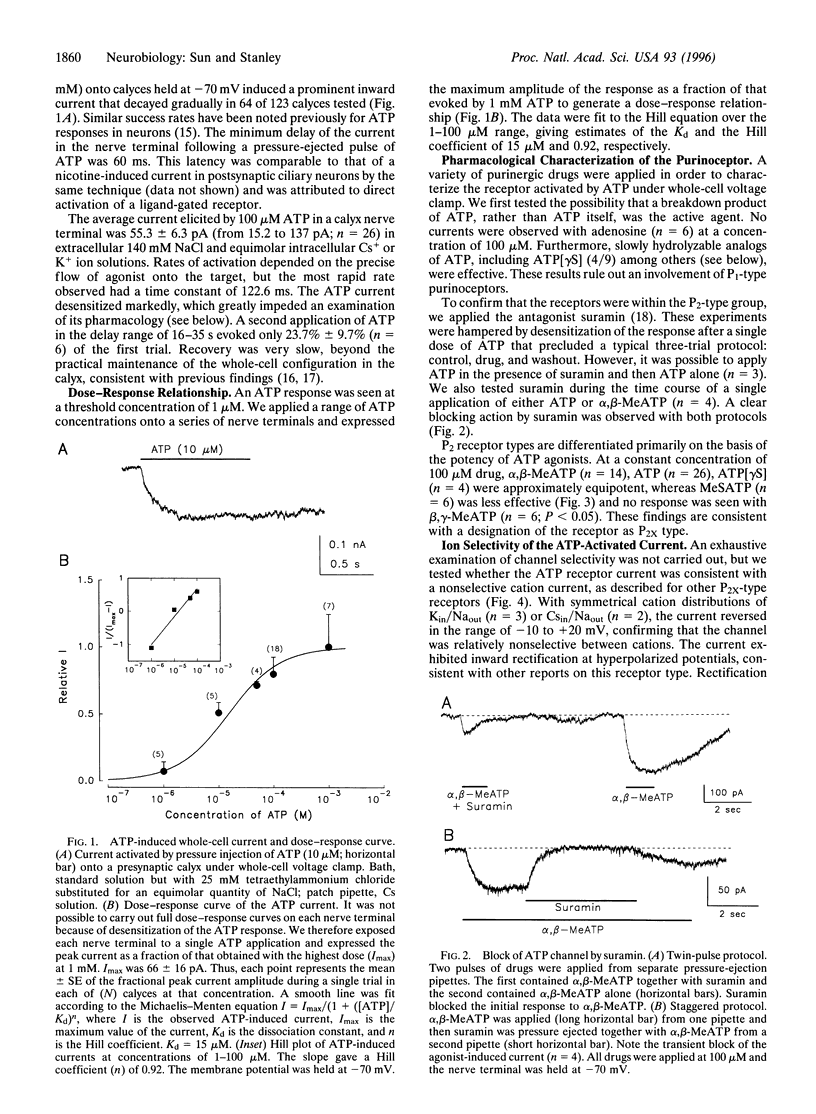
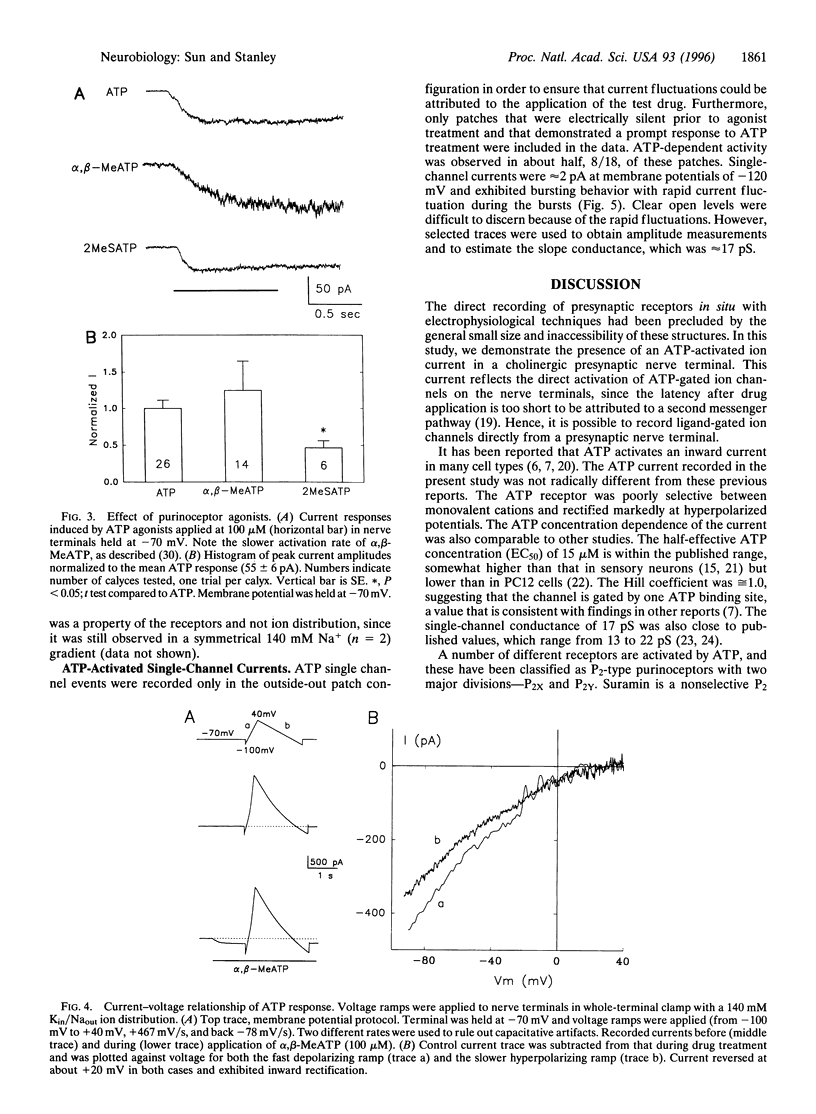
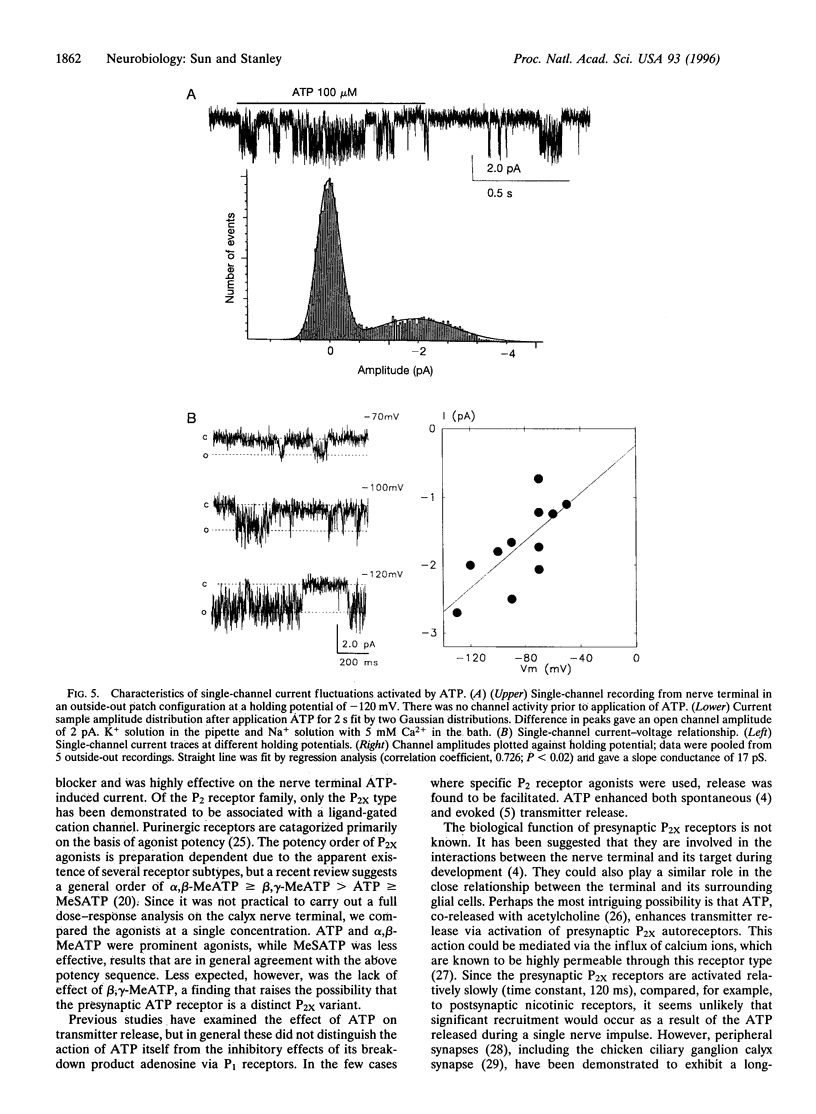
Abstract
ATP has recently been identified as a fast neurotransmitter in both the central and peripheral nervous systems. Several studies have suggested that ATP can also affect the release of classical neurotransmitters, including acetylcholine with which it is co-released. We have searched for ATP receptors on a cholinergic presynaptic nerve terminal using the calyx-type synapse of the chicken ciliary ganglion. ATP was pulsed onto the terminals under voltage clamp and induced a short latency cation current that exhibited inward rectification and marked desensitization. This current was not seen with adenosine but was mimicked by several sterically restricted ATP analogs and was blocked by suramin. ATP-activated single ion channels exhibited prominent flickering and had a conductance of approximately 17 pS. Our results demonstrate a ligand-gated P2X-like purinergic receptor on a cholinergic presynaptic nerve terminal.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbracchio M. P., Burnstock G. Purinoceptors: are there families of P2X and P2Y purinoceptors? Pharmacol Ther. 1994;64(3):445–475. doi: 10.1016/0163-7258(94)00048-4. [DOI] [PubMed] [Google Scholar]
- Bean B. P. ATP-activated channels in rat and bullfrog sensory neurons: concentration dependence and kinetics. J Neurosci. 1990 Jan;10(1):1–10. doi: 10.1523/JNEUROSCI.10-01-00001.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P. Pharmacology and electrophysiology of ATP-activated ion channels. Trends Pharmacol Sci. 1992 Mar;13(3):87–90. doi: 10.1016/0165-6147(92)90032-2. [DOI] [PubMed] [Google Scholar]
- Bennett M. R., Ho S. Probabilistic secretion of quanta from nerve terminals in avian ciliary ganglia modulated by adenosine. J Physiol. 1991;440:513–527. doi: 10.1113/jphysiol.1991.sp018722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Kennedy C. Is there a basis for distinguishing two types of P2-purinoceptor? Gen Pharmacol. 1985;16(5):433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- Dubyak G. R., el-Moatassim C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am J Physiol. 1993 Sep;265(3 Pt 1):C577–C606. doi: 10.1152/ajpcell.1993.265.3.C577. [DOI] [PubMed] [Google Scholar]
- Dunn P. M., Blakeley A. G. Suramin: a reversible P2-purinoceptor antagonist in the mouse vas deferens. Br J Pharmacol. 1988 Feb;93(2):243–245. doi: 10.1111/j.1476-5381.1988.tb11427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F. A. ATP receptors. Curr Opin Neurobiol. 1994 Jun;4(3):347–352. doi: 10.1016/0959-4388(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Fu W. M., Poo M. M. ATP potentiates spontaneous transmitter release at developing neuromuscular synapses. Neuron. 1991 May;6(5):837–843. doi: 10.1016/0896-6273(91)90179-4. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hume R. I., Honig M. G. Excitatory action of ATP on embryonic chick muscle. J Neurosci. 1986 Mar;6(3):681–690. doi: 10.1523/JNEUROSCI.06-03-00681.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue R., Brading A. F. The properties of the ATP-induced depolarization and current in single cells isolated from the guinea-pig urinary bladder. Br J Pharmacol. 1990 Jul;100(3):619–625. doi: 10.1111/j.1476-5381.1990.tb15856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh B. S., Surprenant A., Humphrey P. P. A study on P2X purinoceptors mediating the electrophysiological and contractile effects of purine nucleotides in rat vas deferens. Br J Pharmacol. 1995 May;115(1):177–185. doi: 10.1111/j.1476-5381.1995.tb16336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishtal O. A., Marchenko S. M., Pidoplichko V. I. Receptor for ATP in the membrane of mammalian sensory neurones. Neurosci Lett. 1983 Jan 31;35(1):41–45. doi: 10.1016/0304-3940(83)90524-4. [DOI] [PubMed] [Google Scholar]
- Nakazawa K., Inoue K., Fujimori K., Takanaka A. ATP-activated single-channel currents recorded from cell-free patches of pheochromocytoma PC12 cells. Neurosci Lett. 1990 Oct 30;119(1):5–8. doi: 10.1016/0304-3940(90)90741-q. [DOI] [PubMed] [Google Scholar]
- Nakazawa K., Inoue K., Fujimori K., Takanaka A. Effects of ATP antagonists on purinoceptor-operated inward currents in rat phaeochromocytoma cells. Pflugers Arch. 1991 Apr;418(3):214–219. doi: 10.1007/BF00370517. [DOI] [PubMed] [Google Scholar]
- Scott T. R., Bennett M. R. The effect of ions and second messengers on long-term potentiation of chemical transmission in avian ciliary ganglia. Br J Pharmacol. 1993 Sep;110(1):461–469. doi: 10.1111/j.1476-5381.1993.tb13833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky E. M., Gerzanich V. On the excitatory effects of ATP and its role as a neurotransmitter in coeliac neurons of the guinea-pig. J Physiol. 1993 May;464:197–212. doi: 10.1113/jphysiol.1993.sp019630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky E. M., Ginsborg B. L. Inhibition of acetylcholine release from preganglionic frog nerves by ATP but not adenosine. Nature. 1983 Sep 22;305(5932):327–328. doi: 10.1038/305327a0. [DOI] [PubMed] [Google Scholar]
- Silinsky E. M., Hunt J. M., Solsona C. S., Hirsh J. K. Prejunctional adenosine and ATP receptors. Ann N Y Acad Sci. 1990;603:324–334. doi: 10.1111/j.1749-6632.1990.tb37683.x. [DOI] [PubMed] [Google Scholar]
- Sperlagh B., Vizi E. S. Effect of presynaptic P2 receptor stimulation on transmitter release. J Neurochem. 1991 May;56(5):1466–1470. doi: 10.1111/j.1471-4159.1991.tb02039.x. [DOI] [PubMed] [Google Scholar]
- Stanley E. F. Calcium currents in a vertebrate presynaptic nerve terminal: the chick ciliary ganglion calyx. Brain Res. 1989 Dec 29;505(2):341–345. doi: 10.1016/0006-8993(89)91465-0. [DOI] [PubMed] [Google Scholar]
- Stanley E. F., Goping G. Characterization of a calcium current in a vertebrate cholinergic presynaptic nerve terminal. J Neurosci. 1991 Apr;11(4):985–993. doi: 10.1523/JNEUROSCI.11-04-00985.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley E. F. Single calcium channels on a cholinergic presynaptic nerve terminal. Neuron. 1991 Oct;7(4):585–591. doi: 10.1016/0896-6273(91)90371-6. [DOI] [PubMed] [Google Scholar]
- Surprenant A., Buell G., North R. A. P2X receptors bring new structure to ligand-gated ion channels. Trends Neurosci. 1995 May;18(5):224–229. doi: 10.1016/0166-2236(95)93907-f. [DOI] [PubMed] [Google Scholar]
- Thomas S. A., Hume R. I. Irreversible desensitization of ATP responses in developing chick skeletal muscle. J Physiol. 1990 Nov;430:373–388. doi: 10.1113/jphysiol.1990.sp018296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann H. Signalling via ATP in the nervous system. Trends Neurosci. 1994 Oct;17(10):420–426. doi: 10.1016/0166-2236(94)90016-7. [DOI] [PubMed] [Google Scholar]



