Abstract
Expression of hypoxia-inducible factor (HIF)1α increases the risk of castrate-resistant prostate cancer (CRPC) and metastases in patients on androgen deprivation therapy (ADT) for prostate cancer (PC). We aimed to investigate the effects of nonspecific HIF1α inhibitors (Digoxin, metformin, and angiotensin-2 receptor blockers) on development of CRPC and metastases while on ADT. A retrospective review of prospectively collected medical records was conducted of all men who had continuous ADT as first-line therapy for CRPC at the Austin Hospital from 1983 to 2011. Association between HIF1α inhibitor medications and time to develop CRPC was investigated using actuarial statistics. Ninety-eight patients meeting the criteria were identified. Eighteen patients (21.4%) were treated with the nonspecific HIF1α inhibitors. Both groups had similar characteristics, apart from patients on HIF1α inhibitors being older (70 years vs. 63.9 years). The median CRPC-free survival was longer in men using HIF1α inhibitors compared to those not on inhibitors (6.7 years vs. 2.7 years, P = 0.01) and there was a 71% reduction in the risk of developing CRPC (HR 0.29 [95% CI 0.10–0.78] P = 0.02) after adjustment for Gleason score, age, and prostate-specific antigen (PSA). The median metastasis-free survival in men on HIF1α inhibitors was also significantly longer compared to those on no inhibitors (5.1 years vs. 2.6 years, P = 0.01) with an 81% reduction in the risk of developing metastases (HR 0.19 [CI 0.05–0.76] P = 0.02) after adjustment for Gleason score, age, and PSA. Nonspecific HIF1α inhibitors appear to increase the progression-free survival and reduce the risk of developing CRPC and metastases in patients on continuous ADT.
Keywords: Castrate resistance, Hypoxia-inducible factor, inhibitors, metastases, prostate cancer
Introduction
Androgen deprivation therapy (ADT) is a standard treatment for advanced prostate cancer (PC). However, the disease progresses and castrate-resistant prostate cancer (CRPC) develops in a significant proportion of men while on ADT 1. CRPC is a lethal form of PC that progresses and metastasizes with a median survival of 1–3 years 2. In some series greater than 84% of CRPC patients have metastases at diagnosis, while a further 33% of patients who do not have metastases at the diagnosis of CRPC may be expected to develop metastases within 2 years 3. Treatment of CRPC can be difficult as the majority of these tumors become unresponsive to chemotherapy and radiotherapy. Thus, novel therapeutic strategies targeted at delaying or preventing CRPC are of crucial importance in combating this disease.
Currently, there is no accepted method to determine which PCs will develop resistance to androgen deprivation. Furthermore, to our knowledge no current agents have been shown to prevent or delay development of castrate resistance. We previously demonstrated that the expression of hypoxia-inducible factor 1α (HIF1α) increases the risk of CRPC and metastases in patients on ADT for PC 4, and therefore hypothesized that inhibition of HIF1α may delay or prevent development of these adverse consequences.
To overcome the exorbitant costs of new drug development and to speed up the discovery of novel drug targets, one approach is to find drugs with well-established toxicologic, pharmacokinetic, and pharmacodynamic profiles that may be effective against an unrelated indication. Digoxin, metformin, and angiotensin-2 receptor blockers (ARB) are three commonly used cardiovascular medications, which have all been shown to inhibit HIF1α by different mechanisms 5–7. The effects of these nonspecific HIF1α inhibitors on the development of CRPC and metastases were therefore investigated in this study.
Patients and Methods
Patient characteristics
A retrospective analysis was performed on a prospectively maintained database of all men who received chemotherapy subsequent to CRPC development and who were previously treated with continuous ADT at the Austin Hospital, Melbourne, Australia from 1983 to 2011. ADT as primary or salvage therapy following radiotherapy was allowed. Drug histories were obtained from the hospital records to identify men being treated with the nonspecific HIF1α inhibitors: digoxin, metformin, and ARBs at the time of starting ADT. All outcome and pathological data were obtained through the Victoria Biobank and the Department of Anatomical Pathology at the Austin Hospital, Victoria, Australia. Approval for this study was obtained from the Austin Health Human Research Ethics Committee.
CRPC was defined as two consecutive rises of prostate-specific antigen (PSA) of over 50% PSA nadir at least 1 week apart while on ADT. Time to CRPC was determined from the date of initiation of primary ADT or any form of salvage ADT to the date of the second PSA rise. Patients without metastatic disease at the time of commencing ADT were eligible for analysis of time to metastases. In this case, patients were investigated at their physician's discretion, and the date of initial identification of metastatic disease defined the endpoint.
Immunohistochemistry
All available paraffin-embedded tissue samples were obtained for this cohort of men and were stained for HIF1α using a previously published protocol 4.
Statistics
The Wilcoxon and Mann–Whitney U tests were used to compare any differences in characteristics between the patient groups (those on HIF1α inhibitors vs. those not). CRPC-free survival and metastasis-free survival were assessed using the Kaplan–Meier method and log-rank test. The impact of HIF1α inhibition, age, Gleason score, and PSA at the time of starting ADT on outcomes was assessed using Cox proportional hazards regression. Two-sided P-values were utilized, and a P-value of <0.05 was considered statistically significant. All analyses were performed with SPSS software (version 17.0; SPSS Inc, Chicago, IL).
Results
Ninety-eight patients meeting the criteria were identified of whom 81 received ADT as primary therapy and 17 received ADT for biochemical recurrence post radiotherapy. Thirty-eight men (45%) were excluded from the time to metastases analysis as they had developed bony metastases prior to starting ADT.
Eighteen patients (21.4%) were on nonspecific HIF1α inhibitors prior to androgen deprivation. Of this cohort, there were eight patients on metformin, nine on digoxin, and four on ARBs (irbesartan (3/4) and candesartan (1/4). Three patients were on dual inhibitors with one person in each pair of digoxin and metformin, digoxin and ARB, and metformin and ARB. The dosages used were standard with six out of eight men on metformin on 500 mg twice daily and two on 1 g twice daily. All men on digoxin had 62.5 μg daily, all men on irbesartan had 300 mg daily, and one man on candesartan 16 mg daily. On analysis of the baseline patient characteristics (Table 1), both groups had similar median Gleason scores (7.5 and 8). The median time of follow-up in both groups was also similar. However, the patients on HIF1α inhibitors were significantly older (70 years vs. 63.9 years, P = 0.009). Although both the median PSA score and the proportion of D'Amico high-risk disease (46% vs. 59%) were lower in the patients on HIF1α inhibitors, there was no statistically significant difference, apart from age, between any of these variables in the two groups.
Table 1.
Patient characteristics.
| HIF1α inhibitors | No HIF1α inhibitors | P-value | |
|---|---|---|---|
| No of patients | 18 | 66 | |
| Average age | 70 (54–84) | 63.9 (44–81) | 0.009 |
| Median Gleason score | 7.5 (6–9) | 8 (6–10) | 0.747 |
| Median PSA at start of androgen deprivation | 97 (0–5705) | 407 (0–4780) | 0.08 |
| Percentage D'Amico high-risk disease (%) | 46 | 59 | 0.50 |
| Median follow-up (years) | 3.2 (0.34–9.5) | 2.3 (1.0–8.5) | 0.099 |
HIF, hypoxia-inducible factor; PSA, prostate-specific antigen. The baseline characteristics of the patients with and without HIF1α inhibitor treatment are shown. Differences between the two groups were analyzed using Wilcoxon and Mann–Whitney U tests and P-values were calculated. The range is demonstrated within brackets.
HIF1α expression in tumors of men on nonspecific HIF1α inhibitors
Tissue samples were available for 28 men, but of these only four had been on nonspecific HIF1α inhibitors (metformin in all cases). There was an obvious reduction in HIF1α expression in the samples from men on metformin (Fig. 1), when compared with matched samples from men with similar Gleason score, age, and procedure.
Figure 1.
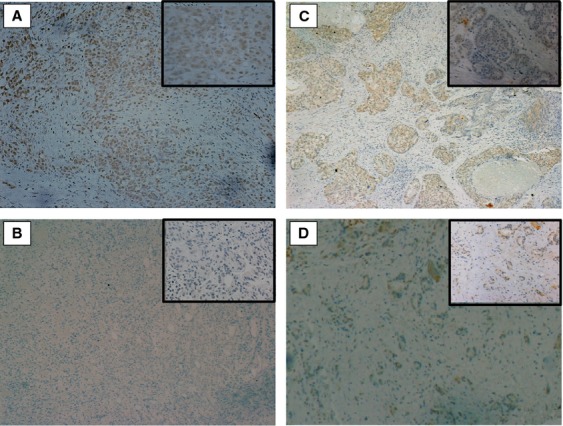
Hypoxia-inducible factor (HIF)1α expression is lower in tumors from men on metformin. All available tissue samples were stained immunohistochemically for HIF1α protein, and compared with samples matched for Gleason score. HIF1α expression was greater in trans urethral resection of prostate (TURP) specimens (A, C) from men who had Gleason 5 + 4 tumors when compared with samples from men on metformin with tumors of the same Gleason score (B, D). Inset magnification: 20×.
Time to CRPC
On a univariate analysis, men on HIF1α inhibitors had a 73% lower risk of developing castrate resistance compared to the men receiving no inhibitors, while a high Gleason score increased the risk by 1.4-fold (Table 2). The median time for developing CRPC (Fig. 2) in patients not on HIF1α inhibitors was 2.7 years compared to 6.7 years in patients using HIF1α inhibitors (log-rank test P = 0.005).
Table 2.
Univariate and multivariate regression analyses.
| Univariate analysis |
Multivariate analysis |
||||||
|---|---|---|---|---|---|---|---|
| No. of patients (%) | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value | |
| Time to CRPC | 84 (100) | ||||||
| HIF1α inhibitor | 0.37 | (0.18–0.76) | 0.007 | 0.29 | (0.10–0.78) | 0.016 | |
| Gleason score | 1.35 | (1.04–1.74) | 0.024 | 1.31 | (0.99–1.72) | 0.057 | |
| Age | 0.96 | (0.93–1.00) | 0.014 | 1.00 | (0.98–1.04) | 0.553 | |
| PSA | 1.00 | (1.00–1.00) | 0.370 | 1.00 | (1.00–1.00) | 0.403 | |
| Time to metastases | 46 (54) | ||||||
| HIF1α inhibitor | 0.29 | (0.11–0.77) | 0.013 | 0.19 | (0.05–0.76) | 0.019 | |
| Gleason score | 1.44 | (1.03–2.01) | 0.035 | 1.50 | (1.05–2.13) | 0.025 | |
| Age | 0.97 | (0.93–1.01) | 0.220 | 1.00 | (0.96–1.06) | 0.866 | |
| PSA | 1.00 | (1.00–1.00) | 0.947 | 1.00 | (1.00–1.00) | 0.284 | |
CRPC, castrate-resistant prostate cancer; HIF, hypoxia-inducible factor; PSA, prostate-specific antigen. Cox proportional univariate analyses of time to castrate resistance and time to metastases are presented. Multivariate regression analyses adjusting for each of the other factors are also presented. Fourteen men (14.3%) were excluded from the analysis as they had missing data.
Figure 2.
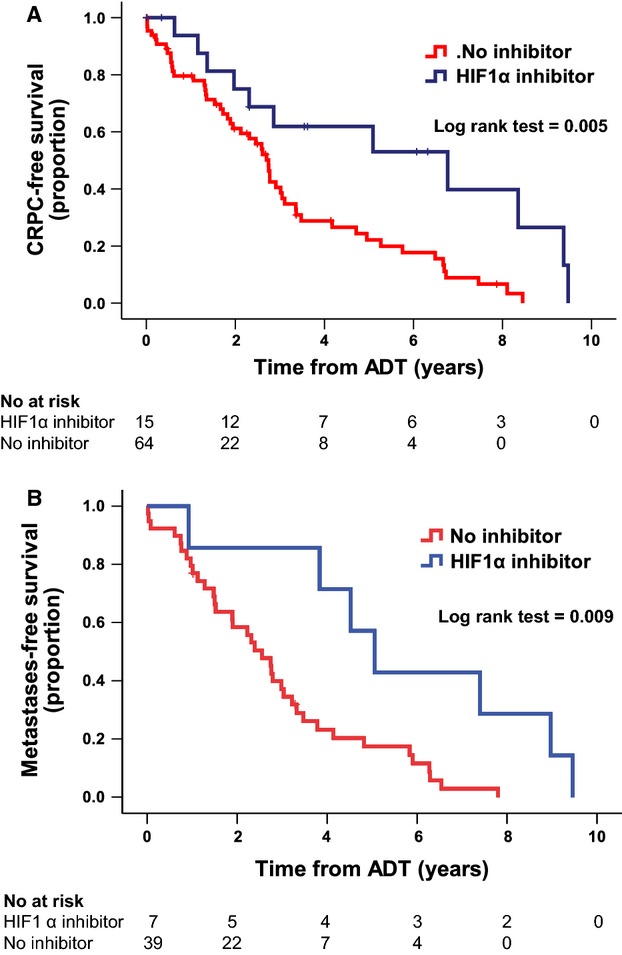
Kaplan–Meier analyses of time to CRPC and time to metastases. The survival curves for CRPC-free survival (A) and metastases-free survival (B) versus the time from starting androgen deprivation therapy are significantly better for patients treated with HIF1α inhibitors. Fourteen men (14.3%) were excluded from the analysis as they had missing data. CRPC, castrate-resistant prostate cancer; HIF, hypoxia-inducible factor.
On a multivariate regression analysis adjusting for Gleason score, age, and PSA level at the time of starting androgen deprivation, there was a 71% reduction (HR 0.29 [CI 0.10–0.78] P = 0.02) in the risk of developing CRPC in patients who were on HIF1α inhibitors. Gleason score was an independent risk factor for development of CRPC (Table 2).
Time to metastases
There were no differences in baseline Gleason scores between those men included in the time to metastases analysis and those excluded from it, or in those men on HIF1α inhibitors and those not on HIF1α inhibitors.
Men on HIF1α inhibitors and on androgen deprivation had a significantly lower risk of developing metastases (71%). A high Gleason score was a predictor of developing metastases in patients starting HIF1α inhibitors (Table 2). The median metastases-free survival time (Fig. 2) in men on HIF1α inhibitors was significantly longer than in those not on inhibitors (5.1 years vs. 2.6 years, P = 0.009).
There was an 81% reduction in the risk of developing metastases in men on HIF1α inhibitors (HR 0.19 [CI 0.05–0.76] P = 0.02) after adjusting for age, Gleason score, and PSA level (Table 2).
The average time from commencing HIF1α inhibitor use to metastases was 54 months (range 3–116 months) and the duration of inhibitor use did not have any effect on the outcomes. In order to establish if these effects were specific to the medication used, further analyses were performed on men taking either aspirin, statins, β blockers or an ACE-1 inhibitor. There was no significant reduction in time in metastases (HR 0.71 [95% CI 0.4–1.4] P = 0.3) or time to castrate resistance (HR 0.69 [95% CI 0.4–1.1] P = 0.2) on a univariate analysis.
Discussion
Expression of androgen receptors has been linked with development of castrate resistance 8, and shown to be upregulated by HIF1α 9. Furthermore, HIF1α is upregulated in prostate tumors 10,11 and is a potent defensive mechanism used by tumor cells against oxidative stress or destruction by androgen deprivation, chemotherapy or radiation cytotoxicity 12. These results are supported by our previously published data, which demonstrated that overexpression of HIF1α increases the risk of castrate resistance and metastases in PC 4, and confirmed the importance of HIF1α as a molecular target in PC.
The aim of this pilot study was to investigate the effects common to the nonspecific HIF1α inhibitors digoxin, metformin, and ARB on the development of CRPC and metastases. Our results demonstrate a significant increase in median metastases-free survival of 2.5 years and CRPC-free survival of 4 years in men using HIF1α inhibitors. While the number of patients on HIF1α inhibitors was too small to analyze further the effects of the individual drugs, all three medications individually demonstrated similar trends toward the benefits seen above. Since all three drugs inhibit HIF1α expression, this observation is consistent with the suggestion that the increased survival is the result of a reduction in HIF1α expression. Furthermore, the side effects of these nonspecific HIF1α inhibitors are generally more tolerable compared with those of the commonly used chemotherapeutic agents. Thus, the use of nonspecific HIF1α inhibitors to delay castrate resistance and metastases is likely to be more advantageous to patients starting androgen deprivation, rather than following development of CRPC. These results, seen even in a small sample size, are in agreement with our previous data that linked HIF1α overexpression with decreased survival 4. Larger prospective studies are warranted to confirm our findings.
The objective of this study was to analyze the effect of HIF1α inhibitors as a class rather than as individual agents. All three of the agents investigated have been shown to reduce the risk of developing various cancers 13–15. Digoxin, a universal protein translation inhibitor that inhibits HIF1α expression in vitro and in vivo 5, reduces the risk of PC, including potentially lethal disease, by 75% 16. Metformin, on the other hand, inhibits HIF1α by an indirect mechanism 6, and increases apoptosis and the anti-proliferative effects of bicalutamide in PC 17. Although Bansal et al. demonstrated that there was a 14% reduction in the incidence of PC in men with type 2 diabetes 18, other studies do not provide a clear consensus on whether there is a link between these conditions. Furthermore, while some studies showed a potential link between diabetes and disease progression 19, metformin treatment of men with diabetes has been shown to improve PSA-recurrence-free survival, distant metastases-free survival, PC-specific mortality, overall survival, and development of CRPC, compared to men with diabetes not on metformin 20. Metformin also reduces the incidence of high-grade PCs and their progression 21, and confers some survival benefit by reducing metabolic syndrome in men with PC 22,23. As angiotensin 2 has been shown to increase HIF1α in vitro by a posttranslational mechanism 7, ARBs would be expected to reduce HIF1α expression. Although ARBs have been shown to reduce proliferation of PCs in vitro 24, the inhibitory effects of different ARBs on HIF1α expression have not been compared and should be studied further.
Although this study did not directly demonstrate that the effects of digoxin, metformin, and ARBs are mediated only via inhibition of the HIF1α pathway, a feature common to all three drugs is that they inhibit HIF1α expression in cell lines in vitro. Thus, a reasonable working hypothesis is that the main actions of these agents are via the HIF1α pathway. Apart from one study demonstrating HIF1α inhibition at 2 g daily of metformin (in keeping with the doses used by some patients in our study) 25, there are no clinical studies looking at the optimal dosage at which these medications inhibit HIF1α, and only preclinical studies observing the inhibition of HIF1α by digoxin or ARBs. This study demonstrates that there may be some benefit even at the doses usually taken by patients and these could be used as a pilot for further studies.
Many men with CRPC would also develop resistance to chemotherapy and would subsequently require additional hormonal or immunotherapeutic agents. However, the currently reported survival advantages in men started on newer hormonal or immunotherapeutic agents such as abiratarone acetate or sipuleucel-T following development of CRPC is less than a year 26. Treatment with nonspecific HIF1α inhibitors might also give an added survival benefit when used with the newer hormonal agents after development of CRPC. A major obstacle in designing trials using HIF1α inhibitors is the length of the study, since many years might elapse prior to reaching the endpoints of CRPC and metastases. Thus, the evidence from prospectively maintained databases is of great interest.
There would be several advantages in using nonspecific HIF1α inhibitors to reduce the risk of developing castrate resistance and metastases in patients on androgen deprivation. Because of the availability of generic brands, the costs to the consumer would be negligible compared to new cancer drugs. Furthermore, due to their widespread long-term use, their side effects are well established in a population of patients who maybe concurrently on these drugs while on ADT. However, the use of these drugs may give rise to new issues such as the use of metformin in nondiabetic patients, and potential cardiac toxicity with digoxin. The need for monitoring of drug levels and the lack of familiarity of urologists and oncologists in the routine prescription of these medications will also require further consideration prior to starting these medications for PC. In order to negate these issues, the future development of specific HIF1α inhibitors would be desirable.
The design of this study only identified men who underwent chemotherapy for CRPC, which may have led to an element of selection bias. Thus, the present results cannot be extrapolated to those who were not medically fit for chemotherapy, or did not survive to the point of considering it. The original indication for the use of drugs was cardiovascular disease and/or hypertension but the severity in the HIF1α inhibitor group, and the presence of cardiovascular disease in men not on HIF1α inhibitors, was not recorded. Thus, these factors could not be adjusted for in the analysis, and the results may have been influenced accordingly. In order to exclude the effects of the severity of cardiovascular disease on mortality, our aims were focussed on cancer-specific outcomes, which did not include mortality. Another limitation of the study is that the medication history was recorded at the start of ADT, and it is possible that the drug doses or compliance with these medications may have changed during the course of therapy. In addition, these findings could be influenced by sample bias due to the small numbers available for this study. Therefore, larger prospective studies should be designed to investigate these findings further.
Conclusions
Nonspecific HIF1α inhibitors appear to increase the progression-free survival and reduce the risk of developing CRPC and metastases in patients on continuous ADT. Larger prospective studies are warranted to confirm these findings. Future trials may be able to utilize these nonspecific inhibitors, pending the development of agents specifically targeting HIF1α.
Acknowledgments
We thank Carmel Murone and Lewis Lee of the Victorian Bio Bank for their tireless efforts in data collection.
Conflict of Interest
None declared.
Funding Information
This work was supported by National Health and Medical Research Council of Australia grant 1020983 (GSB) and by the Austin Medical Research Foundation.
References
- 1.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat. Rev. Cancer. 2001;1:34–45. doi: 10.1038/35094009. Epub 20 March 2002. [DOI] [PubMed] [Google Scholar]
- 2.Lee DJ, Cha EK, Dubin JM, Beltran H, Chromecki TF, Fajkovic H, et al. Novel therapeutics for the management of castration-resistant prostate cancer (CRPC) BJU Int. 2012;109:968–985. doi: 10.1111/j.1464-410X.2011.10643.x. Epub 01 November 2011. [DOI] [PubMed] [Google Scholar]
- 3.Kirby M, Hirst C, Crawford ED. Characterising the castration-resistant prostate cancer population: a systematic review. Int. J. Clin. Pract. 2011;65:1180–1192. doi: 10.1111/j.1742-1241.2011.02799.x. Epub 15 October 2011. [DOI] [PubMed] [Google Scholar]
- 4.Ranasinghe WK, Xiao L, Kovac S, Chang M, Michiels C, Bolton D, et al. The role of hypoxia-inducible factor 1alpha in determining the properties of castrate-resistant prostate cancers. PLoS One. 2013;8:e54251. doi: 10.1371/journal.pone.0054251. Epub 24 January 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin J, Denmeade S, Carducci MA. HIF-1alpha and calcium signaling as targets for treatment of prostate cancer by cardiac glycosides. Curr. Cancer Drug Targets. 2009;9:881–887. doi: 10.2174/156800909789760249. Epub 23 December 2009. [DOI] [PubMed] [Google Scholar]
- 6.Takiyama Y, Harumi T, Watanabe J, Fujita Y, Honjo J, Shimizu N, et al. Tubular injury in a rat model of type 2 diabetes is prevented by metformin: a possible role of HIF-1alpha expression and oxygen metabolism. Diabetes. 2011;60:981–992. doi: 10.2337/db10-0655. Epub 02 February 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolf G, Schroeder R, Stahl RA. Angiotensin II induces hypoxia-inducible factor-1 alpha in PC 12 cells through a posttranscriptional mechanism: role of AT2 receptors. Am. J. Nephrol. 2004;24:415–421. doi: 10.1159/000080086. Epub 17 August 2004. [DOI] [PubMed] [Google Scholar]
- 8.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, et al. Molecular determinants of resistance to antiandrogen therapy. Nat. Med. 2004;10:33–39. doi: 10.1038/nm972. Epub 02 January 2004. [DOI] [PubMed] [Google Scholar]
- 9.Mitani T, Yamaji R, Higashimura Y, Harada N, Nakano Y, Inui H. Hypoxia enhances transcriptional activity of androgen receptor through hypoxia-inducible factor-1alpha in a low androgen environment. J. Steroid Biochem. Mol. Biol. 2011;123:58–64. doi: 10.1016/j.jsbmb.2010.10.009. Epub 09 November 2010. [DOI] [PubMed] [Google Scholar]
- 10.Kimbro KS, Simons JW. Hypoxia-inducible factor-1 in human breast and prostate cancer. Endocr. Relat. Cancer. 2006;13:739–749. doi: 10.1677/erc.1.00728. Epub 07 September 2006. [DOI] [PubMed] [Google Scholar]
- 11.Du Z, Fujiyama C, Chen Y, Masaki Z. Expression of hypoxia-inducible factor 1alpha in human normal, benign, and malignant prostate tissue. Chin. Med. J. (Engl.) 2003;116:1936–1939. Epub 23 December 2003. [PubMed] [Google Scholar]
- 12.Marignol L, Coffey M, Lawler M, Hollywood D. Hypoxia in prostate cancer: a powerful shield against tumour destruction? Cancer Treat. Rev. 2008;34:313–327. doi: 10.1016/j.ctrv.2008.01.006. Epub 13 March 2008. [DOI] [PubMed] [Google Scholar]
- 13.Zhang H, Qian DZ, Tan YS, Lee K, Gao P, Ren YR, et al. Digoxin and other cardiac glycosides inhibit HIF-1alpha synthesis and block tumor growth. Proc. Natl. Acad. Sci. USA. 2008;105:19579–19586. doi: 10.1073/pnas.0809763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Currie CJ, Poole CD, Jenkins-Jones S, Gale EA, Johnson JA, Morgan CL. Mortality after incident cancer in people with and without type 2 diabetes: impact of metformin on survival. Diabetes Care. 2012;35:299–304. doi: 10.2337/dc11-1313. Epub 24 January 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang CH, Lin JW, Wu LC, Lai MS. Angiotensin receptor blockade and risk of cancer in type 2 diabetes mellitus: a nationwide case-control study. J. Clin. Oncol. 2011;29:3001–3007. doi: 10.1200/JCO.2011.35.1908. Epub 22 June 2011. [DOI] [PubMed] [Google Scholar]
- 16.Platz EA, Yegnasubramanian S, Liu JO, Chong CR, Shim JS, Kenfield SA, et al. A novel two-stage, transdisciplinary study identifies digoxin as a possible drug for prostate cancer treatment. Cancer Discov. 2011;2011:68–77. doi: 10.1158/2159-8274.CD-10-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colquhoun AJ, Venier NA, Vandersluis AD, Besla R, Sugar LM, Kiss A, et al. Metformin enhances the antiproliferative and apoptotic effect of bicalutamide in prostate cancer. Prostate Cancer Prostatic Dis. 2012;15:346–352. doi: 10.1038/pcan.2012.16. Epub 23 May 2012. [DOI] [PubMed] [Google Scholar]
- 18.Bansal D, Bhansali A, Kapil G, Undela K, Tiwari P. Type 2 diabetes and risk of prostate cancer: a meta-analysis of observational studies. Prostate Cancer Prostatic Dis. 2013;16:151–158. doi: 10.1038/pcan.2012.40. S1. Epub 04 October 2012. [DOI] [PubMed] [Google Scholar]
- 19.Wu C, Aronson WJ, Terris MK, Presti JC, Jr, Kane CJ, Amling CL, et al. Diabetes predicts metastasis after radical prostatectomy in obese men: results from the SEARCH database. BJU Int. 2013;111:E310–E318. doi: 10.1111/j.1464-410X.2012.11687.x. Epub 12 January 2013. [DOI] [PubMed] [Google Scholar]
- 20.Spratt DE, Zhang C, Zumsteg ZS, Pei X, Zhang Z, Zelefsky MJ. Metformin and prostate cancer: reduced development of castration-resistant disease and prostate cancer mortality. Eur. Urol. 2013;63:709–716. doi: 10.1016/j.eururo.2012.12.004. Epub 05 January 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hitron A, Adams V, Talbert J, Steinke D. The influence of antidiabetic medications on the development and progression of prostate cancer. Cancer Epidemiol. 2012;36:e243–e250. doi: 10.1016/j.canep.2012.02.005. Epub 16 March 2012. [DOI] [PubMed] [Google Scholar]
- 22.Clements A, Gao B, Yeap SH, Wong MK, Ali SS, Gurney H. Metformin in prostate cancer: two for the price of one. Ann. Oncol. 2011;22:2556–2560. doi: 10.1093/annonc/mdr037. Epub 23 March 2011. [DOI] [PubMed] [Google Scholar]
- 23.Nobes JP, Langley SE, Klopper T, Russell-Jones D, Laing RW. A prospective, randomized pilot study evaluating the effects of metformin and lifestyle intervention on patients with prostate cancer receiving androgen deprivation therapy. BJU Int. 2012;109:1495–1502. doi: 10.1111/j.1464-410X.2011.10555.x. Epub 22 September 2011. [DOI] [PubMed] [Google Scholar]
- 24.Funao K, Matsuyama M, Kawahito Y, Sano H, Chargui J, Touraine JL, et al. Telmisartan is a potent target for prevention and treatment in human prostate cancer. Oncol. Rep. 2008;20:295–300. Epub 19 July 2008. [PubMed] [Google Scholar]
- 25.Ece H, Cigdem E, Yuksel K, Ahmet D, Hakan E, Oktay TM. Use of oral antidiabetic drugs (metformin and pioglitazone) in diabetic patients with breast cancer: how does it effect serum Hif-1 alpha and 8Ohdg levels? Asian Pac. J. Cancer Prev. 2012;13:5143–5148. doi: 10.7314/apjcp.2012.13.10.5143. Epub 19 December 2012. [DOI] [PubMed] [Google Scholar]
- 26.Ezzell EE, Chang KS, George BJ. New agents in the arsenal to fight castrate-resistant prostate cancer. Curr. Oncol. Rep. 2013;15:239–248. doi: 10.1007/s11912-013-0305-9. Epub 27 February 2013. [DOI] [PubMed] [Google Scholar]


