Abstract
Aim:
The aim of the study was to evaluate a novel 5 HT3 receptor antagonist (6g) on chronic stress induced changes in behavioural and brain oxidative stress parameter in mice. A complicated relationship exists among stressful stimuli, body's reaction to stress and the onset of clinical depression. Chronic unpredictable stressors can produce a situation similar to human depression, and such animal models can be used for the preclinical evaluation of antidepressants.
Materials and Methods:
In the present study, a novel and potential 5-HT3 receptor antagonist (4-benzylpiperazin-1-yl)(3-methoxyquinoxalin-2-yl) methanone (6g) with good Log P (3.08) value and pA2(7.5) values, synthesized in our laboratory was investigated to study the effects on chronic unpredictable mild stress (CUMS)-induced behavioural and biochemical alterations in mice. Mice were subjected to different stress paradigms daily for a period of 28 days to induce depressive-like behaviour.
Results:
The results showed that CUMS caused depression-like behaviour in mice, as indicated by the significant (P < 0.05) decrease in sucrose consumption and locomotor activity and increase in immobility the forced swim test. In addition, it was found that lipid peroxidation and nitrite levels were significantly (P < 0.05) increased, whereas glutathione levels, superoxide dismutase and catalase activities decreased in brain tissue of CUMS-treated mice. ‘6g’ (1 and 2 mg/kg, p.o., 21 days) and fluoxetine treatment (20 mg/kg, p.o., 21 days) significantly (P < 0.05) reversed the CUMS-induced behavioural (increased immobility period, reduced sucrose preference and decreased locomotor activity) and biochemical (increased lipid peroxidation; decreased glutathione levels, superoxide dismutase and catalase activities). However fluoxetine treatment (20 mg/kg, p.o., 21 days) significantly decreased the nitrite level in the brain while ‘6g’ (1 and 2 mg/kg, p.o., 21 days) did not show significant (P < 0.05) effect on the nitrite levels in brain.
Conclusion:
Compound ‘6g’ exerted antidepressant-like effects in behavioural despair paradigm in chronically stressed mice by restoring antioxidant mechanisms.
KEY WORDS: 5-HT3 receptor antagonist, chronic unpredictable mild stress, depression, oxidative stress
Introduction
Depression is a serious disorder that, according to the World Health Organization (WHO), is one of the leading causes of disability, worldwide. It is one of the most prevalent and costly psychiatric disorder of the developed world with a lifetime prevalence of, between 7.5 and 17%.[1] Stress sensitivity in depression is partly gender-specific. While men and women are, in general, equally sensitive to the depressogenic effects of stressful life events, their responses vary depending upon the type of stressor.[2] The pharmacological treatments for depression currently available include tricyclic antidepressants (TCAs), selective serotonin reuptake inhibitors (SSRIs), serotonin– noradrenergic reuptake inhibitors (SNRIs), and other atypical antidepressant drugs such as monoamine oxidase inhibitors (MAOIs).[3] However, the efficacy of these antidepressants is often inconsistent, and many of them frequently produce side effects such as sedation, apathy and fatigue, sleep disturbance, cognitive impairment, sexual dysfunction, etc. Hence, there remains a pressing need for new, effective, and better tolerated antidepressants. Stress has an important role in the development of human depression.[4]
Chronic unpredictable mild stress (CUMS) model of depression is widely used in preclinical antidepressants screening for investigating the pathophysiology of depression and the associated therapeutic interventions.[5] This model was developed in an attempt to mimic/resemble a variety of behavioural, neurochemical, neuroendocrine and neuroimmune alterations observed in human depressive disorders.[6] In addition increasing evidence that stress increases inflammation, a known mediator of many diseases in humans and animals such as interleukin-6 (IL-6) and nuclear factor kappa B (NF-B) DNA-binding relative to non-depressed controls.[7]
Past research studies suggested, that the repeated and unpredictable stress has a significant impact on reactive oxygen species (ROS) formation in brain, that in turn results in oxidative damage and dysregulation in normal physiology of the central nervous system (CNS).[8] Moreover, increased oxidative stress level in brain is considered as major factor for neurotoxicity and neuronal death toward the progression of chronic stress-induced depressive disorders.[9] At the present situation, the aspiration of developing better antidepressants has stimulated research on novel targets of depression. In our previous studies ‘6g’ showed antidepressant-like and anxiolytic-like effect in various behavioural and chronic models of depression and anxiety (unpublished data). The proposed mechanism for antidepressant effect of ‘6g’ is postsynaptic 5-HT3 receptor antagonism in serotonergic neurons can facilitate specific binding of 5-HT to other postsynaptic receptors such as 5-HT1B, 5-HT2A and 5-HT2C, thereby aiding in serotonergic transmission.[10] The involvement of 5-HT3 receptors in depression and anxiety is complemented by studies of 5-HT3 knockout mice which revealed the regulation of 5-HT3 (3A subtype) in anxiety-related behaviour.[11] Evidence for the relevance of 5-HT3 antagonists in the treatment of depression stems from clinical trials in which patients suffering from complex disorders such as fibromyalgia and bulimia showed improvement of the co-morbid depression.[12]
Despite increased interest among the clinical neurosciences, information regarding the antidepressant activity of serotonin type-3 modulators is still lacking. However, the effect of ‘6g’ in chronic unpredictable stress-induced depression needs to be explored to identify its potential usefulness in the treatment or prevention of depression disorder. Therefore, the present study was designed to investigate the antidepressant-like effect of ‘6g’ in CUMS paradigm by investigating chronic stress-induced behavioural and biochemical alterations in mice.
Materials and Methods
Experimental Animals
The present research works were carried out using male Swiss Albino mice (22 to 25 g), procured from Chaudhary Charan Singh Haryana Agricultural University, Hisar, India. The animals were maintained in standard laboratory conditions (temperature 22 ± 2°C and room humidity, 60 ± 10%) with a 12:12 hour light/dark cycle. The animals were fed with standard diet and filtered water ad libitum. The experimental procedures on animals were in compliance with the Institutional Animal Ethics Committee (Protocol No. IAEC/RES/14/04).
Drugs and Treatment
Fluoxetine (Flx), was obtained as gift samples from Cipla Pharmaceuticals Ltd. The tested molecule ‘6g’ was synthesized at in house medicinal chemistry laboratory, selected from a series of compounds based on Log P and pA2 value. Fluoxetine and ‘6g’ were freshly prepared in distilled water and administered per oral (p.o.) in a constant volume of 10 ml/kg. The drugs were administered per-oral (p.o.) once a day during the last 21 days of the CUMS procedure - The doses and treatment duration of the drugs used were selected according to the previous antidepressant studies conducted in our laboratory.[13]
Chronic Unpredictable Mild Stress Procedure
The CUMS procedure was performed as described by Ducottet et al., (2003),[14] with slight modifications. Briefly, CUMS consisted of exposure to a variety of unpredictable stressors (randomly); as follows: (1) 24 h food deprivation (FD), (2) 24 h water deprivation (WD), (3) 1 h exposure to a empty bottle (EB), (4) 7 h cage tilt (CT) (45°), (5) overnight illumination (OI), (6) 24 h soiled cage (SC) (200ml water in 100g sawdust bedding), (7) 6 min forced swimming (FS) at 12°C, (8) 2 h physically restraint (PR), and (9) 24 h exposure to a foreign object (FO) (e.g. a piece of plastic).

These stressors were randomly scheduled over a 1-week period and repeated throughout the 4-week experiment. Control animals were undisturbed except for necessary housekeeping procedures.
Behavioural Assessments
Spontaneous locomotor activity
In order to identify the association of immobility in the FST with changes in motor activity, the spontaneous locomotor activity of mice was assessed using the actophotometer which contains a square arena (30 × 30 cm) with walls that are fitted with photocells just above the floor level.[13] The photocells were checked before the beginning of the experiment. The mice were then individually placed in the arena. After a two minute acclimatization period, the digital locomotor scores were recorded for the next 8 min in a dimly lit room.
Forced swim test
The FST described earlier by Porsolt et al., (1977)[15] was slightly modified Mahesh et al., (2007).[16] In brief, each mouse was placed individually in a glass cylinder (diameter: 22.5 cm, height: 30 cm) containing 15 cm of water at 23 ± 2°C. The mice were placed in the water and forced to swim for 6 min. The duration of immobility was recorded during the last 4 of the 6 min test. A mouse was considered to be immobile when it stopped struggling and passively moved to remain floating and keep its head above water. Water was changed between trials and temperature was maintained at 23 ± 2°C.
Sucrose preference test
Sucrose preference test was carried out at the end of 4 weeks CUMS exposure. The test was performed as described earlier Casarotto and Andreatini (2007) with slight modifications.[17] In brief, before the test, mice were trained to adapt to sucrose solution (1%, w/v) by placing two bottles of sucrose solution in each cage for a period of 24 h; then one bottle of sucrose solution was replaced with water for 24 h. After the adaptation, mice were deprived of water and food for 24 h. Sucrose preference test was conducted at 9:30 a.m. The mice were housed in individual cages and were free to access to two bottles containing 100 ml of sucrose solution (1% w/v) and 100ml of water, respectively. After 24 h, the volumes of consumed sucrose solution and water were recorded. Then percentage of sucrose consumption was calculated as ratio of the amount of sucrose solution to that of total solution (sucrose and water) ingested within 24 h.
Biochemical analysis
Biochemical estimations in the brain homogenate were carried out 24 h after completion of all behavioural assessments.
Brain homogenate preparation
Animals were sacrificed by decapitation and the brains were quickly removed and washed with ice-cold sterile saline (0.9%). The whole brain samples were then homogenized with ice-cold 0.1 M phosphate buffer (pH 7.4) 10 times (w/v). The homogenate was centrifuged at 2500 × g (4°C) for 15 min to remove cellular debris and aliquots of supernatant were separated and used for biochemical estimations.
Estimation of lipid peroxidation level
The malondialdehyde (MDA) content, a quantitative measurement of lipid peroxidation, was assayed in the form of thiobarbituric acid reactive substances (TBARS) by the method of Wills (1966).[18] In this 0.1ml of supernatant was incubated with 0.5 ml tris hydrochloric acid (0.1 M, pH 7.4) for 2 h. To this, 1ml of trichloroacetic acid (10% w/v) was added and centrifuged at 1000 × g for 10 min. To 1 ml supernatant, 1ml (0.67% w/v) thiobarbituric acid (TBA) was added and kept in the boiling water bath for 10 min, cooled and then 1ml distilled water was added. The amount of lipid peroxidation products was measured by reaction with thiobarbituric acid at 532 nm using the spectrophotometer (UV-1700 Shimadzu, Japan). The values were expressed as nmol/mg protein.
Estimation of reduced glutathione level
Reduced glutathione in the brain was estimated according to the method described by Ellman, (1959).[19] 1ml supernatant was precipitated with 1ml of 4% sulfosalicylic acid and cold digested at 4°C for 1 h. The samples were centrifuged at 1200 × g for 15 min at 4°C. To 1 ml of this supernatant, 2.7 ml of phosphate buffer (0.1 mol/l, pH 8) and 0.2 ml of 5, 5-dithio-bis (2-nitrobenzoic acid) were added. The colour developed was measured immediately at 412 nm (UV-1700 Spectrophotometer, Shimadzu, Japan). Results were expressed as μmol/mg protein.
Estimation of superoxide dismutase activity
Superoxide dismutase activity was measured by the method of Misra and Fridovich (1972).[20] Auto oxidation of epinephrine at pH 10.4 was spectrophotometrically measured. In this method, supernatant of the tissue was mixed with 0.8 ml of 50 mM glycine buffer, pH 10.4 and the reaction was started by the addition of 0.02 ml (−)-epinephrine. After 5 min the absorbance was measured at 480 nm (UV-1700 Spectrophotometer, Shimadzu, Japan). The activity of SOD was expressed as % activity of vehicle-treated control.
Estimation of catalase activity
Brain CAT activity was assayed by the method described earlier.[21] The reaction mixture (1.5 ml) contained 1.0 ml of 0.01 mol/l phosphate buffer (pH 7), 0.1 ml of brain homogenate supernatant and 0.4 ml of 2 mol/l hydrogen peroxide. The reaction was stopped by the addition of 2 ml of dichromate-acetic acid reagent (5% potassium dichromate and glacial acetic acid were mixed in a 1:3 ratio). The absorbance was measured at 620 nm and expressed as micro moles of hydrogen peroxide consumed/min/mg protein.
Estimation of nitrite level
The accumulation of nitrite in the supernatant, an indicator of nitric oxide (NO) production was determined by a colorimetric assay using Greiss reagent (0.1% N-(1-naphthyl) ethylenediamine dihydrochloride, 1% sulfanilamide and 2.5% phosphoric acid) as described by Green et al., (1982).[22] Equal volumes of supernatant and Greiss reagent were mixed, the mixture incubated for 10 min at room temperature in the dark and the absorbance determined at 540nm (UV-1800 Spectrophotometer, Shimadzu, Japan). The concentration of nitrite in the brain was calculated using a sodium nitrite standard curve and expressed as micromole per milligram of protein.
Protein estimation
The protein content was measured in all brain samples for oxidant and antioxidant activity by the biuret method using bovine serum albumin as standard.
Statistical analysis
All data were expressed as mean ± S.E.M. The data obtained in studies from various groups were statistically analyzed using one way analysis of variance (ANOVA) followed by the post-hoc Dunnett's test in Graph pad prism 3 software. The value of P < 0.05 was considered as statistically significant.
Results
Behavioural Observations
Spontaneous locomotor activity
The locomotor activity was evaluated after 28 day of CUMS procedure using actophotometer. There was no significant difference observed in the locomotor scores in mice of different groups as presented in Figure 1.
Figure 1.
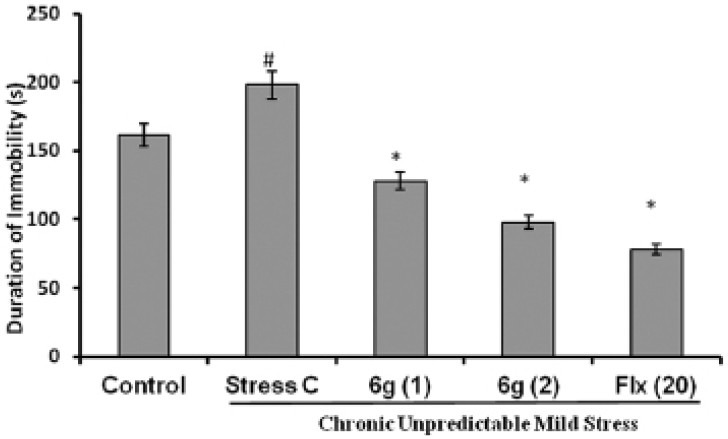
Effect of ‘6g’ (1 and 2 mg/kg, i.p.) and fluoxetine (20 mg/kg, i.p.) treatment on locomotor scores in stressed mice. Each column represents mean locomotor scores recorded in 8 min observation period. The error bars indicate SEM; n= 8/group. Fluoxetine = Flx
Effect of ‘6g’ on immobility time in forced swim test
The effect of ‘6g’ treatment on the duration of immobility in mice FST is shown in Figure 2. The mice subjected to CUMS showed significant increase in duration of immobility as compared to control mice. Chronic treatment with ‘6g’ (1 and 2 mg/kg, p.o.) significantly decreased the immobility duration of stressed mice as compared to the stress vehicle treated group. The positive control, fluoxetine (20 mg/kg, p.o.) also significantly (P < 0.05) inhibited the increase in duration of immobility in stressed mice as compared to the stress vehicle treated group.
Figure 2.
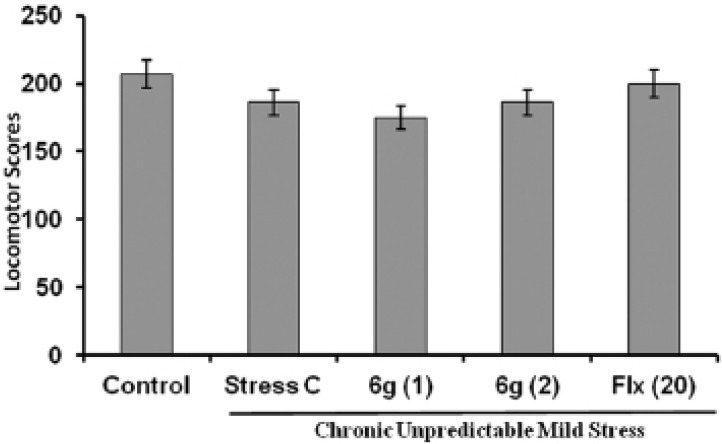
Effect of ‘6g’ (1 and 2 mg/kg, i.p.) and fluoxetine (20 mg/kg, i.p.) treatment on duration of immobility in stressed mice. Each column represents mean duration of immobility(s). The error bar indicates SEM, #P < 0.05 when compared with normal control; *P < 0.05 when compared with stress vehicle treated group; n= 8/group. Fluoxetine = Flx
Effect of ‘6g’ on the percentage of sucrose consumption
The effect of ‘6g’ treatment on the sucrose consumption is shown in Figure 3. The stressed mice showed a significant reduction in the percentage of sucrose consumption when compared to control mice. The chronic administration of ‘6g’ (1 and 2 mg/kg, p.o.) significantly restored the percentage of sucrose consumption in stressed mice as compared to stress vehicle treated group. The positive control, fluoxetine (20 mg/kg, p.o.) also significantly (P < 0.05) reversed the reduction in the percentage of sucrose consumption in stressed mice as compared to the stress vehicle treated group.
Figure 3.
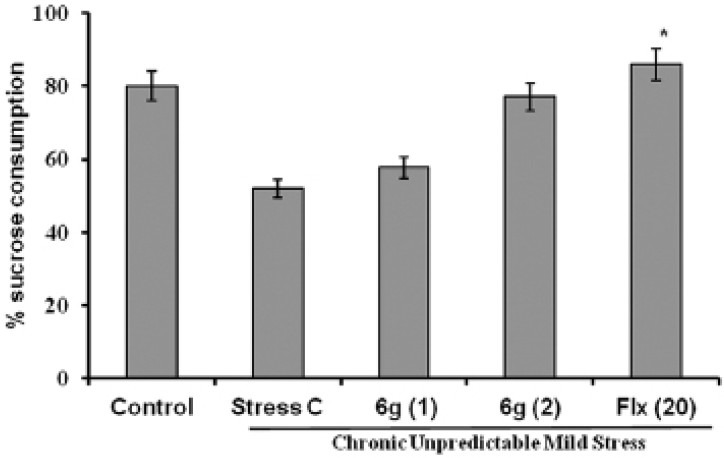
Effect of ‘6g’ (1 and 2 mg/kg, i.p.) and fluoxetine (20 mg/kg, i.p.) treatment on sucrose preference (%) in stressed mice. The error bar indicates SEM, #P < 0.05 when compared with normal control; *P < 0.05 when compared with stress vehicle treated group; n= 8/ group. Fluoxetine = Flx
Biochemical Analysis
Effect of ‘6g’ treatment on brain lipid peroxidation levels
The thiobarbituric acid reactive substances (TBARS) level was significantly increased in brain of stressed mice as compared to the control mice as shown in Table 1. Repeated treatment with ‘6g’ (1 and 2 mg/kg, p.o.) produced a significant (P < 0.05) reduction in TBARS levels in the brain of stressed mice as compared to stress vehicle treated group. Moreover, fluoxetine (20mg/kg, i.p.) treatment also significantly reduced TBARS levels in the brain of stressed mice.
Table 1.
Effect of 6g (1 and 2 mg/kg, p.o.) and Fluoxetine (20 mg/kg, p.o.) on lipid peroxidation and reduced glutathione, superoxide dismutase, catalase and nitrite levels
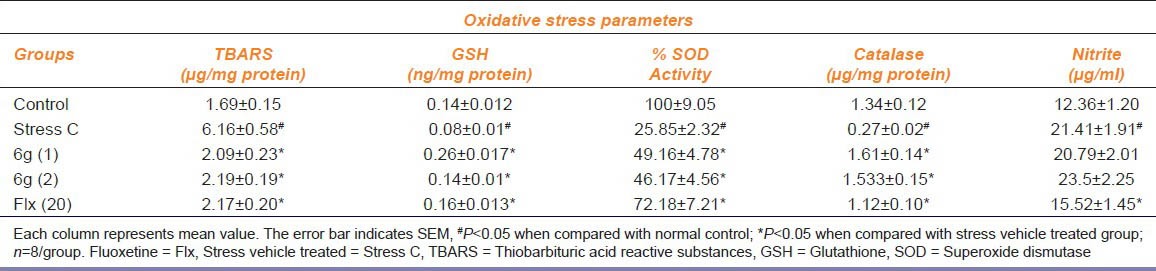
Effect of ‘6g’ treatment on brain glutathione levels
The brain GSH levels were found to be significantly depleted in brain of stressed mice compared to control mice as shown in Table 1. Chronic treatment with ‘6g’ (1 and 2 mg/kg, p.o.) and fluoxetine (20 mg/kg, p.o.) showed a significant (P < 0.05) increased in brain GSH level as compared to the stress vehicle treated group.
Effect of ‘6g’ treatment on brain catalase levels
As depicted in as shown in Table 1, CUMS subjected mice showed a significant (P < 0.05) reduction in brain CAT activity of as compared to control mice. Chronic treatment with ‘6g’ (1 and 2 mg/kg, p.o.) and fluoxetine (20 mg/kg, p.o.) significantly (P < 0.05) restored CAT activity in the brain as compared to stress vehicle treated group.
Effect of ‘6g’ treatment on brain superoxide dismutase levels
The effect of ‘6g’ on the brain SOD activity is shown in as shown in Table 1. The enzymatic activity of SOD was significantly decreased in brain of stressed mice as compared to control mice. Repeated treatment with ‘6g’ (1 and 2 mg/kg, p.o.) and fluoxetine (20 mg/kg, p.o.) significantly (P < 0.05) improved the SOD activity in stressed mice brain as compared to stress vehicle treated group.
Effect of ‘6g’ treatment on brain nitrite level in stressed mice
Stressed mice showed a significant elevated brain nitrite levels as compared to control group mice as shown in Table 1. The chronic administration of ‘6g’ (1 and 2 mg/kg, p.o.) did not produce any significant (P < 0.05) reduction in elevated brain nitrite level in stressed mice when compared with stressed vehicle treated group. The positive control fluoxetine (20 mg/kg, p.o.) showed a significant (P < 0.05) decrease in brain nitrite level.
Discussion
The present study demonstrated the effects of 5-HT3 receptor antagonist, ‘6g’, on mice subjected to CUMS, using the spontaneous locomotor activity, forced swim and sucrose preference tests to assess their behavior and estimating oxidative stress parameters in brain. The results of the present study divulged that chronic treatment with ‘6g’ reversed the CUMS-induced depressive-like behavior in mice. Moreover, ‘6g’ enhanced the beneficial effects against CUMS-induced changes in oxidative stress-related parameters in the mice brain.
The chronic administration of various uncontrollable stressors in an unpredictable manner is a well-documented animal model for the preclinical evaluation of antidepressants.[23] CUMS model has a good interest due to its potential of combining three types of validity criteria such as face validity, construct validity and predictive validity, requested for an animal model of depression.
The changes in locomotor activity have been suggested to mimic antidepressant/depressant-like effect of rodents in behavioural paradigms. However, in this study no significant difference in spontaneous locomotor activity was observed in any of the experimental groups suggested that changes in behavior in vehicle treated and ‘6g’ treated stressed mice was not having any effect on locomotor activity.[24]
Consistent with previous reports, in the present study CUMS-induced the depressive-like behaviour as indicated by FST and sucrose preference test in mice.[24] The FST, also well addressed as the “behavior despair” test is most frequently used to determine depression-like behavioural parameters in animals after exposure to various stressors and situations.[25] The data of present study is in agreement with previous reports that rodents exposed to chronic stress exhibited increase duration of immobility in FST. Porsolt and colleagues (1977) have been reported that increased in the duration of immobility in FST reflects the depression-like symptoms. Prolong administration of ‘6g’ significantly decreased the duration of immobility in stressed mice, which indicates the antidepressant-like action. Moreover, our previous study has shown that ‘6g’ produced antidepressant-like effect by decreasing the duration of immobility in mice FST.[13] Furthermore, numerous studies have been shown that 5-HT3 receptor antagonists activity decreased the duration of immobility in FST and could be used for antidepressant-like effect.[16]
Sucrose preference test is valuable and valid behavioural indicator of chronic stress in a rodent paradigm. Sucrose preference test is regarded as useful and valid behavioural marker of chronic stress in an animal paradigm. Sucrose preference is regarded as an indicator of a key symptom of depression, i.e. anhedonia, indicating loss of interest or pleasure. Studies suggest that chronic unpredictable stress damages nerve cells in the neural reward system. This damage is -thought to be related to the serotonergic (5-HT) and dopaminergic (DA) systems, and to induce a loss of the ability to experience happiness or pleasure.[26]
In the present study, stressed mice showed a decreased sucrose preference compared with unstressed mice. We investigated the influence of ‘6g’ on sucrose consumption, an indicator of anhedonia-like behavioural change in CUMS paradigm. In the present study, stressed mice consume less sucrose solution compared with unstressed mice. This result is consistent with earlier findings that mice exposed to CUMS consume less sucrose compared to non stressed mice.[17] Whereas, treatment with ‘6g’ significantly reversed this behavioural change, which represents the antidepressant-like action of ‘6g’ in CUMS model of depression. Studies suggest that chronic unpredictable stress damages nerve cells in the neural reward system. Moreover, considerable research has been shown that antidepressants treatment has an ability to reverse the chronic stress-induced reduction in sucrose consumption.
Recently, a growing body of data has shown that ROS also play a role in the pathogenesis of neuropsychiatric disorders including depression.[27] Depression is characterized by a significantly decreased antioxidant status, as evidenced by lowered tryptophan, tyrosine, albumin, and zinc, which are all antioxidants. In addition, it is extensively reported that CUMS impaired the antioxidant status of brain tissue, presumably by generating excessive ROS.
Co-existence of increased oxidative stress with symptoms of depression, as evidenced by increased lipid.[27] In the present study, TBARS level that is proportional to lipid peroxidation and oxidative stress was significantly increased in brain of stressed mice. Previous studies have been shown that, MDA a by-product of lipid peroxidation was found to be increased in plasma and serum of depressive patients as compared to control subjects.[27] In the present study repeated treatment with ‘6g’ which inhibited CUMS-induced depressive-like behavior also restored the CUMS-induced lipid peroxidation, suggesting a potential relationship between both events.
Numerous studies have reported that there are also antioxidant defence systems (SOD, CAT and GSH) in brain tissue for scavenging ROS to prevent neuronal damage. An alteration in the antioxidant defence systems induced depressive-like behaviour.[28] GSH, an intracellular non-enzymatic thiol antioxidant system involves in the neutralization of free radicals/ROS. The present study showed that stressed mice have lower brain GSH level indicating an alteration in antioxidant brain defences in CUMS model of depression.[28] Compound ‘6g’ restored the GSH level in brain of stressed mice.
The present study shows a significant reduction in SOD and CAT antioxidant defence systems were also found in the brain of stressed mice. Catalase is the very common enzyme that catalyzes the reduction of hydrogen peroxide in to water and oxygen.[29] SOD promotes dismutation of superoxide into oxygen and hydrogen peroxide. Considerable research have showed a decrease in CAT and SOD activities in the prefrontal cortex, the hippocampus and the striatum of stressed mice, indicating an alteration in antioxidant defences in CUS-induced depressive-like behavior.[29] The present study found a significant increased in both SOD and CAT activities in the brain of stressed mice, in response to chronic ‘6g’ treatment. The stressed mice also showed a nitrosative stress as evidenced by elevated brain nitrite level. A growing body of data has been suggested that depressed patients show elevated nitrite level.[30] ’6g’ also significantly reduced nitrosative stress by decreasing the elevated nitrite level in brain of stressed mice.
Conclusion
Based on the aforementioned findings it is concluded that ‘6g’ showed antidepressant-like effect in chronic unpredictable mild stress-induced depression in mice. The symptomatic improvement of depressive-like symptom by ‘6g’ in CUMS may be related to the neuroprotective effects by the increasing antioxidant system.
Footnotes
Source of Support: Nil
Conflict of Interest: No
References
- 1.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: Results from the national comorbidity survey replication (NCS-R) JAMA. 2003;289:3095–105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 2.Kendler KS, Thornton LM, Prescott CA. Gender differences in the rates of exposure to stressful life events and sensitivity to their depressogenic effects. Am J Psychiatry. 2001;158:587–93. doi: 10.1176/appi.ajp.158.4.587. [DOI] [PubMed] [Google Scholar]
- 3.Nemeroff CB. The burden of severe depression: A review of diagnostic challenges and treatment alternatives. J Psychiatr Res. 2007;41:189–206. doi: 10.1016/j.jpsychires.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 4.Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 5.Garcia LS, Comim CM, Valvassori SS, Réus GZ, Stertz L, Kapczinski F, et al. Ketamine treatment reverses behavioural and physiological alterations induced by chronic mild stress in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:450–55. doi: 10.1016/j.pnpbp.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 6.McEwen BS. Glucocorticoids, depression, and mood disorders: Structural remodeling in the brain. Metabolism. 2005;54:20–3. doi: 10.1016/j.metabol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, et al. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. 2006;163:1630–3. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- 8.Madrigal JL, Olivenza R, Moro MA, Lizasoain I, Lorenzo P, Rodrigo J, et al. Glutathione depletion, lipid peroxidation and mitochondrial dysfunction are induced by chronic stress in rat brain. Neuropsychopharmacology. 2001;24:420–9. doi: 10.1016/S0893-133X(00)00208-6. [DOI] [PubMed] [Google Scholar]
- 9.Tsuboi H, Tatsumi A, Yamamoto K, Kobayashi F, Shimoi K, Kinae N. Possible connections among job stress, depressive symptoms, lipid modulation and antioxidants. J Affect Disord. 2006;91:63–70. doi: 10.1016/j.jad.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 10.Rajkumar R, Mahesh R. The auspicious role of the 5-HT3 receptor in depression: A probable neuronal target? J Psychopharmacol. 2010;24:455–69. doi: 10.1177/0269881109348161. [DOI] [PubMed] [Google Scholar]
- 11.Kelley SP, Bratt AM, Hodge CW. Targeted gene deletion of the 5-HT3A receptor subunit produces an anxiolytic phenotype in mice. Eur J Pharmacol. 2003;461:19–25. doi: 10.1016/s0014-2999(02)02960-6. [DOI] [PubMed] [Google Scholar]
- 12.Haus U, Varga B, Stratz T, Spath M, Muller W. Oral treatment of fibromyalgia with tropisetron given over 28 days. Influence on functional and vegetative symptoms, psychometric parameters and pain. Scand J Rheumatol Suppl. 2000;113:55–8. doi: 10.1080/030097400446652. [DOI] [PubMed] [Google Scholar]
- 13.Mahesh R, Bhatt S, Devadoss T, Jindal AK, Gautam BK, Dhar AK, et al. Anti-depressant Like Effect of Novel 5-HT3 Receptor Antagonist, (4-benzylpiperazin-1-yl) (3-methoxyquinoxalin-2-yl) methanone (6g) in Acute and Chronic Animal Models of Depression. IJPER. 2013;47:71–81. [Google Scholar]
- 14.Ducottet C, Griebel G, Belzung C. Effects of the selective nonpeptide corticotrophin releasing factor receptor 1 antagonist antalarmin in the chronic mild stress model of depression in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:625–31. doi: 10.1016/S0278-5846(03)00051-4. [DOI] [PubMed] [Google Scholar]
- 15.Porsolt RD, Bertin A, Jalfre M. Behavioural despair in mice. A primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229:327–36. [PubMed] [Google Scholar]
- 16.Mahesh R, Rajkumar R, Minasri B, Venkatesha Perumal R. Potential antidepressants: Pharmacology of 2-(4-methylpiperazin-1-yl)-1,8-naphthyridine-3carbonitrile in rodent behavioural models. Pharmazie. 2007;62:919–24. [PubMed] [Google Scholar]
- 17.Casarotto PC, Andreatini R. Repeated paroxetine treatment reverses anhedonia induced in rats by chronic mild stress or dexamethasone. Eur Neuropsychopharmacol. 2007;17:735–42. doi: 10.1016/j.euroneuro.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Wills ED. Mechanism of lipid peroxide formation in animal. Biochem J. 1966;99:667–76. doi: 10.1042/bj0990667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellman GL. Tissue sulfidryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 20.Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–5. [PubMed] [Google Scholar]
- 21.Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–94. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 22.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem. 1982;126:131–8. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 23.Willner P, Muscat R, Papp M. Chronic mild stress-induced anhedonia: A realistic animal model of depression. Neurosci Biobehav Rev. 1992;16:525–34. doi: 10.1016/s0149-7634(05)80194-0. [DOI] [PubMed] [Google Scholar]
- 24.Mahesh R, Bhatt S, Devadoss T, Jindal AK, Gautam BK, Pandey DK. Antidepressant Potential of 5-HT3 Receptor Antagonist, N-n-propyl-3-ethoxyquinoxaline-2-carboxamide (6n) J Young Pharm. 2012;4:235–44. doi: 10.4103/0975-1483.104367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naitoh N, Nomura S, Kunimi Y, Yamaoka K. Swimming-induced “head twitching” in rats in the forced swimming test induced by overcrowding stress: A new marker in the animal model of depression? Keio J Med. 1992;41:221–4. doi: 10.2302/kjm.41.221. [DOI] [PubMed] [Google Scholar]
- 26.Bekris S, Antoniou K, Daskas S, Papadopoulou-Daifoti Z. Behavioural and neurochemical effects induced by chronic mild stress applied to two different rat strains. Behav Brain Res. 2005;161:45–59. doi: 10.1016/j.bbr.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Maes M, De Vos N, Pioli R, Demedts P, Wauters A, Neels H, et al. Lower serum vitamin E concentrations in major depression. Another marker of lowered antioxidant defenses in that illness. J Affect Disord. 2000;58:241–6. doi: 10.1016/s0165-0327(99)00121-4. [DOI] [PubMed] [Google Scholar]
- 28.Ng F, Berk M, Dean O, Bush AI. Oxidative stress in psychiatric disorders: Evidence base and therapeutic implications. Int J Neuropsychopharmacol. 2008;11:851–76. doi: 10.1017/S1461145707008401. [DOI] [PubMed] [Google Scholar]
- 29.Zhang D, Wen XS, Wang XY, Shi M, Zhao Y. Antidepressant effect of Shudihuang on mice exposed to unpredictable chronic mild stress. J Ethnopharmacol. 2009;12:55–60. doi: 10.1016/j.jep.2009.02.029. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki E, Yagi G, Nakaki T, Kanba S, Asai M. Elevated plasma nitrate levels in depressive states. J Affect Disord. 2001;63:221–4. doi: 10.1016/s0165-0327(00)00164-6. [DOI] [PubMed] [Google Scholar]


