Abstract
Aim:
The aqueous extract of leaves of Murraya koenigii was studied for its renoprotective potential against unilateral renal ischemia reperfusion (RIR) injury in male Wistar rats.
Materials and Methods:
Healthy adult male Wistar rats were divided into five groups (n = 8) and were treated with 200 mg/kg., p.o. of aqueous extract of M. koenigii (AEMK) for 30 days to assess both preventive and curative effects of AEMK. Except Group I, RIR was induced to all the groups by clamping the left renal artery using artery clamp for 1 h followed by reperfusion by removing the clamp. Groups II and III underwent RIR at 30th day whereas RIR was induced in Groups IV and V at 1st day of treatment schedule. Biochemical parameters (serum creatinine, blood urea nitrogen, serum total protein and serum Na+), urinary parameters (urine output, urinary creatinine, urinary urea, urinary total protein, urinary Na+), in vivo anti-oxidants, renal myeloperoxidase (MPO) activity and histopathology of kidneys were monitored. Statistical significance was set at P < 0.05.
Results:
Rats were treated with AEMK significantly (P < 0.05) restored the serum and urinary parameters with significant (P < 0.05) improvement in endogenous anti-oxidants such as superoxide dismutase, catalase and reduced glutathione and decreased levels of malondialdehyde and renal MPO when compared with the control groups. Histopathological examination also supported the biochemical and urinary tests.
Conclusions:
Aqueous extract of M. koenigii possesses both preventive and curative effects against RIR injury.
KEY WORDS: Aqueous extract, ischemia reperfusion injury, kidney, Murraya koenigii
Introduction
Acute renal failure (ARF) remains a common and critical clinical entity affecting 5-7% of all hospitalized patients.[1] Despite advances in medical care, ARF still carries a significant morbidity and mortality of 20-70% respectively. Renal ischemia is one of the most common causes of ARF, initiating a complex and interrelated sequence of events resulting in injury and the eventual death of renal cells.[2] Restoration of blood flow is essential for the survival of ischemic renal tissue however it causes reperfusion injury.[3] Reactive oxygen species and inflammatory leukocytes play a vital role in the pathogenesis of renal ischemia reperfusion (RIR) injury.[2] The morphological characteristics of ischemia-induced ARF in animals include loss of the proximal tubular brush border, blebbing of apical membranes, cellular and mitochondrial swelling and apoptosis.[4]
Since no effective therapy is available in the modern system of medicine for the treatment of renal failure, an increasing exploration of complementary and alternative medicine from natural sources is highlighted. The plant Murraya koenigii (Family – Rutaceae) has been used in Indian recipe preparations since several centuries and is reported to have anti-oxidant, anti-diabetic,[5] anti-fungal, anti-bacterial[6] and anti-inflammatory[7] properties. The extract of the leaves of M. koenigii was found to be useful in the treatment of kidney infirmities.[8] Carbazole alkaloids a major phytochemical constituent of plant possess various biological activities. Phytochemical investigations on this plant revealed presence of alkaloids, essential oils, calcium, phosphorus, iron, thiamine, riboflavin, niacin, vitamin A, vitamin C, carotene and oxalic acid in leaves.[9]
Few earlier studies demonstrated that leaves of M. koenigii improved renal function and anti-oxidant status in streptozotocin-induced diabetic rats.[10] A survey of the literature revealed that no methodical reports are available on renoprotective effect of M. koenigii against RIR injury. Hence, the aim of the present study was to evaluate the renoprotective potential of aqueous extract of M. koenigii (AEMK) against unilateral RIR injury.
Materials and Methods
Animals
Adult male Wistar rats (200-250 g) were purchased from Raghavendra Enterprises, Bangalore. The animals were housed in an air-conditioned room under standard conditions (22 ± 2°C) and 12-h light/dark cycle. Rats had free access to standard diet and purified drinking water. All experiments and protocols described in the present study were approved by the Institutional Animal Ethical Committee of Sri Padmavathi School of Pharmacy (No: 1016/a/06/CPCSEA/006/2012), Tiruchanoor, Tirupathi, India.
Chemicals
Ethylenediaminetetraacetic acid, epinephrine bitartrate, hydrogen peroxide, trichloroacetic acid, 5, 5-dithiobis (2-nitrobenzoic acid), thiobarbituric acid, hexadecyltrimethylammonium bromide and O-dianisidine were procured from Merck and S.D. Fine chemicals private limited. Creatinine, blood urea nitrogen (BUN), total protein and sodium assay kits (auto analyzer) were procured from Span Diagnostics Ltd., Gujarat, India.
Instruments
Ultraviolet-visible spectrophotometer (analytical systems, model no: AUV 2060), electronic balance (Shimadzu, model no: DS-852J), homogenizer (Ever shine, model no: 607), auto analyzer (Mispa Excel, Version: 1.4e, Agappe Diagnostics) and Cooling centrifuge (Remi, model no: C-24 BL) were used for estimation of biochemical parameters.
Preparation of Plant Extract
The leaves of M. koenigii were collected locally in Tirupathi, Andhra Pradesh during the month of January, 2012 and authenticated by Dr. B. Seetharam, Associate Professor, Sri Venkateswara Ayurvedic Medical College, Tirupathi. The collected plant material was shade dried and milled to coarse powder (sieve no 40/40) by a mechanical grinder. The powdered plant material and distilled water were taken in the ratio of 1:6 and boiled for half an hour on heating mantle. The decoction was squeezed through muslin cloth to obtain AEMK. This extract was subjected to loss on drying and weight/ml was estimated randomly. Daily the AEMK was prepared freshly and administered to the experimental animals, as per the dose required.
Dose Selection
Due to the documented evidence of safety of AEMK[10] the acute toxicity studies were not repeated in the present study and dose of 200 mg/kg, p.o. of AEMK was selected as per the earlier reports.[5]
Experimental Design
The study was planned to assess ability of AEMK to prevent and also to cure RIR. After acclimatization for 14 days, male adult Wistar rats were randomly divided into five groups containing eight animals each. Group I (normal) was treated with vehicle alone for 30 days. Group II was also treated with vehicle for 30 days but RIR was performed on day 30, served as preventive control. Group III received 200 mg/kg, p.o. of AEMK for 30 days and RIR was performed on day 30, served as preventive. In Groups IV and V, RIR was performed on day 1 followed by treatment with vehicle and 200 mg/kg, p.o. of AEMK respectively for 30 days and served as curative control and curative group respectively.
Induction of RIR Injury
Male Wistar rats were anesthetized by intramuscular injection of ketamine (50 mg/kg) and xylazine (5 mg/kg). A midline abdominal incision was made to expose the left kidney. Blood supply to the kidney was interrupted by clamping the left renal artery using artery clamp for 1 h. Ischemia was confirmed by the blanching of the kidney. After 1 h, the clamp was removed and reperfusion was confirmed visually. The wound was then closed in two layers with silk suture and the animals were allowed to recover with free access to food and water.[11]
Estimation of Biochemical Parameters
At the end of treatment schedule i.e.,72 h after RIR in Groups II and III and 30 days after RIR in Groups IV and V blood samples were withdrawn from the retro orbital venous plexus for estimating serum parameters namely creatinine, BUN,[12] total protein[13] and sodium levels.[14]
Urine samples were collected at 72 h after RIR in Groups II and III and 30 days after RIR in Groups IV and V for 24 h by housing the rats in individual metabolic cages after administration of distilled water (5 ml/animal) orally. The urine output for 24 h was measured followed by estimation of urinary parameters including urinary creatinine, urinary urea, urinary total protein and urinary sodium levels.
At the end, rats were sacrificed by decapitation. The kidneys were excised carefully and washed with normal saline. One kidney (kidney that underwent renal clamping) from each group was sliced into two equal halves. One half was stored in 10% formalin which was further used for histopathological studies. The other half was homogenized for estimating in vivo anti-oxidant parameters and renal myeloperoxidase (MPO) activity.
Preparation of Kidney Homogenate
The excised kidneys were weighed and chilled in ice cold 0.25 M sucrose and homogenized in ice cold 10 mM tris HCl buffer (pH: 7.4) at a concentration of 10% w/v at a speed of 2500 rpm. It was then centrifuged at 5000 rpm at 20°C temperature to get the supernatant which was used for estimation of superoxide dismutase (SOD),[15] catalase (CAT),[16] reduced glutathione (GSH)[17] and lipid peroxidation.[18]
Estimation of Renal MPO
MPO activity in renal homogenate was measured according to the procedure documented previously.[19]
Statistical Analysis
All the data were expressed as mean ± standard error of mean. Statistical significance between >2 groups was tested using one-way analysis of variance followed by the Turkey multiple comparison test using computer based fitting program (Prism Graph pad, version: 5.04 for windows, Graph Pad Software). Statistical significance was set at P < 0.05.
Results
Effect of AEMK on Serum Parameters
In both preventive control (II) and curative control (IV), RIR caused a significant increase in serum creatinine and BUN when compared with the normal Group I depicting the renal failure (P < 0.05). Treatment with AEMK prior and after RIR i.e. both preventive and curative Groups III and V significantly decreased the serum creatinine and BUN as compared to their respective ischemic control Groups II and IV (P < 0.05) [Table 1].
Table 1.
Effect of AEMK on biochemical parameters in RIR rats
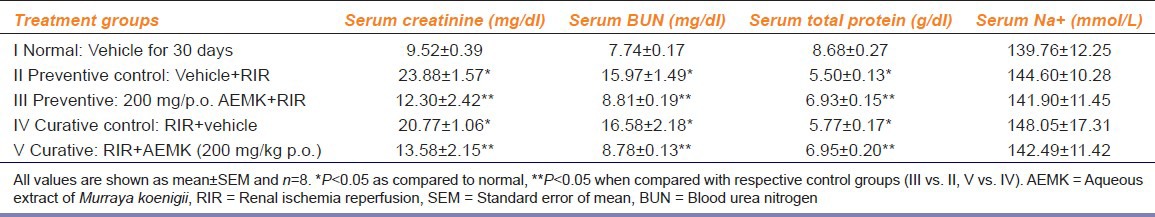
A significant decrease in serum total protein was observed after RIR in preventive control (II) and curative control (IV) when compared with the normal Group I indicating proteinuria due to renal impairment (P < 0.05). A significant increase in the serum total protein was observed in both preventive (III) and curative (V) groups when compared to their control Groups II and IV indicating that the AEMK reduced the urinary leakage of proteins by ameliorating the renal injury (P < 0.05) [Table 1].
However, serum Na+ levels were not affected after either RIR or treatment with AEMK as no significant changes were observed in all groups under treatment or control [Table 1].
Effect of AEMK on Urinary Parameters
Induction of RIR injury produced a significant reduction in urinary output, urinary creatinine and urea levels in preventive control (II) and curative control (IV) groups when compared with the normal Group I (P < 0.05). A significant increase in the urinary output, creatinine and urea were observed in preventive (III) and curative (V) groups when compared with their respective control groups (P < 0.05) [Table 2].
Table 2.
Effect of AEMK on urinary parameters in RIR rats

When compared with the normal Group I, RIR injury significantly increases proteinuria in both preventive control (II) and curative control (IV) groups (P < 0.05). While treatment with AEMK prior to RIR and after RIR significantly decreased the urinary excretion of protein in preventive and curative Groups III and V when compared with their respective control Groups II and IV reflecting preservation of glomerular function (P < 0.05) [Table 2].
Urinary Na+ levels were significantly increased in preventive control (II) group when compared with the normal Group I. When treated with AEMK a significant restoration of urinary excretion of Na+ was observed in preventive Group III (P < 0.05).
After RIR no significant changes in urinary excretion of Na+ was observed in curative control (IV) and curative (V) groups (P < 0.05) [Table 2].
Effect of AEMK on In Vivo Anti-oxidant Parameters
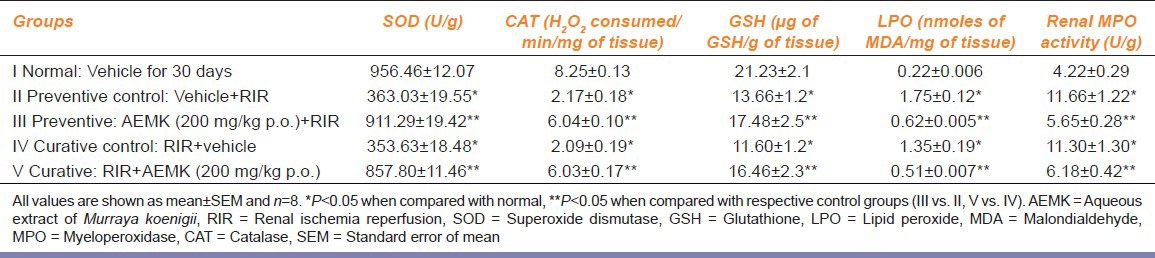
After RIR injury, significant decrease in the levels of enzymatic anti-oxidants namely SOD, CAT, GSH and a significant increase in the malondialdehyde (MDA) levels were observed in both preventive control (II) and curative control (IV) groups when compared with the normal Group I indicating the oxidative stress (P < 0.05).
After treatment with AEMK a significant increase in the levels of SOD, CAT, GSH and decrease in the levels of MDA were observed in both preventive and curative groups when compared with their control Groups II and IV respectively (P < 0.05) [Table 3].
Table 3.
Effect of AEMK on in vivo anti-oxidant parameters and renal MPO activity in RIR rats
Effect of AEMK on Renal MPO Activity
When compared with the normal group, significant increase in MPO levels were observed in the renal tissue homogenate of Groups II and IV indicating the infiltration of neutrophils during ischemia reperfusion (P < 0.05).
Group III pre-treated with AEMK and Group V which received AEMK after RIR, showed significant decrease in MPO activity over the preventive control (II) and curative control (IV) groups indicating that the AEMK had inhibited the neutrophil infiltration (P < 0.05) [Table 3].
Effect of AEMK on Histopathology of Kidney
Normal Group I did not show any morphological changes in kidney. Preventive control and curative control Groups II and IV showed significant tubular defects, interstitial hemorrhage and damage of glomerular apparatus and dilated spaces. However, more severe damage was observed in curative control. Pre-treatment and post-treatment with AEMK restored the tubular and glomerular apparatus with normal morphology in preventive and curative Groups III and V [Figure 1].
Figure 1.

Photo micrographs of kidney sections stained in H and E at × 10. (a) Normal – showing regular renal morphology, (b) preventive control showing interstitial hemorrhage (star) and dilated spaces (arrows), (c) preventive showed minimal glomerular congestion and dilated spaces, (d) curative control showing severe tubular degeneration, dilation (arrow) and interstitial hemorrhage (star), (e) curative showed mild degeneration and tubular dilation
Discussion
Renal artery occlusion model was used in present study as an experimental model of RIR, as it is the most widely applied in vivo model of human ischemic ARF. In this model, persistent vasoconstriction and cellular swelling causes poor blood reflow into the deep cortex and outer medulla, after the arterial clamp is released.[20] Pathological similarities between ischemic ARF and human acute tubular necrosis include injury to the proximal brush border and the presence of cast formation.
Rats that underwent renal ischemia followed by reperfusion showed characteristic signs of renal dysfunction and inflammation. The decline in renal function after RIR was reflected in the results, showing decreased urine output, increased levels of serum creatinine, BUN and decreased levels of serum total protein. Similar decreased levels of urinary creatinine, urinary urea and increased levels of urinary total protein were observed. Both preventive and curative treatments with AEMK restored the biochemical parameters which indicate that AEMK has both preventive and curative effects.
Clinically, renal failure causes increased urinary excretion of Na+ due to decreased tubular reabsorption. In the present study, similar increased urinary excretion of Na+ was observed in preventive control group, which was found to be rectified in preventive group upon treatment with AEMK. However, no significant changes in urinary excretion of Na+ were noted in both curative control and curative groups. Similarly, no significant changes were observed in serum Na+ levels in the Groups II, III, IV and V. Further studies are necessary to clarify this observation as the relation between tubular damage and Na+ excretion is quite complex and affected by many factors.
During reperfusion, large amount of free radicals are generated. Previous studies demonstrated that lipid peroxidation is involved in ischemic ARF and that peroxynitrite contributes to lipid peroxidation in an in vivo ischemia reperfusion model.[21]
In the present study, groups treated with AEMK showed significant increase in endogenous anti-oxidants such as SOD, CAT, GSH and decreased MDA levels compared with the control groups. These results indicated that the protective and curative effects of M. koenigii against RIR may relate to improvements in the endogenous anti-oxidant system and decreased lipid peroxidation due to nitric oxide scavenging property. These results are in agreement with earlier reports for other plants with reported nephroprotector activity like Benincasa cerifera,[22] Linum usitatissimum.[23]
Reperfusion of ischemic tissues induces an inflammatory response and this stimulates the rapid infiltration of neutrophils. When the neutrophils are activated, MPO is released with other tissue-damaging substances from the cells. Therefore, MPO can be used as an indirect measure of tissue polymorphonuclear infiltration.[24] Treatment with AEMK showed decreased MPO activity. These results imply that inflammation associated with ischemia reperfusion of the kidney may be alleviated by M. koenigii.
The histological changes in the ischemia reperfusion groups included degeneration of tubular architecture, tubular dilation, proteinaceous debris, swelling and necrosis and luminal congestion with a loss of brush border indicating severe renal injury in both preventive and curative control groups. The microphotographs of preventive and curative groups clearly shows the regeneration of tubular architecture, decreased cellular damage, congestion and necrosis, which supports the biochemical parameters evaluated in the present study. Leaf extract of M. koenigii have been reported to have strong anti-oxidative,[5] anti-inflammatory,[7] anti-platelet[25] and nitric oxide scavenging activities[26] which might be responsible for its renoprotective potential against RIR.
The present study shows that AEMK possesses both preventive and curative effects against RIR injury. Further identification of active constituents with nephroprotective potential from AEMK will be useful to treat renal disorders.
Acknowledgments
The authors are thankful to Dr. D. Ranganayakulu, Principal, Sri Padmavathi School of Pharmacy, Tiruchanoor, Tirupati for providing facilities to carry out this research work.
Footnotes
Source of Support: Nil
Conflict of Interest: No
References
- 1.Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39:930–6. doi: 10.1053/ajkd.2002.32766. [DOI] [PubMed] [Google Scholar]
- 2.Lieberthal W, Levine JS. Mechanisms of apoptosis and its potential role in renal tubular epithelial cell injury. Am J Physiol. 1996;271:F477–88. doi: 10.1152/ajprenal.1996.271.3.F477. [DOI] [PubMed] [Google Scholar]
- 3.Weight SC, Bell PR, Nicholson ML. Renal ischaemia – Reperfusion injury. Br J Surg. 1996;83:162–70. [PubMed] [Google Scholar]
- 4.Racusen LC. The morphologic basis of acute renal failure. In: Molitoris BA, Finn WF, editors. Acute Renal Failure – A Companion to Brenner and Rector's. The Kidney. Philadelphia: Saunders; 2001. pp. 1–12. [Google Scholar]
- 5.Arulselvan P, Subramanian SP. Beneficial effects of Murraya koenigii leaves on antioxidant defense system and ultra structural changes of pancreatic beta-cells in experimental diabetes in rats. Chem Biol Interact. 2007;165:155–64. doi: 10.1016/j.cbi.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 6.Khuntia TK, Panda DS. Evaluation of antibacterial, antifungal and anthelmintic activity of Murraya koenigii Spreng. Pharma Sci Monit. 2011;2:105–10. [Google Scholar]
- 7.Ramsewak RS, Nair MG, Strasburg GM, DeWitt DL, Nitiss JL. Biologically active carbazole alkaloids from Murraya koenigii. J Agric Food Chem. 1999;47:444–7. doi: 10.1021/jf9805808. [DOI] [PubMed] [Google Scholar]
- 8.Parmar C, Kaushal MK. Murraya koenigii. In: Parmar C, Kaushal MK, editors. Wild Fruits [M] New Delhi: Kalyani Publishers; 1982. pp. 45–8. 80, 413. [Google Scholar]
- 9.Salikutty J, Peter KV. Curry leaf. In: Peter KV, editor. Handbook of Herbs and Spices. Cambridge, England: Wood Head Publishing Limited; 2001. pp. 168–72. [Google Scholar]
- 10.Yankuzo H, Ahmed QU, Santosa RI, Akter SF, Talib NA. Beneficial effect of the leaves of Murraya koenigii (Linn.) Spreng (Rutaceae) on diabetes-induced renal damage in vivo. J Ethnopharmacol. 2011;135:88–94. doi: 10.1016/j.jep.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 11.Savransky V, Molls RR, Burne-Taney M, Chien CC, Racusen L, Rabb H. Role of the T-cell receptor in kidney ischemia-reperfusion injury. Kidney Int. 2006;69:233–8. doi: 10.1038/sj.ki.5000038. [DOI] [PubMed] [Google Scholar]
- 12.Murray RL. Non protein nitrogen compounds: Creatinine. In: Kaplan LA, Pesce AJ, editors. Clinical Chemistry: Theory, Analysis, and Correlation. 2nd ed. St. Louis: The C.V. Mosby Company; 1989. pp. 1015–21. [Google Scholar]
- 13.Koller A, Kaplan LA. Total serum protein. In: Kaplan LA, Pesce AJ, editors. Clinical Chemistry: Theory, Analysis, and Correlation. 2nd ed. St. Louis: The C.V. Mosby Company; 1989. pp. 1057–60. [Google Scholar]
- 14.Terri AE, Sesin PJ. Colorimetric method of potassium estimation using sodium tetraphenylboron. Am J Clin Pathol. 1958;29:86–9. [Google Scholar]
- 15.Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–5. [PubMed] [Google Scholar]
- 16.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–6. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 17.Moron MS, Depierre JW, Mannervik B. Levels of glutathione glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta. 1979;582:67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 18.Slater TF, Sawyer BC. The stimulatory effects of carbon tetrachloride and other halogenoalkanes on peroxidative reactions in rat liver fractions in vitro. General features of the systems used. Biochem J. 1971;123:805–14. doi: 10.1042/bj1230805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taoka Y, Okajima K, Uchiba M, Murakami K, Harada N, Johno M, et al. Activated protein C reduces the severity of compression-induced spinal cord injury in rats by inhibiting activation of leukocytes. J Neurosci. 1998;18:1393–8. doi: 10.1523/JNEUROSCI.18-04-01393.1998. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Frega NS, DiBona DR, Guertler B, Leaf A. Ischemic renal injury. Kidney Int Suppl. 1976;6:S17–25. [PubMed] [Google Scholar]
- 21.Grace PA. Ischaemia-reperfusion injury. Br J Surg. 1994;81:637–47. doi: 10.1002/bjs.1800810504. [DOI] [PubMed] [Google Scholar]
- 22.Bhalodia Y, Kanzariya N, Patel R, Patel N, Vaghasiya J, Jivani N, et al. Renoprotective activity of Benincasa cerifera fruit extract on ischemia/reperfusion-induced renal damage in rat. Iran J Kidney Dis. 2009;3:80–5. [PubMed] [Google Scholar]
- 23.Bodhankar SL, Ghule AE, Jadhav SS. Renoprotective effect of Linum usitatissimum seeds through haemodynamic changes and conservation of antioxidant enzymes in renal ischaemia-reperfusion injury in rats. Arab J Urol. 2011;9:215–21. doi: 10.1016/j.aju.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Donovan DA, Kelly CJ, Abdih H, Bouchier-Hayes D, Watson RW, Redmond HP, et al. Role of nitric oxide in lung injury associated with experimental acute pancreatitis. Br J Surg. 1995;82:1122–6. doi: 10.1002/bjs.1800820838. [DOI] [PubMed] [Google Scholar]
- 25.Jessie SW, Krishnakantha TP. Antiplatelet activity of coriander and curry leaf spices. Pharm Biol. 2005;43:230–3. [Google Scholar]
- 26.Baliga MS, Jagetia GC, Rao SK, Babu K. Evaluation of nitric oxide scavenging activity of certain spices in vitro: A preliminary study. Nahrung. 2003;47:261–4. doi: 10.1002/food.200390061. [DOI] [PubMed] [Google Scholar]


