Abstract
Objective
Physicians often defer obtaining a voiding cystourethrogram (VCUG) after the diagnosis of urinary tract infection (UTI) due to concerns regarding increased health risks and inflated rates of vesicoureteral reflux (VUR). This study examines the health risks and accuracy of VCUG testing after diagnosis of a febrile UTI.
Patients and methods
A retrospective review was conducted of children aged 0–18 years admitted to Nationwide Children’s Hospital with a febrile UTI in 1995–2000. Children were divided into two cohorts – those who had a VCUG performed within 1 week of diagnosis (early VCUG cohort) and those who had a VCUG performed more than 1 week after diagnosis (late VCUG cohort). All children were followed for an additional 5 years after hospital discharge.
Results
The incidence and severity of VUR were similar in patients that underwent early and late VCUG testing. Patients who underwent early VCUG testing showed no sign of worsening illness after the test was performed. During the 5-year follow up, these patients did not have higher rates of return emergency department visits or hospital readmission compared to those who received late VCUG testing.
Conclusions
The rate of VUR detection does not increase with early VCUG testing. Early VCUG testing does not lead to increased risk of bacterial dissemination or urosepsis.
Keywords: Urinary tract infection, Voiding cystourethrogram, Vesicoureteral reflux
Introduction
UTI is one of the most common and serious types of bacterial infection in children [1] and [2]. Estimates on cumulative incidence in American children indicate that up to 180,000 of the annual birth cohort will be diagnosed with a UTI by 6 years of age (3–7% of females and 1–2% of males) [3] and [4]. In 1999, the American Academy of Pediatrics (AAP) issued practice guidelines for the management of UTI. These guidelines recommend that young children undergo a renal ultrasound and VCUG to evaluate for the presence of anatomic abnormalities and VUR after they are diagnosed with their first UTI [5]. The combination of VUR and UTI may predispose children to pyelonephritis, renal scarring, hypertension, and chronic kidney disease [6]. Despite these AAP recommendations, many patients do not undergo VCUG testing. In a recent study of infants <1 year of age who were diagnosed with a febrile UTI, roughly 40% were not evaluated for VUR [7].
The AAP guidelines do not suggest when a VCUG should be performed. The guidelines state that a VCUG should be obtained ‘at the earliest convenient time’, once a child is free of infection and bladder irritability is absent [5]. Traditionally, there have been concerns about the accuracy and safety of a VCUG performed during an active infection. Because UTIs can cause ureteral dilatation and inflammatory changes at the ureterovesical junction, they may cause transient VUR [8] and [9]. Therefore, a VCUG performed during infection may falsely overestimate clinically relevant VUR. Moreover, there are concerns that catheterization during a VCUG may damage the already inflamed urinary tract mucosa, and prolong a UTI or lead to bacterial dissemination and sepsis [10]. Consequently, many physicians defer VCUG testing until the UTI resolves. The aim of this study was to explore the issues of timing, safety, and accuracy of VCUG testing.
Patients and methods
Patients and demographics
A retrospective review was conducted of children admitted to Nationwide Children’s Hospital (NCH) with a febrile UTI from 1995 to 2000. Included were patients aged 0–18 years who were diagnosed with their first culture-proven febrile UTI as defined by AAP guidelines [5]. A fever was defined as a temperature ≥38.3 °C at presentation. Exclusion criteria included complicated medical history and prior VCUG. Data were collected on gender, age, race, presenting symptoms, maximum temperature during admission, and length of admission. The presence, grade, and location of VUR (unilateral vs bilateral) were also noted.
The patients were stratified into two cohorts: (1) those who had VCUGs performed during the inpatient admission, after the initiation of antibiotics (early VCUG cohort), and (2) those who were scheduled to have a VCUG performed after hospital discharge (late VCUG cohort). Early VCUG testing was performed within 7 days of UTI diagnosis. If the VCUG was not performed at NCH, the primary care physician was contacted to determine if the test was performed.
In the early VCUG cohort, complications associated with early VCUG testing were evaluated by monitoring temperature changes 12 h after testing, and recording additional emergency department (ED) evaluation after discharge or readmission within 1 week after discharge.
All patients, regardless of whether or not they had a VCUG, were followed for an additional 5 years after hospital discharge. From 2000 to 2006, it was recorded if patients developed another positive urine culture, presented to the ED with an additional UTI, or were admitted to the hospital for UTI management.
Statistical analysis
Differences between patient cohorts were analyzed using the Chi-square test for proportions and the Mann–Whitney U-test for medians. The differences in the incidence of VUR in the early VCUG vs late VCUG male cohorts were analyzed by the Fisher exact test secondary to the small sample size. Within the early VCUG cohort, differences between median maximal temperatures 12 h before and after a VCUG were compared using the Mann–Whitney U-test. Statistical significance was achieved with a P < 0.05.
Results
Patient demographics
From 1995 to 2000, 251 patients were admitted to NCH with a febrile UTI; 99 patients from this cohort were excluded (Table 1). Among the 152 patients that met the study criteria, 67 underwent early VCUG testing and 85 were scheduled to have an outpatient VUCG. Of the 85 patients scheduled for an outpatient VCUG, 77% (n = 65) obtained the test. Thus, the percentage of patients who had VCUG testing performed as inpatients was significantly higher than the percentage of patients who completed the test as an outpatient (100% vs 77%) (P = 0.005).
Table 1.
| Patients excluded from analysis., |
|---|
| Reason,n |
| Complicated medical history,36 |
| Cultures negative,21 |
| Myelomeningocele,16 |
| Previous diagnosis of reflux,10 |
| Congenital kidney malformation,11 |
| Previous urinary tract infection with negative studies,7 |
| Work up done at another hospital,2 |
| No culture obtained,2 |
When examining patient and cohort demographics, there was seen to be a female preponderance in the outpatient group compared to the inpatient group (P < 0.001) (Table 2). There were no significant differences in age, race, presenting symptoms, or maximal temperature between the two groups (Table 2). The mean length of hospital stay was 2.4 ± 1.4 days for the inpatient group compared to 2.0 ± 0.9 days for those who did not receive an inpatient VCUG (P < 0.001).
Table 2.
| Characteristics of patients that had VCUG test performed.,,, |
|---|
| Characteristic,Inpatients,Outpatients,P |
| Sample size<comma> n,67,65, |
| Female gender<comma> n (%),39 (58),57 (88),<0.001 |
| Age (months),,, |
| Median,2,2,0.62 |
| Range,0.3‰Ûὸ85,0.3‰Ûὸ101, |
| Race or ethnic group<comma> n (%),,,0.392 |
| Caucasian,45 (67),43 (66), |
| African American,12 (18),10 (15), |
| Hispanic,2 (3.0),0 (0), |
| Other or not recorded,8 (12),12 (19), |
| Presenting symptoms,,,0.746 |
| Vomiting<comma> n (%),20 (30),25 (38), |
| Diarrhea<comma> n (%),11 (16),13 (20), |
| Dysuria<comma> n (%),1 (2),1 (2), |
| Gross hematuria<comma> n (%),1 (2),0 (0), |
| Maximum temperature (å¡F),,, |
| Median,102.1,102.2,0.658 |
| Range,98.6‰Ûὸ105,100‰Ûὸ105, |
| Admission interval (days),,, |
| Mean,2.4,2,<0.001 |
| Range,1‰Ûὸ7,1‰Ûὸ7, |
Detection of VUR
Regarding the incidence of reflux, 21 children (31%) in the inpatient cohort and 33 children (51%) in the outpatient cohort had VUR (P = 0.023) (Table 3). When analyzing the cohorts by gender, the incidence of VUR in the inpatient and outpatient cohorts was similar. Among the patients who had VUR, the percentages of males and females were similar. The severity of VUR and the proportion of bilateral VUR were similar between cohorts (Table 4).
Table 3.
| Finding in patients at time of VCUG.,,, |
|---|
| Findings,Inpatients,Outpatients,P |
| Total patients tested<comma> n,67,65,0.023 |
| Reflux present<comma> n (%),21 (31),33 (51), |
| Total females tested<comma> n,39,57,0.483 |
| Reflux present<comma> n (%),17 (44),29 (52), |
| Total males tested<comma> n,28,8,0.054 |
| Reflux present<comma> n (%),4 (14),4 (50), |
Table 4.
| VCUG findings in patients with VUR.,,, |
|---|
| Findings,Inpatients,Outpatients,P |
| Reflux present<comma> n,21,33, |
| Female gender<comma> n (%),17 (81),29 (88),0.484 |
| Reflux grade,,,0.683 |
| I<comma> n (%),2 (10),5 (15), |
| II<comma> n (%),8 (38),15 (46), |
| III<comma> n (%),8 (38),11 (3), |
| IV<comma> n (%),3 (14),2 (6), |
| V<comma> n (%),0 (0),0 (0), |
| Bilateral reflux<comma> n (%),8 (38),16 (49),0.454 |
VCUG risks
To determine whether there were increased health risks associated with early VCUG testing, several criteria were examined. There were no statistical differences detected when comparing the median maximal temperature over the 12 h prior to the VCUG and 12 h after the VCUG (Fig. 1). Moreover, none of the inpatients who had early VCUG testing returned to the NCH ED, developed a subsequent UTI, or were readmitted within a week of discharge.
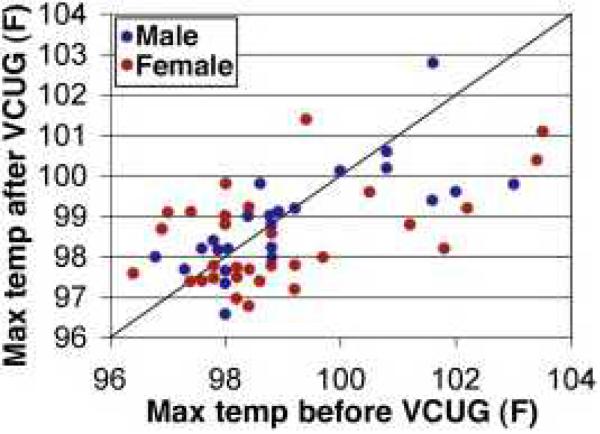
Figure 1.
Scatter graph comparing maximum temperatures of inpatients over 12 h before and after VCUG testing. Differences in median maximal temperature between the 12 h prior to the VCUG (98.6 °F, range 96.4–103.5 °F) and 12 h after the VCUG (98.5 °F, range 96.6–102.8 °F) were not statistically significant (P = 0.492).
VCUG risks
To determine whether there were increased health risks associated with early VCUG testing, several criteria were examined. There were no statistical differences detected when comparing the median maximal temperature over the 12 h prior to the VCUG and 12 h after the VCUG (Fig. 1). Moreover, none of the inpatients who had early VCUG testing returned to the NCH ED, developed a subsequent UTI, or were readmitted within a week of discharge.
Compliance and outcomes
In our study, all patients were followed for 5 years after hospital discharge. After hospital discharge, 24% of patients scheduled for an outpatient VCUG did not undergo testing. McDonald et al. reported a similar pattern of patient non-compliance [9]. When comparing the rates of subsequent UTI, additional ED evaluation, or hospital readmission between patients who had a VCUG and those who did not, we did not find a significant difference. These results support published evidence suggesting that VUR does not increase the risk of recurrent UTIs [3], [12] and [17]. Although small, prospective follow-up studies of children with primary VUR suggest that most children do well [3], [12], [14] and [18].
Given the good prognosis for children with VUR, the practice of detecting reflux after a UTI has been recently questioned [19], [20], [21], [22], [23] and [24]. Currently, no evidence clearly indicates that interventions (medical or surgical) for VUR confer benefit, albeit there is also a lack of evidence suggesting that interventions are not beneficial [12], [25], [26], [27], [28], [29], [30], [31], [32] and [33]. Consequently, we are currently in an evidence-poor environment, trading off possible benefits against probable harms. If it can be shown that intervention for VUR decreases comorbidities, the necessity and importance of the VCUG will be confirmed.
Limitations
The authors recognize that this study is limited by its small sample size and retrospective nature. Because of the retrospective nature of this study and the available data, we cannot report whether patients required more sedation with early VCUG testing or developed less serious complications like increased anxiety, increased pain, hematuria, dysuria, urinary frequency, urgency, or flank pain. We were not able to determine if the resolution of VUR is affected by timing of VCUG. We also cannot report if patients presented to other hospitals for further evaluation after VCUG testing. Finally, we were not able to determine if there is a higher incidence of renal scarring, renal insufficiency, or hypertension in patients that did not have a VCUG.
Conclusion
Our results indicate that early VCUG testing for a first febrile UTI is accurate and does not lead to significant health risks in healthy children.
Acknowledgments
Funding
None.
Footnotes
Conflict of interest statement
No author has a conflict of interest or anything to disclose.
Ethical approval
This study has met ethical approval.
References
- 1.Bachur RG, Harper MB. Predictive model for serious bacterial infections among infants younger than 3 months of age. Pediatrics. 2001;108(2):311–316. doi: 10.1542/peds.108.2.311. [DOI] [PubMed] [Google Scholar]
- 2.Byington CL, et al. Serious bacterial infections in febrile infants younger than 90 days of age: the importance of ampicillin-resistant pathogens. Pediatrics. 2003;111:964–968. doi: 10.1542/peds.111.5.964. 5 Pt 1. [DOI] [PubMed] [Google Scholar]
- 3.Beetz R. May we go on with antibacterial prophylaxis for urinary tract infections? Pediatr Nephrol. 2006;21(1):5–13. doi: 10.1007/s00467-005-2083-6. [DOI] [PubMed] [Google Scholar]
- 4.Chesney RW, et al. Randomized intervention for children with vesicoureteral reflux (RIVUR): background commentary of RIVUR investigators. Pediatrics. 2008;122(Suppl. 5):S233–S23. doi: 10.1542/peds.2008-1285c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Practice parameter: the diagnosis, treatment, and evaluation of the initial urinary tract infection in febrile infants and young children. American Academy of Pediatrics. Committee on Quality Improvement. Subcommittee on urinary tract infection Pediatrics. 1999;103:843–852. doi: 10.1542/peds.103.4.843. 4 Pt 1. [DOI] [PubMed] [Google Scholar]
- 6.Mak RH, Kuo HJ. Pathogenesis of urinary tract infection: an updateCurr. Opin Pediatr. 2006;18(2):148–152. doi: 10.1097/01.mop.0000193276.39495.0d. [DOI] [PubMed] [Google Scholar]
- 7.Cohen AL, et al. Compliance with guidelines for the medical care of first urinary tract infections in infants: a population-based study. Pediatrics. 2005;115(6):1474–1478. doi: 10.1542/peds.2004-1559. [DOI] [PubMed] [Google Scholar]
- 8.Andrich MP, Majd M. Diagnostic imaging in the evaluation of the first urinary tract infection in infants and young children. Pediatrics. 1992;90(3):436–441. [PubMed] [Google Scholar]
- 9.McDonald A, et al. Voiding cystourethrograms and urinary tract infections: how long to wait? Pediatrics. 2000;105(4):E50. doi: 10.1542/peds.105.4.e50. [DOI] [PubMed] [Google Scholar]
- 10.Agrawalla S, Pearce R, Goodman TR. How to perform the perfect voiding cystourethrogram. Pediatr Radiol. 2004;34(2):114–119. doi: 10.1007/s00247-003-1073-8. [DOI] [PubMed] [Google Scholar]
- 11.Smellie J, et al. Vesico-ureteric reflux and renal scarring. Kidney Int Suppl. 1975;4:S65–S72. [PubMed] [Google Scholar]
- 12.Williams G, et al. Vesicoureteral reflux. J Am Soc Nephrol. 2008;19(5):847–862. doi: 10.1681/ASN.2007020245. [DOI] [PubMed] [Google Scholar]
- 13.Mahant S, To T, Friedman J. Timing of voiding cystourethrogram in the investigation of urinary tract infections in children. J Pediatr. 2001;139(4):568–571. doi: 10.1067/mpd.2001.118188. [DOI] [PubMed] [Google Scholar]
- 14.Kassis I, et al. Early performance of voiding cystourethrogram after urinary tract infection in children. Isr Med Assoc J. 2008;10(6):453–456. [PubMed] [Google Scholar]
- 15.Chand DH, et al. Incidence and severity of vesicoureteral reflux in children related to age, gender, race and diagnosis. J Urol. 2003;170:1548–1550. doi: 10.1097/01.ju.0000084299.55552.6c. 4 Pt 2. [DOI] [PubMed] [Google Scholar]
- 16.Silva JM, et al. Features of primary vesicoureteral reflux and renal damage in children at a single institution in Brazil from 1969 to 1999. Int Urol Nephrol. 2003;35(2):161–168. doi: 10.1023/b:urol.0000020293.62258.11. [DOI] [PubMed] [Google Scholar]
- 17.Craig JC, et al. Vesicoureteric reflux and timing of micturating cystourethrography after urinary tract infection. Arch Dis Child. 1997;76(3):275–277. doi: 10.1136/adc.76.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.A, Hannula, et al. Vesicoureteral reflux in children with suspected and proven urinary tract infection. Pediatr Nephrol. 25(8):1463–1469. doi: 10.1007/s00467-010-1542-x. [DOI] [PubMed] [Google Scholar]
- 19.Elder JS. Imaging for vesicoureteral reflux–is there a better way? J Urol. 2005;174(1):7–8. doi: 10.1097/01.ju.0000167220.30217.40. [DOI] [PubMed] [Google Scholar]
- 20.Stefanidis CJ, Siomou E. Imaging strategies for vesicoureteral reflux diagnosis. Pediatr Nephrol. 2007;22(7):937–94. doi: 10.1007/s00467-006-0396-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Venhola M, Uhari M. Vesicoureteral reflux, a benign condition. Pediatr Nephrol. 2009;24(2):223–226. doi: 10.1007/s00467-008-0912-0. [DOI] [PubMed] [Google Scholar]
- 22.Edwards D, et al. Disappearance of vesicoureteric reflux during long-term prophylaxis of urinary tract infection in children. Br Med J. 1977;2(6082):285–288. doi: 10.1136/bmj.2.6082.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gelfand MJ, et al. Vesicoureteral reflux: subpopulations of patients defined by clinical variables. Pediatr Radiol. 2000;30(2):121–124. doi: 10.1007/s002470050028. [DOI] [PubMed] [Google Scholar]
- 24.Sargent MA. What is the normal prevalence of vesicoureteral reflux? Pediatr Radiol. 2000;30(9):587–593. doi: 10.1007/s002470000263. [DOI] [PubMed] [Google Scholar]
- 25.Craig JC, et al. Antibiotic prophylaxis and recurrent urinary tract infection in children. N Engl J Med. 2009;361(18):1748–1759. doi: 10.1056/NEJMoa0902295. [DOI] [PubMed] [Google Scholar]
- 26.Garin EH, et al. Clinical significance of primary vesicoureteral reflux and urinary antibiotic prophylaxis after acute pyelonephritis: a multicenter, randomized, controlled study. Pediatrics. 2006;117(3):626–632. doi: 10.1542/peds.2005-1362. [DOI] [PubMed] [Google Scholar]
- 27.Hodson EM, et al. Interventions for primary vesicoureteric reflux. Cochrane Database Syst Rev. 2007;3:CD001532. doi: 10.1002/14651858.CD001532.pub3. [DOI] [PubMed] [Google Scholar]
- 28.Lendvay TS, et al. The evolution of vesicoureteral reflux management in the era of dextranomer/hyaluronic acid copolymer: a pediatric health information system database study. J Urol. 2006;176:1864–1867. doi: 10.1016/j.juro.2006.04.088. 4 Pt 2. [DOI] [PubMed] [Google Scholar]
- 29.Montini G, et al. Prophylaxis after first febrile urinary tract infection in children? A multicenter, randomized, controlled, noninferiority trial. Pediatrics. 2008;122(5):1064–1071. doi: 10.1542/peds.2007-3770. [DOI] [PubMed] [Google Scholar]
- 30.Pennesi M, et al. Is antibiotic prophylaxis in children with vesicoureteral reflux effective in preventing pyelonephritis and renal scars? A randomized, controlled trial Pediatrics. 2008;121(6):e1489–194. doi: 10.1542/peds.2007-2652. [DOI] [PubMed] [Google Scholar]
- 31.Roussey-Kesler G, et al. Antibiotic prophylaxis for the prevention of recurrent urinary tract infection in children with low grade vesicoureteral reflux: results from a prospective randomized study. J Urol. 2008;179(2):674–679. doi: 10.1016/j.juro.2007.09.090. discussion 679. [DOI] [PubMed] [Google Scholar]
- 32.Brandstrom P, et al. The Swedish reflux trial in children: III. Urinary tract infection pattern. J Urol. 2010;184(1):286–291. doi: 10.1016/j.juro.2010.01.061. [DOI] [PubMed] [Google Scholar]
- 33.G, Holmdahl, et al. The Swedish reflux trial in children: II. Vesicoureteral reflux outcome. J Urol. 184(1):280–285. doi: 10.1016/j.juro.2010.01.059. [DOI] [PubMed] [Google Scholar]