Abstract
We report the case of a young woman who presented with progressive dysphagia and swelling in the anterior aspect of the neck of short duration. On evaluation, she was diagnosed with amelanotic malignant melanoma of the cervical oesophagus. She underwent total laryngopharyngo-oesophagectomy with gastric transposition with bilateral modified radical neck dissection with feeding jejunostomy and a permanent tracheostomy with postoperative combined chemoradiation therapy. However, in spite of aggressive treatment, the patient expired 8 months after initial presentation with distant metastasis.
Background
Primary malignant melanoma is an uncommon tumour in the upper gastrointestinal (GI) tract and accounts for 0.1–0.2% of all primary malignancies of the oesophagus.1–4 It was first described by Baur5 in 1906. The first case of primary malignant melanoma of the oesophagus (PMME) was reported by Garfinkle and Cahan6 in 1952. PMME is commonly seen in the elderly and usually in the distal oesophagus.1 2 4 7 8 Presentation is usually late with poor prognosis despite advances in treatment modalities.1 Three hundred and thirty-seven cases of PMME have been reported in the world literature till 2011.8 We present a case of an amelanotic variant of malignant melanoma of the upper one-third of the oesophagus in a young woman with rapid progression of the disease with eventual mortality and the review of literature regarding the malignancy and its management.
Case presentation
A 24-year-old woman presented to the outpatient department of our tertiary care hospital with progressive dysphagia and swelling in front of the neck of 1-month duration. Dysphagia initially started for solids, which however over a month progressed to absolute dysphagia. She also reported a swelling in the front of the neck which initially started off as a diffuse swelling in the lower part of the neck and gradually progressed over a period of 1 month to involve the entire anterior aspect of the neck. There was no history of odynophagia, fever, hoarseness of voice, cough on intake of food, loss of appetite, loss of weight or referred otalgia. There was no history of smoking, tobacco chewing or alcohol consumption. The patient's history and family history were unremarkable.
On examination, there was no evidence of skin or eye melanomas. The patient was pale. Examination of the neck revealed a diffuse bulge in the anterior aspect of the neck extending from the hyoid bone above, to the sternal notch below, and the entire laryngeal framework appeared to be pushed forward. There was splaying of the thyroid ala on both sides (figure 1). The skin over the swelling appeared normal. There was no local rise of temperature. The swelling was firm and non-tender. There were no palpable lymph nodes. Laryngeal crepitus was absent. The carotid pulse was bilaterally normal and equal. Indirect laryngoscopy examination revealed a diffuse bulge in the posterior pharyngeal wall with intact mucosa in the hypopharynx. The lower extent of the posterior pharyngeal wall bulge could not be visualised. There was pooling of saliva in both pyriform fossae. The endolarynx was normal with mobile vocal cords and an adequate glottis chink. Ear, nose and oral cavity examination was normal. Systemic examination was normal.
Figure 1.
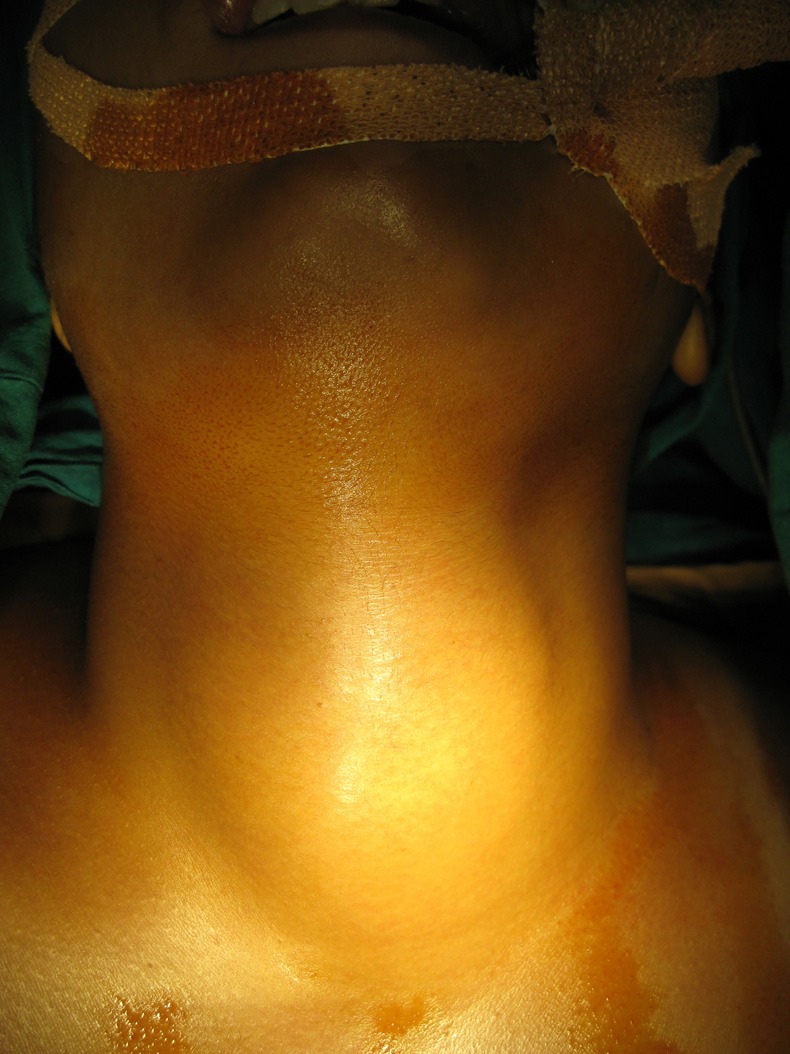
Swelling in the neck with splaying of the thyroid ala.
Investigations
Complete blood count revealed low haemoglobin. Upper GI endoscopy revealed a hard mass in the posterior pharyngeal wall region (figure 2). The lower extent of the mass could not be ascertained since the scope could not be negotiated beyond the cricopharyngeal sphincter. MRI of the neck and chest revealed a well-defined polypoidal lesion arising from the anterior wall of the proximal cervical oesophagus (C5-D1 vertebra) measuring 4.3×4.2×2.6 cm that was mildly hyperintense to the muscle on T1-weighted, heterogeneously hyperintense on T2-weighted and showed heterogeneous contrast enhancement with non-enhancing necrotic areas within it. Anteriorly, the lesion was abutting the cervical tracheal air column without infiltrating it, and posteriorly it was abutting the prevertebral muscles with focal effacement of the fat planes. Laterally, the lesion was abutting the carotid sheath without infiltration. Enlarged lymph nodes were noted in level VI and level II bilaterally (figures 3 and 4).
Figure 2.
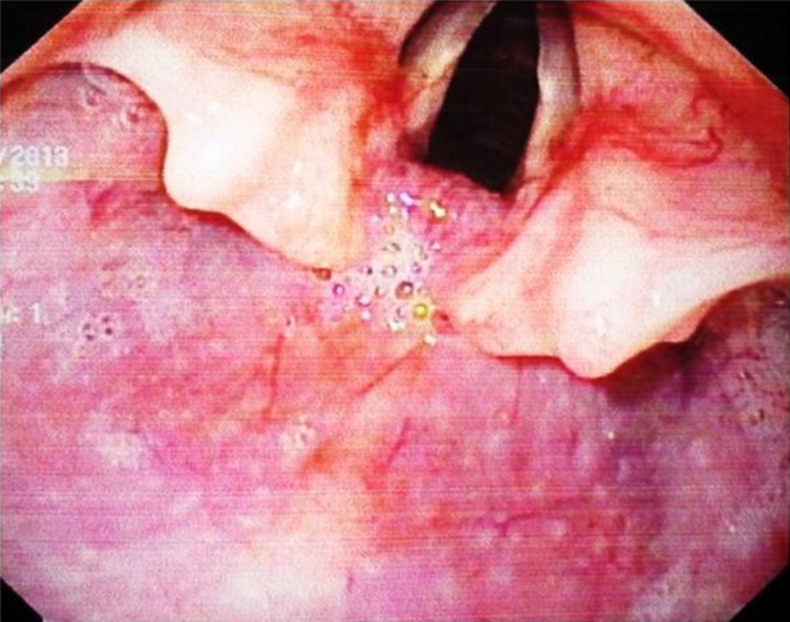
Upper gastrointestinal scopy showing the bulge in the posterior pharyngeal wall.
Figure 3.
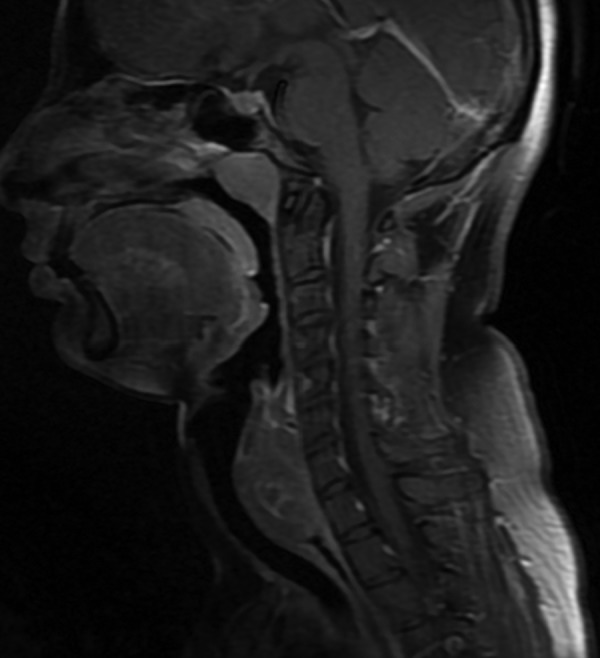
Sagittal T1-weighted MRI showing the tumour extending from C5 to D1 vertebra.
Figure 4.
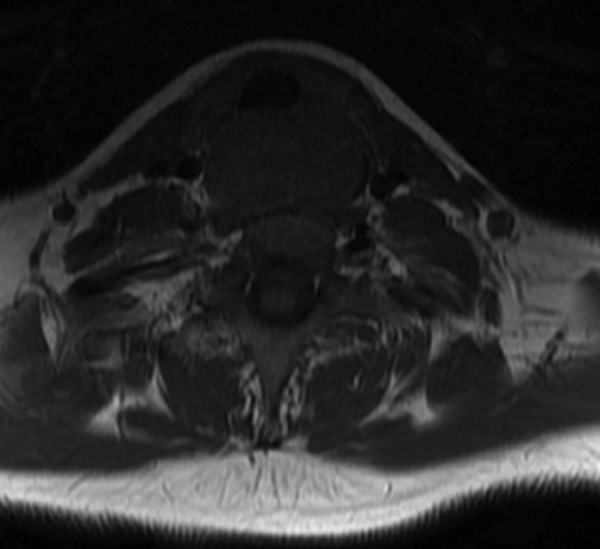
Axial T1-weighted MRI showing the tumour abutting the trachea anteriorly, carotid sheaths laterally and prevertebral area posteriorly.
The patient underwent hypopharyngoscopy and incision biopsy of the lesion and the tissue obtained was sent for histopathological examination. Histopathological examination revealed sheets and masses of malignant cells with pleomorphic hyperchromatic nuclei, and prominent nucleoli with eosinophilic cytoplasm consistent with malignant melanoma (figure 5). Immunohistochemical staining (IHC) of the specimen was positive for S100 (figure 6), HMB45 (figure 7) and negative for CK, CD117 and leukocyte common antigen. Hence, a final diagnosis of amelanotic malignant melanoma of the oesophagus was made.
Figure 5.
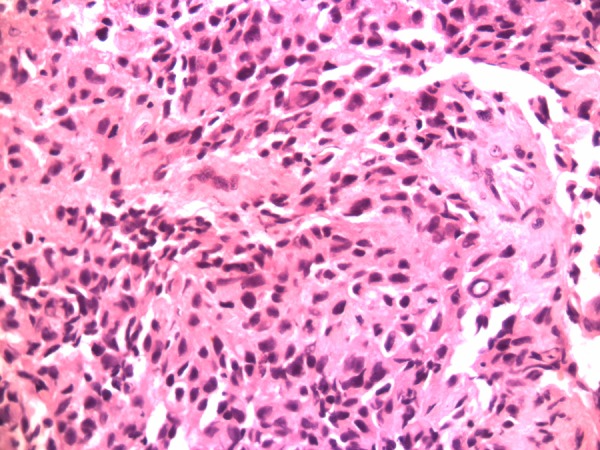
H&E staining (×40 magnification) showing sheets of malignant cells with pleomorphic hyperchromatic nuclei, prominent nucleoli with eosinophilic cytoplasm.
Figure 6.
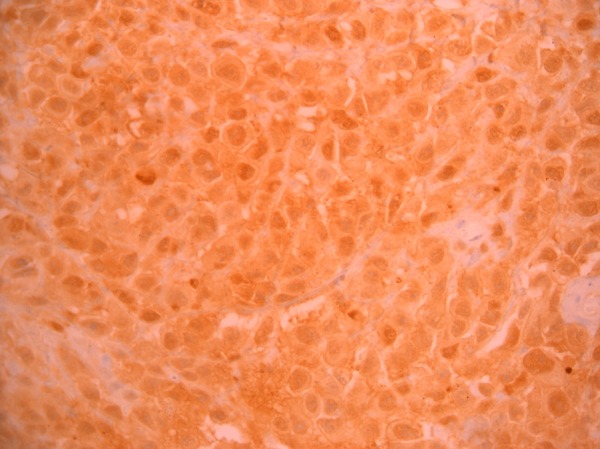
S100 immunohistochemical staining (×40 magnification) showing positivity.
Figure 7.
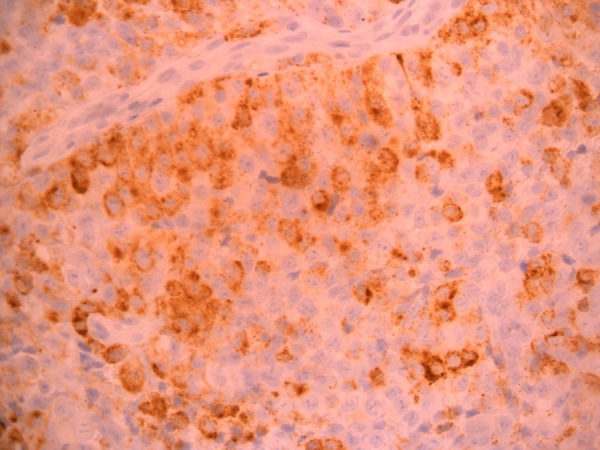
HMB45 immunohistochemical staining (×40 magnification) showing positivity.
Further, the patient underwent ultrasound of the abdomen and pelvis and a bone scan to detect other foci of melanoma but no other foci were found.
Differential diagnosis
Malignancy postcricoid area.
Treatment
The patient was advised interferon therapy with a guarded prognosis, but she refused due to financial constraints. She reviewed 1 week later with mild stridor and consented to a surgical procedure. She underwent a preliminary endoscopic examination to determine the lower extent of the tumour; however, the lower extent could not be ascertained and surgical exposure of the neck was carried out using a Gluck-Sorenson incision and she underwent total laryngopharyngo-oesophagectomy (TLPE) with gastric transposition with bilateral modified radical neck dissection with feeding jejunostomy and a permanent tracheostomy. Intraoperatively, the tumour was seen to extend from the postcricoid area to the cervical oesophagus (figure 8), which affirmed the decision to do a TLPE with gastric transposition. The main operative specimen (figure 9) showed invasion into the thyroid on histopathology and lymph node metastasis was observed in the bilateral level II, level V and level VI lymph nodes. She underwent external beam radiation therapy of 60 Gy in 30 fractions over 6 weeks to the neck and chest along with oral temozolomide-based chemotherapy of dose 100 mg daily over 6 weeks. She was advised to take oral temozolomide chemotherapy of dose 250 mg for 5 days every following month.
Figure 8.
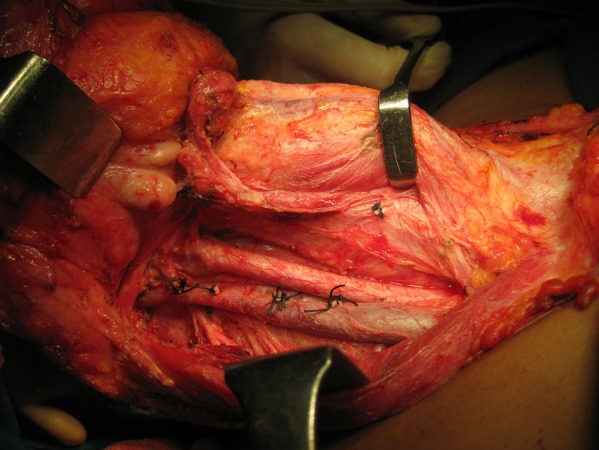
Intraoperatively, the tumour was visualised in the cervical oesophagus.
Figure 9.
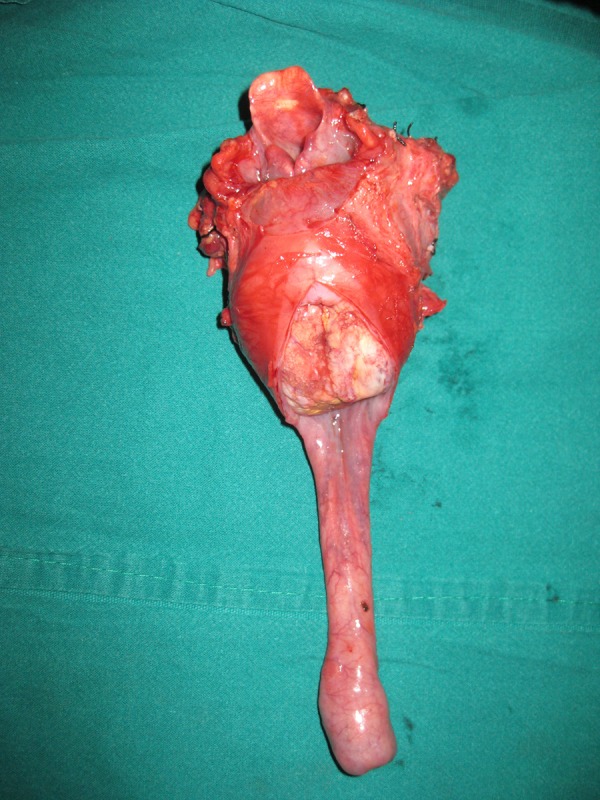
Total laryngopharyngo-oesophagectomy specimen showing the tumour in the cervical oesophagus.
Outcome and follow-up
The patient was advised regular monthly follow-up in view of the poor prognosis of amelanotic malignant melanoma of the oesophagus. The bone scan at 4-month follow-up revealed extensive osteolytic and osteoblastic metastasis in the ribs, ischium, parietal bones, systemic metastasis to the liver and regional metastasis to supraclavicular nodes for which she was advised dacarbazine-based palliative chemotherapy along with external beam radiation therapy to the hip which she refused. The patient died 8 months after initial presentation.
Discussion
PMME forms 0.1–0.2% of primary malignancies of the oesophagus1–4 and the amelanotic variant forms 10–25% of all PMMsE.1 2 PMME is extremely rare and has been described largely in the Japanese population due to the evidence of a high number of melanocytes in the oesophagus in that population.2 4 9 Male-to-female ratio is 2:1 and it has been classically seen in the 6th and 7th decades of life.1 2 4 7 8 The migration of neural crest cells to the middle and lower third of the oesophagus classically accounts for the presence of PMME in this location1 2 7 8; however, there have been reports of PMME in the upper third of the oesophagus.10 In our case, we had a young lady presenting to us with an amelanotic variant of PMME in the proximal one-third of the oesophagus. Tobacco and alcohol are important and common predisposing factors in malignancies of the oesophagus; however, their role in the development of PMME is unknown. Melanosis plays a role and has been reported as a predisposing factor in 23–25% of the cases in the world literature.4
Melanocytes have been demonstrated at the epitheliostromal junction of the oesophageal mucosa in 2.5–8% of the individuals.4 It has been found that melanoblasts originate and further differentiate into melanocytes in the oesophageal wall after migrating from the neural crest cells.1 2 4 8 The role of NRAS and BRAF mutations with the presence of KIT and PDGFRA gene has been demonstrated in the pathogenesis of PMME.11 Allen and Spitz12 constructed a diagnostic criteria for PMME; however, it was noted that only 40% of oesophageal melanomas fulfilled these criteria.2 4 13
A majority of patients with PMME present with non-specific symptoms of dysphagia, odynophagia, loss of appetite, weight loss, substernal epigastric discomfort and heartburn and, due to the rare nature of the disease, the level of suspicion is low.1 2 4 8 The median time of presentation is usually 3–3.5 months from the time of onset of symptoms.1 2 In our case, the patient presented with rapidly progressive dysphagia and swelling in the anterior aspect of the neck and the time of presentation from the onset of symptoms was 1 month. On examination, PMME appears to be commonly polypoidal; however, ulcerative variants have been seen.4 8 9 13 The level of pigmentation due to the submucosal component varies depending on the presence of the quantity of melanin.1 4 8 Solitary intraluminal tumours are commonly seen, although the presence of multiple tumours with satellite lesions have been reported.4 8 In our patient, there was a smooth bulge in the posterior hypopharyngeal wall with extension into the upper oesophagus.
Amelanotic PMME grows radially and is extremely aggressive.4 8 13 Majority of the patients (around 40%) have metastasis by the haematogenous or lymphatic route at the time of presentation. Perioesophageal chain of lymph nodes and supraclavicular lymph nodes are involved initially followed by a distant lymphatic spread.2 4 8 13 Distant metastasis to organs like the liver, lungs, brain, pleura, peritoneum, bone and heart is by the haematogenous route.2 4 8 13 Our patient presented with locoregional metastasis at first presentation and developed distant metastasis to the bone and liver following surgical treatment and postoperative chemoradiation therapy.
Various diagnostic modalities have been used for the diagnosis of PMME. They include contrast oesophagography, endoluminal ultrasound, contrast-enhanced CT (CECT) scan, MRI, endoscopy of the GI tract with biopsy with IHC of the biopsy specimen, fludeoxyglucose-positron emission tomography (FDG-PET), Gallium-67 and single-photon emission CT (SPECT).1 2 Contrast oesophagography shows the characteristic gross appearance of the tumour.1 2 4 8 9 13 CECT and MRI identify the site and extent of the tumour growth along with detection of locoregional and distant metastasis of the tumour.1 2 8 13 Endoscopy and biopsy with IHC form the mainstay of confirmatory diagnosis of PMME.1–3 8 IHC helps to differentiate poorly differentiated carcinomas from malignant melanoma.1 2 9 HMB-45, S-100 protein, Vimentin, Melan A, neuron-specific enolase positivity and cytokeratin, P-63 and CEA negativity confirm the presence of melanoma and exclude carcinoma.1 2 4 8 CD117 excludes the presence of GI tumours. Desmin is used to differentiate amelanotic melanomas from muscle tumours.2 Endoluminal ultrasound acts as an adjunctive tool in the diagnosis of tumour extent and extraction of biopsies for histopathological examination.1 FDG-PET has excellent sensitivity for detecting lymph node metastasis and serves as an excellent tool for detecting local and distant metastasis and to monitor the activity of the tumour during treatment.1 14 15 Gallium-67 and SPECT can also assess the tumour extent, size and metastasis.1 A review of the recent literature shows the application of KIT and PDGFRA transmembrane oncoproteins to detect PMME and differentiate it from melanoma of the skin.11
The poor prognosis of amelanotic PMME, cost effectiveness and morbidity associated with different modalities of therapy often poses a dilemma in the treatment decision of this aggressive tumour. Surgical resection forms the mainstay of therapy for amelanotic PMME.1 2 4 8 9 13 Adjunctive therapies include radiation therapy, chemotherapy and immunotherapy which, when used as a single modality, have proven ineffective with high recurrence rates.1 2 4 8 Surgical resection usually includes a total/near total oesophagectomy with wide clearance margins.1 2 4 8 9 13 Endoscopic laser resection can be used for small tumours or for palliation therapy.4 8 Preoperative chemoradiation has been advocated for downstaging the tumour and improving the survival rates.1 9 13 Presence of metastatic disease warrants the role of radiation therapy.2 4 Chemotherapy with drugs in combination or when used alone has varying results. Dacarbazine and interferon-β, when used preoperatively, have shown favourable responses in PMME.1 8 9 13 Development of interferons to specific antigens of melanoma as immunotherapy has evolved over the past decade and shows promise for the future therapy in melanoma.8 Our patient underwent radical resection of the tumour with postoperative chemoradiation therapy. In spite of this treatment, she died 8 months after initial presentation.
Prognosis of PMME is extremely poor and death is usually due to disseminated disease. Five-year survival rate is less than 5% and median time of survival in PMME is less than 1 year.1 2 4 7 8 13
Learning points.
Amelanotic malignant melanoma is a rare, aggressive tumour of the oesophagus.
Endoscopy, biopsy and immunohistochemical staining form the mainstay of diagnosis.
Surgical clearance is the treatment of choice in amelanotic primary malignant melanoma of the oesophagus.
Prognosis is poor with a median time of survival of less than 1 year and 5-year survival less than 5%.
Acknowledgments
The authors would like to thank Dr Dipak Ranjan Nayak and Dr Suresh Pillai for their constant support.
Footnotes
Contributors: BR was the principal investigator and reviewed the manuscript. AMB prepared the manuscript and reviewed the literature. SVV collected the case data. ST documented the imaging data.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Kransfelder M, Seidl S, Dobritz M, et al. Amelanotic esophageal malignant melanoma: case report and review of literature. Case Rep Gastroenterol 2008;2:224–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stringa O, Valdez R, Beguerie JR, et al. Primary amelanotic melanoma of the esophagus. Int J Dermatol 2006;45:1207–10 [DOI] [PubMed] [Google Scholar]
- 3.Terada T. A clinicopathologic study of esophageal 860 benign and malignant lesions in 910 cases of consecutive esophageal biopsies. Int J Clin Exp Pathol 2013;6:191–8 [PMC free article] [PubMed] [Google Scholar]
- 4.Lee SH, Park SH, Kim HG, et al. Primary malignant melanoma of the esophagus. Yonsei Med J 1998;39:468–73 [DOI] [PubMed] [Google Scholar]
- 5.Baur EH. Ein fall von primaerem melanoma de oesophagus. Arb Geb Pathol Anat Inst Tuebingen 1906;5:343–54 [Google Scholar]
- 6.Garfinkle J, Cahan W. Primary melanocarcinoma of the esophagus. First histopathologically proven case. Cancer 1952;5:921–6 [DOI] [PubMed] [Google Scholar]
- 7.Sabanathan S, Enj J, Pradhan G. Primary malignant melanoma of the esophagus. Am J Gastroenterol 1989;84:1475–81 [PubMed] [Google Scholar]
- 8.Morita FHA, Ribeiro U, Jr, Sallum RAA, et al. Primary malignant melanoma of the esophagus – a rare and aggressive disease. World J Surg Oncol 2013;11:210–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suzuki Y, Aoyama N, Minamide J, et al. Amelanotic malignant melanoma of the esophagus: report of a patient with recurrence successfully treated with chemoendocrine therapy. Int J Clin Oncol 2005;10:204–7 [DOI] [PubMed] [Google Scholar]
- 10.Chalkiadakis G, Wihlm J, Morand G, et al. Primary malignant melanoma of the esophagus. Ann Thorac Surg 1985;39:472–5 [DOI] [PubMed] [Google Scholar]
- 11.Terada T. Amelanotic malignant melanoma of the esophagus: report of two cases with immunohistochemical and molecular genetic study of KIT and PDGFRA. World J Gastroenterol 2009;15:2679–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allen AC, Spitz S. Malignant melanoma: a clinicopathological analysis of the criteria for diagnosis and prognosis. Cancer 1953;6:1–45 [DOI] [PubMed] [Google Scholar]
- 13.Naomoto Y, Perdomo JA, Kamikawa Y, et al. Primary malignant melanoma of the esophagus: report of a case successfully treated with pre- and post-operative adjuvant hormone-chemotherapy. Jpn J Clin Oncol 1998;28:758–61 [DOI] [PubMed] [Google Scholar]
- 14.Vandewoude M, Cornelis A, Wyndaele D, et al. (18)FDG-PET-scan in staging of primary malignant melanoma of the oesophagus: a case report. Acta Gastroenterol Belg 2006;69:12–14 [PubMed] [Google Scholar]
- 15.Crippa F, Leutner M, Belli F, et al. Which kind of lymph node metastases can FDG-PET detect? A clinical study on melanoma. J Nucl Med 2000; 41:1491–4 [PubMed] [Google Scholar]


