Abstract
Aims:
Male sub-fertility and infertility are major complications of diabetes mellitus. The non-selective β-blocker carvedilol has been reported to have favorable effects on some of the diabetic complications based on its antioxidant and anti-apoptotic effects. This study aims to evaluate the possible testicular protective effect of carvedilol in streptozotocin (STZ)-induced diabetic rat model and its possible mechanisms.
Materials and Methods:
Diabetes was induced by a single i.p. dose of 65 mg/kg of STZ. In parallel groups of diabetic rats, carvedilol in low and high doses (1 and 10 mg/kg/day orally) were administered for 4 weeks. Oxidative stress markers as reduced glutathione (GSH) and the product of lipid peroxidation; malondialdehyde (MDA) were evaluated in testicular homogenate. The level of expression of the apoptotic marker; caspase 3, was assessed using western blot, followed by densitometric analysis.
Results:
Induction of diabetes caused distortion of histological normal testicular structure, with decrease (P < 0.05) in GSH and increase (P < 0.05) in MDA, as well as induction of caspase 3 expression. Carvedilol in low or high doses reverted diabetes-induced histological damage, restored antioxidant activity and ameliorated caspase 3 expression.
Conclusion:
Carvedilol confers testicular protection against diabetes-induced damage through antioxidant and anti-apoptotic mechanisms.
KEY WORDS: Apoptosis, carvedilol, diabetes, oxidative stress, testes
Introduction
Diabetes is considered one of the largest health problems globally.[1] Male patients may suffer from sub-fertility or infertility as a diabetic complication,[2] through multifactorial mechanisms.[3] Carvedilol is α1/β-adrenoceptors blocker used in the treatment of hypertension and congestive heart failure.[4] Despite that β-blockers are prescribed cautiously to diabetics due to undesirable effects on glucose control and insulin sensitivity, carvedilol is not associated with such effects.[5] Contrarily, carvedilol treatment ameliorates signs of diabetes in hypertensive patients[6] and have beneficial effects compared with other β-blockers.[7] Hence, the aim of this study is to evaluate the possible protective effects of carvedilol on testicular dysfunction in streptozotocin (STZ)-induced diabetic rats.
Materials and Methods
Chemicals
Carvedilol was a generous gift from Global Napi Pharmaceuticals (Egypt). STZ was purchased from Sigma-Aldrich Co., (USA). Polyclonal rabbit/anti-rat caspase 3 antibody was supplied by Lab Vision (USA).
Experimental Animals and Protocol Design
Adult male Wistar rats of 160-190 g weight were obtained from the National Research Centre, Giza, Egypt. Animals were housed in the standard animal facility, distributed five rats/cage throughout the experiments. Tap water and laboratory chow (supplemented with 18% proteins, 3% lipids and 10% fibers) were supplied ad libitum. The board of our faculty approved the procedures of animal experiment protocol (REC1/2013/Pharm/1). Animals were left to acclimatize for 1 week before starting the experiment, after which animals were fasted for 12 h and then divided into four groups, each of eight rats. The groups were control group, STZ-induced diabetic group and STZ-induced diabetic group treated with low or high dose of carvedilol of 1 or 10 mg/kg/day orally for 4 weeks (Carv1 or Carv10, respectively).[8] Diabetes was induced via a single i.p. dose of 65 mg/kg STZ that was freshly prepared in 0.01 mM citrate buffer, pH 4.5 and used within 10 min of preparation.[9] All rats receiving STZ were given 10% sucrose in water in the first 48 h after injection to prevent hypoglycemia. Diabetes was confirmed by the drop of blood taken from the tail for glucose level estimation via blood glucose meter (ACCU-CHEK® Active, Roche Diagnostics, Germany). Only rats with fasting blood glucose concentrations more than 250 mg/dl were included in this study. Carvedilol was freshly prepared in 1% carboxymethylcellulose vehicle and treatment was started after 72 h of diabetes induction by STZ. Groups not receiving either carvedilol or STZ received the corresponding vehicles.
Sample Preparation
After 4 weeks of carvedilol therapy, there was no mortality among experimental groups. Glucose level was determined by blood glucose meter and then rats were sacrificed. Both testes were rapidly excised. One testis was fixed in 10% formalin for histopathological examination. The other testis was snap-frozen in liquid nitrogen and kept at −80°C. Testes were homogenized in ice-cold phosphate buffer (0.01 M, pH 7.4; 20% w/v) for various biochemical analyses.
Biochemical Analysis
Assessment of testicular antioxidant defense mechanisms was carried out in tissue homogenate, evaluating glutathione (GSH) concentration, as well as that of malondialdehyde (MDA) that reflects the level of lipid peroxide content. For GSH, a spectrophotometric kit (Biodiagnostic, Egypt) was used. In brief, the method is based on that the sulfhydryl component of GSH reacts with 5,5-dithio-bis-2-nitrobenzoic acid (Ellman's reagent) producing 5-thio-2-nitrobenzoic acid having a yellow color, that was measured colorimetrically at 405 nm (absorbance [sample] ×2.22/g tissue used). Results were expressed as μmol/g tissue. The testicular content of lipid peroxides was determined by biochemical assessment of thiobarbituric acid reacting substance through spectrophotometric measurement of color at 535 nm, using 1,1,3,3-tetramethoxypropane as standard (absorbance [sample] +0.004/0.0466) as previously shown.[10] The results were expressed as equivalents of MDA in testicular tissue homogenate in nmol/g tissue.
Histopathological Examination
Testes fixed in 10% formalin and embedded in paraffin were sectioned at 5 μm thickness and stained with Hematoxylin and Eosin for routine histopathological assessment. Slides were microscopically analyzed using light microscopy. Testicular histologic parameters were studied and scored from 0 to 4 for each parameter, with the higher numbers indicating larger distribution for that particular parameter.[11]
Western Blot and Densitometric Analysis
Testicular tissue samples were homogenized in phosphate buffered saline buffer containing 1% Triton X-100, 1 mM ethylenediaminetetraacetic acid and protease inhibitor cocktail. The protein concentration of the tissue homogenate was determined[12] and 25 μg was applied in each lane on 10% sodium dodecyl sulfate-polyacrylamide gel. After electrophoresis and transfer, the membrane was incubated in blocking buffer containing 0.1% Tween-20 in Tris-buffered saline (TBST) and 5% non-fat milk powder, after which they were incubated with anti-caspase 3 antibody diluted 1:1000. After three washes, 10 min each, in TBST, the membrane was incubated with secondary antibody (anti-rabbit immunoglobulin G alkaline phosphatase conjugate) for 1 h at room temperature. After membrane was washed three times in TBST, detection solution containing substrate was added and incubated for 5 min at room temperature. After washing, the membrane was dried and photographed. Densitometric analysis of western blot was done to quantify the relative density of the protein bands using Image J (MD, USA) according to the manufacturer's instructions as described.[13]
Statistical Analysis
The data were analyzed by one-way ANOVA followed by Dunnett multiple comparison test. The values are represented as means ± standard error of the mean. All statistical analysis was carried out using GraphPad Prism version 5.00 (San Diego, CA, USA). The differences were considered to be significant when the calculated P < 0.05.
Results
Effect of Carvedilol on Testicular Histopathology in STZ-Induced Diabetic Rats
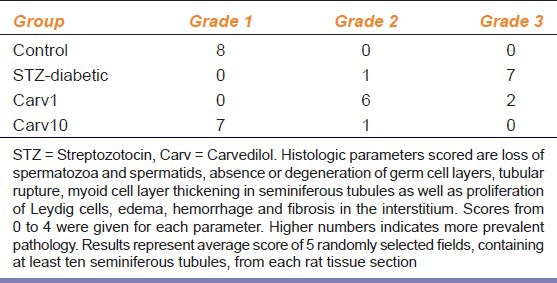
Induction of diabetes via STZ altered the normal histological picture of rat testis compared with controls [Figure 1a and b], as it caused abnormal architecture of seminiferous tubules with necrotic cells evident in the adluminal portion and lack of spermatogonia in the basal portion, as well as evident increase of extracellular matrix in the interstitial areas. Grading of the level of testicular histopathology is shown in Table 1. Low dose carvedilol (1 mg/kg/day; Carv1 group) showed minor improvement represented by reappearance of spermatogonia, moderate decrease in extracellular matrix [Figure 1c]. On the other hand, high dose carvedilol (10 mg/kg/day; Carv10 group) improved testicular structure, presenting with normal seminiferous tubules, spermatids, spermatogonia and extracellular matrix [Figure 1d]. Improvement of testicular histology in Carv1 and Carv10 groups was not accompanied by any significant alteration in blood glucose level compared with STZ-diabetic rats not receiving carvedilol (data not shown).
Figure 1.
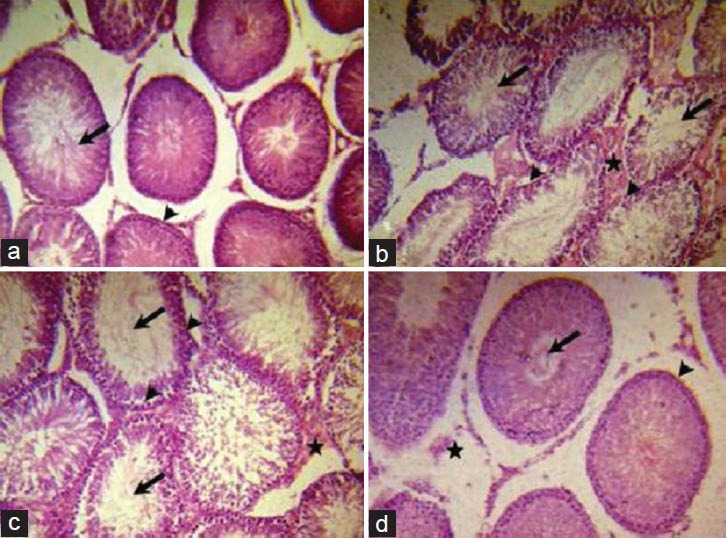
Effect of carvedilol on testicular histopathology in streptozotocin (STZ)-induced diabetic rats. A photomicrograph of a section in rat testis (H and E, ×200) of: (a) Untreated control group, having normal structure of testis with normal adluminal tubular pattern of spermatids (arrow) and normal spermatogonia in the basal compartment (arrowhead), (b) STZ-induced diabetic rat testes (single i.p. injection of 65 mg/kg), showing abnormal structure of seminiferous tubules, with its adluminal part either empty or filled with necrotic shredded cells (arrows), absence of spermatogonia in the basal area (arrowheads) and interstitial compartment filled with extracellular matrix (star), (c) low dose carvedilol group receiving daily single oral dose of 1 mg/kg/day for 4 weeks showing minor improvement in the tubular basal compartment with presence of some spermatogonia (arrowheads), decrease in extracellular matrix in interstitial space (star), but the adlunimal part of the tubules is still either lacking spermatids or filled with necrotic cells (arrows) and (d) high dose carvedilol group receiving daily single oral dose of 10 mg/kg/day for 4 weeks showing normal appearance of seminiferous tubules with normal spermatids in the adluminal compartment (arrow), normal spermatogonia in the basal area (arrowhead) and normal extracellular matrix in the interstitial space (star)
Table 1.
Grading the effect of Carv on testicular histopathology in STZ-induced diabetic rats
Effect of Carvedilol on Testicular Oxidative Stress Markers in STZ-Induced Diabetic Rats
Protective effect of carvedilol on one of the most powerful endogenous anti-oxidants, GSH, was evaluated in testicular tissue of diabetic rats [Figure 2a]. Induction of diabetes by STZ caused significant reduction (P < 0.05) in GSH level compared with control. Treatment with carvedilol in low (1 mg/kg/day) or high (10 mg/kg/day) dosages (Carv1 and Carv10 groups, respectively) significantly restored (P < 0.05) GSH level in STZ-induced diabetic rats. The effect of carvedilol on lipid peroxidation was assessed by evaluating MDA level in testicular tissue [Figure 2b]. STZ caused marked increase (P < 0.05) in MDA compared with controls, which was significantly reversed in Carv1 and Carv10 groups compared with STZ group (P < 0.05). Interestingly, Carv10 group receiving high dose of carvedilol decreased MDA to levels even not significantly different from control.
Figure 2.
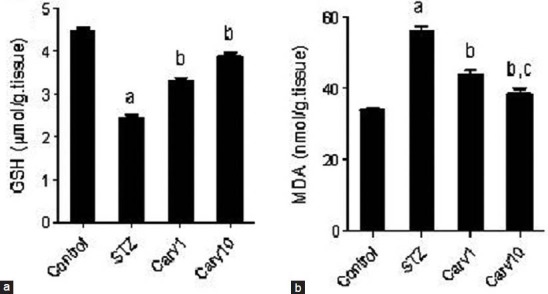
Effect of carvedilol on testicular (a) reduced glutathione (GSH) and (b) malondialdehyde (MDA) in streptozotocin (STZ)-induced diabetic rat. Carvedilol was given in low dose of 1 mg/kg/day (Carv1) and high dose of 10 mg/kg/day (Carv10) as a single daily oral dose for 4 weeks. Values are represented as means ± standard error of the mean of 8 observations. Significant difference is reported when P < 0.05. aSignificant difference compared to control, bsignificant difference compared with STZ-induced diabetic group, cno significant difference compared to control
Effect of Carvedilol on Caspase 3 Expression in STZ-Induced Diabetic Rat Testis
The expression of the apoptotic marker; caspase 3, was assessed in testes of STZ-treated rats via western blot [Figure 3], with densitometric evaluation of relative levels of caspase 3 expression. Diabetic rats exhibited significantly higher levels of caspase 3 expression compared with the control (P < 0.05). Both low (Carv1) and high (Carv10) doses of carvedilol succeeded in reverting caspase 3 expression to levels not significant from normal control.
Figure 3.
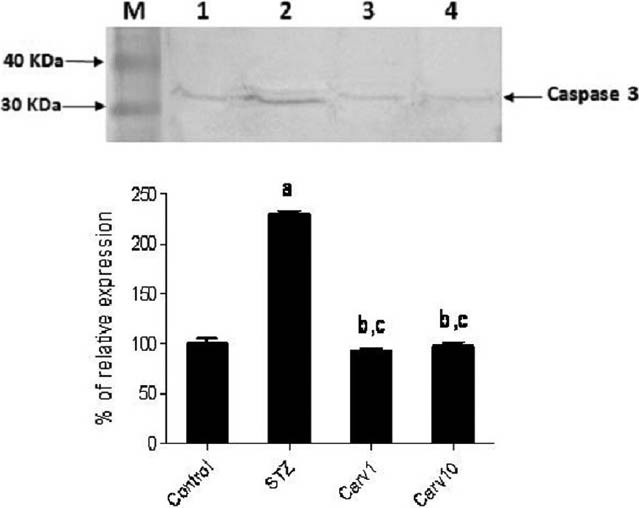
Effect of carvedilol on caspase 3 expression in testis of streptozotocin (STZ)-induced diabetic rats. Upper panel shows western blot expression of caspase 3 in testes of STZ-treated rats, with or without carvedilol 1 (Carv1) and 10 (Carv10) mg/kg/day orally for 4 weeks. Lower panel shows densitometric relative caspase 3 expression. Results are represented as percent of average of 3 different analyses compared with the control. Significant difference is reported when P < 0.05. aSignificant difference compared to control, bsignificant difference compared to STZ-induced diabetic group, cno significant difference compared with control
Discussion
Diabetes may affect testicular function through several pathways. The initiative diabetic assault is disruption of glucose transport from the blood to testicular germ cells through the blood-testis-barrier.[14] This barrier is mainly composed of Sertoli cells that have a pivotal role in controlling testicular glucose homeostasis as they divide the testis into two physically and functionally independent areas; the basal and the adluminal compartments. Impairment of glucose transport is followed by an increase in free radical formation and oxidative stress[15] as a result, sperm nuclear and mitochondrial deoxyribonucleic acid damage occur.[2] In consequence, an apoptotic response arises in sperm cells causing higher percentage of spermatic cellular death.[16]
Here, induction of diabetes using STZ in rats caused distortion of normal histological seminiferous tubular structure. This was accompanied by increased testicular oxidative stress markers, as decreased level of GSH and increased lipid peroxidation content, as well as by increased expression of the apoptotic marker; caspase 3. Previous studies have shown that several compounds possessing antioxidant properties might confer testicular protection against diabetes-induced oxidative stress and/or apoptotic markers, as sulfurous mineral water and sodium hydrosulfide,[17] curcumin,[18] quercetin,[19] ferulic acid,[20] pioglitazone,[21] and melatonin.[22]
To the best of our knowledge, carvedilol has not been reported to have any testicular beneficial effects, whether under diabetic conditions or otherwise. This report shows that 4 weeks carvedilol therapy in low or high dose succeeded in restoring, at least in part, testicular structure to normal. This was attained via carvedilol-induced elevation of testicular GSH and reduction of the product of lipid peroxidation; MDA, as well as down-regulation of the expression of the apoptotic marker; caspase 3. Such effects of carvedilol were not attributed to alteration of blood glucose. Carvedilol has been previously reported to have anti-apoptotic effect in organs other than the testis, as on the heart[23] and kidney.[24] In addition, carvedilol ameliorated oxidative stress in serum of hypertensive patients,[25] carbon tetrachloride liver damage[26] and arthritis.[27] The reported dose of carvedilol for antioxidant actions in healthy volunteers was 6.25-25 mg/day,[28] which corresponds to 1 and 10 mg/kg/day in rats,[9] which we chose as low and high dose in the current study.
Conclusion
Carvedilol, in low or high dosage, protects against diabetes-induced testicular damage. The mechanisms involved include carvedilol antioxidant and anti-apoptotic effects. Further studies are necessary to assess the usefulness of carvedilol on the level of fertility in diabetic patients.
Footnotes
Source of Support: Nil
Conflict of Interest: No
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.La Vignera S, Condorelli R, Vicari E, D’Agata R, Calogero AE. Diabetes mellitus and sperm parameters. J Androl. 2012;33:145–53. doi: 10.2164/jandrol.111.013193. [DOI] [PubMed] [Google Scholar]
- 3.Shrilatha B, Muralidhara Early oxidative stress in testis and epididymal sperm in streptozotocin-induced diabetic mice: Its progression and genotoxic consequences. Reprod Toxicol. 2007;23:578–87. doi: 10.1016/j.reprotox.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Leonetti G, Egan CG. Use of carvedilol in hypertension: An update. Vasc Health Risk Manag. 2012;8:307–22. doi: 10.2147/VHRM.S31578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Del Sindaco D, Pulignano G, Cioffi G, Tarantini L, Di Lenarda A, De Feo S, et al. Safety and efficacy of carvedilol in very elderly diabetic patients with heart failure. J Cardiovasc Med (Hagerstown) 2007;8:675–82. doi: 10.2459/01.JCM.0000285316.71057.26. [DOI] [PubMed] [Google Scholar]
- 6.Bakris GL, Fonseca V, Katholi RE, McGill JB, Messerli FH, Phillips RA, et al. Metabolic effects of carvedilol vs metoprolol in patients with type 2 diabetes mellitus and hypertension: A randomized controlled trial. JAMA. 2004;292:2227–36. doi: 10.1001/jama.292.18.2227. [DOI] [PubMed] [Google Scholar]
- 7.Jawa A, Nachimuthu S, Pendergrass M, Asnani S, Fonseca V. Beta-blockers have a beneficial effect upon endothelial function and microalbuminuria in African-American subjects with diabetes and hypertension. J Diabetes Complications. 2008;22:303–8. doi: 10.1016/j.jdiacomp.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fu GS, Huang H, Chen F, Wang HP, Qian LB, Ke XY, et al. Carvedilol ameliorates endothelial dysfunction in streptozotocin-induced diabetic rats. Eur J Pharmacol. 2007;567:223–30. doi: 10.1016/j.ejphar.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 9.Tunali S, Yanardag R. Protective effect of vanadyl sulfate on skin injury in streptozotocin-induced diabetic rats. Hum Exp Toxicol. 2013;32:1206–12. doi: 10.1177/0960327113478445. [DOI] [PubMed] [Google Scholar]
- 10.Morsy MA. Protective effect of lisinopril on hepatic ischemia/reperfusion injury in rats. Indian J Pharmacol. 2011;43:652–5. doi: 10.4103/0253-7613.89820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cosentino MJ, Nishida M, Rabinowitz R, Cockett AT. Histological changes occurring in the contralateral testes of prepubertal rats subjected to various durations of unilateral spermatic cord torsion. J Urol. 1985;133:906–11. doi: 10.1016/s0022-5347(17)49278-0. [DOI] [PubMed] [Google Scholar]
- 12.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 13.Miller L. USA: University of Missouri; [Serial online]; 2007. [Last cited on 2013 July 20]. Quantifying western blots without expensive commercial quantification software. Dalton Cardiovascular Research Center. Available from: https://www.support.dalton.missouri.edu/index.php/wiki/Public: Quantifying_Color_Intensity . [Google Scholar]
- 14.Alves MG, Martins AD, Rato L, Moreira PI, Socorro S, Oliveira PF. Molecular mechanisms beyond glucose transport in diabetes-related male infertility. Biochim Biophys Acta. 2013;1832:626–35. doi: 10.1016/j.bbadis.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Zhao Y, Zhao H, Zhai X, Dai J, Jiang X, Wang G, et al. Effects of Zn deficiency, antioxidants, and low-dose radiation on diabetic oxidative damage and cell death in the testis. Toxicol Mech Methods. 2013;23:42–7. doi: 10.3109/15376516.2012.731437. [DOI] [PubMed] [Google Scholar]
- 16.Roessner C, Paasch U, Kratzsch J, Glander HJ, Grunewald S. Sperm apoptosis signalling in diabetic men. Reprod Biomed Online. 2012;25:292–9. doi: 10.1016/j.rbmo.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Sadik NA, El-Seweidy MM, Shaker OG. The antiapoptotic effects of sulphurous mineral water and sodium hydrosulphide on diabetic rat testes. Cell Physiol Biochem. 2011;28:887–98. doi: 10.1159/000335803. [DOI] [PubMed] [Google Scholar]
- 18.Kanter M, Aktas C, Erboga M. Curcumin attenuates testicular damage, apoptotic germ cell death, and oxidative stress in streptozotocin-induced diabetic rats. Mol Nutr Food Res. 2013;57:1578–85. doi: 10.1002/mnfr.201200170. [DOI] [PubMed] [Google Scholar]
- 19.Kanter M, Aktas C, Erboga M. Protective effects of quercetin against apoptosis and oxidative stress in streptozotocin-induced diabetic rat testis. Food Chem Toxicol. 2012;50:719–25. doi: 10.1016/j.fct.2011.11.051. [DOI] [PubMed] [Google Scholar]
- 20.Thyagaraju BM, Muralidhara Ferulic acid supplements abrogate oxidative impairments in liver and testis in the streptozotocin-diabetic rat. Zoolog Sci. 2008;25:854–60. doi: 10.2108/zsj.25.854. [DOI] [PubMed] [Google Scholar]
- 21.Gumieniczek A, Hopkała H, Zabek A. Protective effects of a PPARgamma agonist pioglitazone on anti-oxidative system in testis of diabetic rabbits. Pharmazie. 2008;63:377–8. [PubMed] [Google Scholar]
- 22.Armagan A, Uz E, Yilmaz HR, Soyupek S, Oksay T, Ozcelik N. Effects of melatonin on lipid peroxidation and antioxidant enzymes in streptozotocin-induced diabetic rat testis. Asian J Androl. 2006;8:595–600. doi: 10.1111/j.1745-7262.2006.00177.x. [DOI] [PubMed] [Google Scholar]
- 23.Hassan F, Meduru S, Taguchi K, Kuppusamy ML, Mostafa M, Kuppusamy P, et al. Carvedilol enhances mesenchymal stem cell therapy for myocardial infarction via inhibition of caspase-3 expression. J Pharmacol Exp Ther. 2012;343:62–71. doi: 10.1124/jpet.112.196915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carvalho Rodrigues MA, Gobe G, Santos NA, Santos AC. Carvedilol protects against apoptotic cell death induced by cisplatin in renal tubular epithelial cells. J Toxicol Environ Health A. 2012;75:981–90. doi: 10.1080/15287394.2012.696512. [DOI] [PubMed] [Google Scholar]
- 25.Zepeda RJ, Castillo R, Rodrigo R, Prieto JC, Aramburu I, Brugere S, et al. Effect of carvedilol and nebivolol on oxidative stress-related parameters and endothelial function in patients with essential hypertension. Basic Clin Pharmacol Toxicol. 2012;111:309–16. doi: 10.1111/j.1742-7843.2012.00911.x. [DOI] [PubMed] [Google Scholar]
- 26.Hamdy N, El-Demerdash E. New therapeutic aspect for carvedilol: Antifibrotic effects of carvedilol in chronic carbon tetrachloride-induced liver damage. Toxicol Appl Pharmacol. 2012;261:292–9. doi: 10.1016/j.taap.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 27.Arab HH, El-Sawalhi MM. Carvedilol alleviates adjuvant-induced arthritis and subcutaneous air pouch edema: Modulation of oxidative stress and inflammatory mediators. Toxicol Appl Pharmacol. 2013;268:241–8. doi: 10.1016/j.taap.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 28.Dandona P, Karne R, Ghanim H, Hamouda W, Aljada A, Magsino CH., Jr Carvedilol inhibits reactive oxygen species generation by leukocytes and oxidative damage to amino acids. Circulation. 2000;101:122–4. doi: 10.1161/01.cir.101.2.122. [DOI] [PubMed] [Google Scholar]


