Abstract
Objective:
The present study aimed at investigate the therapeutic effects of delayed puerarin treatment in neurological outcomes after middle cerebral artery occlusion (MCAO) in rats.
Materials and Methods:
Male Wistar rats were subjected to MCAO for 120 min followed by reperfusion for 14 days. Puerarin (0, 50, 100, 200 mg/kg, intra-peritoneally) was administered at 24 h after stroke onset and repeated daily for 14 days. Neurological deficits were evaluated at 1, 4, 7, 14 days after stroke. Brain infarct volume and peri-infarct context vessel density were examined at 14 days after stroke.
Results:
Puerarin significantly improved neurological functions up to 14 days after stroke and decreased the infarct volume with doses of 50 mg/kg and 100 mg/kg compared with saline controls. Puerarin treatment also significantly increased peri-infarct context vessel density at 14 days after stroke.
Conclusions:
Delayed treatment of puerarin initiated at 24 h after stroke is beneficial with improved long-term neurological outcomes and reduced infarction volume in focal ischemic stroke in rats. Enhanced vascular remodeling by puerarin might at least partially contribute to its beneficial effects.
KEY WORDS: Cerebrovascular remodeling, focal cerebral ischemia, long-term neurological outcomes, puerarin, rats
Introduction
Puerarin (daidzein-8-C-glucoside) is the major isoflavonoid derived from the Chinese medical herb Radix puerariae (kudzu root). In China, R. puerariae is known as Ge Gen and has been used for a long time as a traditional Chinese medicine for treating various diseases including cardiovascular disorders, such as angina and myocardial infarction.[1,2,3] Interestingly, experimental studies also showed puerarin may reduce acute tissue damage from cerebral ischemia in rats.[4,5] However, investigations on puerarin's long-term therapeutic effects, particularly with delayed treatment to ischemic stroke remain largely lacking but is considered of highly translational significance for evaluation of preclinical efficacy.[6]
Materials and Methods
Focal Cerebral Ischemia in Rats
The puerarin used in this study was purchased from Sigma (St. Louis, MO; Product Number 82435); its molecular weight is 416.36 (C21H20O9) with purity ≥98.0% (high-performance liquid chromatography). All experiments were performed following an institutionally approved protocol in accordance with the guide for the care and use of laboratory animals. Adult male Wistar rats weighing 280-320 g were employed in all experiments. Transient right middle cerebral artery occlusion was induced by following the standard method.[7] All animals were randomly selected into four experimental groups (n = 22 rats per group), each group on animals were treated intraperitoneally at 24 h after stroke with puerarin at 0 (saline control), 50, 100 and 200 mg/kg, respectively; thereafter received the same treatment daily for 14 days. Assessment to treatment groups was blinded to investigators. The dose selection was based previously published work from others in range 25-400 mg/kg.[4,8]
Neurobehavioral Tests
Two neurobehavioral tests modified neurological severity score (mNSS) and adhesive-removal test were assessed before surgery and at 1, 4, 7 and 14 days after stroke by independent investigators blinded to experimental groups. mNSS was adopted to evaluate the comprehensive functions of motor, sensor, reflex and balance of animals as previously described by others.[9] These neurological functions were graded on a scale of 0-18 and 1 score point was awarded for the inability of performing each test or for the loss of a specific reflex. The higher score means the worse neurological condition. We also used adhesive-removal test to measure the somatosensory deficit of animals by following a standard protocol as described by others.[10] Briefly, sticky paper dot of identical size were used as tactile stimuli to adhere to the distal-radial area of animal's each forelimb wrist. Then we started to record the time for the animal to remove the dots from each wrist.
Measurement of Brain Infarction
At 14 days after stroke, rats were killed followed by cardio-perfusion. We used Hematoxylin and Eosin staining for seven 20-mm coronal sections at +4.7, +2.7, +0.7, −1.3, −3.3, −5.3 and −7.3 mm from bregma. Sections were digitalized and infarct area was measured using US National Institutes of Health Image 1.62 software (National Institutes of Health, Bethesda, Md.) as previously described.[11]
Immunohistochemistry for Cerebrovascular Density Quantification
Immunohistochemistry was performed by following standard method[11] on brain sections at −0.7 mm and −1.3 mm from bregma (axial sections 3 and 4 for quantification, because the two sections represented the location of maximal cortical infarction).[11] Primary antibody of von Willebrand factor (vWF) (1:100, Abbiotec, San Diego, CA, USA) was used. immunoreactive signals were examined and digitized by Olympus Microscope (Olympus B × 51, Tokyo, Japan). We measured numbers of vWF-positive vessels on 3 low-power fields (×10) of peri-infarct cortex per section. Vascular density was quantitated and presented as total number of vWF-positive vessels per square millimeter area.[12]
Statistical Analysis
Data are presented as mean ± standard error. Multi-group comparisons were performed using analysis of variance followed by Fisher protected least significant difference tests. P <0.05 was considered to be significant.
Results
Delayed puerarin treatment improved long-term neurological outcomes and reduced brain infarction after focal stroke in rats. For mNSS, both 50 and 100 mg/kg of puerarin significantly reduced mNSS at day 4, day 7 and day 14 after stroke compared with saline treated control animals [Figure 1a]. For adhesive-remove test, puerarin treatment also significantly improved performance of the adhesive-remove tests at day 14 after stroke in 50 mg/kg of puerarin treatment group, at day 7 and day 14 after stroke in 100 mg/kg of puerarin treatment group compared with saline treatment group, respectively [Figure 1b]. However, 200 mg/kg puerarin had no significant effects in the both neurological function tests after stroke [Figure 1a and b].
Figure 1.
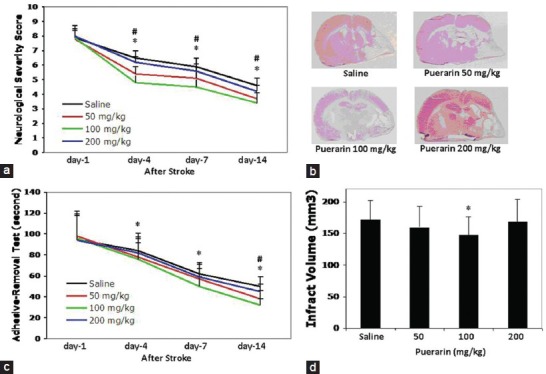
Effects of puerarin treatment in long-term neurological outcomes and brain infarction after focal stroke in rats. (a) Modified neurological severity score was assessed at day 1, day 4, day 7 and day 14 after stroke compared to saline treated control animals. (b) Adhesive-remove test was assessed at day 1, day 4, day 7 and day14 after stroke. (c) Representative H and E stained brain sections for brain infarction determination at 14 days after stroke. (d) Quantitation of brain infarction volume. Data are expressed as mean ± standard deviation. #P < 0.05 for 50 mg/kg of puerarin versus saline, *P < 0.05 for 100 mg/kg of puerarin versus saline. n = 15-18 rats per group
At 14 days after stroke, brain infarction volume was significantly reduced by 100 mg/kg puerarin treatment, but no significant difference was detected in animals treated with 50 or 200 mg/kg puerarin compared with saline controls [Figure 1c and d]. No significant difference in mortality was detected between groups. Mortalities were 22.7% for saline, 27.3% for 50 mg/kg puerarin, 18.1% for 100 mg/kg puerarin and 31.8% for 100 mg/kg puerarin treated animals, respectively.
Delayed puerarin treatment increased peri-infarct brain vascular density at 14 days after focal stroke in rats. At 14 days after stroke, brain vascular density at peri-infarct cortex area was significantly increased in animals treated with 50 or 100 mg/kg of puerarin, not 200 mg/kg of puerarin compared with saline controls [Figure 2a and b].
Figure 2.
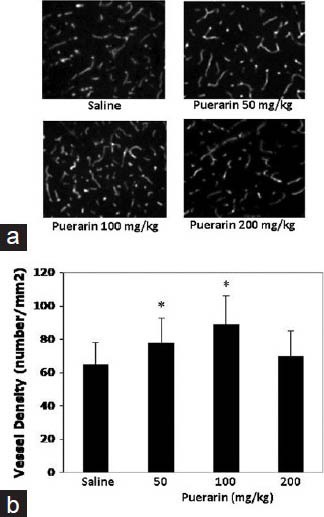
Effects of puerarin treatment in peri-infarct brain vascular density at 14 days after focal stroke in rats. (a) Representative immunohistochemistry image stained peri-infarct cortex microvessels. (b) Quantitation of vascular density in peri-infarct cortex area. Data are expressed as mean ± standard deviation. *P < 0.05 versus saline. n = 6 rats per group
Discussion
Traditional Chinese medicine has been used in countries of South and East Asia for treating stroke.[13] The lack of effective and widely applicable pharmacological treatments for ischemic stroke patients have resulted in a growing interest in traditional medicine.[13] Puerarin, a form of herbal medicine, which has shown that it attenuates brain damage after ischemic stroke in rats.[4,5,8,14] Previous studies have demonstrated that puerarin has multiple therapeutic effects involving anti-apoptosis,[14] anti-inflammation[4] anti-oxidative stress,[15,16] anti-excitotoxicity,[16] activated potassium channel[3] and increase cerebral blood flow,[17] indicating puerarin might be a novel candidate for new drug development. However, all these published studies focused only on pre-stroke or early puerain treatment in acute brain tissue damage.
It is worthy of noting that, nearly 200 clinical trials in the United States that all attempts at neuroprotection for ischemic stroke have failed, except for the National Institute of Neurological Disorders and Stroke recombinant tissue plasminogen activator trial.[18] One of key reasons of the failure is over 80% of stroke patients came to hospital at 8 h or later after stroke onset, result in missing the very short (a few hours) effective neuroprotection treatment time window.[18,19] To overcome this barrier, the Stroke Progress Review Group of National Institute of Health in 2011 suggested that development of novel effective stroke therapy with delayed administration is a high priority for stroke research.[20] In general, delayed administration of therapeutic agents aims to restore neurological functional outcome by promoting brain endogenous remodeling and plasticity when treatment is initiated at a delayed time after stroke. Thus, there is a compelling need to develop and test delayed neurological therapeutic approaches of stroke.[20,21,22] In this study, we applied this delayed administration after stroke according to the recent recommendations on translational stroke research.[6,23,24] Puerarin treatment was initiated at delayed 24 h after stroke and tested in clinically relevant long-term neurological outcomes 14 days after stroke. Our results showed, lower doses of puerarin at 50 and 100 mg/kg significantly improved neurological function and reduced brain infarction, 100 mg/kg was the optimal dose among the three tested doses. No significant effects were detected in animals treated with 200 mg/kg puerarin, suggested possible high dose-associated side-effects that neutralized its benefits.
Recovery mechanisms play dominant roles in late phase after stroke and vascular remodeling is one of key recovery mechanisms. It has been reported that puerarin induces angiogenesis in myocardium of rat with myocardial infarction[25] and increases number and activity of cultured endothelial progenitor cells from peripheral blood.[26] In this study, we found 50 or 100 mg/kg puerarin significantly increased vascular density around peri-infarct cortex area, suggesting enhanced vascular remodeling might partially contribute beneficial effects of puerarin. However, we are aware that further preclinical efficacy investigations are needed to define optimal treatment time window and regimen, test in other stroke models and animals with morbidities and determine underlying mechanisms and safety profile.[6,24]
Conclusion
Delayed treatment of puerarin initiated at 24 h after stroke is beneficial with improved long-term neurological outcomes and reduced infarction volume in focal ischemic stroke of rats. Enhanced vascular remodeling by puerarin might at least partially contribute to its beneficial effects.
Acknowledgment
The authors would like to thank Jiangsu Province Administration of Traditional Chinese Medicine (No. LJ200912) for their support for this study.
Footnotes
Source of Support: Nil
Conflict of Interest: No
References
- 1.Zhang Z, Lam TN, Zuo Z. Radix Puerariae: An overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J Clin Pharmacol. 2013;53:787–811. doi: 10.1002/jcph.96. [DOI] [PubMed] [Google Scholar]
- 2.Yeung DK, Leung SW, Xu YC, Vanhoutte PM, Man RY. Puerarin, an isoflavonoid derived from Radix puerariae, potentiates endothelium-independent relaxation via the cyclic AMP pathway in porcine coronary artery. Eur J Pharmacol. 2006;552:105–11. doi: 10.1016/j.ejphar.2006.08.078. [DOI] [PubMed] [Google Scholar]
- 3.Gao Q, Yang B, Ye ZG, Wang J, Bruce IC, Xia Q. Opening the calcium-activated potassium channel participates in the cardioprotective effect of puerarin. Eur J Pharmacol. 2007;574:179–84. doi: 10.1016/j.ejphar.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 4.Chang Y, Hsieh CY, Peng ZA, Yen TL, Hsiao G, Chou DS, et al. Neuroprotective mechanisms of puerarin in middle cerebral artery occlusion-induced brain infarction in rats. J Biomed Sci. 2009;16:9. doi: 10.1186/1423-0127-16-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang L, Zhao A, Wang F, Chai Q, Chai X. Protective effect of puerarin on acute cerebral ischemia in rats. Zhongguo Zhong Yao Za Zhi. 1997;22:752–4. 765. [PubMed] [Google Scholar]
- 6.Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40:2244–50. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guan W, Kozak A, El-Remessy AB, Johnson MH, Pillai BA, Fagan SC. Acute treatment with candesartan reduces early injury after permanent middle cerebral artery occlusion. Transl Stroke Res. 2011;2:179–85. doi: 10.1007/s12975-010-0061-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao L, Ji X, Song J, Liu P, Yan F, Gong W, et al. Puerarin protects against ischemic brain injury in a rat model of transient focal ischemia. Neurol Res. 2009;31:402–6. doi: 10.1179/174313209X444017. [DOI] [PubMed] [Google Scholar]
- 9.Shohami E, Novikov M, Bass R. Long-term effect of HU-211, a novel non-competitive NMDA antagonist, on motor and memory functions after closed head injury in the rat. Brain Res. 1995;674:55–62. doi: 10.1016/0006-8993(94)01433-i. [DOI] [PubMed] [Google Scholar]
- 10.Chen J, Li Y, Chopp M. Intracerebral transplantation of bone marrow with BDNF after MCAo in rat. Neuropharmacology. 2000;39:711–6. doi: 10.1016/s0028-3908(00)00006-x. [DOI] [PubMed] [Google Scholar]
- 11.Zhao BQ, Wang S, Kim HY, Storrie H, Rosen BR, Mooney DJ, et al. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med. 2006;12:441–5. doi: 10.1038/nm1387. [DOI] [PubMed] [Google Scholar]
- 12.Chen J, Cui X, Zacharek A, Roberts C, Chopp M. eNOS mediates TO90317 treatment-induced angiogenesis and functional outcome after stroke in mice. Stroke. 2009;40:2532–8.12. doi: 10.1161/STROKEAHA.108.545095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen YF. Traditional Chinese herbal medicine and cerebral ischemia. Front Biosci (Elite Ed) 2012;4:809–17. doi: 10.2741/E420. [DOI] [PubMed] [Google Scholar]
- 14.Xu X, Zhang S, Zhang L, Yan W, Zheng X. The Neuroprotection of puerarin against cerebral ischemia is associated with the prevention of apoptosis in rats. Planta Med. 2005;71:585–91. doi: 10.1055/s-2005-871261. [DOI] [PubMed] [Google Scholar]
- 15.Chen F, Zhang HQ, Zhu J, Liu KY, Cheng H, Li GL, et al. Puerarin enhances superoxide dismutase activity and inhibits RAGE and VEGF expression in retinas of STZ-induced early diabetic rats. Asian Pac J Trop Med. 2012;5:891–6. doi: 10.1016/S1995-7645(12)60166-7. [DOI] [PubMed] [Google Scholar]
- 16.Xu X, Zheng X. Potential involvement of calcium and nitric oxide in protective effects of puerarin on oxygen-glucose deprivation in cultured hippocampal neurons. J Ethnopharmacol. 2007;113:421–6. doi: 10.1016/j.jep.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 17.Chen L, Chai Q, Zhao A, Chai X. Effect of puerarin on cerebral blood flow in dogs. Zhongguo Zhong Yao Za Zhi. 1995;20:560–2. [PubMed] [Google Scholar]
- 18.Minnerup J, Sutherland BA, Buchan AM, Kleinschnitz C. Neuroprotection for stroke: Current status and future perspectives. Int J Mol Sci. 2012;13:11753–72. doi: 10.3390/ijms130911753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grotta JC, Jacobs TP, Koroshetz WJ, Moskowitz MA. Stroke program review group: An interim report. Stroke. 2008;39:1364–70. doi: 10.1161/STROKEAHA.107.510776. [DOI] [PubMed] [Google Scholar]
- 20.Hermann DM, Chopp M. Promoting brain remodelling and plasticity for stroke recovery: Therapeutic promise and potential pitfalls of clinical translation. Lancet Neurol. 2012;11:369–80. doi: 10.1016/S1474-4422(12)70039-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morimoto T, Yasuhara T, Kameda M, Baba T, Kuramoto S, Kondo A, et al. Striatal stimulation nurtures endogenous neurogenesis and angiogenesis in chronic-phase ischemic stroke rats. Cell Transplant. 2011;20:1049–64. doi: 10.3727/096368910X544915. [DOI] [PubMed] [Google Scholar]
- 22.Kumar P, Moon LD. Therapeutics targeting Nogo-A hold promise for stroke restoration. CNS Neurol Disord Drug Targets. 2013;12:200–8. doi: 10.2174/1871527311312020006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tajiri N, Dailey T, Metcalf C, Mosley YI, Lau T, Staples M, et al. In vivo animal stroke models: A rationale for rodent and non-human primate models. Transl Stroke Res. 2013;4:308–21. doi: 10.1007/s12975-012-0241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lapchak PA, Zhang JH, Noble-Haeusslein LJ. RIGOR Guidelines: Escalating STAIR and STEPS for effective translational research. Transl Stroke Res. 2013;4:279–85. doi: 10.1007/s12975-012-0209-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang S, Chen S, Shen Y, Yang D, Liu X, Sun-Chi AC, et al. Puerarin induces angiogenesis in myocardium of rat with myocardial infarction. Biol Pharm Bull. 2006;29:945–50. doi: 10.1248/bpb.29.945. [DOI] [PubMed] [Google Scholar]
- 26.Zhu JH, Wang XX, Chen JZ, Shang YP, Zhu JH, Guo XG, et al. Effects of puerarin on number and activity of endothelial progenitor cells from peripheral blood. Acta Pharmacol Sin. 2004;25:1045–51. [PubMed] [Google Scholar]


