Abstract
Objectives:
To evaluate the cardioprotective effects of trapidil on myocardial ischemia-reperfusion injury (MIRI) in rabbits.
Materials and Methods:
Rabbits were subjected to 40 min of myocardial ischemia followed by 120 min of reperfusion. Blood for superoxide dismutase (SOD) and malondialdehyde (MDA) were estimated. At the end of reperfusion, the rabbits were sacrificed and the hearts were isolated for histological examination. An apoptotic index (AI) was determined using the terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick-end-labeling (TUNEL) method. The expression of apoptosis-related proteins Bax and Bcl-2 was analyzed using immunohistochemistry. Statistical analyses were performed by one-way analysis of variance (ANOVA), P < 0.05 considered statistically significant
Results:
Trapidil caused a significant (P < 0.05) increase in SOD activity, as decreased MDA levels and significantly (P < 0.05) reduced the expression of Bax as compared with the ischemia-reperfusion (IR) control group.
Conclusion:
Trapidil may attenuate the myocardial damage produced by IR injury and offer potential cardioprotective action.
KEY WORDS: Apoptosis index, ischemia-reperfusion, myocardial ischemia-reperfusion injury, trapidil
Introduction
Ischemia-reperfusion (IR) injury occurs due to blood restoration after a critical period of coronary artery obstruction. It is associated with clinical problems, such as thrombolysis, angioplasty, and coronary bypass surgery.[1] However, reperfusion may lead to accelerated and additional myocardial injury, resulting in a spectrum of reperfusion-associated pathologies.[2] In addition, reperfusion of the ischemic myocardium results in irreversible cell damage. Interestingly, many of the features of IR injury are related to impaired structure. Following ischemic preconditioning, the myocardium is transiently more resistant to deleterious effect of prolonged ischemia.[3] While many pharmacological interventions have been used to render cardioprotection against oxidative stress caused by IR injury, there is still a need for more effective therapies that can ameliorate IR injury and delay the development of irreversible cell injury.[4,5]
Trapidil (C10H15N5), an antiplatelet drug with broad biological activities, has been demonstrated to reduce restenosis after angioplasty in animals, as well as in humans.[6] The clinical dosage of trapidil is 150-300 mg/day/person. According to a dose algorithm by Xu Shu-yun et al.,[7] a relevant dosage of trapidil in rabbits is 7-14 mg/kg/day.
This study investigated to evaluate the cardioprotective effect of trapidil in rabbit models of myocardial ischemia-reperfusion injury (MIRI) and the relationship between dose and action. We
found that there was a correlation between the cardioprotective effects of trapidil in an IR-induced myocardial injury model and the expression of Bax and Bcl-2 proteins.
Materials and Methods
Male Japanese white rabbits, weighing 2.5-3.0 kg were used in this study. The animal experiments were conducted in accordance with guidelines of the Institutional Animal Care and Use Committee of Shenyang Pharmaceutical University, and the study protocol was approved by the Institutional Ethics Committee.
Drugs and chemicals
tablets were a generous gift from Wuhan Di’ao Pharmacy Co., Ltd (Wuhan, Hubei province, China). SABC immunohistochemical staining kits were purchased from Wuhan Boster Bioengineering limited company (Wuhan, Hubei province, China). Superoxide dismutase (SOD) and malondialdehyde (MDA) assay kits were purchased from the Nanjing Jiancheng Bioengineering Institute (Nanjing, Jiangsu province, China). An in situ Cell Death Detection Kit was purchase from Roche (Basel, Switzerland)
Experimental protocol
Twenty-four rabbits were randomly divided into four groups of six animals. Group A (Sham group) consisted of sham-operated rabbits that underwent the same operative procedures as the other groups, but without occluding blood flow. Group B (IR group), C and D underwent 40 min of ischemia followed by 120 min of reperfusion. Groups A and B were administered distilled water (5 ml/kg/day) intragastrically daily for 7 days. Groups C and D received oral trapidil (7 and 14 mg/kg/day, respectively) for 7 days.
Heart preparations
The rabbits were anesthetized, placed in a supine position, and then intubated with endotracheal, femoral artery, and carotid artery tube. Left thoracotomy was performed and the left main artery was exposed according to the method described by Asgeri et al.[8,9,10] A silk thread was passed around the left anterior descending coronary artery (LAD). The end of the tie was threaded through a small vinyl tube to form a snare.[11] Electrocardiogram, blood pressure, and heart rate were recorded using a 16-physiograph (Stoelting Co., IL, USA).
Determination of the MDA level and SOD activities
At 120 min after IR, MDA concentration and SOD activity in serum samples were measured using commercially available assay kits according to the manufacturer's instructions.[12]
Observation under optical microscope
Myocardial tissue from the left ventricular anterior wall (LVAW) of sacrificed rabbits (approximately 3 mm in thickness) was removed upon euthanasia. Samples were fixed in 4% precooled paraformaldehyde for 72 h and embedded in paraffin for histological studies. Sections were processed with hematoxylin and eosin (H and E) stain. Images were visualized under an optical microscope at ×400 magnification.[13]
Cardiomyocyte apoptotic index
An area under the ligature of the LVAW (about 1 cm2) was isolated and fixed in 4% paraformaldehyde. Three slides from each block were evaluated for the percentage of apoptotic cells using a TUNEL assay according to the manufacturer's instructions. Both total and TUNEL-positive myocytes were counted in each field. The results are expressed as apoptotic index (AI): (number of TUNEL-positive myocytes/total myocytes) ×100%.[14]
Expressions of Bax and Bcl-2 proteins
Tissue sections (3 μm thickness) were mounted on poly-L-lysine-coated glass slides and then deparaffinized with xylene. The expression of Bax and Bcl-2 was determined according to the manufacturer's instructions.[15,16]
Transmission electron microscope
The specimens of the LVAW (about 1 mm3) were fixed in 2.5% glutaraldehyde at 4°C. After citric lead plus uranyl acetate double staining, samples were cut (70 nm thick) and observed with a JEM-1200EM (JEOL Ltd.), and photographs were taken.
Statistical analysis
Data were compared by analysis of variance (ANOVA) using IBM SPSS Statistics 19 software. Statistical analyses were performed by one-way ANOVA. Values are presented as mean ± standard deviation (SD). P < 0.05 was considered statistically significant.
Results
MDA and SOD levels in serum
After 120 min of reperfusion, the MDA and SOD levels in the trapidil groups was significantly different when compared with the IR group. Moreover, MDA and SOD was significantly different between groups C and D (P < 0.05) [Table 1].
Table 1.
Changes of MDA, SOD, Bax, Bcl-2, and AI
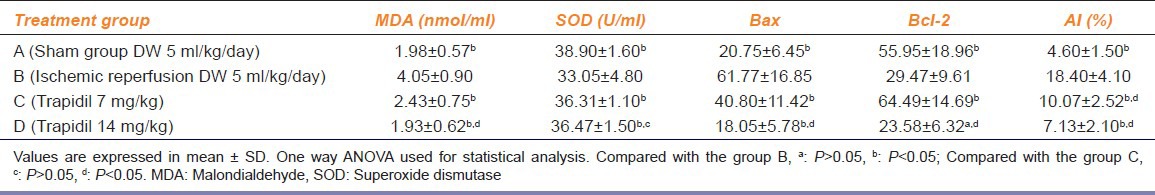
Cardiomyocyte morphology
Structural changes within the LVAW from rabbit hearts were observed under an optical microscope. In Group A, there was normal morphology of myocardial cells and regular arrangement. The LVAW in Group B had an abnormal cardiocyte structure, and the myocardial fibers were partially fractured. Myocardial cells in groups C and D had a normal long fusiform shape, the nuclei were ovoid in shape, and the cells were regularly arranged with the nuclei centrally located [Figure 1].
Figure 1.

Pathological changes of cardiac cells under the light microscope in four groups: A: Sham group; B: IR group; C: Trapidil group (7 mg/kg); D: Trapidil group (14 mg/kg), (H and E, ×400)
Expression of Bax and Bcl-2
Apoptotic proteins Bax and Bcl-2 were assessed by optical density (OD). Trapidil pretreatment significantly improved the expression of Bax and Bcl-2 as compared with Group B (P < 0.05) [Table 1].
Myocardial apoptosis
The number of TUNEL-positive cells in myocardial heart tissues was manually counted in five randomly selected fields, and AI was expressed as a percentage of total cells counted. AI in groups C and D was reduced when compared with that of group B. Moreover, AI was increased significantly in Group C compared with Groups D (P < 0.05) [Table 1 and Figure 2].
Figure 2.
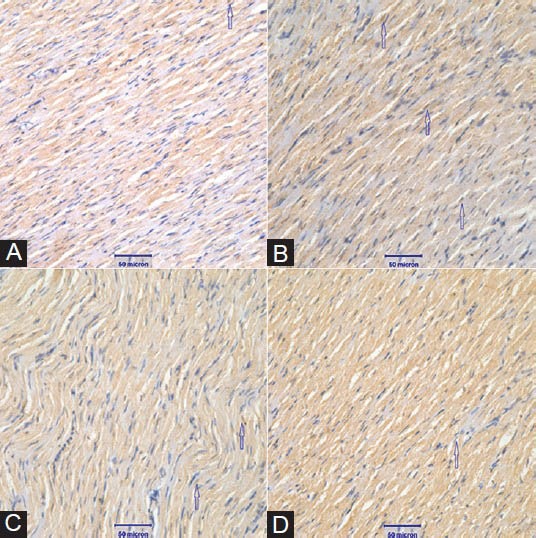
(A-D) Myocardial apoptosis determined by TUNEL staining (×400)
Transmission electron microscope
Representative electron micrographs of the ischemic border zone exposed to coronary artery ligation are shown in Figure 3. Group A had myocardial fibers that were organized into neat bundles. Mitochondria had a normal morphology and were arranged along the myocardial fiber axis, and mitochondrial membranes were integrated. Damaged mitochondria accumulated in Group B. Abnormal mitochondria are defined by marked swelling, vacuolation (loss of electron density) or disrupted cristae. Myocardial fibers in group B were fuzzy and disordered, with severe swelling of mitochondria and the presence of vacuoles. Mitochondria in the myocardial fibers showed disorder, and mitochondrial cristae were markedly reduced. Myocardial cells in groups C and D exhibited partial shrinkage. Mitochondria cristae were lucid, but a population of the mitochondria still displayed vacuolar degeneration [Figure 3].
Figure 3.
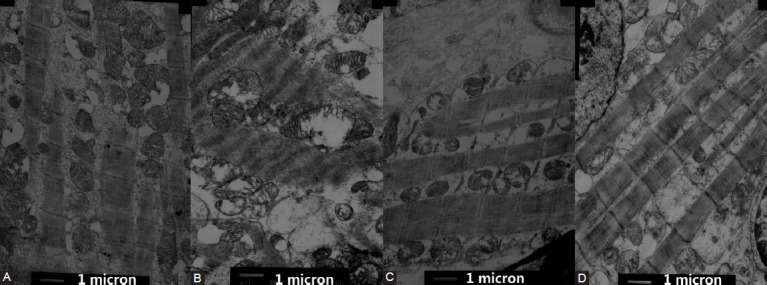
(A-D) Changes of cardiac cells under the electron microscope (×8000)
Discussion
Trapidil was initially developed as a vasodilator and antianginal agent, but was later used as an antiplatelet agent. Trapidil competitively inhibits phosphodiesterase. Additionally, the drug decreases platelet aggregation and activation by increasing prostaglandin synthesis and inhibiting thromboxane synthetase.[17]
A variety of mechanisms and mediators are involved in the pathophysiology of reperfusion injury. A key event in reperfusion is the reintroduction of molecular oxygen and subsequent formation of reactive oxygen species (ROS). A growing body of evidence has shown that ROS is an important mediator of MIRI. ROS can lead to lipid peroxidation, which is a pivotal mechanism involved in oxidative stress. Thus, inhibition of lipid peroxidation may attenuate IR heart injury.[18] As shown in Table 1, in this study, a significant increase in serum SOD activity and a decrease in MDA level were evident in rabbits following IR.
Cell apoptosis is one of the most important pathophysiological processes during acute myocardial ischemia and MIRI.[19] Recent studies have indicated that apoptosis plays an important role in the process of cardiomyocyte damage subsequent to myocardial infarction.[20] The Bcl-2 family consists of pro-apoptotic and antiapoptotic members. The balance between pro-apoptotic (Bax) and antiapoptotic (Bcl-2) proteins determines the population of cells that survive or undergo apoptosis after a certain stimulus or injury.[21] In this study, TUNEL-positive cells correlated with an upregulation of Bax and a downregulation of Bcl-2. In addition, our results suggested that trapidil activated the expression of Bcl-2 and blocked the stimulation of Bax, demonstrating the potent cardioprotective action of trapidil against oxidative stress. Cardiomyocytes in the IR group underwent severe apoptosis, whereas the AI was decreased in the trapidil groups. Taken together, these results suggest that suppression cardiomyocyte apoptosis during IR injury may be an underlying mechanism of trapidil in MIRI.
In this study, irregular cardiomyocyte structures were observed in the IR group. In addition, myocardial cells lost normal structure and featured signs of toxicity, such as rupture of cell membranes, mitochondrial swelling, and vacuolation. Notably, there was an increase in integrated mitochondria in groups C and D. Thus, the protective effect of trapidil in a rabbit model of MIRI correlated with drug dose.
Although the induction of both altered myocardial structure and apoptosis was observed in this in vivo rabbit MIRI model, the association between cardioprotective effects and the extent of myocardial injury was not fully demonstrated. Therefore, it is possible that myocardial injury contributes to cell apoptosis. We hypothesize that trapidil may inhibit apoptosis, which subsequently suppresses the induction of apoptosis.
The protective action of trapidil is related to apoptosis as a result of oxidative injury and IR. These findings highlight the importance of trapidil pretreatment in MIRI. Further studies are needed to investigate the in vivo effects and mechanisms of trapidil treatment following ischemia, as this would more likely mimic the clinical situation.
Conclusion
MIRI causes pronounced oxidative stress and tissue damage in the heart. At the histological level in a rabbit MIRI model, administration of trapidil protects against MIRI-induced cardiotoxicity in a dose-dependent manner.
Footnotes
Source of Support: Nil
Conflict of Interest: No
References
- 1.Zhao L, Peng DQ, Zhang J, Song JQ, Teng X, Yu YR, et al. Extracellular signal-regulated kinase 1/2 activation is involved in intermedin1-53 attenuating myocardial oxidative stress injury induced by ischemia/reperfusion. Peptides. 2012;33:329–35. doi: 10.1016/j.peptides.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 2.Huang GQ, Wang JN, Tang JM, Zhang L, Zheng F, Yang JY, et al. The combined transduction of copper, zinc-superoxide dismutase and catalase mediated by cell-penetrating peptide, PEP-1, to protect myocardium from ischemia-reperfusion injury. J Transl Med. 2011;9:73. doi: 10.1186/1479-5876-9-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar A, Taliyan R, Sharma PL. Evaluation of thyroid hormone induced pharmacological preconditioning on cardiomyocyte protection against ischemic-reperfusion injury. Indian J Pharmacol. 2012;44:68–72. doi: 10.4103/0253-7613.91870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurian GA, Paddikkala J. Role of Mitochondrial Enzymes and Sarcoplasmic ATPase in Cardioprotection Mediated by Aqueous Extract of Desmodium gangeticum (L) DC Root on Ischemic Reperfusion Injury. Indian J Pharm Sci. 2010;72:745–52. doi: 10.4103/0250-474X.84585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen K, Wu L, Wang ZZ. Extrinsic regulation of cardiomyocyte differentiation of embryonic stem cells. J Cell Biochem. 2008;104:119–28. doi: 10.1002/jcb.21604. [DOI] [PubMed] [Google Scholar]
- 6.Ishikura K, Fujita H, Hida M, Awazu M. Trapidil inhibits platelet-derived growth factor-induced migration via protein kinase A and RhoA/Rho-associated kinase in rat vascular smooth muscle cells. Eur J Pharmacol. 2005;515:28–33. doi: 10.1016/j.ejphar.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 7.Xu SY, Bian RL, Chen X. Third Edition. PMPH; 2002. Pharmacological Experiment Method; p. 1861. [Google Scholar]
- 8.Asgeri M, Ahmadpour F, Negargar S, Khadra WZ, Porhomayon J, Nader ND. The comparative myocardial protection by propofol and isoflurane in an in vivo model of ischemia reperfusion. Semin Cardiothorac Vasc Anesth. 2011;15:56–65. doi: 10.1177/1089253211411732. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J, Qi XY, Wan YF, Yuan C. Establishment of a reperfusion model in rabbits with acute myocardial infarction. Cell Biochem Biophys. 2011;60:249–58. doi: 10.1007/s12013-010-9147-3. [DOI] [PubMed] [Google Scholar]
- 10.Liu X, Liu H, Zeng Z, Zhou W, Liu J, He Z. Pharmacokinetics of ligustrazine ethosome patch in rats and antimyocardial ischemia and anti-ischemic reperfusion injury effect. Int J Nanomedicine. 2011;6:1391–8. doi: 10.2147/IJN.S20263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang YH, Xu JJ, Li JX, Cheng XS. Remote postconditioning induced by brief pulmonary ischemia and reperfusion attenuates myocardial reperfusion injury in rabbits. Chin Med J (Engl) 2011;124:1683–8. [PubMed] [Google Scholar]
- 12.Hu X, Zhou X, He B, Xu C, Wu L, Cui B, et al. Minocycline protects against myocardialischemia and reperfusion injury by inhibiting high mobility group box1 protein in rats. Eur J Pharmacol. 2010;638:84–9. doi: 10.1016/j.ejphar.2010.03.059. [DOI] [PubMed] [Google Scholar]
- 13.Song XJ, Yang CY, Liu B, Wei Q, Korkor MT, Liu JY, et al. Atorvastatin Inhibits Myocardial Cell Apoptosis in a Rat Model with Post-myocardial Infarction Heart Failure by Downregulating ER Stress Response. Int J Med Sci. 2011;8:564–72. doi: 10.7150/ijms.8.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang SJ, Wu XS, Han ZH, Zhang XX, Wang CM, Li XY, et al. Neuregulin-1 preconditioning protects the heart against ischemia/reperfusion injury through a PI3K/Akt-drpendent mechanism. Chin Med J (Engl) 2011;124:1683–8. [PubMed] [Google Scholar]
- 15.Charan Sahoo K, Arora S, Goyal S, Kishore K, Ray R, Chandra Nag T, et al. Cardioprotective effects of benazepril, an angiotensin-converting enzyme inhibitor, in an ischaemia-reperfusion model of myocardial infarction in rats. J Renin Angiotensin Aldosterone Syst. 2009;10:201–9. doi: 10.1177/1470320308353059. [DOI] [PubMed] [Google Scholar]
- 16.Kheradmand A, Dezfoulian O, Alirezaei M. Ghrelin regulates Bax and PCNA but not Bcl-2 expressions following scrotal hyperthermia in the rat. Tissue Cell. 2012;44:308–15. doi: 10.1016/j.tice.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 17.Koumura A, Hamanaka J, Kawasaki K, Tsuruma K, Shimazawa M, Hozumi I, et al. Fasudil and ozagrel in combination show neuroprotective effects on cerebral infarction after murine middle cerebral artery occlusion. J Pharmacol Exp Ther. 2011;338:337–44. doi: 10.1124/jpet.110.177675. [DOI] [PubMed] [Google Scholar]
- 18.Zhang S, He B, Ge J, Zhai C, Liu X, Liu P. Characterization of chemical composition of Agaricus brasiliensis polysaccharides and its effect on myocardial SOD activity, MDA and caspase-3 level in ischemia-reperfusion rats. Int J Biol Macromol. 2010;46:363–6. doi: 10.1016/j.ijbiomac.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Yang T, Zhu H, Zhou H, Lin QL, Li WJ, Liu JW. Rice protein hydrolysate attenuates hydrogen peroxide induced apoptosis of myocardiocytes H9c2 through the Bcl-2/Bax pathway. Food Res Int. 2012;48:736–41. [Google Scholar]
- 20.Ishihara Y, Shimamoto N. Sulfaphenazole Attenuates Myocardial Cell Apoptosis Accompanied With Cardiac Ischemia-Reperfusion by Suppressing the Expression of BimEL and Noxa. J Pharmacol Sci. 2012;119:251–9. doi: 10.1254/jphs.12079fp. [DOI] [PubMed] [Google Scholar]
- 21.Ding HS, Yang J, Chen P, Yang J, Bo SQ, Ding JW, et al. The HMGB1-TLR4 axis contributes to myocardial ischemia/reperfusion injury via regulation of cardiomyocyte apoptosis. Gene. 2013;527:389–93. doi: 10.1016/j.gene.2013.05.041. [DOI] [PubMed] [Google Scholar]


