Abstract
Aim:
Two recurrent cases of severe acute liver injury attributed to the use of a wild germander decoction, prepared with some variation in traditional method has been reported. The aim of the present study was to correlate the hepatotoxic effect observed in patients who consumed germander decoction with teucrin A levels. Antioxidant properties were analyzed to assess any possible differences between the decoction used traditionally by the family (without negative consequences) and the decoction taken by the patients.
Materials and Methods:
Different types of germander decoctions were prepared in the laboratory by simulating the same conditions for preparing the decoction by the patients and their family members. The levels of teucrin A, the polyphenols and the antioxidant power were determined. One-way analysis of variance was used to test for differences between the groups.
Results and Conclusions:
The extract consumed by the patients had higher concentration of teucrin A, lower antioxidant activity and lower content of polyphenols compared with the traditional decoction, revealing an inverse relationship between teucrin A content and antioxidant capacity. These case reports emphasize that more information is needed on the safety and quality of these natural products.
KEY WORDS: Antioxidant activity, germander, high-performance liquid chromatography analysis, teucrin A, Teucrium chamaedrys L.
Introduction
Herbal products can be potent or lethal if used improperly.[1,2] The plant can have both beneficial and toxic effects in relation to the many constituents or to the dose utilized.[3,4] Teucrium chamaedrys L. (Lamiaceae), known as germander, is a perennial herb (30-60 cm) native to Europe and South West Asia, blooming in late spring and summer. The flowering aerial parts are traditionally used as a folk medicine for dyspepsia, anorexia, nasal catarrh, chronic bronchitis, gout, rheumatoid arthritis, fever, uterine infections and to promote wound healing, more recently germander is used to reduce body weight.[5,6] Teucrium species are rich in neo-clerodane diterpenoids, which are accepted as chemotaxonomic markers of these species. In addition, aerial parts of T. chamaedrys contain numerous other compound classes including saponins, glycosides and flavonoids.[7,8]
Capsules containing germander, either alone or combined, are marketed as a weight-control supplements and has been associated with hepatotoxicity as reported about thirty cases from France.[9] Toxicological studies showed that one of the major furano neo-clerodane diterpenoids, teucrin A, was implicated in the hepatotoxicity of germander.[10]
A possible mechanism of the germander induced hepatotoxicity is: activation of cytochrome P450 (mainly 3A) into reactive metabolites (probably epoxides). The reactive electrophilic metabolites stimulate apoptosis by decreasing thiols (i.e. by glutathione conjugate formation) and increasing intracellular calcium.[11] In a study by Druckova et al.[12] observed that metabolic activation of teucrin A in rats results in extensive damage of numerous hepatic proteins (involved in lipid, aminoacid and drug metabolism, mitochondrial and peroxisomal enzymes) by covalent modification. The formation of reactive metabolites may also lead to immune reactions.[13] Therefore, it is likely that germander-induced hepatotoxicity may be due to direct and secondary immune reactions.
Despite its toxicity, the use of germander preparations still continues. Recent studies have highlighted the antioxidant activity of T. chamaedrys, which has shown significant free radical scavenger activity in vitro, thus suggesting its possible use as a natural antioxidant and consequently as protection against damage from free radicals.[14,15,16]
We report two cases of a husband and wife with recurrent severe acute liver injury attributed to the ingestion of a wild germander decoction. The preparation was taken for two consecutive periods (2 months and 1 month respectively) in 1 year, as herbal medicine with detoxifying properties, in accordance with a traditional family custom which had previously produced no adverse consequences. History suggested that the patients had introduced two variations with respect to the family tradition: The germander harvest area and the preparation time of the decoction. Hence, a study to identify whether these variations were responsible for the hepatotoxicity was undertaken.
Materials and Methods
The present case is about a 65-year-old male patient and a 64-year-old women patient were hospitalized in the S. Maria alle Scotte Hospital in Siena because of nausea, vomiting, diarrhea, asthenia and with severe acute liver failure. The serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and γ-glutamyltranspeptidase (γ-GT), were 5 times higher than the normal value (N < 41 IU/l).
Hepatitis serological markers, including anti-hepatitis A immunoglobulin M (IgM), hepatitis B surface antigen, anti-hepatitis B IgM, anti-hepatitis C antibodies, anti-hepatitis E immunoglobulin M and Epstein-Barr virus IgM, were negative.
Ultrasound examination was performed and steatosis and hepatomegaly were recognized. The patients had no other cause of liver injury and they denied any recent ingestion of known hepatotoxins, drugs, alcohol, mushrooms or substances of abuse.
After discharge from hospital, the patients followed a dietary regime provided by the hospital, that excluded the administration of drugs. They were checked monthly and 2 months later the hepatic markers had returned to the normal values. At 6 months after the first medical emergency, the same patients were hospitalized again with the above reported symptoms and clinical data. On accurate investigation the medical team discovered that the cause of the liver damage could be correlated to the use of the germander preparation. The patients were monitored and the clinical course was favorable after dechallenge. After 2 months the markers for liver damage ALT, AST and γ-GT had normalized.
To evaluate whether the variations in germander harvest area and preparation of the decoction were responsible for hepatoxicity, four germander decoctions were prepared in the laboratory, using two different samples of germander and following the traditional method used by the patients’ family and the method used by the patients themselves. To verify if the germander decoction was responsible for the hepatotoxic effect observed in these patients, the levels of teucrin A were measured by high-performance liquid chromatography (HPLC) method. In addition, the antioxidant activity was analyzed and the total phenolic content was determined, to assess any possible differences between the decoction used traditionally by the family and the decoction taken by the patients.
Plant Collection
Two different samples of aerial flowering parts of T. chamaedrys L. (Lamiaceae) were collected during the blooming period (June). The first sample was collected near Grosseto (Tuscany), in a pinewood not far from the sea, the same harvesting area used by the patients, the second one was collected in the hills around Siena (Tuscany), in a wood of holm oaks, in the same area chosen for harvesting by the female patient's mother. For each of these samples of T. chamaedrys four different lots in the same habitat were collected for each sample. The identification of both samples of germander was performed and two voucher specimens (Siena - 687411, Siena - 687511) were deposited at the Herbarium, Department of Life Sciences, University of Siena.
Preparation of Extracts
After drying, the flowering tops were ground with a knife-mill and left in boiling water for 5 min to produce a decoction (1 g/300 ml). It was then filtered using two different procedures: (1) Filtering after 10 min, in accordance with the family tradition; (2) filtering after 12 h as in cases of intoxication.
Thus four extracts were prepared:
Extract A: by filtering, after 10 min, the germander collected in a wood of holm oaks (reproduced according to family tradition);
Extract B: by filtering, after 12 h, the germander collected in a wood of holm oaks;
Extract C: by filtering, after 10 min, the germander collected in a pinewood;
Extract D: By filtering, after 12 h, the germander collected in a pinewood (reproduced according to the method used by the patients).
HPLC Analysis
To evaluate the teucrin A content a reversed phase HPLC analysis was performed using a method described by Bosisio et al.[17]
Teucrin A (purity 99%) used as a standard was purchased from Phytolab GmbH and Co.
Extracts (A-D) were dissolved in water at concentrations of 2.5 mg/ml and injected in triplicate. The HPLC analytical procedure was validated (in terms of recovery, precision and accuracy) and its intra- and inter-day performance was established.
Antioxidant Activity
2,2-diphenylpicrylhydrazyl (DPPH) assay
The scavenging activity of the DPPH radical (Sigma-Aldrich) was measured in triplicate as described by Ramadan et al.[18]
The capacity to scavenge the DPPH radical was expressed as scavenging effect (I %) at 0.5 mg/ml, the highest concentration tested.
Ferric reducing/antioxidant power (FRAP) test
The FRAP test assay was used to measure the total antioxidant power.[19] The results of triplicate determinations were expressed as μmol of FRAP (the FRAP value)/gram of extract.
Determination of polyphenol content
The amount of the total phenolics in the Teucrium extracts was determined, in triplicate, with Folin-Ciocalteu reagent according to Koski et al.[20] using Gallic acid (Sigma-Aldrich) as a standard. The results were expressed in milligrams of Gallic acid equivalent (GAE)/gram of extract (mg GAE/gram of extract).
Statistical Analysis
Statistical analysis was performed using SPSS version 17 for Windows software package (SPSS Inc, Chicago, IL, USA). Results are expressed as the mean ± standard error of twelve determinations (performed in triplicate on each of the four lots). Levene's test was used to assess the homogeneity of the variance of the groups. One-way analysis of variance was utilized to evaluate differences among the groups. The Tukey multiple comparison test was used because the variances between the groups were always homogeneous. P <0.05 were considered to be statistically significant.
Results
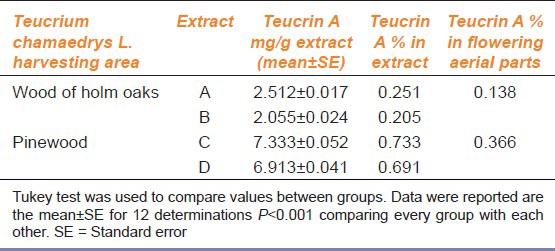
Extracts C and D showed statically significant high content of teucrin A when compared with extracts A and B (P < 0.001) [Table 1]. Moreover, there was an inverse relationship between the content of teucrin A and the extraction time (P < 0.001 A vs. B; C vs. D). The concentration of teucrin A in the flowering aerial parts of the T. chamaedrys collected in the hills around Siena, in a wood of holm oaks, was 0.138% as reported in literature: 0.13% referred to aerial parts;[21] whereas the content of teucrin A in the T. chamaedrys collected in the pinewood was about 3 times higher (0.366%).
Table 1.
Content of teucrin A in extracts and in flowering aerial parts obtained by Teucrium chamaedrys L. harvested in a wood of holm oaks and in a pinewood
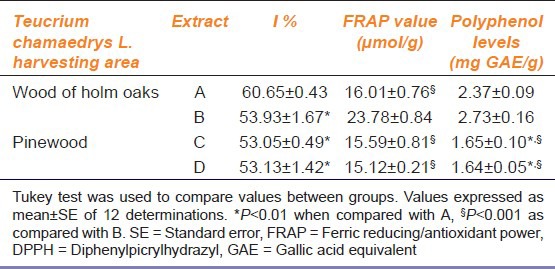
Data relative to in vitro antioxidant analysis are reported in Table 2. The antioxidant activity of extract A was higher than D (P < 0.01 in DPPH) as also the contents of polyphenols (P < 0.01). Consequently extract A, derived from the T. chamaedrys collected in a wood of holm oaks and prepared according to family tradition, had high antioxidant properties, while extract D, derived from the T. chamaedrys collected in the pinewood and prepared as the patients themselves had done, had lower antioxidant activity.
Table 2.
Inhibition percentage (I %) values at 0.5 mg/ml obtained with DPPH test, FRAP values and polyphenol levels in Teucrium chamaedrys L. extracts
A positive correlation was observed between antioxidant activity of Teucrium extracts, evaluated with FRAP tests and polyphenol content. A linear function [Figure 1] was adopted as model of regression (y = 5.61 + 5.728x; r = 0.77 with P < 0.001). In addition, an inverse relationship between the teucrin A content of Teucrium extracts and antioxidant capacity was observed and a power function [Figure 2] was adopted as model of regression because, unlike the linear model, this function assigns positive values of teucrin A for all values of polyphenol content. The co-efficient of determination (R2) was used to evaluate the goodness of fit of model (R2 = 0.834 with P < 0.001).
Figure 1.
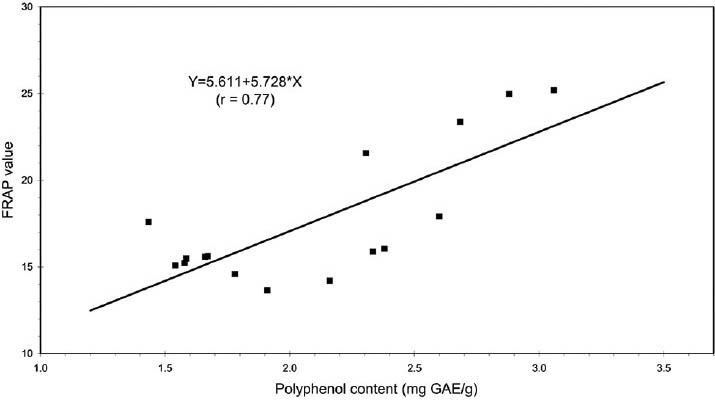
Correlation between antioxidant activity (ferric reducing/ antioxidant power) and polyphenol content of Teucrium extracts. A linear function was adopted as model of regression
Figure 2.
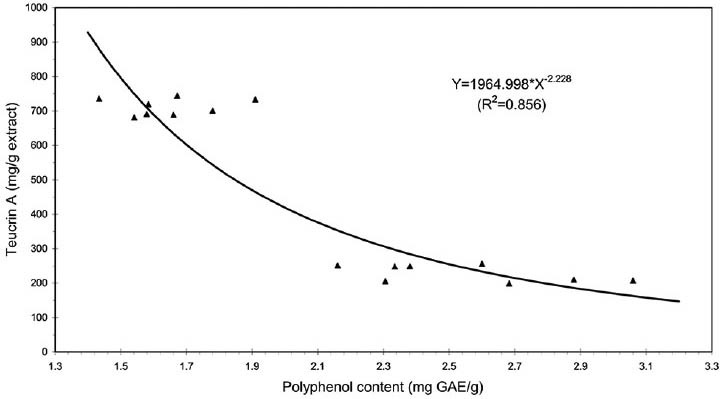
Relationship between levels of teucrin A and polyphenol content of Teucrium extracts. A power function was adopted as model of regression
Discussion
A causal relationship between T. chamaedrys and these severe cases of acute liver failure is supported by the exclusion of viral, autoimmune, or biliary diseases and the time link between the second administration of the herbal preparation and onset of hepatitis; in effect germander readministration was followed by rehospitalization.
It is known that hepatotoxicity generally occurs after approximately 2 months of ingestion of the oral preparation, frequently decoction and consists in a non-specific hepatitis, which usually has a benign course. Typical features include anorexia, nausea, abdominal pain and jaundice associated with a marked rise in serum amino-transferases; recovery usually occurs within 6 weeks-6 months once ingestion of the germander decoction has stopped.[13]
In these case reports, we observed analogies with case reports in literature: the preparation was taken orally over a long period (for 2 months the 1st time and 1 month the 2nd time), the patients presented the same symptoms described in the literature, except jaundice Larrey et al.[22] have reported “a marked increase in serum aminotransferase levels 3-18 weeks after germander administration.”
The germander decoction was consumed as a detoxifying drink for many years, by the patient's mother without any adverse consequences This caused the patients to believe that the herbal preparations was harmless of the harmlessness of this herbal preparation. There are, however, two important differences between family tradition and the use by these two patients: plant collection and plant extraction. First, the aerial flowering parts of T. chamaedrys L. had been collected in a different habitat with respect to the woman's mother: in a pinewood not far from the sea, instead of in a wood of holm oaks on a hill. Secondly, the patients had not followed the family method of preparing the decoction; traditionally, the family had made the decoction using boiling water for 5 min and then filtered after 10 min, while in this case, the users left the decoction overnight and filtered the next morning, thus increasing the contact time between plant and water.
Using HPLC analysis we demonstrated that the different extractive methods (varied length of contact) is not decisive for toxic effect, since an inverse correlation occurs between the content of teucrin A and the extraction time. Undoubtedly, the different climatic/ecological conditions in which the plants grow are crucial and may affect the concentration of the chemical compounds and consequently the pharmacological/toxicological activities.
These differences influence also the antioxidant capacity; the decoction used by the patients (extract D) not only has more hepatotoxic substance (teucrin A) but also had lower antioxidant activity and the lower polyphenol content, which probably results in a lower level of protection. The correlation between the antioxidant activity and the hepatoprotective effect has been demonstrated for other species of Teucrium.[23]
This study showed that the different environmental or geographic location of the plant harvest influenced the content of phytoconstituents in decoction and consequently the pharmacological and toxicological properties. However, the non-traditional method of preparation had little effect on the toxic properties of the decoction.
Conclusion
Different environmental or geographic locations of the plant harvest can influence the content of phytoconstituents and consequently the pharmacological and toxicological properties of traditional herbal medicines. Further investigations are required to identify the precise toxic molecules in herbal medicines to predict and ensure the safety and quality of the plant.
Acknowledgments
This study was supported by University of Siena Grant.
Footnotes
Source of Support: Nil
Conflict of Interest: No
References
- 1.García-Cortés M, Borraz Y, Lucena MI, Peláez G, Salmerón J, Diago M, et al. Liver injury induced by “natural remedies”: An analysis of cases submitted to the Spanish Liver Toxicity Registry. Rev Esp Enferm Dig. 2008;100:688–95. doi: 10.4321/s1130-01082008001100004. [DOI] [PubMed] [Google Scholar]
- 2.Chitturi S, Farrell GC. Hepatotoxic slimming aids and other herbal hepatotoxins. J Gastroenterol Hepatol. 2008;23:366–73. doi: 10.1111/j.1440-1746.2008.05310.x. [DOI] [PubMed] [Google Scholar]
- 3.O’Sullivan HM, Lum K. The poisoning of ’awa: The non-traditional use of an ancient remedy. Pac Health Dialog. 2004;11:211–5. [PubMed] [Google Scholar]
- 4.Galati G, O’Brien PJ. Potential toxicity of flavonoids and other dietary phenolics: Significance for their chemopreventive and anticancer properties. Free Radic Biol Med. 2004;37:287–303. doi: 10.1016/j.freeradbiomed.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 5.Rader JI, Delmonte P, Trucksess MW. Recent studies on selected botanical dietary supplement ingredients. Anal Bioanal Chem. 2007;389:27–35. doi: 10.1007/s00216-007-1254-7. [DOI] [PubMed] [Google Scholar]
- 6.Herrera S, Bruguera M. Hepatotoxicity induced by herbs and medicines used to induce weight loss. Gastroenterol Hepatol. 2008;31:447–53. doi: 10.1157/13125592. [DOI] [PubMed] [Google Scholar]
- 7.Bedir E, Manyam R, Khan IA. Neo-clerodane diterpenoids and phenylethanoid glycosides from Teucrium chamaedrys L. Phytochemistry. 2003;63:977–83. doi: 10.1016/s0031-9422(03)00378-9. [DOI] [PubMed] [Google Scholar]
- 8.Lin LZ, Harnly JM, Upton R. Comparison of the phenolic component profiles of skullcap (Scutellaria lateriflora) and germander (Teucrium canadense and T. chamaedrys), a potentially hepatotoxic adulterant. Phytochem Anal. 2009;20:298–306. doi: 10.1002/pca.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Berardinis V, Moulis C, Maurice M, Beaune P, Pessayre D, Pompon D, et al. Human microsomal epoxide hydrolase is the target of germander-induced autoantibodies on the surface of human hepatocytes. Mol Pharmacol. 2000;58:542–51. doi: 10.1124/mol.58.3.542. [DOI] [PubMed] [Google Scholar]
- 10.Zhou S, Koh HL, Gao Y, Gong ZY, Lee EJ. Herbal bioactivation: The good, the bad and the ugly. Life Sci. 2004;74:935–68. doi: 10.1016/j.lfs.2003.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen XW, Serag ES, Sneed KB, Zhou SF. Herbal bioactivation, molecular targets and the toxicity relevance. Chem Biol Interact. 2011;192:161–76. doi: 10.1016/j.cbi.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 12.Druckova A, Mernaugh RL, Ham AJ, Marnett LJ. Identification of the protein targets of the reactive metabolite of teucrin A in vivo in the rat. Chem Res Toxicol. 2007;20:1393–408. doi: 10.1021/tx7001405. [DOI] [PubMed] [Google Scholar]
- 13.Starakis I, Siagris D, Leonidou L, Mazokopakis E, Tsamandas A, Karatza C. Hepatitis caused by the herbal remedy Teucrium polium L. Eur J Gastroenterol Hepatol. 2006;18:681–3. doi: 10.1097/00042737-200606000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Kadifkova Panovska T, Kulevanova S, Stefova M. In vitro antioxidant activity of some Teucrium species (Lamiaceae) Acta Pharm. 2005;55:207–14. [PubMed] [Google Scholar]
- 15.Gursoy N, Tepe B. Determination of the antimicrobial and antioxidative properties and total phenolics of two “endemic” Lamiaceae species from Turkey: Ballota rotundifolia L. and Teucrium chamaedrys C. Koch. Plant Foods Hum Nutr. 2009;64:135–40. doi: 10.1007/s11130-009-0115-2. [DOI] [PubMed] [Google Scholar]
- 16.Pacifico S, D’Abrosca B, Pascarella MT, Letizia M, Uzzo P, Piscopo V, et al. Antioxidant efficacy of iridoid and phenylethanoid glycosides from the medicinal plant Teucrium chamaedris in cell-free systems. Bioorg Med Chem. 2009;17:6173–9. doi: 10.1016/j.bmc.2009.07.065. [DOI] [PubMed] [Google Scholar]
- 17.Bosisio E, Giavarini F, Dell’Agli M, Galli G, Galli CL. Analysis by high-performance liquid chromatography of teucrin A in beverages flavoured with an extract of Teucrium chamaedrys L. Food Addit Contam. 2004;21:407–14. doi: 10.1080/02652030410001670157. [DOI] [PubMed] [Google Scholar]
- 18.Ramadan MF, Kroh LW, Mörsel JT. Radical scavenging activity of black cumin (Nigella sativa L.), coriander (Coriandrum sativum L.), and niger (Guizotia abyssinica Cass.) crude seed oils and oil fractions. J Agric Food Chem. 2003;51:6961–9. doi: 10.1021/jf0346713. [DOI] [PubMed] [Google Scholar]
- 19.Nencini C, Menchiari A, Franchi GG, Micheli L. In vitro antioxidant activity of aged extracts of some Italian Allium species. Plant Foods Hum Nutr. 2011;66:11–6. doi: 10.1007/s11130-010-0204-2. [DOI] [PubMed] [Google Scholar]
- 20.Koski A, Psomiadou E, Tsimidou M, Hopia A, Kefalas P, Wähälä K, et al. Oxidative stability and minor constituents of virgin olive oil and cold-pressed rapeseed oil. Eur Food Res Technol. 2002;214:294–8. [Google Scholar]
- 21.De Vincenzi M, Maialetti F, Silano M. Constituents of aromatic plants: Teucrin A. Fitoterapia. 2003;74:746–9. doi: 10.1016/s0367-326x(03)00145-x. [DOI] [PubMed] [Google Scholar]
- 22.Larrey D, Vial T, Pauwels A, Castot A, Biour M, David M, et al. Hepatitis after germander (Teucrium chamaedrys) administration: Another instance of herbal medicine hepatotoxicity. Ann Intern Med. 1992;117:129–32. doi: 10.7326/0003-4819-117-2-129. [DOI] [PubMed] [Google Scholar]
- 23.Amini R, Yazdanparast R, Aghazadeh S, Ghaffari SH. Teucrium polium reversed the MCD diet-induced liver injury in rats. Hum Exp Toxicol. 2011;30:1303–12. doi: 10.1177/0960327110388961. [DOI] [PubMed] [Google Scholar]


