Abstract
Objectives:
The present study evaluates the hepatoprotective activity of N-acetyl cysteine (NAC) against carbamazepine (CBZ)-induced hepatotoxicity.
Materials and Methods:
Rats were treated with CBZ (50 mg/kg p.o.) and CBZ supplemented with NAC 50, 100 and 200 mg/kg for 45 days, after which blood samples were collected and subjected to liver function tests. Animals were killed, liver was separated, weighed and the levels of antioxidants and liver enzymes were estimated. In addition, histopathological investigation was also performed.
Results:
Serum glutamate pyruvate transaminase (SGPT), serum glutamate oxaloacetate (SGOT) transaminase, alkaline phosphatase (ALP), bilirubin, lipid peroxidation, absolute and relative liver weights were significantly (P < 0.05) elevated, whereas serum levels of albumin, total protein and body weight were decreased in the CBZ-treated animals. CBZ also produced vacuolar degeneration, centrilobular congestion and hepatic necrosis as evidenced from histopathological report. NAC significantly reduced the levels of serum transaminase, ALP, bilirubin and liver weight and increased the levels of total protein, albumin and body weight.
Conclusion:
It was observed that NAC increased the glutathione (GSH) content, reduced lipid peroxidation and reversed the CBZ-induced histopathological abnormalities. CBZ-induced hepatotoxicity may be due its toxic epoxide metabolite-induced oxidative stress.
KEY WORDS: Carbamazepine, hepatotoxicity, N-acetyl cysteine, oxidative stress
Introduction
Anticonvulsant drugs produce hepatotoxicity and liver function abnormalities.[1] Carbamazepine (CBZ) is an effective antiepileptic drug and a potent inducer of microsomal enzymes in liver. CBZ is metabolized by the hepatic P450 3A family of microsomal enzymes. Hepatic biotransformation is the main route of elimination of CBZ. Epoxidation and hydroxylation are the main metabolic pathways, though conjugation reactions may also have a role.[2] CBZ induces its own metabolism (auto-induction).[3] A transient and asymptomatic elevation of liver enzymes occurs in 25-61% of patients receiving CBZ.[4] The most important metabolic product of CBZ is 10,11-CBZ epoxide, which has been shown to be pharmacologically active.[5] CBZ-associated hepatotoxicity manifests as granulomatous hepatitis, abnormal liver function tests, hepatocellular necrosis, lymphadenopathy and biliary tract infection.[5] Hepatotoxic reactions of CBZ usually occur within 3-4 weeks after the initiation of therapy and are independent of serum CBZ levels. Symptoms usually resolve if the drug is discontinued, however, fatal hepatotoxicity can occur even after early intervention and discontinuation of the drug.[6] CBZ-induced hepatic damage in children and adults. CBZ-induced acute hepatitis and hepatotoxicity is proved to be fatal with parenchymal collapse and bile duct proliferation.[7] CBZ showed pathological liver function tests in children aged 1 year or above.[8] In adults, CBZ showed granulomatous hepatitis, eosinophilic infiltrates, portal inflammation, hepatocellular necrosis, bile duct proliferation, cholangitis and cholestasis.[9] CBZ is a potent enzyme inducer causing increased liver microsomal enzymes, thereby altering the metabolism of lipids, bile acids and bilirubin.[10] CBZ-provoked hepatotoxicity may be due to TNF-α-activated apoptosis, neoantigen formation and genetic or acquired mitochondrial abnormalities.[11] CBZ is found to produce hepatotoxicity shortly after initiating the treatment, usually in 4 weeks, with a range of 1-16 weeks.[12] CBZ-induced hepatotoxicity responds to drug withdrawal and reoccurs with drug rechallenge.[13] The mechanism responsible for aromatic antiepileptic drug (AAED)-induced hepatotoxicity has been attributed to the accumulation of arene oxides, due to defective detoxification by epoxide hydrolase, immune-mediated reactions and by direct toxicity.[14] Despite the mode of cell death (apoptosis, necrosis or autophagy), the different stresses triggered by cytotoxic drugs converge on mitochondria resulting in hepatotoxicity.[15] The idiosyncratic drug-induced hepatotoxicity may be mediated, at least in part, by oxidative stress, characterized by enhanced levels of ROS (e.g. hydroxyl radical, superoxide anion and hydrogen peroxide), due to reduced elimination and/or increased production of these species.[16] In the present study, the oxidative stress as a potential mechanism responsible for AAED-associated hepatotoxicity has been addressed.
Materials and Methods
Animals
The pathogen-free adult male albino wistar rats weighing 150-200 g were used. The rats were housed in polypropylene cages at room temperature (25 ± 3°C) with 12/12 hours light and dark cycle and the animals were fed with a balanced diet and tap water ad libitum. The study protocol was approved by the Institutional Animal Ethical Committee of M.S. Ramaiah College of Pharmacy, Bangalore, Karnataka (Ref. No. 220/abc/CPCSEA).
Study Protocol
The rats were divided into five groups with six animals in each group. The first group served as control and received drinking water orally daily by gavage for 45 days. The second group received 50 mg/kg CBZ dissolved in water daily by oral gavage for 45 days between 11.00 hrs and 12 hrs. Third, fourth and fifth groups received 50, 100 and 200 mg/kg (p.o) of NAC in 0.2% CMC, respectively 1 h prior to administration of 50 mg/kg CBZ for 45 days between 11.00 hours and 12.00 hours. On 45th day of drug administration, the animals were anesthetized under light ether anesthesia and the blood samples were collected from retroorbital plexus for estimation of biochemical parameters such as SGOT, SGPT, ALP, total bilirubin, total protein and albumin. Serum was separated by centrifuging blood at 2500 rpm for 10 min and the levels of SGOT, SGPT, ALP, bilirubin, albumin and total protein were analyzed by using a commercially available enzymatic kit (AGAPPE, India) and an Autoanalyser (Chemistry Analyser (CA 2005), B4B Diagnostic Division, China).
The animals were then killed, liver tissues were isolated and rinsed with cold phosphate buffer (PB, 100 mM, pH 7.4), weighed, sliced for histopathological studies and stored at -40°C. The stored tissues were homogenized and the homogenate was centrifuged at 10,000 Χ g for 10 min at 4°C.
The supernatant was stored at -40°C for further biochemical estimations of endogenous activities of antioxidants such as SOD, catalase and GSH[17] and lipid peroxidation.[18]
Histopathological studies
The histopathological study in liver tissue was conducted according to the method of Li et al., (1998).[19] Rats were anesthetized under ether anesthesia and killed. The liver was fixed in 4% paraformaldehyde overnight. A block was prepared in block preparation unit (Shandon Histocenter-2) and coronal sections (10 μm) were cut with the help of a microtome (Leica RM 2255, Lab India) and picked up on poly-l-lysine-coated slides and were stained with hematoxylin and eosin (HE).
Statistical analysis
The results are expressed as mean ± SEM. Statistical analysis was performed using one-way analysis of variance (ANOVA) with Tukey's post hoc statistical tests using InStat. A P < 0.05 was considered significant.
Results
Effect Of Chronic Treatment Of Cbz And Cbz + Nac On Liver Parameters
SGOT, SGPT, ALP, total bilirubin, total protein and albumin are indicators of hepatic function. The CBZ-treated group showed significantly elevated levels of SGOT, SGPT, ALP and total bilirubin and reduced levels of total protein and albumin (P < 0.05) as compared to the control group. Administration of CBZ along with NAC 50 mg/kg does not show significant difference in the liver parameters. Administration of CBZ along with NAC 100 mg/kg showed mild improvement in the liver parameters. Administration of CBZ along with NAC 200 mg/kg showed significant difference in the liver parameters. Administration of CBZ along with NAC significantly reversed the elevated levels of SGOT, SGPT, ALP and total bilirubin and decreased levels of total protein and albumin (P < 0.05) [Table 1].
Table 1.
Effect of chronic treatment of carbamazepine and carbamazepine+N-acetyl cysteine on liver enzymes, bilirubin, albumin, total protein and liver lipid peroxidation
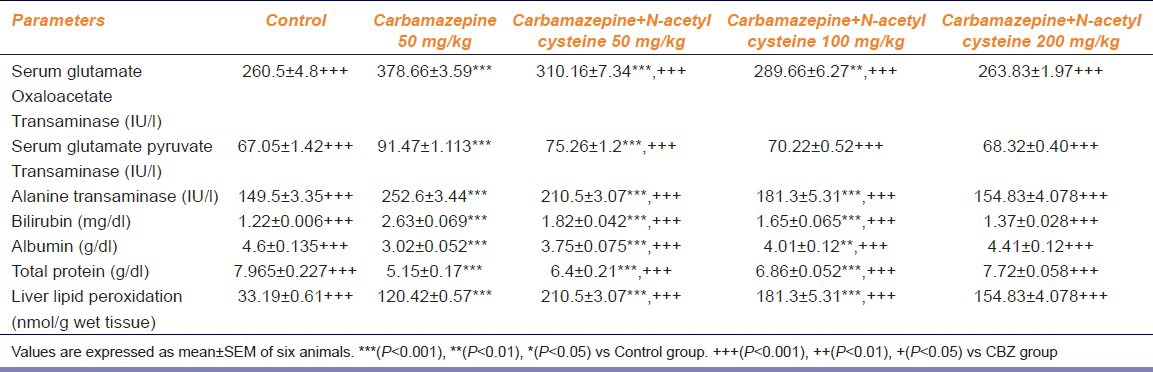
Effect Of Nac On Cbz-Induced Alterations On Liver Lipid Peroxidation
The CBZ treatment significantly increased the liver lipid peroxidation as compared to the control group. CBZ along with NAC 50 and 100 mg/kg does not showed dose-dependent reversal (P < 0.05) in the levels of CBZ-induced elevated lipid peroxidation. CBZ along with NAC (200 mg/kg) showed dose-dependent reversal (P < 0.05) in the levels of CBZ-induced elevated lipid peroxidation [Table 1].
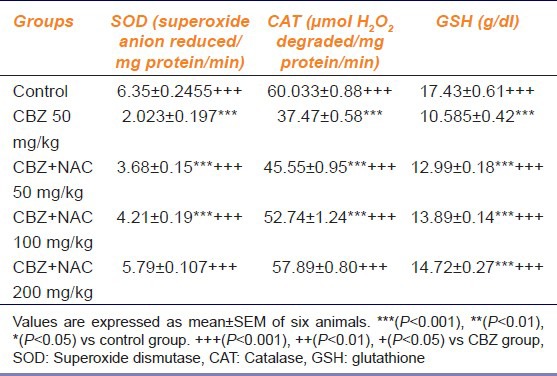
Effect Of Chronic Treatment Of Cbz And Cbz Along With Nac On Sod, Gsh And Catalase
Table 2 shows the effect of chronic treatment of CBZ and CBZ along with NAC on SOD, GSH and catalase. Chronic CBZ treatment significantly decreased the SOD, GSH and catalase levels when compared to control animals. CBZ along with NAC 50 and 100 mg/kg does not showed dose-dependent reversal (P < 0.05) in the levels of CBZ-induced elevated SOD, GSH and catalase. CBZ along with NAC (200 mg/kg) showed dose-dependent reversal (P < 0.05) in the levels of CBZ-induced elevated SOD, GSH and catalase [Table 2].
Table 2.
Effect of carbamazepine and carbamazepine+N-acetyl cysteine on superoxide dismutase, catalase and glutathione
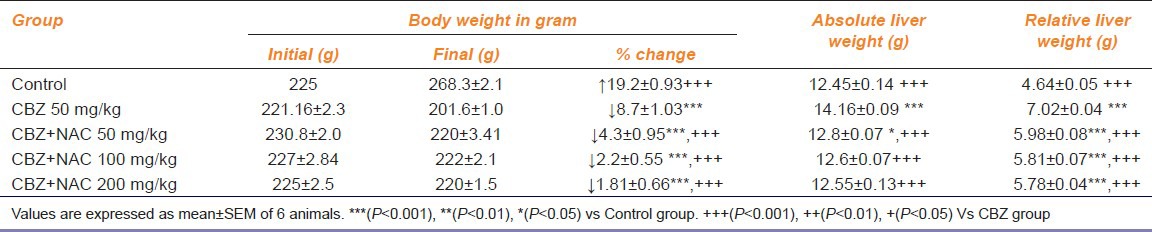
Effect Of Cbz And Cbz Supplemented Nac On Body Weight, Absolute And Relative Liver Weight
the end of 45 days treatment with CBZ, there was a significant decrease in body weight and an increase in the absolute and relative liver weights when compared to the control group (P < 0.05). NAC at the dose of 50, 100 and 200 mg/kg reversed the CBZ decreased body weight and CBZ increased the absolute and relative liver weights compared with the CBZ group (P < 0.05) [Table 3].
Table 3.
Effect of carbamazepine and carbamazepine+N-acetyl cysteine on body weight, absolute and relative liver weight
Histopathological Changes
Figure 1a reveals normal hepatic architecture, which radiates from the central vein to the lobular periphery in the control group. In the CBZ-treated group some of the hepatocytes showed hemorrhage, centrilobular and sinusoidal congestion revealing hepatic damage [Figure 1b]. CBZ + NAC at a dose of 50 mg/kg showed interlobular fibrosis [Figure 1c]. CBZ + 100 mg/kg NAC showed sinusoids with mild congestion [Figure 1d]. CBZ + 200 mg/kg NAC showed a normal periportal area and appeared similar to the control liver [Figure 1e].
Figure 1.
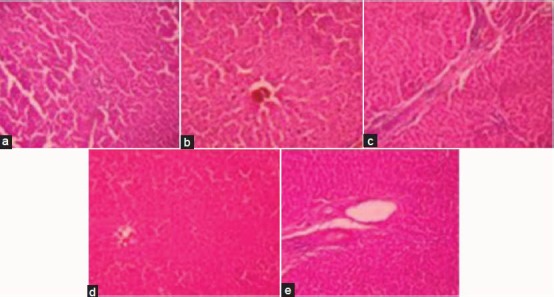
Effect of carbamazepine on liver histopathology a - Control b - CBZ Effect of N-acetyl cysteine on carbamazepine-induced alterations in liver histopathology c - CBZ + NAC (50 mg/kg) d - CBZ + NAC (100 mg/kg) e - CBZ + NAC (200 mg/kg)
Discussion
A study by Eghba et al. showed that CBZ induced oxidative stress, increased ROS formation and lipid peroxidation products and also decreased mitochondrial membrane potential. CBZ caused a decrease in cellular GSH content and an elevation in oxidized GSH levels. Their investigation showed that taurine could alleviate oxidative damages induced by CBZ and melatonin acts as a good antioxidant to protect hepatocytes against cytotoxicity induced by CBZ. It was concluded that taurine and melatonin are effective antioxidants to prevent CBZ-induced hepatotoxicity.[20] Adikwu and Deo concluded that vitamin C has a hepatoprotective effect, owing to its antioxidant property. Vitamin C was reported to normalize the levels of serum alanine aminotransferase, aspartate aminotransferase, gamma glutamine, ALP, lactate dehydrogenase (LDH) and malondialdehyde (MDA) and serum bilirubin in intoxicated animals. So, NAC also possess the same property as antioxidant and exerts hepatoprotection and reduces lipid peroxidation due to its antioxidant activity.[21] In our previous study, it was concluded that the administration of vitamin C exerted hepatoprotective activity against CBZ-induced hepatotoxicity.[22]
In the present study, it was observed that the CBZ treatment significantly increased the levels of SGOT, SGPT and bilirubin, the markers of hepatotoxicity and decreased the levels of albumin and total protein. Supplementation with NAC decreased the markers of hepatotoxicity and exerted significant protection against CBZ-induced toxicity by its ability to decrease the lipid peroxidation and thus oxidative stress through its free radical-scavenging activity, which improved the levels of antioxidant defense system. NAC at 50 and 100 mg/kg showed interlobular fibrosis and mild sinusoidal congestion. NAC at 200 mg/kg improved the hepatic histopathological damages induced by CBZ. This study revealed the hepatoprotective potential of NAC against CBZ-induced liver damage.
Diazinon is an organophosphate compound that is widely used in agriculture; NAC (160 mg/kg, i.p.) significantly reversed the diazion (100 mg/kg)-induced hepatotoxicity as observed by reversal of elevated markers of hepatotoxicity.[23] Manov et al. found that in hepatoma-derived cells, acetaminophen caused cytotoxic effects leading to oxidative stress, mitochondrial dysfunction, alterations of membrane permeability and apoptosis.[24] In the course of acetaminophen cytotoxicity, the generation of ROS appears as an early event, which is preceded by LDH leakage, GSH depletion and apoptosis. NAC protected hepatoma-derived cells from acetaminophen-induced oxidative injury. NAC pretreatment partially decreased the covalent binding of CCl4-reactive metabolites at 1st and 3rd hour of poisoning and to a small extent, the concentration of CCl4 reaching the liver at 3rd hour. NAC also diminished partially the CCl4-induced increase in lipid peroxidation at 3rd hour. The results suggested that early and late protective effects of NAC might be attributable to its prior conversion to cysteine and GSH.[25] Ethanol treatment resulted in steatosis, inflammatory infiltrates, occasional foci of necrosis, elevated ALT, accumulation of MDA and hydroxynonenal protein adducts, decreased antioxidant capacity, and increased antibody titers toward serum hydroxyethyl radical, MDA and hydroxynonenal adducts. NAC treatment increased cytosolic antioxidant capacity, abolished ethanol-induced lipid peroxidation, and inhibited the formation of antibodies toward hydroxynonenal and hydroxyethyl radical adducts.[26] N-acetyl cysteine (NAC) decreased the mortality rate, partly prevented the paracetamol-induced liver damage and partly restored enzyme activities. NAC and S-carboxymethyl cysteine markedly inhibited the rate of covalent binding.[27]
Wong et al. investigated the protective effect of NAC against CCl4 and trichloroethylene-induced hepatotoxicity in rats. Rats administered with higher dose of NAC caused significant reduction in SGPT and SGOT levels. They concluded that NAC effectively protects against CCl4 and trichloroethylene-induced hepatotoxicity in rats.[28] Azathioprine (AZA) decreased the viability of cultured hepatocytes, depleted intracellular GSH, reduced metabolic activity, mitochondrial swelling, increased oxygen consumption of intact mitochondria and LDH release. NAC reversed AZA-induced hepatotoxicity in humans by increasing cytosolic GSH.[29]
AZA (15 mg/kg, i.p.) increased the activity of hepatic aminotransferases, liver lipid peroxides, and reduced the GSH contents in liver. The protective effect of aminoguanidine against the enzyme leakage seems to be through the liver cell membrane permeability restoration.[30] Pretreatment with NAC attenuated the ischemia and reperfusion-induced liver injury by scavenging the radicals and by increasing the SOD and catalase levels.[31]
In the present study NAC decreased the lipid peroxidation by restoring the depleted Cat, SOD and GSH levels, whereby prevented the hepatotoxicity as it is a precursor for GSH.
Conclusion
According to our study results, it could be concluded that NAC acts as an effective antioxidant in the prevention of CBZ-induced hepatotoxicity. Administration of NAC produced a significant hepatoprotective effect and effectively reduced lipid peroxidation. We recommend further clinical trial studies on the antioxidant effect of NAC in patients receiving CBZ.
Footnotes
Source of Support: Nil
Conflict of Interest: No
References
- 1.Wyllie E, Wyllie R. Routine laboratory monitoring for serious adverse effects of antiepileptic medications: The controversy. Epilepsia. 1991;32:S74–9. [PubMed] [Google Scholar]
- 2.Bernus I, Dickinson RG, Hooper WD, Eadie MJ. Dose-dependent metabolism of carbamazepine in humans. Epilepsy Res. 1996;24:163–72. doi: 10.1016/0920-1211(96)00011-3. [DOI] [PubMed] [Google Scholar]
- 3.Scheyer RD, Cramer JA, Mattson RH. A pharmacodynamic approach to the estimate of carbamazepine autoinduction. J Pharm Sci. 1994;83:491–4. doi: 10.1002/jps.2600830409. [DOI] [PubMed] [Google Scholar]
- 4.Benedetti MS, Ruty B, Baltes E. Induction of endogenous pathways by antiepileptics and clinical implications. Fundam Clin Pharmacol. 2005;19:511–29. doi: 10.1111/j.1472-8206.2005.00341.x. [DOI] [PubMed] [Google Scholar]
- 5.Driefus FE, Langer DH. Hepatic considerations in the use of antiepileptic drugs. Epilepsia. 1987;28:S23–9. doi: 10.1111/j.1528-1157.1987.tb05768.x. [DOI] [PubMed] [Google Scholar]
- 6.Hopen G, Nesthus I, Laerum OD. Fatal CBZ-associated hepatitis. Report of two cases. Acta Med Scand. 1981;210:333–5. [PubMed] [Google Scholar]
- 7.Zucker P, Daum F, Cohen MI. Fatal carbamazepine hepatitis. J Pediatr. 1977;91:667–8. doi: 10.1016/s0022-3476(77)80529-5. [DOI] [PubMed] [Google Scholar]
- 8.Pellock JM. Carbamazepine side effects in children and adults. Epilepsia. 1987;28:S64–70. doi: 10.1111/j.1528-1157.1987.tb05780.x. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell MC, Boitnott JK, Arregui A, Maddrey WC. Granulomatous hepatitis associated with carbamazepine therapy. Am J Med. 1981;71:733–5. doi: 10.1016/0002-9343(81)90244-8. [DOI] [PubMed] [Google Scholar]
- 10.Havel RJ, Kane JP. Structure and metabolism of plasma lipoproteins. In: Secriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and molecular basis of inherited disease. New York: McGraw-Hill; 1995. pp. 1841–51. [Google Scholar]
- 11.Santos NA, Medina WS, Martins NM, Mingatto FE, Curti C, Santos AC. Aromatic antiepileptic drugs and mitochondrial toxicity: Effects on mitochondria isolated from rat liver. Toxicol In Vitro. 2008;22:1143–52. doi: 10.1016/j.tiv.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Zaccara G, Franciotta D, Perucca E. Idiosyncratic adverse reactions to antiepileptic drugs. Epilepsia. 2007;48:1223–44. doi: 10.1111/j.1528-1167.2007.01041.x. [DOI] [PubMed] [Google Scholar]
- 13.Horowitz S, Patwardhan R, Marcus E. Hepatotoxic reactions associated with carbamazepine therapy. Epilepsia. 1988;29:149–54. doi: 10.1111/j.1528-1157.1988.tb04411.x. [DOI] [PubMed] [Google Scholar]
- 14.Bavdekar SB, Muranjan MN, Gogtay NJ, Kantharia V, Kshirsagar NA. Anticonvulsant hypersensitivity syndrome: Lymphocyte toxicity assay for the confirmation of diagnosis and risk assessment. Ann Pharmacother. 2004;38:1648–50. doi: 10.1345/aph.1E042. [DOI] [PubMed] [Google Scholar]
- 15.Kass GE. Mitochondrial involvement in drug-induced hepatic injury. Chem Biol Interact. 2006;163:145–59. doi: 10.1016/j.cbi.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 16.Nitti M, Pronzato MA, Marinari UM, Domenicotti C. PKC signaling in oxidative hepatic damage. Mol Aspects Med. 2008;29:36–42. doi: 10.1016/j.mam.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Sedlak J, Lindsay RH. Estimation of total, protein bound and non-protein SH groups in tissue with Ellman's reagent. Anal Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 18.Mihara M, Uchiyama M. Determination of malonaldehyde precursors in tissues by thiobarbituric acid test. Ann Biochem. 1978;86:271–8. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Powers C, Jiang N, Chopp M. Intact, injured, necrotic and apoptotic cells after focal cerebral ischemia in the rat. J Neurol Sci. 1998;156:119–32. doi: 10.1016/s0022-510x(98)00036-7. [DOI] [PubMed] [Google Scholar]
- 20.Eghbal MA, Taziki S, Sattari MR. Protective role of melatonin and taurine against carbamazepine-induced toxicity in freshly isolated rat hepatocytes. Int J Morphol. 2013;1:1081–9. [Google Scholar]
- 21.Adikwu E, Deo O. Hepatoprotective Effect of Vitamin C (Ascorbic Acid) Pharmacol Pharm. 2013;4:84–92. [Google Scholar]
- 22.Santhrani T, Maheswari E, Saraswathy GR. Carbamazepine provoked hepatotoxicity: Attenuation by vitamin C. Oxid Antioxid Med Sci. 2013;2:37–43. [Google Scholar]
- 23.Salehi M, Jafari M, Asgari A. The protective effect of N-Acetyl-Cysteine on DZN-induced hepatotoxicity. Clin Biochem. 2011;44:S123. [Google Scholar]
- 24.Manov I, Hirsh M, Iancu TC. Acetaminophen hepatotoxicity and mechanisms of its protection by N-acetylcysteine: A study of Hep3B cells. Exp Toxicol Pathol. 2002;53:489–500. doi: 10.1078/0940-2993-00215. [DOI] [PubMed] [Google Scholar]
- 25.Valles EG, de Castro CR, Castro JA. N-Acetyl cysteine is an early but also a late preventive agent against carbon tetrachloride-induced liver necrosis. Toxicol Lett. 1994;71:87–95. doi: 10.1016/0378-4274(94)90202-x. [DOI] [PubMed] [Google Scholar]
- 26.Ronis MJ, Butura A, Sampey BP, Shankar K, Prior RL, Korourian S, et al. Effects of N-acetylcysteine on ethanol-induced hepatotoxicity in rats fed via total enteral nutrition. Free Radic Biol Med. 2005;39:619–30. doi: 10.1016/j.freeradbiomed.2005.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ioannides C, Hall DE, Mulder DE, Steele CM, Spickett J, Delaforge M, et al. A comparison of the protective effects of N-acetylcysteine and S-carboxymethylcysteine against paracetamol-induced hepatotoxicity. Toxicology. 1983;28:313–21. doi: 10.1016/0300-483x(83)90005-7. [DOI] [PubMed] [Google Scholar]
- 28.Wong CK, Ooi VE, Wong CK. Protective effects of N-acetylcysteine against carbon tetrachloride and trichloroethylene induced poisoning in rats. Environ Toxicol Pharmacol. 2003;14:109–16. doi: 10.1016/S1382-6689(03)00045-0. [DOI] [PubMed] [Google Scholar]
- 29.Menor C, Cara CJ, Fernandez-Moreno MD, Fueyo JA, Escribano O, Olleros T, et al. Protective role and molecular basis of N-acetyl-L-cysteine usage in azathioprine-induced rat hepatocyte necrosis. Gastroenterology. 2003;124:A723. [Google Scholar]
- 30.Raza M, Ahmad M, Gado A, Al-Shabanah OA. A comparison of hepatoprotective activities of aminoguanidine and N-acetylcysteine in rat against the toxic damage induced by azathioprine. Comp Biochem Physiol C Toxicol Pharmacol. 2003;134:451–6. doi: 10.1016/s1532-0456(03)00022-x. [DOI] [PubMed] [Google Scholar]
- 31.Chen CF, Hsueh CW, Tang TS, Wang D, Shen CY, Pei JS. Reperfusion liver injury-induced superoxide dismutase and catalase expressions and the protective effects of N-acetyl cysteine. Transplant Proc. 2007;39:858–60. doi: 10.1016/j.transproceed.2007.02.018. [DOI] [PubMed] [Google Scholar]


