Abstract
Objective:
The present study investigates the neuroprotective activity of ethanol extract of Tinospora cordifolia aerial parts against 6-hydroxy dopamine (6-OHDA) lesion rat model of Parkinson's disease (PD).
Materials and Methods:
T. cordifolia ethanol extract (TCEE) was standardized with high performance thin layer chromatography using berberine. Experimental PD was induced by intracerebral injection of 6-OHDA (8 μg). Animals were divided into five groups: sham operated, negative control, positive control (levodopa 6 mg/kg) and two experimental groups (n = 6/group). Experimental groups received 200 and 400 mg/kg of TCEE once daily for 30 days by oral gavage. Biochemical parameters including dopamine level, oxidative stress, complex I activity and brain iron asymmetry ratio and locomotor activity including skeletal muscle co-ordination and degree of catatonia were assessed.
Results:
TCEE exhibited significant neuroprotection by increasing the dopamine levels (1.96 ± 0.20 and 2.45 ± 0.40 ng/mg of protein) and complex I activity (77.14 ± 0.89 and 78.50 ± 0.96 nmol/min/mg of protein) at 200 and 400 mg/kg respectively when compared with negative control group. Iron asymmetry ratio was also significantly attenuated by TCEE at 200 (1.57 ± 0.18) and 400 mg/kg (1.11 ± 0.15) when compared with negative control group. Neuroprotection by TCEE was further supported by reduced oxidative stress and restored locomotor activity in treatment groups.
Conclusion:
Results show that TCEE possess significant neuroprotection in 6-OHDA induced PD by protecting dopaminergic neurons and reducing the iron accumulation.
KEY WORDS: Complex I activity, dopamine, iron asymmetry, levodopa, neurodegeneration
Introduction
Parkinson's disease (PD) is the second most common neurodegenerative disorder, primarily affecting people over 55 years of age (approximately 1.5-2%), young adults and even children. PD is characterized by the loss of 50-70% of dopaminergic neurons in the substantia nigra (SN).[1] Levodopa (L-DOPA) is considered as the gold standard drug for treating PD, but multiple complications such as motor fluctuations, hallucinations and frank psychosis may arise from long-term therapy. Degradation of L-DOPA generates many toxic metabolites that cause oxidative stress in neurons. Cultured dopaminergic neurons have undergone necrosis or apoptosis when exposed to L-DOPA, suggesting that L-DOPA becomes toxic at a certain threshold.[2] This led to the belief that treatment with L-DOPA should be delayed as long as possible and has provided the impetus for evaluation of new drugs to treat PD. Recent findings of abnormal protein folding, coupled with oxidative stress, provide scientific rationale for novel therapeutic strategies designed to delay disease progression. To be effective, these disease-modifying and neuroprotective therapies must be instituted early in the course of the disease. A growing understanding of processes leading to cell death elicited the notion that neuroprotection may be a reachable goal in the treatment of PD. Neuroprotective therapy is expected to slow or even halt the underlying progression of degeneration.
Tinospora cordifolia (Willd.) Miers. (Menispermaceae) is a large deciduous climbing shrub found mainly in tropical parts of India and China. It has been widely used in the traditional Indian system of medicine to treat leprosy, diabetes, asthma, anorexia, jaundice, pyrexia, gout and diarrhea. It is also considered as a bitter tonic, astringent, diuretic, aphrodisiac and curative against skin infections.[3] T. cordifolia is categorized as “Rasayana” in Ayurveda, used for its general adaptogenic and prohost immunomodulatory activity in fighting infections. It possesses anti-androgenic,[4] anti-inflammatory,[5] active against throat cancer,[6] stress[7] and improves learning and memory.[8] T. cordifolia has reported non-toxic in an acute toxicity study.[8] Recently, it has been extensively studied and reported to have potent anti-oxidant activity.[9] As anti-oxidants are known to prevent/protect neurodegeneration,[10] the present study was undertaken to evaluate the anti-Parkinson's activity of T. cordifolia ethanol extract (TCEE). Parkinsonism was induced by stereotactic injection of 6-hydroxy dopamine (6-OHDA) into the striatum of the brain. 6-OHDA is one of the most widely used models of PD in rats; it produces neuronal degeneration and enhances oxidative stress due to dopaminergic toxicity.[11]
Materials and Methods
Preparation of Extract
T. cordifolia was collected in the month of July, 2009 from the local areas of Coimbatore District, Tamil Nadu and was authenticated by Dr. S Rajan, Field Botanist, Survey of Medicinal Plants and Collection Unit, Central Council for Research in Homoeopathy, Department of AYUSH, The Nilgiris, Tamil Nadu. A voucher specimen has been deposited at the herbarium of the JSS College of Pharmacy, Ooty (JSSCP/Ph.Cog/137). Aerial parts of the plant were chopped and dried in the shade. Dried plant material was subjected to percolation with 70% ethanol and the yield was 17% w/w.
High Performance Thin Layer Chromatography Standardization
Following preliminary phytochemical analysis, TCEE was subjected to standardization. Standardization was carried out by HPTLC method using berberine as a reference standard according to the method described earlier.[12] The amount of berberine present was determined using the calibration curve plotted between concentration and area of standard.
Experimentation
Male Wistar rats weighing 180-220 g were used in this study. The experimentation on animals was approved on 19th September 2009 by the Institutional Animal Ethical Committee under the regulation of Committee for the Purpose of Control and Supervision of Experiments on Animals, New Delhi, India (JSSCP/IAEC/M.Pharm/PH.Cology/09/2009-10). Animals were divided into five groups of 6 in each. Group I served as sham operated, received normal saline (10 ml/kg, p.o.), Groups II-V were induced with Parkinsonism and assigned as follows: group II served as a negative control, received normal saline (10 ml/kg, p.o), Group III served as a positive control, received L-DOPA (6 mg/kg, p.o.) and Groups IV and V served as TCEE treated, received 200 and 400 mg/kg, p.o. of TCEE respectively. All the animals were treated with TCEE or vehicle once daily for 30 days.
Experimental Parkinsonism was induced by stereotactic surgery using 6-OHDA at a dose of 8 μg in a single intracerebral injection using following co-ordinates: AP −2.5 mm; Lat +2.0 mm; and Dev −8.5 mm by a method described earlier.[13] Desipramine (25 mg/kg, i.p.) was injected 30 min before surgery, to protect noradrenaline terminals from the effects of 6-OHDA. 3 weeks following surgery, animals were treated with L-DOPA or TCEE according to their respective group for a period of 30 days.
Evaluation of Locomotor Activity
The major clinical symptom of PD is muscle rigidity. This was evaluated in an animal model by rotarod and catatonia methods. Rotarod test was carried out similar to a reported method.[14] Briefly the apparatus consist of a 70 cm long rod with diameter 3 cm placed at a height of 50 cm and divided into four sections. Five trials were taken before the main reading to all the groups by adjusting the rate of rotation at 30 rpm. Catatonia was assessed using a method reported earlier.[15] Briefly the animals were placed with their forepaws on a wooden box on height 9 cm and the time spent without deliberate move to step down was determined. An average of three trials was taken with each trail commencement up to maximum 30 s.
Biochemical Estimations
At the end to 30 days treatment with TCEE, animals were sacrificed using lethal dose of anesthesia and brains were extracted for the biochemical estimations listed below.
Dopamine Measurement by High Performance Liquid Chromatography
Dissected brain tissue were immediately frozen on dry ice and stored at −80°C. Tissues were sonicated and centrifuged in 0.1 M perchloric acid (about 100 μl/mg of tissue). The supernatant fluid was collected to measure dopamine levels by HPLC according to the method described earlier.[16] Concentration of dopamine was expressed as ng/mg of protein and protein concentration of tissue homogenates was measured by Lowry's method.
Evaluation of Oxidative Degradation
Brain homogenate was used to measure oxidative degradation in terms of lipid peroxidation according to the procedure described earlier.[17]
Isolation of Mitochondrial Fractions and Complex I Assay
Brain tissue was homogenized in a Dounce tissue grinder (Wheaton, Millville, NJ, USA) in mitochondrial isolation buffer (70 mM sucrose, 210 mM mannitol, 5 mM Tris HCl, 1 mM ethylenediaminetetraacetic acid; pH: 7.4) and suspensions were centrifuged at 800 × g at 4°C for 10 min. The supernatant fluids were centrifuged at 13,000 × g at 4°C for 10 min and the pellets were washed with mitochondrial isolation buffer and centrifuged at 13,000 × g at 4°C for 10 min to obtain the crude mitochondrial fraction. Nicotinamide adenine dinucleotides: ubiquinone oxidoreducase (complex I) activity was measured in the SN as described earlier.[18]
Localization of Iron in SN (Brain Iron Asymmetry Ratio)
Localization of iron in SN was carried out according to Perl's diaminobenzidine (DAB) method[19] with slight modification. The isolated and partially frozen brains were cut horizontally to get 30-40 μm sections on a vibratome and then mounted on a glass slide. The sections were immersed in potassium ferrocyanide (2%) and hydrochloric acid (2%) for 30 min at room temperature and then rinsed with deionized water for 5 min. The Perl's reaction was intensified by placing the tissue in DAB (0.5%) in cold phosphate buffer (pH 7.4) for 15 min. Hydrogen peroxide (1%) was added as 2 ml for every 200 ml of DAB solution. The sections remained in the solution for 25 min. The slides of the section were made using a motic microscope (Motic images plus 2.0), under the magnification of ×40 equipped with a camera. Perl's stained sections were analyzed by densitometry with an IBM AT computer using Jandel scientific image analysis software (JAWA™) (Corte Madera, CA) with a grey scale range of 256 grey levels. Iron asymmetry ratio was measured by taking the ratio of ipsilateral densitometry value and contralateral densitometry value.
Statistical Analysis
Statistical analysis is performed by one-way ANOVA followed by Bonferroni multiple comparisons test, using the statistical package GraphPad Prism 5.0 5.0 (La Jolla, CA) version. The significance of difference between and within various groups was determined. Differences were considered to be significant when P < 0.01.
Results
Preliminary phytochemical analyzes revealed the presence of alkaloids, glycosides, saponins, tannins, flavonoids, steroids, carbohydrates and proteins while quinones were absent. Following preliminary phytochemical analysis TCEE was subjected for standardization using HPTLC. Standardization of herbal extracts is required in terms of quality and quantity before subjecting to any pharmacological evaluation. TCEE was standardized by HPTLC using berberine, one of the major marker compounds present in T. cordifolia. The amount of berberine present in TCEE was 0.28% w/w which reflects the quality of extract.
Rotarod and catalepsy tests were the common locomotor tests and give a brief view about the neuronal loss. Treatment with 6-OHDA led to a significant (P < 0.001) decrease in time of fall in rotarod test and significantly (P < 0.001) increased catalepsy score in catatonia test. Skeletal muscle co-ordination following treatment with TCEE and L-DOPA were depicted in Table 1. The results shown that animals treated with TCEE at 200 (P < 0.01) and 400 (P < 0.001) mg/kg significantly increased the time of fall when compared with 6-OHDA group. The degree of catalepsy following treatment with TCEE and L-DOPA were given in Table 1. The results indicate that animal treated with TCEE at 200 (P < 0.01) and 400 (P < 0.001) significantly reduced the degree of catalepsy when compared with 6-OHDA group.
Table 1.
Effect of TCEE treatment for a period of 30 days on rotarod performance and muscle co-ordination test

In the present study, significant (P < 0.001) decrease in brain dopamine levels was observed when compared with sham control group. The level of dopamine in the brain following treatment with L-DOPA and TCEE was given in Figure 1. The dopamine concentrations in the brain region was regained dose-dependently and significantly (P < 0.01 for 200 and P < 0.001 for 400 mg/kg) following treatment with TCEE when compared with negative control group.
Figure 1.
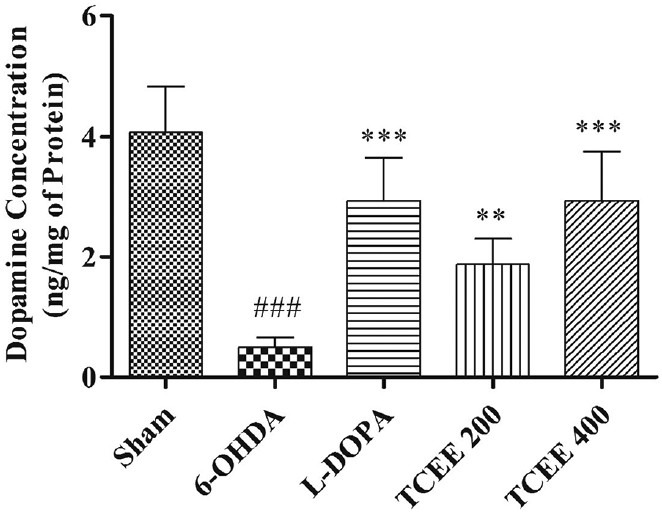
Effect of Tinospora cordifolia ethanol extract treatment for a period of 30 days on brain dopamine levels. Graph represented as mean ± standard deviation (n = 6). ##P < 0.001 when compared with sham operated; ***P < 0.001, **P < 0.01 when compared with 6-OHDA. L-DOPA: Levodopa; 6-OHDA: 6-hydroxy dopamine; TCEE: T. cordifolia ethanol extract
Anti-oxidants levels and oxidative degradation were significantly altered in 6-OHDA group when compared with sham operated group. Animals treated with TCEE for a period of 30 days brought back the levels of lipid peroxidation [Figure 2]. The present study also shows that complex I activity was significantly (P < 0.01) reduced for 6-OHDA treated group when compared with sham operated group [Figure 3]. Treatment with TCEE showed equipotent and significant (P < 0.01) increase in the complex I activity when compared with negative control group.
Figure 2.
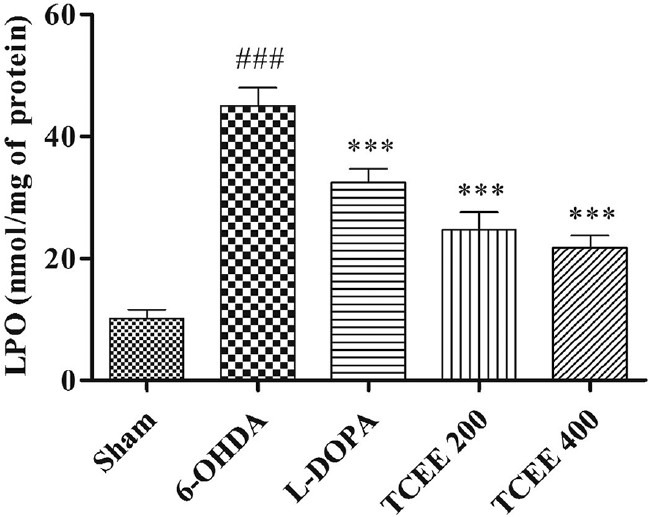
Effect of Tinospora cordifolia ethanol extract treatment for a period of 30 days on lipid peroxidation. Graph represented as mean ± standard deviation (n = 6). ##P < 0.001 when compared with sham operated; ***P < 0.001, **P < 0.01 when compared with 6-OHDA. L-DOPA: Levodopa; 6-OHDA: 6-hydroxy dopamine; TCEE: T. cordifolia ethanol extract
Figure 3.
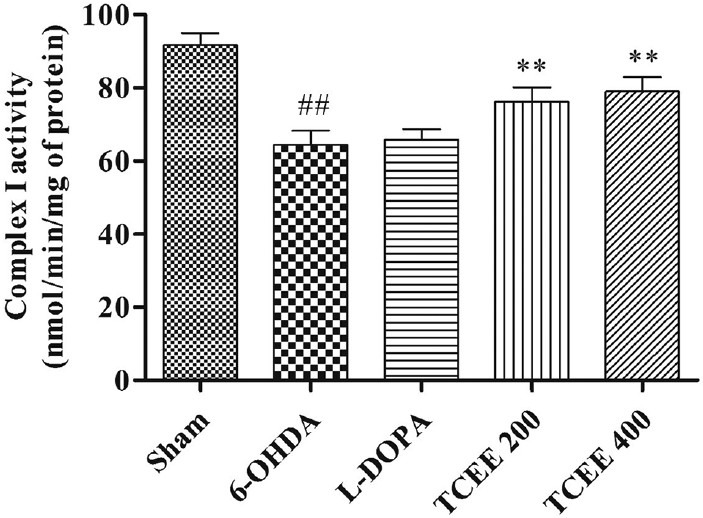
Effect of Tinospora cordifolia ethanol extract treatment for a period of 30 days on mitochondrial complex I activity. Graph represented as mean ± standard deviation (n = 6). ##P < 0.01 when compared with sham operated; **P < 0.01 when compared with 6-OHDA. L-DOPA: Levodopa; 6-OHDA: 6-hydroxy dopamine; TCEE: T. cordifolia ethanol extract
SN iron localization studies clearly indicate that the iron deposition or iron asymmetry ratio for 6-OHDA was significantly (P < 0.001) increased when compared with sham control group. L-DOPA treated groups showed significantly (P < 0.01) increase in iron asymmetry ratio when compared with negative control group. TCEE treated groups dose-dependently and significantly reduced the iron deposition after 30 days treatment when compared with negative control group [Figures 4 and 5].
Figure 4.
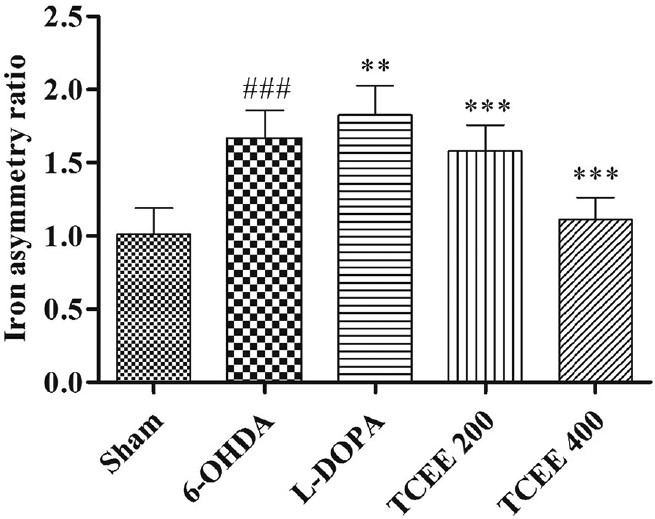
Effect of Tinospora cordifolia ethanol extract treatment for a period of 30 days on brain iron asymmetry ratio. Graph represented as mean ± standard deviation (n = 6). ###P < 0.001 when compared with sham operated; ***P < 0.001, **P < 0.01 when compared with 6-OHDA. L-DOPA: Levodopa; 6-OHDA: 6-hydroxy dopamine; TCEE: T. cordifolia ethanol extract
Figure 5.
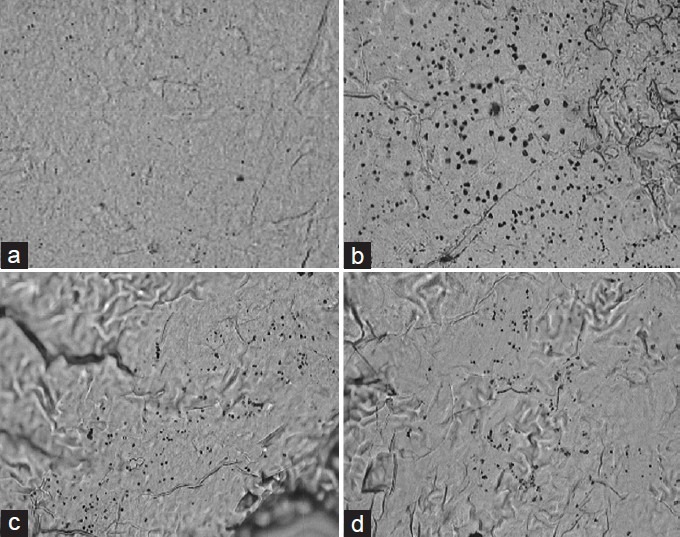
Effect of Tinospora cordifolia ethanol extract (TCEE) on the iron degeneration in substantia nigra. (a) Sham operated, (b) 6-hydroxy dopamine, (c) Levodopa, (d) TCEE (400 mg/kg)
Discussion
PD implicated to neuronal death in SN with increased oxidative stress and mitochondrial respiratory failure.[20] PD treatment has improved over the past quarter of a century and new therapies are emerging. Although treatment with L-DOPA, the gold standard anti-Parkinson's drug, results in symptomatic improvement, mortality rates have remained unchanged[2] and severe side-effects of this therapy appear in about 5 years. In search of a new effective drug in Parkinson's therapy the interest on natural products, especially plants have been growing. Recently, several plants, with different pharmacological properties have been tested for PD treatment.[21]
Our result on the effect of 6-OHDA on locomotor activity in animals is consistent with previous reports.[22] In the present study, rotarod and catalepsy tests were employed for the assessment of locomotor activity. An increase in the fall of time and decrease in catalepsy score following TCEE treatment was observed. In addition, complex I assay and iron degeneration studies revealed that this effect may have been due to reversal of pathological changes following treatment.
Depletion of dopamine levels was the major hallmark as well as a biomarker in the diagnosis of PD. It is believed that intracerebral administration of 6-OHDA intoxicate the dopaminergic neurons in the SN, the possible mechanism that causes PD. Loss of dopaminergic neurons leads to decrease in the levels of dopamine and its metabolites. The increased levels of dopamine by the treatment of TCEE might be due to decreased metabolism of dopamine or due to increased biosynthesis of dopamine by the dopaminergic neurons present in SN. The increased dopamine levels by TCEE is further supported by the anti-stress and anti-depression activity of T. cordifolia root extract in which dopamine levels were normalized after treatment.[23] Based upon our results, it is best evidenced that TCEE could reduce dopaminergic neurodegeneration by reducing iron induced damage.
Reduction in GSH might impair H2O2 clearance and promote OH radical's formation and produces oxidative stress. All anti-oxidant defense mechanisms are related and disturbance on one might damage the balance all. The depletion in GSH content and enhancement of LPO might lead to the degeneration of nigrostriatal neurons and consequently leads to reduction in the content of catecholamine.[10] Animals treated with TCEE for a period of 30 days brought back the oxidative degradation. This might be due to the presence of flavonoids and tannins present in TCEE that are likely responsible for free radical scavenging activity.[24] The anti-oxidant activity was consistent with the previous studies on T. cordifolia.[25]
Altogether we can conclude that the observed results were consistent and pharmacologically suitable results showing the neuroprotective activity of TCEE in experimental PD. The mitochondrial activity retained by TCEE showed a promising way for the treatment of clinical PD. Further pharmacological and clinical investigations are needed to implement it for clinical use.
Acknowledgments
The authors would like to thank All Indian Council for Technical Education (New Delhi, India) under Research Promotion Scheme (8023/BOR/RIP/RPS-198/2008-09) for their support.
Footnotes
Source of Support: This work was supported by All Indian Council for Technical Education (New Delhi, India) under Research Promotion Scheme (8023/BOR/RIP/RPS.198/2008.09)
Conflict of Interest: No
References
- 1.de Lau LM, Breteler MM. Epidemiology of Parkinson's disease. Lancet Neurol. 2006;5:525–35. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- 2.Simpkins N, Jankovic J. Neuroprotection in Parkinson disease. Arch Intern Med. 2003;163:1650–4. doi: 10.1001/archinte.163.14.1650. [DOI] [PubMed] [Google Scholar]
- 3.Sarma DN, Sameksha K, Khosa RL. Alkaloids from Tinospora cordifolia Miers. J Pharm Sci Res. 2009;1:26–7. [Google Scholar]
- 4.Kapur P, Pereira BM, Wuttke W, Jarry H. Androgenic action of Tinospora cordifolia ethanolic extract in prostate cancer cell line LNCaP. Phytomedicine. 2009;16:679–82. doi: 10.1016/j.phymed.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Gulati OD, Pandey DC. Anti-inflammatory activity of Tinospora cordifolia. Rheumatism. 1982;17:76–83. [Google Scholar]
- 6.Chauhan K. Successful treatment of throat cancer with ayurvedic drugs. Suchitra Ayurved. 1995;47:840–2. [Google Scholar]
- 7.Sarma DN, Khosa RL, Chansauria JPN, Sahai M. Antistress activity of Tinospora cordfolia and Centella asiutica extracts. Phytother Res. 1996;10:181–3. [Google Scholar]
- 8.Ashutosh A, Malini S, Bairy KL, Muddana SR. Effect of Tinospora cordifolia on learning and memory in normal and memory deficit rats. Indian J Pharmacol. 2002;34:339–49. [Google Scholar]
- 9.Ramya P, Lakshmidevi N. Studies on anti-oxidant activity of Tinospora cordifolia (Miers.) leaves using in vitro models. J Am Sci. 2010;6:736–43. [Google Scholar]
- 10.Prasad KN, Cole WC, Kumar B. Multiple antioxidants in the prevention and treatment of Parkinson's disease. J Am Coll Nutr. 1999;18:413–23. doi: 10.1080/07315724.1999.10718878. [DOI] [PubMed] [Google Scholar]
- 11.Ahmad M, Saleem S, Ahmad AS, Ansari MA, Yousuf S, Hoda MN, et al. Neuroprotective effects of Withania somnifera on 6-hydroxydopamine induced Parkinsonism in rats. Hum Exp Toxicol. 2005;24:137–47. doi: 10.1191/0960327105ht509oa. [DOI] [PubMed] [Google Scholar]
- 12.Puratchimani V, Jha S. HPTLC standardization of Tinospora cordifolia using tinosporaside. Indian J Pharm Sci. 2007;69:578–81. [Google Scholar]
- 13.Zhen X, Torres C, Cai G, Friedman E. Inhibition of protein tyrosine/mitogen-activated protein kinase phosphatase activity is associated with D2 dopamine receptor supersensitivity in a rat model of Parkinson's disease. Mol Pharmacol. 2002;62:1356–63. doi: 10.1124/mol.62.6.1356. [DOI] [PubMed] [Google Scholar]
- 14.Krishnakumar A, Abraham PM, Paul J, Paulose CS. Down-regulation of cerebellar 5-HT (2C) receptors in pilocarpine-induced epilepsy in rats: Therapeutic role of Bacopa monnieri extract. J Neurol Sci. 2009;284:124–8. doi: 10.1016/j.jns.2009.04.032. [DOI] [PubMed] [Google Scholar]
- 15.Zazpe A, Artaiz I, Innerárity A, Del Olmo E, Castro E, Labeaga L, et al. In vitro and in vivo characterization of F-97013-GD, a partial 5-HT1A agonist with antipsychotic- and antiparkinsonian-like properties. Neuropharmacology. 2006;51:129–40. doi: 10.1016/j.neuropharm.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Cleren C, Calingasan NY, Chen J, Beal MF. Celastrol protects against MPTP- and 3-nitropropionic acid-induced neurotoxicity. J Neurochem. 2005;94:995–1004. doi: 10.1111/j.1471-4159.2005.03253.x. [DOI] [PubMed] [Google Scholar]
- 17.Kraus RL, Pasieczny R, Lariosa-Willingham K, Turner MS, Jiang A, Trauger JW. Antioxidant properties of minocycline: Neuroprotection in an oxidative stress assay and direct radical-scavenging activity. J Neurochem. 2005;94:819–27. doi: 10.1111/j.1471-4159.2005.03219.x. [DOI] [PubMed] [Google Scholar]
- 18.Tieu K, Perier C, Caspersen C, Teismann P, Wu DC, Yan SD, et al. D-beta-hydroxybutyrate rescues mitochondrial respiration and mitigates features of Parkinson disease. J Clin Invest. 2003;112:892–901. doi: 10.1172/JCI18797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall S, Rutledge JN, Schallert T. MRI, brain iron and experimental Parkinson's disease. J Neurol Sci. 1992;113:198–208. doi: 10.1016/0022-510x(92)90247-i. [DOI] [PubMed] [Google Scholar]
- 20.Eriksen JL, Petrucelli L. Parkinson's disease-molecular mechanisms of disease. Drug Discov Today Dis Mech. 2004;1:399–405. [Google Scholar]
- 21.Morais LC, Barbosa JM, Almeida RN. Plants and bioactive compounds for the treatment of Parkinson's disease. Brazilian Arch Sci Fitomed. 2003;1:127–32. [Google Scholar]
- 22.Heuer A, Smith GA, Lelos MJ, Lane EL, Dunnett SB. Unilateral nigrostriatal 6-hydroxydopamine lesions in mice I: Motor impairments identify extent of dopamine depletion at three different lesion sites. Behav Brain Res. 2012;228:30–43. doi: 10.1016/j.bbr.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 23.Mutalik M, Mutalik M. Tinospora cordifolia: Role of depression, cognition, and memory. Aust J Medical Herbalism. 2011;23:168–73. [Google Scholar]
- 24.Sivakumar V, Rajan MS, Riyazullah MS. Preliminary phytochemical screening and evaluation of free radical scavenging activity of Tinospora cordifolia. Int J Pharm Pharm Sci. 2010;2:186–8. [Google Scholar]
- 25.Agrawal SS, Naqvi S, Gupta SK, Srivastava S. Prevention and management of diabetic retinopathy in STZ diabetic rats by Tinospora cordifolia and its molecular mechanisms. Food Chem Toxicol. 2012;50:3126–32. doi: 10.1016/j.fct.2012.05.057. [DOI] [PubMed] [Google Scholar]


