 3):701–706. doi: 10.1042/bj2900701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flier J. S., Mueckler M. M., Usher P., Lodish H. F. Elevated levels of glucose transport and transporter messenger RNA are induced by ras or src oncogenes. Science. 1987 Mar 20;235(4795):1492–1495. doi: 10.1126/science.3103217. [DOI] [PubMed] [Google Scholar]
- Harris J. R., Lippman M. E., Veronesi U., Willett W. Breast cancer (2). N Engl J Med. 1992 Aug 6;327(6):390–398. doi: 10.1056/NEJM199208063270606. [DOI] [PubMed] [Google Scholar]
- Hediger M. A., Rhoads D. B. Molecular physiology of sodium-glucose cotransporters. Physiol Rev. 1994 Oct;74(4):993–1026. doi: 10.1152/physrev.1994.74.4.993. [DOI] [PubMed] [Google Scholar]
- Kayano T., Burant C. F., Fukumoto H., Gould G. W., Fan Y. S., Eddy R. L., Byers M. G., Shows T. B., Seino S., Bell G. I. Human facilitative glucose transporters. Isolation, functional characterization, and gene localization of cDNAs encoding an isoform (GLUT5) expressed in small intestine, kidney, muscle, and adipose tissue and an unusual glucose transporter pseudogene-like sequence (GLUT6). J Biol Chem. 1990 Aug 5;265(22):13276–13282. [PubMed] [Google Scholar]
- Kayano T., Fukumoto H., Eddy R. L., Fan Y. S., Byers M. G., Shows T. B., Bell G. I. Evidence for a family of human glucose transporter-like proteins. Sequence and gene localization of a protein expressed in fetal skeletal muscle and other tissues. J Biol Chem. 1988 Oct 25;263(30):15245–15248. [PubMed] [Google Scholar]
- Mahraoui L., Takeda J., Mesonero J., Chantret I., Dussaulx E., Bell G. I., Brot-Laroche E. Regulation of expression of the human fructose transporter (GLUT5) by cyclic AMP. Biochem J. 1994 Jul 1;301(Pt 1):169–175. doi: 10.1042/bj3010169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantych G. J., James D. E., Devaskar S. U. Jejunal/kidney glucose transporter isoform (Glut-5) is expressed in the human blood-brain barrier. Endocrinology. 1993 Jan;132(1):35–40. doi: 10.1210/endo.132.1.8419132. [DOI] [PubMed] [Google Scholar]
- Minn H., Soini I. [18F]fluorodeoxyglucose scintigraphy in diagnosis and follow up of treatment in advanced breast cancer. Eur J Nucl Med. 1989;15(2):61–66. doi: 10.1007/BF00702620. [DOI] [PubMed] [Google Scholar]
- Mueckler M., Caruso C., Baldwin S. A., Panico M., Blench I., Morris H. R., Allard W. J., Lienhard G. E., Lodish H. F. Sequence and structure of a human glucose transporter. Science. 1985 Sep 6;229(4717):941–945. doi: 10.1126/science.3839598. [DOI] [PubMed] [Google Scholar]
- Nieweg O. E., Kim E. E., Wong W. H., Broussard W. F., Singletary S. E., Hortobagyi G. N., Tilbury R. S. Positron emission tomography with fluorine-18-deoxyglucose in the detection and staging of breast cancer. Cancer. 1993 Jun 15;71(12):3920–3925. doi: 10.1002/1097-0142(19930615)71:12<3920::aid-cncr2820711220>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Rand E. B., Depaoli A. M., Davidson N. O., Bell G. I., Burant C. F. Sequence, tissue distribution, and functional characterization of the rat fructose transporter GLUT5. Am J Physiol. 1993 Jun;264(6 Pt 1):G1169–G1176. doi: 10.1152/ajpgi.1993.264.6.G1169. [DOI] [PubMed] [Google Scholar]
- Shepherd P. R., Gibbs E. M., Wesslau C., Gould G. W., Kahn B. B. Human small intestine facilitative fructose/glucose transporter (GLUT5) is also present in insulin-responsive tissues and brain. Investigation of biochemical characteristics and translocation. Diabetes. 1992 Oct;41(10):1360–1365. doi: 10.2337/diab.41.10.1360. [DOI] [PubMed] [Google Scholar]
- Thorens B., Cheng Z. Q., Brown D., Lodish H. F. Liver glucose transporter: a basolateral protein in hepatocytes and intestine and kidney cells. Am J Physiol. 1990 Dec;259(6 Pt 1):C279–C285. doi: 10.1152/ajpcell.1990.259.2.C279. [DOI] [PubMed] [Google Scholar]
- Thorens B., Sarkar H. K., Kaback H. R., Lodish H. F. Cloning and functional expression in bacteria of a novel glucose transporter present in liver, intestine, kidney, and beta-pancreatic islet cells. Cell. 1988 Oct 21;55(2):281–290. doi: 10.1016/0092-8674(88)90051-7. [DOI] [PubMed] [Google Scholar]
- Threadgold L. C., Kuhn N. J. Monosaccharide transport in the mammary gland of the intact lactating rat. Biochem J. 1984 Feb 15;218(1):213–219. doi: 10.1042/bj2180213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse N. Y., Hoh C. K., Hawkins R. A., Zinner M. J., Dahlbom M., Choi Y., Maddahi J., Brunicardi F. C., Phelps M. E., Glaspy J. A. The application of positron emission tomographic imaging with fluorodeoxyglucose to the evaluation of breast disease. Ann Surg. 1992 Jul;216(1):27–34. doi: 10.1097/00000658-199207000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera J. C., Rivas C. I., Fischbarg J., Golde D. W. Mammalian facilitative hexose transporters mediate the transport of dehydroascorbic acid. Nature. 1993 Jul 1;364(6432):79–82. doi: 10.1038/364079a0. [DOI] [PubMed] [Google Scholar]
- Vera J. C., Rivas C. I., Velásquez F. V., Zhang R. H., Concha I. I., Golde D. W. Resolution of the facilitated transport of dehydroascorbic acid from its intracellular accumulation as ascorbic acid. J Biol Chem. 1995 Oct 6;270(40):23706–23712. doi: 10.1074/jbc.270.40.23706. [DOI] [PubMed] [Google Scholar]
- Vera J. C., Rosen O. M. Functional expression of mammalian glucose transporters in Xenopus laevis oocytes: evidence for cell-dependent insulin sensitivity. Mol Cell Biol. 1989 Oct;9(10):4187–4195. doi: 10.1128/mcb.9.10.4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARBURG O. On the origin of cancer cells. Science. 1956 Feb 24;123(3191):309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- Waddell I. D., Zomerschoe A. G., Voice M. W., Burchell A. Cloning and expression of a hepatic microsomal glucose transport protein. Comparison with liver plasma-membrane glucose-transport protein GLUT 2. Biochem J. 1992 Aug 15;286(Pt 1):173–177. doi: 10.1042/bj2860173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl R. L., Zasadny K., Helvie M., Hutchins G. D., Weber B., Cody R. Metabolic monitoring of breast cancer chemohormonotherapy using positron emission tomography: initial evaluation. J Clin Oncol. 1993 Nov;11(11):2101–2111. doi: 10.1200/JCO.1993.11.11.2101. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Seino Y., Fukumoto H., Koh G., Yano H., Inagaki N., Yamada Y., Inoue K., Manabe T., Imura H. Over-expression of facilitative glucose transporter genes in human cancer. Biochem Biophys Res Commun. 1990 Jul 16;170(1):223–230. doi: 10.1016/0006-291x(90)91263-r. [DOI] [PubMed] [Google Scholar]