Abstract
Oral diseases are a major burden on individuals and health systems. The aim of this study was to determine whether consumption of tobacco and alcohol were associated with the prevalence of oral/dental problems in Mexican adults. Using data from the National Performance Evaluation Survey 2003, a cross-sectional study part of the World Health Survey, dental information from a representative sample of Mexico (n = 22,229, N = 51,155,740) was used to document self-reported oral/dental problems in the 12 months prior to the survey. Questionnaires were used to collect information related to sociodemographic, socioeconomic, and other risk factors. Three models were generated for each age group (18–30, 31–45 and 46–98 years). The prevalence of oral/dental conditions was 25.7%. Adjusting for sex, schooling, socioeconomic position, diabetes, and self-reported health, those who used tobacco (sometimes or daily) (OR = 1.15, p = 0.070; OR = 1.24, p < 0.01; and OR = 1.16, p < 0.05, for each age group respectively) or alcohol (moderate or high) (OR = 1.26, p < 0.001; OR = 1.18, p < 0.01 and OR = 1.30, p < 0.001, for each age group respectively) had a higher risk of reporting oral/dental problems. Because tobacco and alcohol use were associated with self-reported oral/dental problems in one out of four adults, it appears advisable to ascertain how direct is such link; more direct effects would lend greater weight to adopting measures to reduce consumption of tobacco and alcohol for the specific purpose of improving oral health.
Keywords: oral health, epidemiology, smoking, alcohol, adults
1. Introduction
Dental caries and periodontal disease, as well as the possible sequel of both (tooth loss), are major dental public health problems worldwide. Poor oral health and untreated oral diseases have a negative impact on the quality of life of individuals and their overall health. This oral disease burden is markedly higher in developing countries like Mexico [1,2,3,4,5]. Dental caries is a multifactorial infectious and transmissible disease involving an imbalance of normal molecular interactions between the tooth surface/subsurface and the adjacent microbial biofilm where acids are produced [6]. Periodontal disease is a chronic bacterial infection that affects both the gingiva and the bone that supports the teeth; it is caused by anaerobic Gram-negative microorganisms that are present in the bacterial plaque that adheres to the teeth [7]. According to The Global Burden of Disease (GBD) 2010 Study, poor oral conditions remained highly prevalent in 2010, collectively affecting 3.9 billion people. The most common and the greatest burden globally is untreated caries in permanent teeth (global prevalence of 35% for all ages combined), whereas severe periodontitis and untreated caries in deciduous teeth were the 6th and 10th most prevalent conditions, affecting, respectively, 11% and 9% of the global population. Severe tooth loss was the 36th most prevalent condition, with a global estimate of 2% [3]. These diseases are equally highly prevalent and incident in Mexico across various age groups, and are concentrated in those with greater social disadvantage. A diet rich in simple carbohydrates, poor dietary patterns, inappropriate oral hygiene behaviors, restricted access to dental services, tobacco use, and excessive use of alcohol are some risk factors for these diseases [8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24].
Tobacco and alcohol use have considerable impacts on health [25,26,27,28,29,30], being two of the most important lifestyle and addiction considerations in this area. Tobacco use and tobacco smoke exposure remain the leading risk factors of preventable death globally. In Mexico, 21.7% of the 12 to 65 years age group are current smokers (31.4% male and 12.6% female), while 51.9% of the population has never smoked [31]. On the other hand, 71.3% of that population has used alcohol at some point in their lives (80.6% of men and 62.6% of women) [32]. There is considerable epidemiological evidence demonstrating the adverse effects of both tobacco and alcohol contributing to periodontal disease, tooth decay, tooth loss, staining of teeth and dental restorations, reduction of the ability to smell and taste, and oral conditions such as smoker’s palate, smoker’s melanosis, coated tongue, precancerous lesions and cancer, dental implant failures, and possibly oral candidosis [10,15,16,17,18,19,20,21,22,23,24]. In Mexico there are few studies that have addressed oral health conditions at the national level. The aim of this study was to determine whether use of tobacco and alcohol were associated with the prevalence of self-reported oral / dental problems in Mexican adults (18 and older).
2. Material and Methods
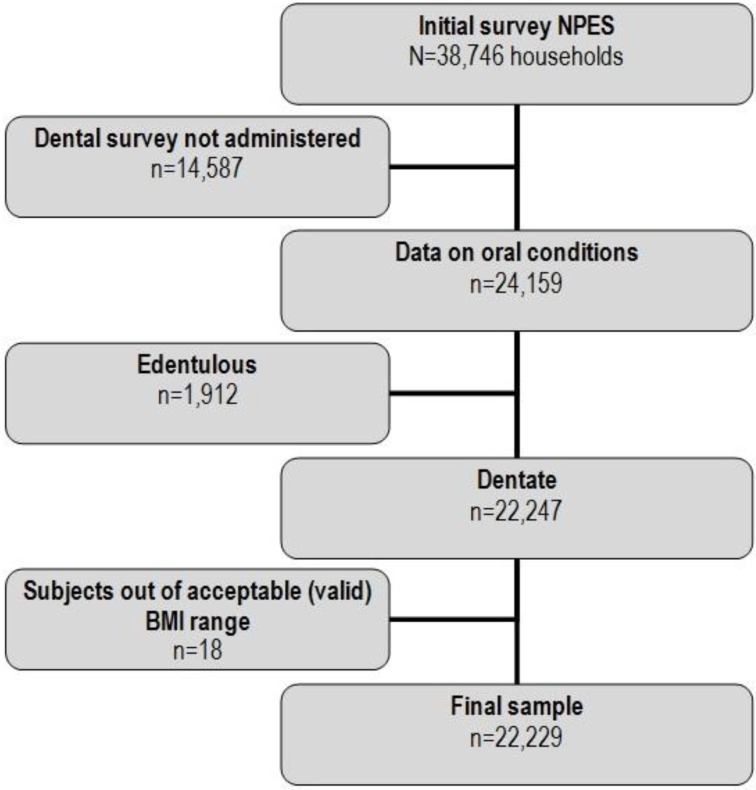
The present study conducted analyses of the National Performance Evaluation Survey 2003 (NPES) in Mexico. This cross-sectional study was part of the technical collaboration between the Ministry of Health and the World Health Organization (WHO), which used the survey instrument and sampling strategies developed by WHO for the World Health Survey (WHS). Further details of the survey methodology and on some oral health indicators are available elsewhere [33,34,35,36]. Information was collected from 38,746 households, with a mean of 1,250 households for each State. The sample design was probabilistic, multistage, stratified, through conglomerates, and was calculated to provide representative information at the State level, and across urban and rural areas. Data on dental conditions are available for only 20 out of the 32 States of Mexico (not every state implemented the entire set of survey modules), leading to a total of 24,159 households. Only subjects with teeth were included in the present analysis, so the final sample comprised 22,229 participants (Figure 1).
Figure 1.
Flow chart for the individuals included in the study.
NPES consisted of two different questionnaires. In the first questionnaire, information was gathered with regard to residential services, income, expenses, and health insurance. The second questionnaire gathered information including health status, health risk factors, prevalence of key diseases, use of health services, non-clinical expectations of the population, and insurance coverage of certain clinical services.
2.1. Dependent Variable
Self-reporting of oral/dental problems in the recent past was our dependent variable; it was gathered through question Q6757 “During the last 12 months, did you have any problems with your mouth and/or teeth?”; the response options were yes or no.
2.2. Independent Variables
A series of socio-demographic and socio-economic factors and variables related to health were included: age (18 to 98 years), sex (male or female), residence (rural or urban), marital status (single, married, divorced, widowed, cohabitating), Indian ethnicity status (“Do you speak an indigenous language?”: no or yes), schooling (less than elementary school, complete elementary, complete middle school, high school/equivalent, college studies/higher), occupation (employed in the public sector, employed outside the public sector, self-employed, or not working or doing volunteer work), health insurance (insured or non-insured), socio-economic level (in tertiles), having a disability (none or yes), physical activity (high activity or low activity), chronic disease (none or any), body mass index (BMI; underweight <18.5, normal 18.5–24.9, overweight 25.0–29.9, or obesity ≥30), tobacco current use (never/not currently, sometimes, or daily) and alcohol current use (Never and low: fewer than 4 servings for women and 5 for men in the last week on one occasion; or high: 4 servings and more for women, and 5 for men in the last week on one occasion). Valid data were included as those BMI values between 10 and 58, and height measurements between 130 and 200 cm [37].
The household survey included general topics, such as building materials of the household and ownership of consumable goods, which were used to construct a Wealth Index (WI) using principal components analysis (PCA). The WI included owning a refrigerator, washing machine, dishwasher, personal computer, car, bicycle, television, etc., where goods were combined into a single estimator [38]. There were some missing data for weight (n = 326), height (n = 521), Indian ethnicity status (n = 196), health insurance (n = 48), and socio-economic level (n = 1), which were imputed through regression imputation [39].
First, we conducted a univariate analysis, reporting the summary measures according to each case for the nominal and ordinal variables (frequency and percentages) and the continuous variables (measures of central tendency and dispersion). A binary logistic regression analysis was used for the bivariate and multivariate analyses. The variance inflation factor was used to analyze and avoid multicollinearity between the independent variables. Variables with a statistical significance of p < 0.25 in the bivariate analysis were included in the final model. Subsequently, the p-values were calculated, and the odds ratios (OR) were calculated with a confidence interval (CI) of 95% and interactions were tested [40]. Due to the design used in the survey sampling, a module for complex samples was used for the statistical analysis (STATA 11.0, StataCorp, College Station, TX, USA).
The Medical Research Committee of the National Institute of Public Health in Mexico granted ethical approval. Participation in the survey was voluntary. All individuals provided written, informed consent.
3. Results
The study included 22,229 individuals, who were a weighted representation of 51,155,740 of the population in the country. Table 1 shows the descriptive results of several sociodemographic and socioeconomic variables, including both crude and weighed percentages. The 18–30 year olds age group made up 37.0% of the survey participants. Women accounted for 57.7% and those who lived in urban/metropolitan locations were 73.5%. Also, 56.9% of respondents were married and 8.5% were classified as being of indigenous ethnicity. As for the variables pertaining to socioeconomic status, 46.7% had completed middle school or higher, 50.2% had no formal employment, and 65.4% did not have any health insurance. The Wealth Index (WI) was divided into quartiles. Equally, in Table 1 we present several health-related variables for the study population: 4.5% reported having diabetes, 45.7% had a BMI in the normal/moderate range, 67.6% reported their general health as very good/good, while 21.7% and 46.3% reported using tobacco and alcohol currently, respectively. The prevalence of self-reported oral problems in the 12 months preceding the survey was 25.7%.
Table 1.
Descriptive characteristics of the study population and prevalence of self-reported oral and dental problems (estimated national population N = 51,155,740) (National Performance Evaluation Survey 2003).
| Variable | n | Percentage | N | % Weighted |
|---|---|---|---|---|
| Age | ||||
| 18–30 years | 7,676 | 34.5 | 18,912,188 | 37.0 |
| 31–45 years | 7,600 | 34.2 | 18,256,912 | 35.7 |
| 46–and more years | 6,953 | 31.3 | 13,986,640 | 27.3 |
| Sex | ||||
| Male | 9,397 | 42.3 | 21,655,360 | 42.3 |
| Female | 12,832 | 57.7 | 29,500,380 | 57.7 |
| Residence | ||||
| Rural | 6,462 | 29.1 | 13,544,848 | 26.5 |
| Urban/Metropolitan | 5,767 | 70.9 | 37,610,892 | 73.5 |
| Marital-status | ||||
| Single | 4,113 | 18.5 | 10,591,127 | 20.7 |
| Married | 12,836 | 57.7 | 29,118,247 | 56.9 |
| Divorced | 1,232 | 5.5 | 2,469,875 | 4.8 |
| Widowed | 1,570 | 7.1 | 2,631,745 | 5.1 |
| Cohabitating | 2,478 | 11.1 | 6,344,746 | 12.4 |
| Indian ethnicity status | ||||
| No | 20,817 | 93.6 | 46,822,650 | 91.5 |
| Yes | 1,412 | 6.4 | 4,333,090 | 8.5 |
| Schooling | ||||
| Less than elementary | 2,924 | 13.1 | 6,196,688 | 12.1 |
| Complete elementary | 9,393 | 42.3 | 21,082,147 | 41.2 |
| Complete secondary | 4,974 | 22.4 | 11,854,773 | 23.2 |
| High School/equivalent | 3,154 | 14.2 | 7,927,560 | 15.5 |
| College and higher | 1,784 | 8.0 | 4,094,572 | 8.0 |
| Occupation | ||||
| Government Employee | 2,212 | 9.9 | 4,297,865 | 8.4 |
| Non-Government Employee | 3,030 | 13.6 | 7,358,914 | 14.4 |
| Self-Employed | 5,796 | 26.1 | 13,494,269 | 26.4 |
| Employer | 58 | 0.3 | 87,830 | 0.2 |
| Voluntary Worker | 96 | 0.4 | 235,224 | 0.5 |
| Does not work | 11,037 | 49.7 | 25,681,638 | 50.2 |
| Health insurance | ||||
| Non-insured | 14,129 | 63.6 | 33,435,760 | 65.4 |
| Social security | 7,561 | 34.0 | 16,433,454 | 32.1 |
| Other insurance | 539 | 2.4 | 1,286,526 | 2.5 |
| Wealth Index | ||||
| 1 quartile (lowest) | 5,559 | 25.0 | 12,912,573 | 25.2 |
| 2 quartile | 5,573 | 25.1 | 11,981,913 | 23.4 |
| 3 quartile | 6,149 | 27.7 | 13,754,179 | 26.9 |
| 4 quartile (highest) | 4,948 | 22.2 | 12,507,075 | 24.4 |
| Diabetes | ||||
| No | 21,223 | 95.5 | 48,862,029 | 95.5 |
| Yes | 1,006 | 4.5 | 2,293,711 | 4.5 |
| BMI | ||||
| Underweight < 18.5 | 544 | 2.4 | 1,153,652 | 2.3 |
| Normal BMI (18.5–24.9) | 9,883 | 44.5 | 23,357,437 | 45.7 |
| Overweight (25.0–29.9) | 8,394 | 37.8 | 19,345,482 | 37.8 |
| Obesity (≥30) | 3,408 | 15.3 | 7,299,169 | 14.3 |
| Self-reported health | ||||
| Very good/good | 14,709 | 66.2 | 34,602,142 | 67.6 |
| Moderate | 6,366 | 28.6 | 14,163,997 | 27.7 |
| Bad/very bad | 1,154 | 5.2 | 2,389,601 | 4.7 |
| Smoking (current) | ||||
| No | 17,555 | 79.0 | 40,029,133 | 78.2 |
| Sometimes | 3,095 | 13.9 | 7,731,460 | 15.1 |
| Daily | 1,579 | 7.1 | 3,395,147 | 6.6 |
| Alcohol Use (current) | ||||
| No | 11,724 | 52.7 | 27,446,858 | 53.7 |
| Low | 9,976 | 44.9 | 22,716,583 | 44.4 |
| High | 529 | 2.4 | 992,299 | 1.9 |
Note: SRODP = Self-report of oral and dental problems.
Table 2 shows the prevalence of reported oral problems across the categories of the independent variables included. In the bivariate logistic regression analysis (Table 2) all variables were significant (p < 0.05), except for education and occupation.
Table 2.
Bivariate analyses of the prevalence of self-report of oral and dental problems and independent variables (National Performance Evaluation Survey 2003).
| Variable | % SRODP | OR | 95% CI | p value * |
|---|---|---|---|---|
| Age | ||||
| 25 years | 18.9 | 1 * | ||
| 25–44 years | 23.8 | 1.33 | 1.15–1.54 | <0.001 |
| 45–64 years | 33.2 | 2.12 | 1.82–2.49 | <0.001 |
| 65 and more years | 33.7 | 2.17 | 1.78–2.64 | <0.001 |
| Sex | ||||
| Male | 22.6 | 1 * | ||
| Female | 27.9 | 1.32 | 1.20–1.46 | <0.001 |
| Residence | ||||
| Rural | 21.5 | 1 * | ||
| Urban/Metropolitan | 27.1 | 1.25 | 1.21–1.50 | <0.001 |
| Marital-status | ||||
| Single | 22.0 | 1 * | ||
| Married | 26.0 | 1.24 | 1.09–1.42 | 0.001 |
| Divorced | 33.0 | 1.74 | 1.37–2.22 | <0.001 |
| Widowed | 33.5 | 1.78 | 1.44–2.20 | <0.001 |
| Cohabitating | 23.8 | 1.11 | 0.92–1.33 | 0.268 |
| Indian ethnicity status | ||||
| No | 26.1 | 1 * | ||
| Yes | 21.2 | 0.76 | 0.63–0.91 | 0.004 |
| Schooling | ||||
| Complete elementary & less | 25.2 | 1 * | ||
| Complete secondary | 25.4 | 1.01 | 0.89–1.14 | 0.874 |
| High School/equivalent | 25.3 | 1.00 | 0.87–1.15 | 0.958 |
| College and higher | 29.7 | 1.24 | 1.04–1.49 | 0.014 |
| Occupation | ||||
| Government Employee | 25.7 | 2.10 | 0.80–5.51 | 0.131 |
| Non-Government Employee | 22.6 | 1.77 | 0.67–4.64 | 0.242 |
| Self-Employed | 24.9 | 2.01 | 0.77–5.24 | 0.152 |
| Employer | 14.1 | 1 * | ||
| Voluntary Worker | 30.5 | 2.66 | 0.84–8.38 | 0.093 |
| Does not work | 26.9 | 2.23 | 0.86–5.80 | 0.099 |
| Health insurance | ||||
| Non-insured | 24.3 | 1 * | ||
| Social security | 27.7 | 1.19 | 1.07–1.31 | 0.001 |
| Other insurance | 35.1 | 1.68 | 1.26–2.24 | <0.001 |
| Wealth Index | ||||
| 1 quartile (lowest) | 20.5 | 1 * | ||
| 2 quartile | 24.1 | 1.23 | 1.07–1.41 | 0.003 |
| 3 quartile | 27.1 | 1.44 | 1.26–1.65 | <0.001 |
| 4 quartile (highest) | 30.9 | 1.74 | 1.51–1.99 | <0.001 |
| Diabetes | ||||
| No | 24.7 | 1 * | ||
| Yes | 45.6 | 2.54 | 2.06–3.14 | <0.001 |
| BMI | ||||
| Underweight < 18.5 | 17.4 | 0.67 | 0.48–0.92 | 0.014 |
| Normal BMI (18.5–24.9) | 23.9 | 1 * | ||
| Overweight (25.0–29.9) | 26.3 | 1.13 | 1.01–1.26 | 0.023 |
| Obesity (≥30) | 30.7 | 1.41 | 1.22–1.62 | <0.001 |
| Self-reported health | ||||
| Very good/good | 21.5 | 1 * | ||
| Moderate | 33.2 | 1.81 | 1.63–2.01 | <0.001 |
| Bad/very bad | 41.3 | 2.56 | 2.08–3.16 | <0.001 |
| Smoking (current) | ||||
| No | 22.8 | 1 * | ||
| Sometimes/Daily | 29.0 | 1.27 | 1.13–1.43 | <0.001 |
| Alcohol Use (current) | ||||
| No | 24.6 | 1 * | ||
| Low/High | 29.4 | 1.38 | 1.25–1.52 | <0.001 |
Notes: SRODP = Self-report of oral and dental problems; * The Pearson chi-squared statistic is corrected for the survey design using the second-order correction of Rao and Scott and converted into an F-statistic.
Finally, three logistic regression multivariate models were fitted, one for each age group (Table 3). Women were 47%, 48%, and 29% (p < 0.05) more likely to report oral problems than men for each age group, respectively. We also found some differences associated with variations in socioeconomic status, except for those survey participants in the 46–98 years old age group: subjects with higher educational attainment (18–30 years: OR = 1.40; 95% CI = 1.23–1.59, and 31–45 years: OR = 1.17; 95% CI = 1.04–1.31) compared with those with less education; and those who were scored higher in the Wealth Index (4th quartile OR = 1.26, OR = 1.18, OR = 1.23 for each age group) compared to those in worst Wealth Index quartiles (1st, 2nd and 3rd), had higher odds of presenting oral/dental problems (p < 0.05).
Table 3.
Results of multivariate logistic regression models for each one of the three age groups on the prevalence of self-reported oral and dental problems and independent variables (National Performance Evaluation Survey 2003).
| Variable | OR (95% CI) 18–30 years |
OR (95% CI) 31–45 years |
OR (95% CI) 46–98 years |
|---|---|---|---|
| Sex | |||
| Male | 1 * | 1 * | 1 * |
| Female | 1.47 (1.30–1.67) † | 1.48 (1.31–1.66) † | 1.29 (1.16–1.45) † |
| Schooling | |||
| Complete elementary & less | 1 * | 1 * | 1 * |
| More than elementary school | 1.40 (1.23–1.59) † | 1.17 (1.04–1.31) ‡ | 1.11 (0.96–1.28) n/s |
| Wealth index | |||
| 1, 2, 3 quartile | 1 * | 1 * | 1 * |
| 4 quartile (highest) | 1.26 (1.09–1.45) ‡ | 1.18 (1.03–1.35) ¶ | 1.23 (1.08–1.40) ‡ |
| Diabetes | |||
| No | 1 * | 1 * | 1 * |
| Yes | 1.00 (0.50–1.99) n/s | 1.63 (1.21–2.19) ‡ | 1.54 (1.32–1.81) † |
| Self-reported health | |||
| Very good/good | 1 * | 1 * | 1 * |
| Moderate | 1.95 (1.71–2.23) † | 1.79 (1.60–2.01) † | 1.47 (1.31–1.64) † |
| Bad/very bad | 1.73 (1.20–2.48) ‡ | 2.29 (1.79–2.94) † | 1.85 (1.56–2.21) † |
| Smoking (current) | |||
| No | 1 * | 1 * | 1 * |
| Sometimes/Daily | 1.15 (0.99–1.34) n/s | 1.24 (1.08–1.42) ‡ | 1.16 (1.01–1.32) ¶ |
| Alcohol Use (current) | |||
| No | 1 * | 1 * | 1 * |
| Low/High | 1.26 (1.11–1.43) † | 1.18 (1.05–1.33) ‡ | 1.30 (1.16–1.45) † |
Notes: * Reference category; † p < 0.001; ‡ p < 0.01, ¶ p < 0.05.
With regard to the variables related to general health we noted that subjects with diabetes had higher odds of self-reporting oral/dental problems in the 12 months preceding the study (p < 0.05), except for the 18–30 years age group (p > 0.05). Also, individuals who reported having moderate and bad/very bad general health were more likely to have oral/dental problems (p < 0.01) than individuals who reported having very good/good health, across all age groups.
As far as tobacco use was concerned, in the 18–30 years age group no significant differences were found for oral/dental conditions (p > 0.05). However, for the 31–45 and 46–98 years age groups the odds ratios of reporting oral/dental conditions were greater among those who admitted to using tobacco (sometimes or daily) (OR = 1.24; 95% CI = 1.08–1.42 and OR = 1.16; 95% CI = 1.01–1.32, respectively) than those who reported not using tobacco. As far as alcohol use was concerned, there were significant differences (p < 0.05) in the three age groups. The odds ratios of reporting oral/dental problems were greater among those who used alcohol (low or high) compared to those who denied using alcohol (OR = 1.26; p < 0.001, OR = 1.18; p < 0.01, and OR = 1.30; p < 0.001, respectively for each age group).
4. Discussion
This study was undertaken to determine whether the use of alcohol and tobacco were associated with the prevalence of reported oral/dental problems in Mexican adults with teeth, using data from a national representative survey. Although oral health is an essential component of overall health and wellbeing, and treatment of oral diseases is expensive and poses a financial impact on families and society, oral health does not always hold an important place in the priorities of individuals or healthcare systems [3,4]. From an epidemiological point of view, the study of oral health needs across diverse population groups can help to focus human and financial resources toward solving health problems that occur in the general population [41]. We found an overall prevalence of self-reported oral/dental problems of 25.7% in the 12 months preceding the study. In South Africa, 16% of respondents reported having had problems with their mouth and/or teeth [42], however the study used a reference period of six months for self-reporting.
We observed that tobacco and alcohol use were associated with self-reported oral/dental problems. Excessive consumption of tobacco and alcohol are considered serious public health problems in Mexico and around the world due to their high social and economic costs; also because some perspectives argue these substances are the first entry points to multiple risk behaviors. Their overall health impacts in terms of morbidity, disability and mortality include not only harm but also health care direct costs and indirect costs due to inability, disability and loss of production; they also entail intangible costs such as suffering, pain and family impacts [31,32,43]. In relation to oral health, several studies have linked excessive use of these substances with oral diseases such as caries, periodontal disease, and dental needs: our results are partially consistent with those findings [15,16,17,18,19,20,21,22,23,24,33], emphasizing that these two substances may be associated with worse oral health status. Given the format of the questions in the national survey it is important to note that excessive use in the prior week does not necessarily equate to addiction. There are multiple hypothesized links, such as the suggestion that smoking can promote caries by fostering increased formation of S. mutans biofilm on tooth surfaces [44]. There is sufficient evidence to infer a causal relationship with smoking for cancers in the oral cavity and pharynx, as well as periodontitis. Evidence is suggestive but not sufficient to infer a causal relationship between smoking and root-surface caries and between maternal smoking and oral clefts. Furthermore, a negative response to periodontal treatment has consistently been reported [45,46]. The most obvious biological connection between substances in tobacco smoke and oral/dental health is the destruction of tooth-supporting tissue [47]. The destructive influences of smoking on the periodontal tissues span from suppression of inflammation through interference with vascular and immune reactions to undermining bone support, leading to bone loss, pocket formation, and tooth loss [16]. In relation to alcohol use, one of the effects may be increased cariogenic potential derived from sweet drinks high in simple sugars and acid content in alcoholic drinks. Additionally, combined with a cariogenic, nutritionally poor diet, poor oral hygiene measures, decreased salivary flow, and a high incidence of smoking in those persons with high alcohol intake, alcohol use may provide an environment conducive to rapid progression of periodontal disease and caries [48]. Another mechanism contributing to this link could be lower utilization of health services generally by persons who use alcohol (in particular those who abuse it) since it is reasonable to propose that high risk behaviors and low utilization of preventive/curative services lead to higher and earlier morbidity [49]. As members of the health team, dentists have an obligation to promote oral health and healthy lifestyles among their patients, and this responsibility includes increasing awareness of the harmful effects of alcohol and tobacco on general health and oral health [50]. Although estimators for tobacco and alcohol use were different, confidence intervals suggested differences were not significant. It seems more salient the individual effects on oral/dental health.
Epidemiologic evidence worldwide confirms the relationship between socioeconomic status and oral health of individuals, regardless of the indicators used; those with higher status have better oral health, a feature known as social health gradient. While most studies have found this effect in clinical settings, the linkage with self-reported health measurements is not entirely consistent [51,52]. There are multiple possible explanations for this contradictory finding: (1) although individuals with more social disadvantage have worse oral health conditions, those with better socioeconomic position could better identify oral health needs through enhanced access to information and health education. On the other hand, (2) people with worse socioeconomic position may have more urgent priorities in life and oral health may not be one of them; under this interpretation, symptoms associated with oral diseases could not represent “meaningful” health needs. Finally, (3) it has been observed that people of lower socioeconomic position are less likely to receive dental care even in the presence of pain [53] and this trend could be primarily due to barriers in access to care. Many complex relationships undoubtedly moderate the interactions between variables.
Socio-demographic variables such as age and sex have also been linked to health status; as age increases, disease experience also increases, just as in oral health needs. It has been found that women in Mexico have oral health and higher oral health needs [33]. In terms of health, the variables of sex (biological construct) and gender (behavioral and social construct) are recognized as useful parameters for research and action, as differences determine specific diseases for men and for women [54]. In this regard, the observed differences between men and women have been well documented by other authors in several health topics [55], as well as in our own study, with women reporting worse health status than men. One of the main explanations for such differential is that women may recognize pain and discomfort more easily than men [55]. Other explanations that have been proposed are (1) the specific conditions of women (maternal conditions, risk exposures, poverty and social exclusion, empowerment), especially in developing countries; (2) conditions associated with increased longevity in women (arising from aging and chronic diseases); (3) conditions resulting from the interaction of sex and gender (depressive symptoms); and (4) gender-based conditions (violence, for example) [54]. Finally, although our study was based on self-report of oral health/dental problems, our findings agree with previous observations in which subjects with diabetes have poor oral health, manifested especially as periodontal disease, tooth loss and caries [33,56,57].
The limitations of the present study are mainly related to its design; a cross-sectional study, in which the cause and the effect are measured at the same time, emphasizes the need to avoid making extrapolations to other population groups because we cannot distinguish between cause and effect in our observations. Another limitation is the lack of clinical confirmation by normative evaluations of dental and periodontal disease, which would have provided a more detailed investigation rather than solely relying on self-reported health conditions. It is also possible that our results may be somewhat dated, as the survey accrued information from several years ago and hence disease profiles may have suffered some changes. However, to our knowledge, this is the first study conducted in Mexico and Latin America based on the World Health Survey data using this indicator as a response variable. Based on the results we conclude that one out of four adults in Mexico reported having had oral problems on the 12 months prior to the study data collection. Tobacco and alcohol use were associated with increased prevalence of oral disease. Besides its confirmatory value using national level data, in the larger scheme of things our results lend further credence to the need to adopting measures to reduce consumption of tobacco and alcohol for the specific purpose of improving oral health. This approach would be a separate consideration from implementing an overall common risk factor approach to support public health and health promotion measures to reduce excessive use of tobacco and alcohol, in order to improve both general health and oral health outcomes.
Acknowledgments
The valuable editorial and scientific input by Michael Kowolik is gratefully acknowledged.
Author Contributions
Carlo Eduardo Medina-Solís, América Patricia Pontigo-Loyola, Leticia Avila-Burgos and Gerardo Maupomé—conceived and performed the study/analysis, interpretation of data and drafted the manuscript. Eduardo Pérez-Campos, Pedro Hernández-Cruz and Martha Mendoza-Rodríguez—helped in the interpretation of data and final drafting of the manuscript. All authors read, revised it critically for important intellectual content, commented upon, and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Do L.G. Distribution of caries in children: Variations between and within populations. J. Dent. Res. 2012;91:536–543. doi: 10.1177/0022034511434355. [DOI] [PubMed] [Google Scholar]
- 2.Petersen P.E., Ogawa H. The global burden of periodontal disease: Towards integration with chronic disease prevention and control. Periodontol. 2000. 2012;60:15–39. doi: 10.1111/j.1600-0757.2011.00425.x. [DOI] [PubMed] [Google Scholar]
- 3.Marcenes W., Kassebaum N.J., Bernabé E., Flaxman A., Naghavi M., Lopez A., Murray C.J.L. Global burden of oral conditions in 1990–2010: A systematic analysis. J. Dent. Res. 2013;92:592–597. doi: 10.1177/0022034513490168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Medina-Solís C.E., Pontigo-Loyola A.P., Pérez-Campos E., Hernández-Cruz P., de la Rosa-Santillana R., Navarete-Hernández J.J., Maupomé G. Principal reasons for extraction of permanent tooth in a sample of Mexicans adults. Rev. Invest. Clin. 2013;65:141–149. [PubMed] [Google Scholar]
- 5.Casanova-Rosado A.J., Medina-Solís C.E., Casanova-Rosado J.F., Vallejos-Sánchez A.A., Minaya-Sánchez M., Mendoza-Rodríguez M., Marquez-Rodriguez S. Toothbrushing frequency in Mexican schoolchildren and associated socio-demographic, socioeconomic and dental variables. Med. Sci. Monitor. 2014 doi: 10.12659/MSM.890106. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pitts N.B., Stamm J.W. International consensus workshop on caries clinical trials (ICW-CCT)—Final consensus statements: Agreeing where the evidence leads. J. Dent. Res. 2004;83:C125–C128. doi: 10.1177/154405910408301S27. [DOI] [PubMed] [Google Scholar]
- 7.Negrato C.A., Tarzia O., Jovanovič L., Chinellato L.E. Periodontal disease and diabetes mellitus. J. Appl. Oral Sci. 2013;21:1–12. doi: 10.1590/1678-7757201302106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Padilla-Suzuki B.E., Llodra-Calvo J.C., Belío-Reyes I.A., García-Jau R.A., Osuna-Ramírez I., Ramírez-Alvarez M., Loyola-Rodríguez J.P. Predicting risk of caries in schoolchildren from northwestern Mexico: Longitudinal study. Rev. Invest. Clin. 2013;65:24–29. [PubMed] [Google Scholar]
- 9.Villalobos-Rodelo J.J., Medina-Solís C.E., Verdugo-Barraza L., Islas-Granillo H., García-Jau R.A., Escoffié-Ramírez M., Maupomé G. Experience of non-reversible and reversible carious lesions in 11 and 12 years old Mexican schoolchildren: A negative binomial regression analysis. Biomedica. 2013;33:88–98. doi: 10.1590/S0120-41572013000100011. [DOI] [PubMed] [Google Scholar]
- 10.Irigoyen-Camacho M.E., Sanchez-Perez L., Molina-Frechero N., Velazquez-Alva C., Zepeda-Zepeda M., Borges-Yanez A. The relationship between body mass index and body fat percentage and periodontal status in Mexican adolescents. Acta Odontol. Scand. 2014;72:48–57. doi: 10.3109/00016357.2013.797100. [DOI] [PubMed] [Google Scholar]
- 11.Costa S.M., Vasconcelos M., Abreu M.H. High dental caries among adults aged 35 to 44 years: Case-control study of distal and proximal factors. Int. J. Environ. Res. Public Health. 2013;10:2401–2411. doi: 10.3390/ijerph10062401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costa S.M., Martins C.C., Bonfim M.L., Zina L.G., Paiva S.M., Pordeus I.A., Abreu M.H. A systematic review of socioeconomic indicators and dental caries in adults. Int. J. Environ. Res. Public Health. 2012;9:3540–3574. doi: 10.3390/ijerph9103540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costa L.R., Daher A., Queiroz M.G. Early childhood caries and body mass index in young children from low income families. Int. J. Environ. Res. Public Health. 2013;10:867–878. doi: 10.3390/ijerph10030867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minaya-Sánchez M., Medina-Solís C.E., Vallejos-Sánchez A.A., Marquez-Corona M.D., Pontigo-Loyola A.P., Islas-Granillo H., Maupomé G. Gingival recession and associated factors in a homogeneous Mexican adult male population: A cross-sectional study. Med. Oral Patol. Oral Cir. Bucal. 2012;17:807–813. doi: 10.4317/medoral.17815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benedetti G., Campus G., Strohmenger L., Lingström P. Tobacco and dental caries: A systematic review. Acta Odontol. Scand. 2013;71:363–371. doi: 10.3109/00016357.2012.734409. [DOI] [PubMed] [Google Scholar]
- 16.Bergström J. Tobacco smoking and chronic destructive periodontal disease. Odontology. 2004;92:1–8. doi: 10.1007/s10266-004-0043-4. [DOI] [PubMed] [Google Scholar]
- 17.Minaya-Sánchez M., Medina-Solís C.E., Maupomé G., Vallejos-Sánchez A.A., Casanova-Rosado J.F., Márquez-Corona M.L. Prevalence of and risk indicators for chronic periodontitis in males from Campeche, Mexico. Rev. Salud Pública (Bogotá) 2007;9:388–398. doi: 10.1590/S0124-00642007000300007. [DOI] [PubMed] [Google Scholar]
- 18.Polk D.E., Wang X., Feingold E., Shaffer J.R., Weeks D.E., Weyant R.J., Crout R.J., McNeil D.W., Marazita M.L. Effects of smoking and genotype on the PSR index of periodontal disease in adults aged 18–49. Int. J. Environ. Res. Public Health. 2012;9:2839–2850. doi: 10.3390/ijerph9082839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zee K.Y. Smoking and periodontal disease. Aust. Dent. J. 2009;54:S44–S50. doi: 10.1111/j.1834-7819.2009.01142.x. [DOI] [PubMed] [Google Scholar]
- 20.Warnakulasuriya S., Dietrich T., Bornstein M.M., Casals-Peidró E., Preshaw P.M., Walter C., Wennström J.L., Bergström J. Oral health risks of tobacco use and effects of cessation. Int. Dent. J. 2010;60:7–30. [PubMed] [Google Scholar]
- 21.César-Neto J.B., Rosa E.F., Pannuti C.M., Romito G.A. Smoking and periodontal tissues: A review. Braz. Oral. Res. 2012;26:25–31. doi: 10.1590/S1806-83242012000700005. [DOI] [PubMed] [Google Scholar]
- 22.Reibel J. Tobacco and oral diseases. Update on the evidence, with recommendations. Med. Princip. Pract. 2003;12:22–32. doi: 10.1159/000069845. [DOI] [PubMed] [Google Scholar]
- 23.Agbor M., Azodo C., Tefouet T. Smokeless tobacco use, tooth loss and oral health issues among adults in Cameroon. Afr. Health Sci. 2013;13:785–790. doi: 10.4314/ahs.v13i3.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ando A., Ohsawa M., Yaegashi Y., Sakata K., Tanno K., Onoda T., Itai K., Tanaka F., Makita S., Omama S., et al. Factors related to tooth loss among community-dwelling middle-aged and elderly Japanese men. J. Epidemiol. 2013;23:301–306. doi: 10.2188/jea.JE20120180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schnoll R.A., Goren A., Annunziata K., Suaya J.A. The prevalence, predictors and associated health outcomes of high nicotine dependence using three measures among US smokers. Addiction. 2013;108:1989–2000. doi: 10.1111/add.12285. [DOI] [PubMed] [Google Scholar]
- 26.Durante A.S., Pucci B., Gudayol N., Massa B., Gameiro M., Lopes C. Tobacco smoke exposure during childhood: Effect on cochlear physiology. Int. J. Environ. Res. Public Health. 2013;10:5257–5265. doi: 10.3390/ijerph10115257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Winkler V., Ott J.J., Cowan M., Becher H. Smoking prevalence and its impacts on lung cancer mortality in Sub-Saharan Africa: An epidemiological study. Prev. Med. 2013;57:634–640. doi: 10.1016/j.ypmed.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 28.Petković G., Barišić I. Prevalence of fetal alcohol syndrome and maternal characteristics in a sample of schoolchildren from a rural province of Croatia. Int. J. Environ. Res. Public Health. 2013;10:1547–1561. doi: 10.3390/ijerph10041547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roerecke M., Rehm J. Alcohol use disorders and mortality: A systematic review and meta-analysis. Addiction. 2013;108:1562–1578. doi: 10.1111/add.12231. [DOI] [PubMed] [Google Scholar]
- 30.Ikehara S., Iso H., Yamagishi K., Kokubo Y., Saito I., Yatsuya H., Inoue M., Tsugane S. Alcohol consumption and risk of stroke and coronary heart disease among Japanese women: The Japan public health center-based prospective study. Prev. Med. 2013;57:505–510. doi: 10.1016/j.ypmed.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 31.Reynales-Shigematsu L.M., Guerrero-López C.M., Lazcano-Ponce E., Villatoro-Velázquez J.A., Medina-Mora M.E., Fleiz‐Bautista C. Instituto Nacional de Psiquiatría Ramón de la Fuente Muñiz; Instituto Nacional de Salud Pública; Secretaría de Salud. Encuesta Nacional de Adicciones 2011: Reporte de Tabaco. INPRFM; Mexico: 2012. [(accessed on 30 June 2013)]. Available online: http://encuestas.insp.mx/ena/ena2011/ENA2011_tabaco.pdf. [Google Scholar]
- 32.Medina-Mora M.E., Villatoro-Velázquez J.A., Fleiz-Bautista C., Téllez-Rojo M.M., Mendoza-Alvarado L.R., Romero-Martínez M. Instituto Nacional de Psiquiatría Ramón de la Fuente Muñiz; Instituto Nacional de Salud Pública; Secretaría de Salud. Encuesta Nacional de Adicciones 2011: Reporte de Alcohol. INPRFM; Mexico: 2012. [(accessed on 30 June 2013)]. Available online: http://encuestas.insp.mx/ena/ena2011/ENA2011_tabaco.pdf. [Google Scholar]
- 33.Medina-Solis C.E., Pérez-Núñez R., Maupomé G., Casanova-Rosado J.F. Edentulism among Mexicans 35 years old and older, and associated factors. Amer. J. Public Health. 2006;96:1578–1581. doi: 10.2105/AJPH.2005.071209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Medina-Solís C.E., Pérez-Núñez R., Maupomé G., Avila-Burgos L., Pontigo-Loyola A.P., Patiño-Marín N., Villalobos-Rodelo J.J. National survey on edentulism and its geographic distribution, among Mexicans 18 years of age and older (with emphasis in WHO age groups) J. Oral Rehabil. 2008;35:237–244. doi: 10.1111/j.1365-2842.2007.01767.x. [DOI] [PubMed] [Google Scholar]
- 35.Medina-Solís C.E., Pontigo-Loyola A.P., Pérez-Campos E., Hernández-Cruz P., Ávila-Burgos L., Kowolik M.J., Maupomé G. Association between edentulism and angina pectoris in Mexican adults 35 years of age and older: A multivariate analysis of a population-based survey. J. Periodontol. 2013 doi: 10.1902/jop.2013.130186. [DOI] [PubMed] [Google Scholar]
- 36.Medina-Solís C.E., Pontigo-Loyola A.P., Pérez-Campos E., Hernández-Cruz P., Avila-Burgos L., Mendoza-Rodríguez M. Edentulism and other variables associated with self-reported health status in Mexican adults. Med. Sci. Monitor. 2014 doi: 10.12659/MSM.890100. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gutierrez J.P., Rivera-Dommarco J., Shamah-Levy T., Villalpando-Hernandez S., Franco A., Cuevas-Nasu L. Encuesta Nacional de Saludy Nutricion 2012. Resultados Nacionales, Instituto Nacional de Salud Publica; Cuernavaca, Mexico: 2012. [Google Scholar]
- 38.Kolenikov S., Angeles G. Working Paper No. WP-04-85. Chapel Hill; CPC/MEASURE, Chapel Hill, NC, USA: 2004. The Use of Discrete Data in Principal Component Analysis with Applications to Socio-economic Indices. [Google Scholar]
- 39.McKnight P.E., McKnight K.M., Sidani S., Figueredo A.J. Missing Data: A Gentle Introduction. Guilford; New York, NY, USA: 2007. [Google Scholar]
- 40.Hosmer D.W., Lemeshow S. Applied Logistic Regression. 2nd ed. Jonh Wiley & Sons Interscience Publication; New York, NY, USA: 2000. [Google Scholar]
- 41.Pontigo-Loyola A.P., Medina-Solís C.E., Márquez-Corona M.L., Vallejos-Sánchez A.A., Minaya-Sánchez M., Escoffié-Ramirez M., Maupomé G. Influence of predisposing, enabling, and health care need variables on the use of dental health services among Mexican adolescents from a semi-rural location. Gac. Med. Mex. 2012;148:218–226. [PubMed] [Google Scholar]
- 42.South African Department of Health South African Demographic and Health Survey (SADHS) [(accessed on 29 June 2013)];2003 Chapters 11–15. Available online: http://www.info.gov.za/view/DownloadFileAction?id=90140.
- 43.Guerrero-López C.M., Reynales-Shigematsu L.M., Jiménez-Ruiz J.A., Karam-Araujo R., Maldonado-Cruz C.A., Camacho-Solis R. Absenteeism attributable to smoking in the Mexican Social Security Institute, 2006–2009. Salud Publica Mex. 2012;54:233–241. doi: 10.1590/S0036-36342012000300005. [DOI] [PubMed] [Google Scholar]
- 44.Huang R., Li M., Gregory R.L. Effect of nicotine on growth and metabolism of Streptococcus mutans. Eur. J. Oral. Sci. 2012;120:319–325. doi: 10.1111/j.1600-0722.2012.00971.x. [DOI] [PubMed] [Google Scholar]
- 45.Chambrone L., Preshaw P.M., Rosa E.F., Heasman P.A., Romito G.A., Pannuti C.M., Tu Y.K. Effects of smoking cessation on the outcomes of non-surgical periodontal therapy: A systematic review and individual patient data meta-analysis. J. Clin. Periodontol. 2013;40:607–615. doi: 10.1111/jcpe.12106. [DOI] [PubMed] [Google Scholar]
- 46.Sohn W. Smokers may experience poorer bone regeneration than nonsmokers after periodontal treatment. J. Amer. Dent. Assn. 2013;144:531–532. doi: 10.14219/jada.archive.2013.0156. [DOI] [PubMed] [Google Scholar]
- 47.Hanioka T., Ojima M., Tanaka K., Matsuo K., Sato F., Tanaka H. Causal assessment of smoking and tooth loss: A systematic review of observational studies. BMC Public Health. 2011;11:221. doi: 10.1186/1471-2458-11-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rifkind J.B. What should I look for when treating an alcoholic patient (current or recovered) in my office? [(accessed on 22 January 2014)];J. Can. Dent. Assoc. 2011 77 b114. Available online: http://www.jcda.ca/article/b114. [PubMed] [Google Scholar]
- 49.Pinkhasov R.M., Wong J., Kashanian J., Lee M., Samadi D.B., Pinkhasov M.M., Shabsigh R. Are men shortchanged on health? Perspective on health care utilization and health risk behavior in men and women in the United States. Int. J. Clin. Pract. 2010;64:475–487. doi: 10.1111/j.1742-1241.2009.02290.x. [DOI] [PubMed] [Google Scholar]
- 50.Doni B.R., Patil S., Peerapur B.V., Kadaganchi H., Bhat K.G. Estimation and comparison of salivary immunoglobulin a levels in tobacco chewers, tobacco smokers and normal subjects. Oral Health Dent. Manag. 2013;12:105–111. [PubMed] [Google Scholar]
- 51.Tsakos G., Demakakos P., Breeze E., Watt R.G. Social gradients in oral health in older adults: Findings from the English longitudinal survey of aging. Amer. J. Public Health. 2011;101:1892–1899. doi: 10.2105/AJPH.2011.300215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aida J., Kondo K., Kondo N., Watt R.G., Sheiham A., Tsakos G. Income inequality, social capital and self-rated health and dental status in older Japanese. Soc. Sci. Med. 2011;73:1561–1568. doi: 10.1016/j.socscimed.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 53.Vargas C.M., Macek M.D., Marcus S.E. Sociodemographic correlates of tooth pain among adults: United States, 1989. Pain. 2000;85:87–92. doi: 10.1016/S0304-3959(99)00250-X. [DOI] [PubMed] [Google Scholar]
- 54.Buvinić M., Medici A., Fernández E., Torres A.C. Gender Differentials in Health. In: Jamison D.T., Breman J.G., Measham A.R., Alleyne G., Claeson M., Evans D.B., editors. Disease Control Priorities in Developing Countries. 2nd ed. World Bank; Washington, DC, USA: 2006. [Google Scholar]
- 55.Szwarcwald C.L., Souza-Júnior P.R., Esteves M.A., Damacena G.N., Viacava F. Socio-demographic determinants of self-rated health in Brazil. Cad. Saude Publica. 2005;21:S54–S64. doi: 10.1590/S0102-311X2005000700007. [DOI] [PubMed] [Google Scholar]
- 56.Patel M.H., Kumar J.V., Moss M.E. Diabetes and tooth loss: An analysis of data from the national health and nutrition examination survey, 2003–2004. J. Amer. Dent. Assn. 2013;144:478–485. doi: 10.14219/jada.archive.2013.0149. [DOI] [PubMed] [Google Scholar]
- 57.Klein B.E., Klein R., Knudtson M.D. Life-style correlates of tooth loss in an adult Midwestern population. J. Public Health Dent. 2004;64:145–150. doi: 10.1111/j.1752-7325.2004.tb02744.x. [DOI] [PubMed] [Google Scholar]