Abstract
Objectives
To establish normal ranges for HE4 serum levels in healthy women.
Study Design
HE4 levels were measured in healthy women and analyzed by age, menopausal status and pregnancy status. Upper 95th percentiles were determined for normal ranges.
Results
Serum samples from 1101 healthy women and 67 pregnant women were analyzed. Above the age of 40 significant elevations in HE4 concentrations emerged with advancing age. The upper 95th percentile for HE4 levels for premenopausal women was 89 pM, for postmenopausal women 128 pM, and for all women 115 pM. There was a significant difference in the median serum HE4 levels in premenopausal women (46.6 pM) compared with postmenopausal women (57.6 pM) (p<0.001). In pregnant women, median HE4 concentrations were significantly lower than their premenopausal counterparts (p<0.001).
Conclusions
HE4 serum concentrations vary significantly based on age. These variations must be considered when determining the upper limit of normal for HE4.
Keywords: Biomarker, HE4, healthy women, pregnancy
Introduction
Over the past 3 decades CA125 has provided a biomarker for monitoring women diagnosed with ovarian cancer during treatment and prior to disease recurrence (1;2). CA125 has also been studied extensively for a possible role in early detection and screening for ovarian cancer. Although promising the role of CA125 in this area has yet to be defined (3–6).
Although CA125 is the current standard biomarker for the management of ovarian cancer it is not without limitations. CA125 is elevated in only 50 to 60% of early stage cases and is not expressed by up to 20% of all ovarian cancers (7). CA125 specificity is also limited as levels can be elevated in several benign gynecologic disorders such as endometriosis, pelvic inflammatory disease and benign neoplasms of the ovaries and uterus (7–11). As well, CA125 can be elevated in many common non gynecologic conditions such as congestive heart failure, hepatic disease and inflammatory diseases that affect pleural, peritoneal and pericardial surfaces.
The novel serum biomarker Human Epididymal Protein 4 (HE4) has be shown to be over-expressed in serous, endometrioid and clear cell epithelial ovarian cancers (12). HE4 has also been demonstrated to be a sensitive and specific serum biomarker for ovarian cancer that is less frequently elevated by benign conditions that occur in premenopausal women (13;14). Recently it was shown the addition of HE4 to CA125 increased the sensitivity and specificity of either marker alone for the detection of ovarian cancer (14–17).
The HE4 protein is a whey acid protein (WAP) with a four disulfide core originally isolated in epithelial cells of the human epididymis and is expressed in numerous tissues throughout the body, including the female reproductive tract (18). Importantly, HE4 circulates in the bloodstream and can be detected through an immunosorbent assay (EIA) using a monoclonal mouse antibody directed at an HE4 epitope.
In 2009 the United States Food and Drug Agency (FDA) approved HE4 for monitoring women diagnosed with epithelial ovarian cancer with similar indications to the use of CA125. To date, however, there are no large trials examining serum HE4 levels in healthy premenopausal and postmenopausal women and healthy pregnant women. The upper 95th percentile of 150 pM for both premenopausal and postmenopausal women is reported in the FDA package insert for the HE4 EIA Kit (Fujirebio Diagnostics Inc, Malvern PA, USA). This value does not take into consideration patient age or menopausal status and what actually constitutes normal levels in healthy women and whether these levels vary by subgroups have not been clearly evaluated and published. The purpose of this study was to examine serum levels of HE4 in healthy women based on age, menopausal status and pregnancy status to refine normative data for this novel biomarker.
Materials and Methods
A meta-analysis was performed using data collected in three independent trials measuring HE4 levels in healthy females utilizing the HE4 EIA kit (Fujirebio Diagnostics Inc. Malvern, PA). The studies included 1) an IRB approved study at Women and Infant’s Hospital to obtain residual serum from healthy premenopausal women (N=101) and postmenopausal women (N=91) and residual serum samples from women during their first, second and third trimesters of pregnancy (n = 67); 2) An IRB approved trial through MD Anderson Cancer Center (MDACC) enrolling postmenopausal women in a multicenter low-risk ovarian cancer screening trial through an ovarian SPORE P50 grant, 143 samples were obtained from this trial and; 3) Serum collected from IRB approved repositories obtained by Fujirebio Diagnostics Inc. in which samples from 374 premenopausal and 392 postmenopausal healthy women were banked (Protocol FDI-53. IRB review of the FDI-53 protocol found the data was unlinked and de-identified and therefore did not require IRB approval. All blood samples were centrifuged and the serum was collected and frozen at −80°C until testing.
Menopausal status was determined with the following criteria for each of the individual studies: For the Women and Infants samples women age 55 years or older were considered postmenopausal and women age 45 years or younger were considered premenopausal. No samples were obtained from WIH between the ages of 46 to 54 years. All women entered from the MDACC trial were postmenopausal as determined by a medical interview and a history of amenorrhea for greater than one year. The menopausal status for the patient samples obtained from the Fujirebio clinical trial sample banks was determined through medical history or chart review as reported by the serum banks supplying these samples. Serum levels for HE4 were tested at each institution respectively using an HE4 EIA assay kit (Fujirebio Diagnostics Inc, Malvern PA).
Statistical Analysis
The primary endpoint of this study was to describe serum concentrations of HE4 (pM) to determine the normal ranges in healthy premenopausal and postmenopausal women, and pregnant women. In each group the median, range, mean, standard deviation, percent coefficient of variation, and the 90th, 95th, 97.5th and the 99th percentile for serum HE4 levels were determined. Normal serum levels for HE4 were defined using a cut point at the upper 95th percentile. P values for medians were derived using a continuity corrected Pearson’s chi-square median test and the Wilcoxon rank sum method. Log base 2 transformed scatter plots also were generated for HE4 levels by decadal age group and menopausal status; standard scatter plots were generated for HE4 levels in pregnant women. All HE4 values were derived using cubic spline interpolation.
Results
This study included serum samples from 1168 women with a total of 1101 healthy, nonpregnant women and 67 pregnant women. There were 475 premenopausal women (101 from WIHRI and 374 from Fujirebio) with a mean age of 34.3 years (range: 15 – 57 years) and 626 postmenopausal women (91 from WIH, 143 from MDACC and 392 from Fujirebio) with a mean age of 62.8 (range: 34 – 94). In addition, the study included 67 healthy pregnant women from WIH, with 25 women in the 1st trimester, 25 women in the 2nd trimester, and 17 women in the 3rd trimester of pregnancy.
HE4 levels by menopausal status
The mean, standard deviation, median and ranges for serum HE4 levels by age groups and menopausal status are shown in Table 1.
Table 1.
Statistical characteristics of serum HE4 levels (pM) for premenopausal and postmenopausal women by age group.
| N=1101 | Premenopausal | Postmenopausal | |||||
|---|---|---|---|---|---|---|---|
| Age Group |
<30 | 30 – 39 | >=40 | <60 | 60 – 69 | 70 – 79 | >=80 |
| N | 170 | 159 | 146 | 256 | 236 | 102 | 32 |
| Mean | 55.5 | 49.9 | 61.4 | 56.0 | 66.9 | 76.6 | 137.8 |
| Std Dev | 66.8 | 28.6 | 54.6 | 26.1 | 49.3 | 34.1 | 87.7 |
| Median | 46.2 | 43.5 | 50.5 | 50.7 | 59.9 | 66.9 | 113.4 |
| Range | 24.5–656.4 | 22.4–293.5 | 22.6–645.6 | 18.7–285.8 | 12.0–690.8 | 21.7–228.9 | 48.5–430.8 |
The median serum HE4 levels for all premenopausal women (N=475) combined was 46.6 pM. A comparison of the median HE4 serum levels for premenopausal women by decade of age showed no significant differences between the age group less than 30 years (N=170), median of 46.2 pM, and age 30 to 39 (N=159), median of 43.5 pM (median p=0.204, ranksum p = 0.1600). When comparing the age group 30 to 39 (N=159) to the age group of 40 and over (N=146), there was a statistically significant difference in the median HE4 serum levels (43.5 vs. 50.5 pM, respectively, median p=0.007, ranksum p<0.0001). When comparing premenopausal women less than 40 years old (N=329) to those 40 years or older (N=146), a statistically significant difference in the median HE4 levels was observed (44.9 pM vs. 50.5 pM, respectively, median p = 0.010, ranksum p<0.0001).
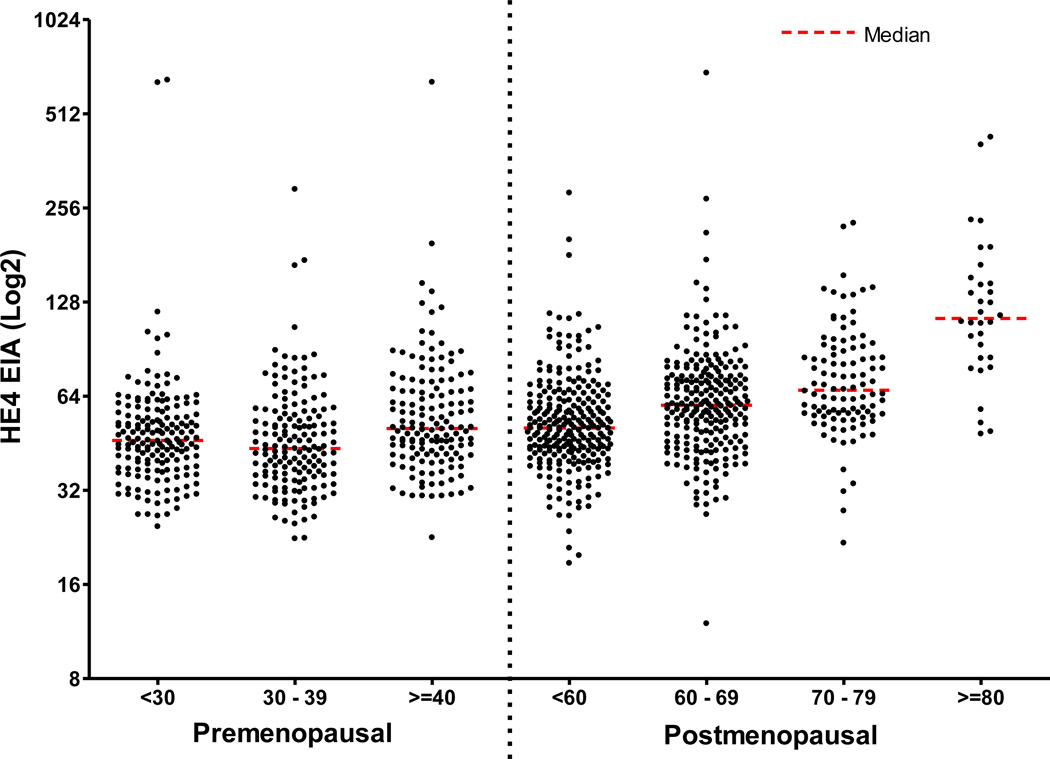
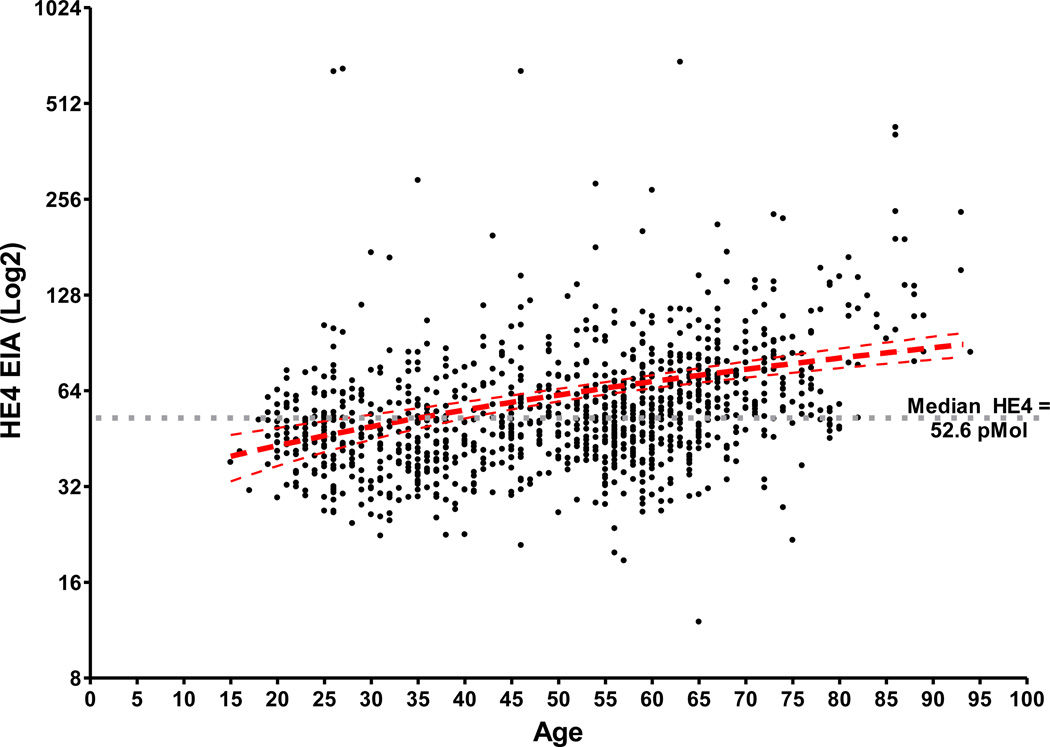
The median serum HE4 levels for all postmenopausal women combined (N=626) was 57.6 pM. A comparison of the HE4 serum levels for postmenopausal women by decade of age showed statistically significant differences in median serum HE4 levels between each age group, with increasing HE4 levels up to the age of 80 years. The postmenopausal age group less than 60 (N=256) had a median of HE4 level of 50.7 pM, which was significantly lower compared to the age group of 60 to 69 (N=236), which had a median HE4 level of 59.9 pM (p<0.001). The age group 60 to 69 had a significantly lower median serum HE4 level compared with a median of 66.9 pM for the age group 70 to 79 (N=102) (median p=0.024, ranksum p = 0.0005). Comparison of the age group 80 and older (N=32) showed significant elevation of the median serum HE4 level (113.4 pM) when compared to all other postmenopausal age groups (p<0.001). The median serum HE4 level for all premenopausal women was 46.6 pM, which was significantly lower compared to the median HE4 level for all postmenopausal women (57.6 pM, p<0.001) and to the median HE4 level for postmenopausal women under the age of 80 (N=594, 56.8 pM, p<0.001). However the median HE4 level for premenopausal women age 40 and older (50.5 pM) and postmenopausal women age less than 60 years (50.7 pM) showed no significant difference indicating that differences in HE4 levels may not be related to menopausal status but age. Taking this into consideration the most significant age cut off with regard to the difference between the HE4 concentrations was at the age of 60 years. For all women less than 60 years of age the median HE4 level was 48.2 pM and for all women 60 years or older the median HE4 level was 63.4 (p<0.001). Figure 1 displays a scatterplot of the serum HE4 levels for all 1101 samples grouped by menopausal status and age. The median serum HE4 levels clearly rise consistently with age regardless of menopausal status (Figure 2).
Figure 1.
Scatterplot of the serum HE4 levels for premenopausal and postmenopausal women by age groups.
Figure 2.
Serum HE4 levels increase with age and are independent of menopausal status.
HE4 upper limits of normal
The upper 90th, 95th, 97.5th and 99th percentiles for premenopausal, postmenopausal and all women combined were determined and shown in Table 2. The 95th percentile is often used as the upper limit of normal in biomarker analysis. Using the 95th percentile as the upper limit of normal cut point; premenopausal women had a cut point 89.1 pM and postmenopausal women had a cut point of 128.0 pM. Combining all women pre and postmenopausal the 95th percentile for serum HE4 was 114.8 pM. Examination of all women (pre and postmenopausal) using an age cut point of 60 years, women less than 60 years of age had an 95th percentile cut point of 93.3 pM and women older than 60 years of age had a 95th percentile cutpoint of 141.7 pM.
Table 2.
Distribution of serum HE4 levels in women, stratified by menopausal status.
| All | Premenopausal | Postmenopausal | |
|---|---|---|---|
| N | 1101 | 475 | 626 |
| 90th Percentile (pM) | 90.9 | 75.1 | 101.2 |
| 95th Percentile (pM) | 114.8 | 89.1 | 128.0 |
| 97.5th Percentile (pM) | 145.8 | 118.6 | 160.1 |
| 99th Percentile (pM) | 228.9 | 179.7 | 231.8 |
The international Federation of Clinical Chemistry and Laboratory Medicine and the Clinical and Laboratory Standards Institute have described methods for determining biomarker cut points (19). This method uses the upper 95% reference limit of the 95th percentile reference interval with 90% confidence intervals. These values for serum HE4 levels are displayed in Table 3. With this method, the upper limit for premenopausal women is 118.9 pM (90% CI: 97.7 – 167.4) and for postmenopausal women the upper limit is 167.8 pM (90% CI: 140.8 – 212.7). Combining both pre and postmenopausal women, the upper limit of normal is 146.3 pM (90% CI 138.0 –191.5) (Table 3), which is equivalent to the 150 pM threshold reported in the FDA package insert for the HE4 EIA assay. When examining the cut point of 60 years of age for all women (pre and postmenopausal), women less than 60 years of age had an upper limit of normal of 116.9 pM (90% CI: 102.6 – 138.0) and women older than 60 years of age had an upper limit of normal of 212.7 (90% CI: 152.7 – 234.3).
Table 3.
Confidence interval ranges for the 95th percentile for serum HE4 levels (pM) for premenopausal women, postmenopausal women and all women.
| All | Premenopausal | Postmenopausal | |
|---|---|---|---|
| N | 1101 | 475 | 626 |
| 95% Reference Interval | 28.2–146.3 | 26.8–118.9 | 29–155.4 |
| Lower 95% Reference Limit (90% CI) | 28.2 (26.5–29) | 26.8 (25.7–29) | 29 (27.5 – 30.9) |
| Upper 95% Reference Limit (90% CI) | 146.3 (138–191.5) | 118.9 (97.7–67.4) | 167.8 (140.8–212.7) |
| Average | 62.4 | 55.4 | 67.6 |
| Std. Deviation | 49.2 | 52.9 | 45.6 |
| Variance | 2424.5 | 2796.5 | 2082.2 |
| Median | 52.6 | 46.6 | 57.6 |
HE4 Levels by Pregnancy Status
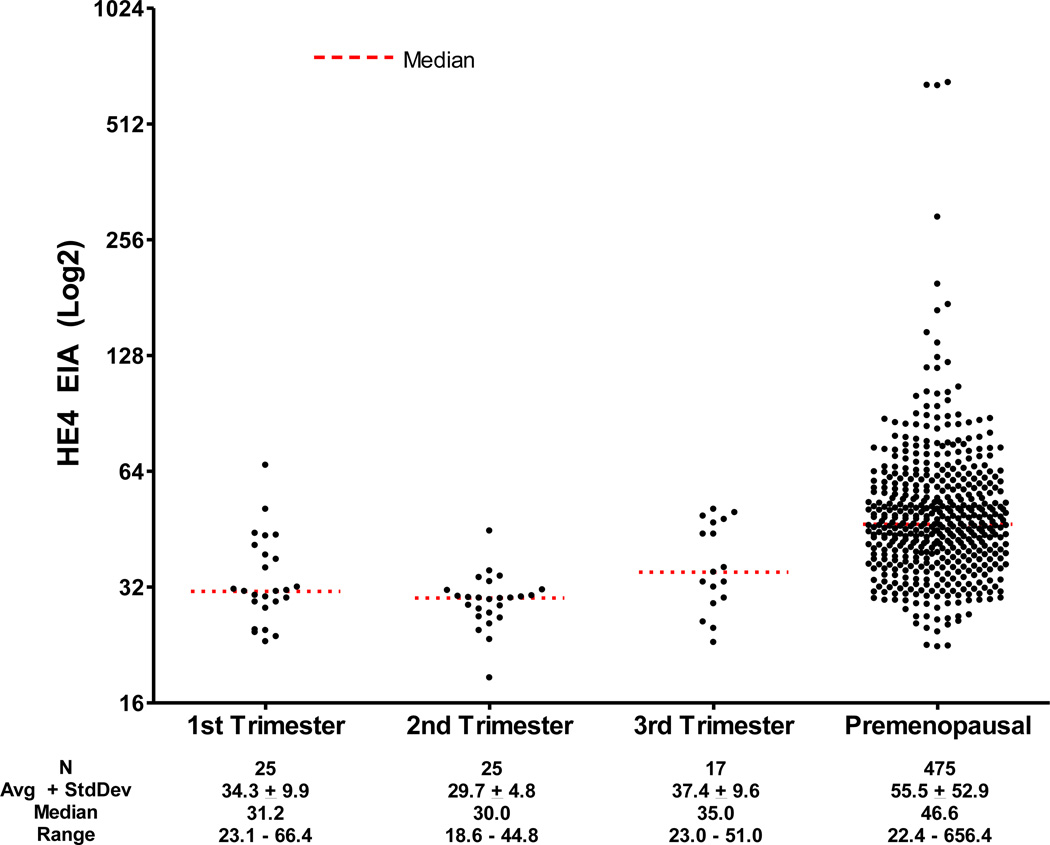
In a separate group of 67 pregnant women, no statistically significant differences were noted in median HE4 levels by trimester (Table 4). However, the difference in median HE4 levels between the 2nd and 3rd trimesters approached statistical significance (30.0 vs 35.0 pM; P = 0.059). Moreover, the distribution of the HE4 serum values between the 2nd and 3rd trimester groups did differ significantly (Wilcoxon rank sum P = 0.0116), most likely due to a larger number of higher HE4 values in the 3rd trimester group. The distributions of the 1st and 2nd and the 1st and 3rd trimesters did not differ significantly (Wilcoxon rank sum P = 0.0990 and 0.1826, respectively). The 95th percentile HE4 cutoff for all pregnant women was 49.7 pM, ranging from 35.1pM to 50.2 pM in the 2nd and 3st trimesters, respectively.
Table 4.
Statistical characteristic for serum HE4 Levels (pM) during pregnancy by trimester
| 1st Trimester | 2nd Trimester | 3rd Trimester | All | |
|---|---|---|---|---|
| N | 25 | 25 | 17 | 67 |
| Mean | 34.3 | 29.7 | 37.4 | 33.4 |
| Median | 31.2 | 30.0 | 35.0 | 30.5 |
| Std Dev | 9.9 | 4.8 | 9.6 | 8.7 |
| 95th percentile | 49.6 | 35.1 | 50.2 | 49.7 |
| 97.5th percentile | 57.2 | 39.1 | 50.6 | 51.0 |
| 99th percentile | 62.7 | 42.5 | 50.8 | 56.2 |
When women in any trimesters of pregnancy were compared with all premenopausal women, median HE4 values were significantly lower in pregnant women (30.5 pM vs 46.6 pM; P < 0.001) and serum sample distributions differed significantly (Wilcoxon rank sum P < 0.0001) (Figure 3).
Figure 3.
Scatterplot of pregnant women by trimester and all premenopausal women.
Comment
HE4 has demonstrated utility as a marker for the detection and management of ovarian cancer, especially in combination with CA 125, the current gold standard marker for this malignancy (14–16;20). However, to date no clear normative values have been published for the serum biomarker in healthy women and pregnant women. The results of our study elucidate normal HE4 serum levels in healthy women without malignant or benign gynecological disorders, and provide insight into how HE4 levels vary in different populations of healthy women.
Our findings show a significant difference in serum HE4 concentrations by age and with a significant rise starting at age 60 years. When combining all menopausal women with a comparison to all premenopausal women there is a significantly higher serum HE4 level in the postmenopausal patient population. However, interestingly when comparing premenopausal women age 40 year or greater to postmenopausal women age 60 years or younger there was no statistical difference in the median serum HE4 levels for the two groups. This suggests that menopausal status does not play a role in the increasing median serum levels; rather, age is the critical factor. These findings are consistent with a study examining a cohort of women at high risk for ovarian cancer which showed increasing HE4 levels with age(21). Among premenopausal healthy women, no statistically significant differences in median HE4 serum concentrations emerged based on decadal age when comparing women aged less than 30 years with women 30 to 39 years and women greater than or equal to 40. However, when comparing premenopausal women less than 40 years old with women 40 years and over, a statistically significant difference emerged again supporting age as a determinant for increasing HE4 levels in healthy females. Among postmenopausal women, steady increases in median serum HE4 concentrations were seen across the different age groups: less than 60, 60 to 69, 70 to 79, and 80 years and older. In postmenopausal women, increasing CA125 levels also have been linked to advancing age in healthy women (22;23). In our study, the most notable increase occurred after age 79 years. Increases in HE4 observed in our study of aging postmenopausal women elevates the risk for false positive tumor marker findings, underscoring the clinical importance of recognizing these trends in normal values when using HE4 for cancer diagnosis. While the precise reason for these increases remains uncertain, they are likely secondary to age-related declines in renal function, or perhaps an increased prevalence of co-morbid conditions.
An upper limit of normal for serum HE4 levels of 150 pM has been reported in the FDA package insert for the HE4 EIA kit (Fujirebio Diagnostics Inc. Malvern, PA) for premenopausal and postmenopausal women combined. The current study achieved similar results using the 97.5th percentile resulting in a threshold of 145.8 pM. As well, employing the international Federation of Clinical Chemistry and Laboratory Medicine and the Clinical and Laboratory Standards Institute method of determining cut points which uses the upper confidence interval for the 95th percentile, a threshold of 146.3 pM was observed in this study, which is similar to that reported in the FDA package insert. However, a single cutpoint for both menopausal groups may lead to inaccurate patient evaluations. Clinically important variations in HE4 serum concentration occur based on age among healthy women, underscoring the need to employ normative data that addresses these specific subgroups. In our study, normal HE4 serum levels, defined using a cut point at the 95th percentile, were 114.8 pM for all women, 89.1 pM for premenopausal women, and 125.6 pM for postmenopausal women, illustrating the need to use age or menopausal specific cut points. When using specific cut points for biomarkers clinicians need to be familiar with the patient population, the laboratories and the methods by which the biomarkers are being measured and reported. For instance, using a cut point at the 99th percentile, HE4 values for healthy premenopausal women were 180 pM, for postmenopausal women 232 pM and for all women 229 pM. With this in mind, when HE4 levels are obtained in otherwise healthy individuals, it is important to realize that values higher than the FDA approved normal of 150 pM can occur. The same holds true for CA125 levels where some manufactures platform CA125 assays have a 95th percentile cut point of 21 U/ml and others a 35 U/ml upper limit of normal. In monitoring known ovarian cancer, it will be important to utilize individual patient baselines, particularly in postmenopausal women. Further studies will be required to determine whether the trend of HE4 values provides even greater specificity, as is the case with CA125 (24).
Median HE4 concentrations were not significantly different among trimesters but were significantly lower when compared with their premenopausal counterparts (p<0.001). Only slight increases in HE4 serum levels were noted between the second and third trimesters. In pregnant individuals, the 95th percentile upper limits of normal for HE4 serum concentration were 49.6 pM, 35.1 pM, and 50.2 pM during the first, second, and third trimesters, respectively, with an overall upper limit of 49.7 pM. Moreover, median HE4 serum levels were significantly lower (approximately 16 pM) in pregnant women versus premenopausal women. In contrast, serum levels of CA125, and other tumor markers such as CA19-9, carcinoembryonic antigen, squamous cell carcinoma antigen, mucin-like carcinoma-associated antigen, and tissue polypeptide-specific antigen, increase notably during pregnancy (25). Elevations in CA125 during pregnancy occur predominantly during the first trimester, perhaps because of its role in early fetal development (26;27). The lower concentrations of serum HE4 in pregnancy maybe a result of increase renal clearance associated with pregnancies. In our study, no elevations in median HE4 serum concentrations were seen during the first trimester vis-à-vis the second and third trimesters or all trimesters combined. These findings suggest that in pregnant women, HE4 will remain a relatively reliable and robust marker of ovarian cancer and may be useful for the evaluation of ovarian cysts and pelvic masses in pregnant patients, while CA125 serum measures could yield an increased number of false positives. Our results further bolster the rationale for using dual marker combination of HE4 and CA125 to maintain optimal levels of specificity(14;16;28).
The current study was limited by the absence of demographic data, which would allow conclusions about HE4 concentration variability based on such factors as race and ethnicity. Nonetheless, our findings show that HE4 serum concentrations display clinically relevant variability by age in healthy women that should be weighed when considering what constitutes the upper limit of normal for HE4 serum concentrations. Furthermore, our findings demonstrate that, in pregnant healthy women, HE4 concentrations do not manifest clinically important variability among trimesters. Indeed, HE4 concentrations were not elevated during any trimester of pregnancy. Additionally, all HE4 serum levels in this study were measured with an HE4 EIA assay kit from Fujirebio Diagnostics Inc. Currently the only other available platforms to measure HE4 serum levels are provided by Roche and Abbott Diagnostics. Although these platforms were designed to provide comparable measurements, direct comparative studies need to be performed and the findings of the present study should be limited to the HE4 EIA assay.
Our findings should provide clinically relevant normative HE4 serum concentrations for healthy women based on age, menopausal and pregnancy status.
Acknowledgements
RGM is partially supported by NCI 1 RO1 CA136491-01 grant and philanthropic support from Swim Across America. RCB is partially supported by funds from the M.D. Anderson SPORE in Ovarian Cancer NCI P50 CA83639, and philanthropic support from Golfers Against Cancer, the Tracey Jo Wilson Foundation and the Mossy Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential Conflict of interest:
Richard G. Moore, MD: Receives research funding from Fujirebio Diagnostics Inc. and Abbott Diagnostics Inc.
Geralyn Lambert-Messerlian, PhD: Receives research funding from Fujirebio Diagnostics Inc., Abbott Diagnostics Inc. and Beckman Coulter Inc.
Michael Craig Miller, BSn: Receives consulting fees from Fujirebio Diagnostics Inc.
Robert C. Bast, MD: Has served on the Scientific Advisory Boards of Fujirebio Diagnostics Inc., Vermillion Inc. and Illumina Inc. and receives royalties from Fujirebio Diagnostics Inc. for CA125.
Elizabeth E. Eklund, BSn: No conflict of interest to report.
Karen H Lu, MD: No conflict of interest to report.
Reference List
- 1.Bast RC, Jr, Feeney M, Lazarus H, Nadler LM, Colvin RB, Knapp RC. Reactivity of a monoclonal antibody with human ovarian carcinoma. J Clin Invest. 1981 Nov;68(5):1331–1337. doi: 10.1172/JCI110380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bast RC, Jr, Klug TL, St John E, Jenison E, Niloff JM, Lazarus H, et al. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N Engl J Med. 1983 Oct 13;309(15):883–887. doi: 10.1056/NEJM198310133091503. [DOI] [PubMed] [Google Scholar]
- 3.Bast RC, Jr, Badgwell D, Lu Z, Marquez R, Rosen D, Liu J, et al. New tumor markers: CA125 and beyond. Int J Gynecol Cancer. 2005 Nov;15(Suppl 3):274–281. doi: 10.1111/j.1525-1438.2005.00441.x. [DOI] [PubMed] [Google Scholar]
- 4.Moore RG, Maclaughlan S, Bast RC., Jr Current state of biomarker development for clinical application in epithelial ovarian cancer. Gynecol Oncol. 2009 Oct 29; doi: 10.1016/j.ygyno.2009.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menon U, Skates SJ, Lewis S, Rosenthal AN, Rufford B, Sibley K, et al. Prospective study using the risk of ovarian cancer algorithm to screen for ovarian cancer. J Clin Oncol. 2005 Nov 1;23(31):7919–7926. doi: 10.1200/JCO.2005.01.6642. [DOI] [PubMed] [Google Scholar]
- 6.Menon U, Gentry-Maharaj A, Hallett R, Ryan A, Burnell M, Sharma A, et al. Sensitivity and specificity of multimodal and ultrasound screening for ovarian cancer, and stage distribution of detected cancers: results of the prevalence screen of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) Lancet Oncol. 2009 Apr;10(4):327–340. doi: 10.1016/S1470-2045(09)70026-9. [DOI] [PubMed] [Google Scholar]
- 7.Niloff JM, Knapp RC, Schaetzl E, Reynolds C, Bast RC., Jr CA125 antigen levels in obstetric and gynecologic patients. Obstet Gynecol. 1984 Nov;64(5):703–707. [PubMed] [Google Scholar]
- 8.Buamah PK, Skillen AW. Serum CA 125 concentrations in patients with benign ovarian tumours. J Surg Oncol. 1994 Jun;56(2):71–74. doi: 10.1002/jso.2930560204. [DOI] [PubMed] [Google Scholar]
- 9.Buamah P. Benign conditions associated with raised serum CA-125 concentration. J Surg Oncol. 2000 Dec;75(4):264–265. doi: 10.1002/1096-9098(200012)75:4<264::aid-jso7>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 10.Fuith LC, Daxenbichler G, Dapunt O. CA 125 in the serum and tissue of patients with gynecological disease. Arch Gynecol Obstet. 1987;241(3):157–164. doi: 10.1007/BF00931312. [DOI] [PubMed] [Google Scholar]
- 11.Meden H, Fattahi-Meibodi A. CA 125 in benign gynecological conditions. Int J Biol Markers. 1998 Oct;13(4):231–237. doi: 10.1177/172460089801300411. [DOI] [PubMed] [Google Scholar]
- 12.Drapkin R, von Horsten HH, Lin Y, Mok SC, Crum CP, Welch WR, et al. Human epididymis protein 4 (HE4) is a secreted glycoprotein that is overexpressed by serous and endometrioid ovarian carcinomas. Cancer Res. 2005 Mar 15;65(6):2162–2169. doi: 10.1158/0008-5472.CAN-04-3924. [DOI] [PubMed] [Google Scholar]
- 13.Hellstrom I, Raycraft J, Hayden-Ledbetter M, Ledbetter JA, Schummer M, McIntosh M, et al. The HE4 (WFDC2) protein is a biomarker for ovarian carcinoma. Cancer Res. 2003 Jul 1;63(13):3695–3700. [PubMed] [Google Scholar]
- 14.Moore RG, Brown AK, Miller MC, Skates S, Allard WJ, Verch T, et al. The use of multiple novel tumor biomarkers for the detection of ovarian carcinoma in patients with a pelvic mass. Gynecol Oncol. 2007 Nov 30;108:402–408. doi: 10.1016/j.ygyno.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 15.Huhtinen K, Suvitie P, Hiissa J, Junnila J, Huvila J, Kujari H, et al. Serum HE4 concentration differentiates malignant ovarian tumours from ovarian endometriotic cysts. Br J Cancer. 2009 Apr 21;100(8):1315–1319. doi: 10.1038/sj.bjc.6605011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore RG, McMeekin DS, Brown AK, Disilvestro P, Miller MC, Allard WJ, et al. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol Oncol. 2009 Jan;112(1):40–46. doi: 10.1016/j.ygyno.2008.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nolen B, Velikokhatnaya L, Marrangoni A, De GK, Lomakin A, Bast RC, Jr, et al. Serum biomarker panels for the discrimination of benign from malignant cases in patients with an adnexal mass. Gynecol Oncol. 2010 Jun;117(3):440–445. doi: 10.1016/j.ygyno.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galgano MT, Hampton GM, Frierson HF., Jr Comprehensive analysis of HE4 expression in normal and malignant human tissues. Mod Pathol. 2006 Jun;19(6):847–853. doi: 10.1038/modpathol.3800612. [DOI] [PubMed] [Google Scholar]
- 19.Horowitz GL, Altaie S, Boyd JC, Ceriotti F, Garg U, Horn P, et al. Defining, Establishing, and Verifying Reference Intervals in the Clinical Laboratory; Approved Guideline. Clinical and Laboratory Standards Institute / International Federation of Clinical Chemisty and Laboratory Medicine. 2008 Jan 1;28(30):1–76. [Google Scholar]
- 20.Anastasi E, Marchei GG, Viggiani V, Gennarini G, Frati L, Reale MG. HE4: a new potential early biomarker for the recurrence of ovarian cancer. Tumour Biol. 2010 Apr;31(2):113–119. doi: 10.1007/s13277-009-0015-y. [DOI] [PubMed] [Google Scholar]
- 21.Lowe KA, Shah C, Wallace E, Anderson G, Paley P, McIntosh M, et al. Effects of personal characteristics on serum CA125, mesothelin, and HE4 levels in healthy postmenopausal women at high-risk for ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2008 Sep;17(9):2480–2487. doi: 10.1158/1055-9965.EPI-08-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pauler DK, Menon U, McIntosh M, Symecko HL, Skates SJ, Jacobs IJ. Factors influencing serum CA125II levels in healthy postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2001 May;10(5):489–493. [PubMed] [Google Scholar]
- 23.Dehaghani AS, Ghiam AF, Hosseini M, Mansouri S, Ghaderi A. Factors influencing serum concentration of CA125 and CA15-3 in Iranian healthy postmenopausal women. Pathol Oncol Res. 2007;13(4):360–364. doi: 10.1007/BF02940317. [DOI] [PubMed] [Google Scholar]
- 24.Skates SJ, Menon U, MacDonald N, Rosenthal AN, Oram DH, Knapp RC, et al. Calculation of the Risk of Ovarian Cancer From Serial CA-125 Values for Preclinical Detection in Postmenopausal Women. J Clin Oncol. 2003 May 15;21(10 Suppl):206–210. doi: 10.1200/JCO.2003.02.955. [DOI] [PubMed] [Google Scholar]
- 25.Sarandakou A, Protonotariou E, Rizos D. Tumor markers in biological fluids associated with pregnancy. Crit Rev Clin Lab Sci. 2007;44(2):151–178. doi: 10.1080/10408360601003143. [DOI] [PubMed] [Google Scholar]
- 26.Seki K, Kikuchi Y, Uesato T, Kato K. Increased serum CA 125 levels during the first trimester of pregnancy. Acta Obstet Gynecol Scand. 1986;65(6):583–585. doi: 10.3109/00016348609158392. [DOI] [PubMed] [Google Scholar]
- 27.Fendrick JL, Staley KA, Gee MK, McDougald SR, Quirk JG, Jr, O'Brien TJ. Characterization of CA 125 synthesized by the human epithelial amnion WISH cell line. Tumour Biol. 1993;14(5):310–318. doi: 10.1159/000217844. [DOI] [PubMed] [Google Scholar]
- 28.Moore RG, Miller MC, Disilvestro P, Landrum LM, Gajewski W, Ball JJ, et al. Evaluation of the Diagnostic Accuracy of the Risk of Ovarian Malignancy Algorithm in Women With a Pelvic Mass. Obstet Gynecol. 2011 Aug;118(2, Part 1):280–288. doi: 10.1097/AOG.0b013e318224fce2. [DOI] [PMC free article] [PubMed] [Google Scholar]