Abstract
A 45-year-old Indian woman presented in neurosurgery outpatient with seizures, headache and vomiting for the past 1 month. MRI of the brain was suggestive of a malignant central nervous system (CNS) tumour. Histological and immunohistochemical examinations of stereotactic biopsy of the tumour were diagnostic of a low-grade diffuse small lymphocytic lymphoma of the CNS. No evidence of any occult systemic lymphoma was observed, confirming its ‘primary’ origin in the CNS. The diagnosis of a low-grade primary CNS lymphoma (PCNSL) is difficult as clinical and neuroradiological features are wide and variable. The clinical course is more indolent than a high-grade PCNSL and thus, a less aggressive and localised targeted treatment could be sufficient rather than the high dose, neurotoxic methotrexate-based chemotherapeutic treatment, recommended for high-grade PCNSL. Histological and immunohistological confirmation is therefore mandatory for early, appropriate treatment and prognostic implications.
Background
Primary central nervous system lymphoma (PCNSL) is a rare extra-nodal non-Hodgkin’s lymphoma (NHL) that arises in the central nervous system (CNS) without evidence of systemic lymphoma.1 It is a rare aggressive disease, reported to account for approximately 1% tumours of the CNS.2 A low-grade PCNSL is extremely rare and constitutes only about 3–4% of PCNSLs.3 4 Histologically, it is characterised by predominance of mature lymphocytes and a proliferation index of less than 20%.5 We report a case of a primary low-grade diffuse small lymphocytic lymphoma (SLL) of the CNS in a 45-year-old woman, rarely reported in Indian population, with emphasis on its wide differential diagnosis and the diagnostic challenges.
Case presentation
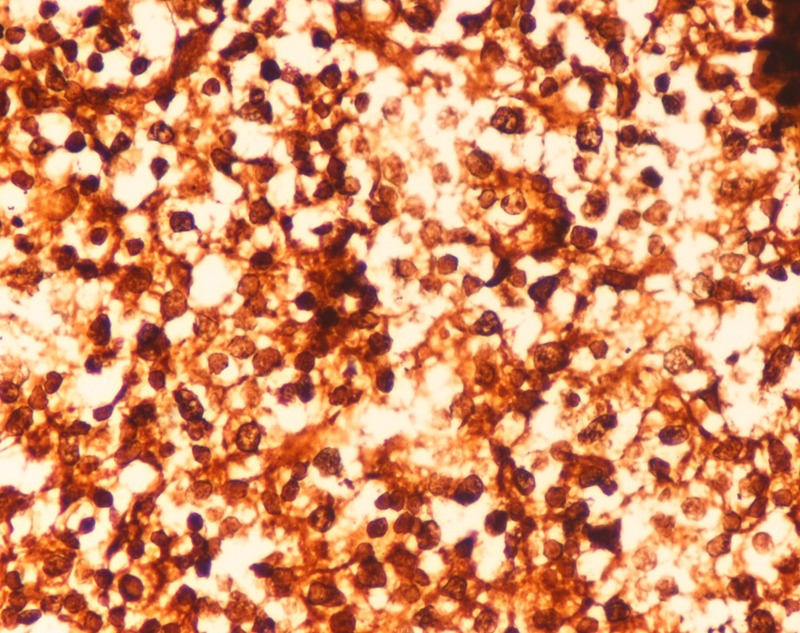
A 45-year-old Indian woman presented to the neurosurgery outpatient department with seizures, headache and vomiting for the past 1 month. There was no history of cognitive impairment, loss of coordination and gait abnormalities, peripheral neuropathy, cranial nerve dysfunction, ocular complaints, drug-intake (immunosuppressive drugs), radiation exposure, systemic infection or any autoimmune disease. Her family history was unremarkable. The patient's HIV screening tests were negative. On clinical examination, no lymphadenopathy or organomegaly was present. The patient was admitted and evaluated with MRI of the brain. T2-weighted MRI showed a well-defined, rounded hyperintense lesion with minimal oedema, involving the right caudate and anterior limb of the internal capsule, causing effacement of the ipsilateral lateral ventricle (figure 1). Another smaller similar lesion was seen in the region of the posterior limb of the internal capsule (figure 1). Postcontrast T1-weighted MRI showed a heterogeneous irregular enhancement of the tumour (figure 2). Radiological findings were suggestive of a malignant CNS tumour. A stereotactic biopsy of the brain tumour was performed and submitted for histopathological examination. H&E-stained biopsy tissue showed diffuse proliferation of monomorphic small mature lymphocytes (figure 3). The lymphocytes were seen in cellular aggregates and also as single, diffusely infiltrating cells (figure 3). These cells were predominantly small with occasional intermediate-sized variants. No foci or area of geographic necrosis was present. Mitosis was not appreciable. Reticulin stain demonstrated framework of fine reticulin fibres around groups and cellular aggregates and also around individual lymphocytes (figure 4). The fibrillary processes were not appreciable. Van-gieson stain (VG) was found to be negative. Immunohistochemical staining demonstrated positivity to leucocyte common antigen (LCA) CD-45 (figure 5A) and B-cell immunomarkers CD-20 (figure 5B) and CD-19 and negativity to T-cell marker CD-3. Further, immunostaining showed positivity to CD-5 (figure 5C) and CD-23 (figure 5D) and non-reactivity to CD-10, CD-138, bcl-6, multiple myeloma oncogene 1 (MUM-1). Glial fibrillary acidic protein (GFAP) was found to be negative in tumour cells. Epithelial membrane antigen (EMA) marker and synaptophysin were also non-reactive. Proliferation index was assessed by applying Ki-67 antibody and was found to be low (less than 10%; figure 6). Based on the clinical, radiological, histological and immunohistochemical findings, a diagnosis of a low-grade diffuse small lymphocytic of CNS lymphoma was made. Further, cerebrospinal fluid (CSF) analysis, bone marrow aspiration cytology and histology examinations, thoracic and abdominal CT and ophthalmology examination with slit-lamp were performed to detect any foci of occult systemic lymphoma but were found to be unremarkable, confirming the ‘primary’ origin of lymphoma in the CNS.
Figure 1.
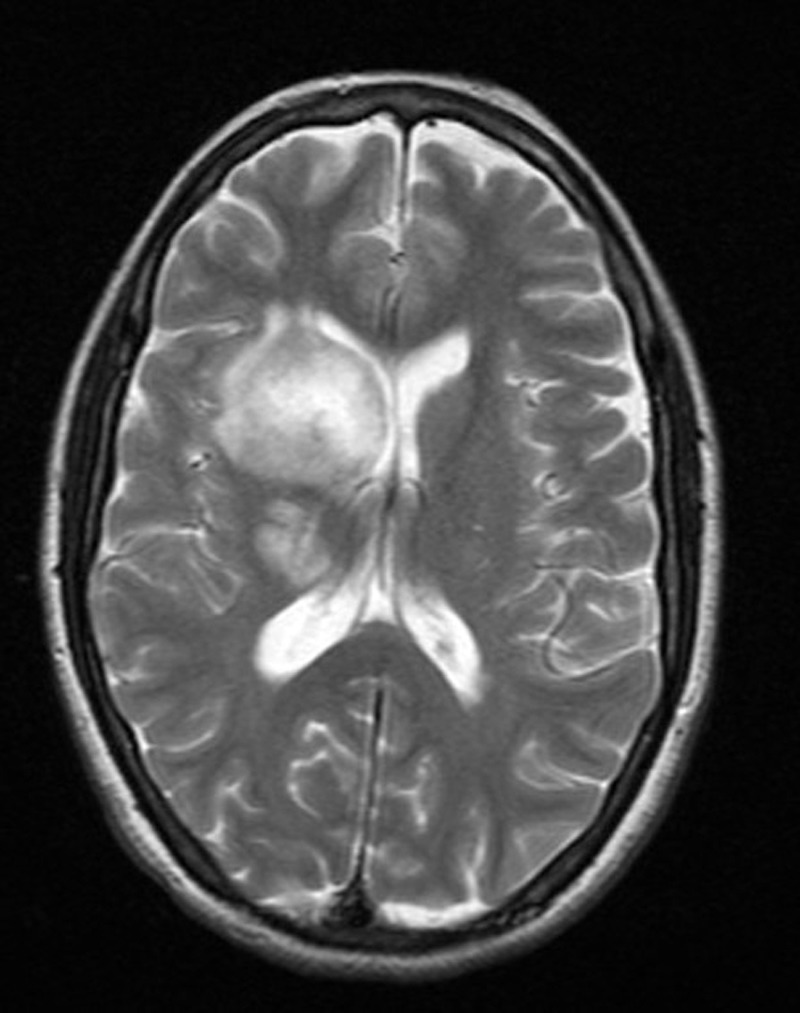
Axial T2-weighted MRI showing a round hyperintense tumour involving right caudate and anterior limb of internal capsule causing effacement of the ipsilateral lateral ventricle, another smaller lesion is noted in the region of posterior limb of internal capsule.
Figure 2.
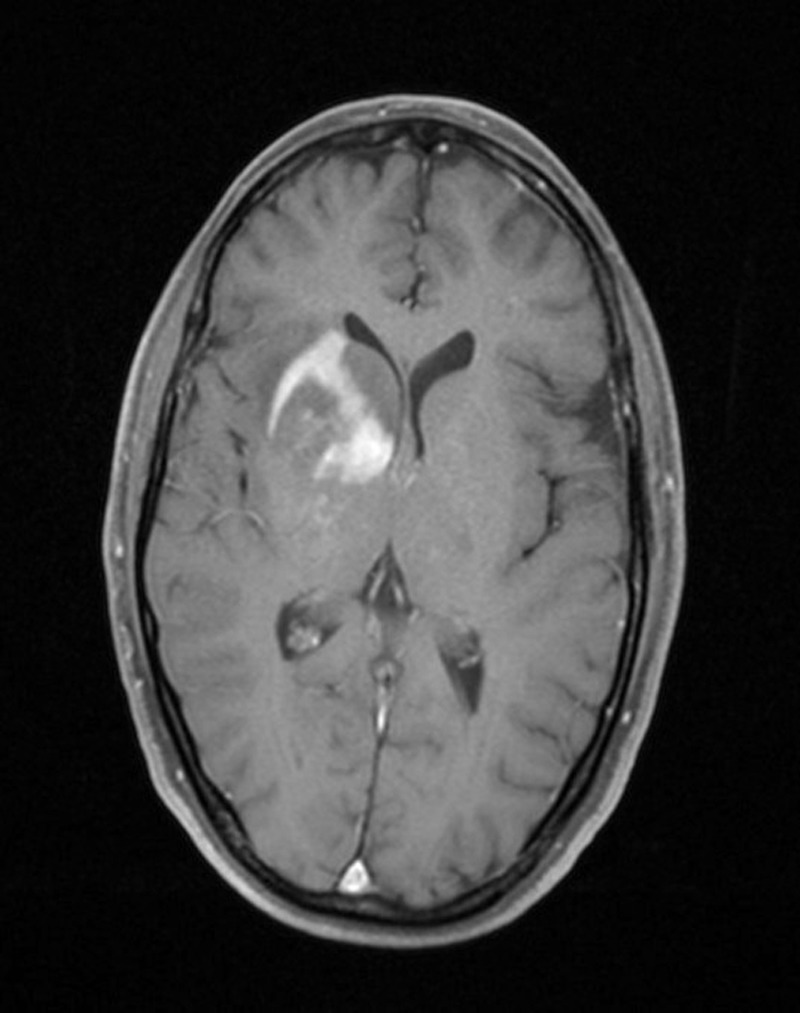
Postcontrast T1-weighted MRI showing heterogeneous irregular enhancement of the tumour.
Figure 3.
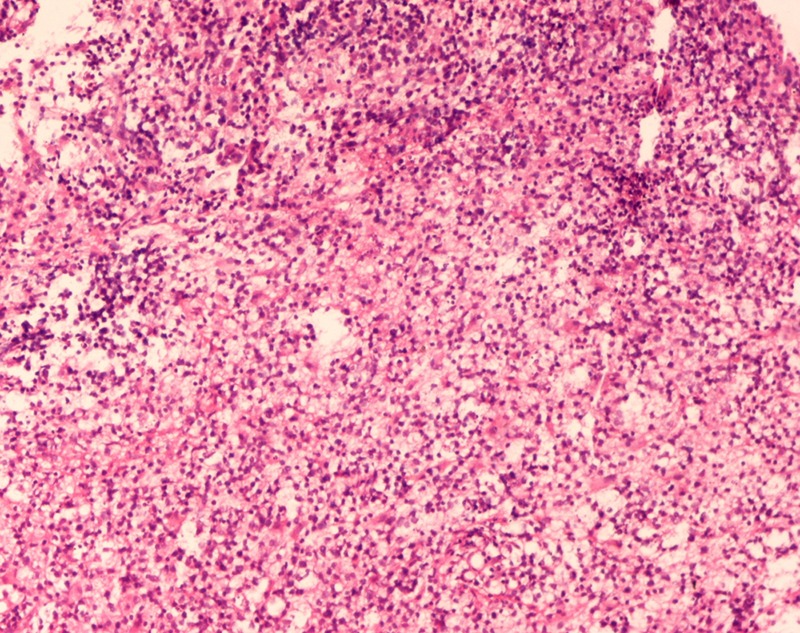
Stereotactic biopsy of the tumour showing diffusely proliferating monomorphic lymphocytes (H&E ×50).
Figure 4.
Tumour cells showing fine reticulin fibres around groups and cellular aggregates and also around individual cells (reticulin ×500).
Figure 5.
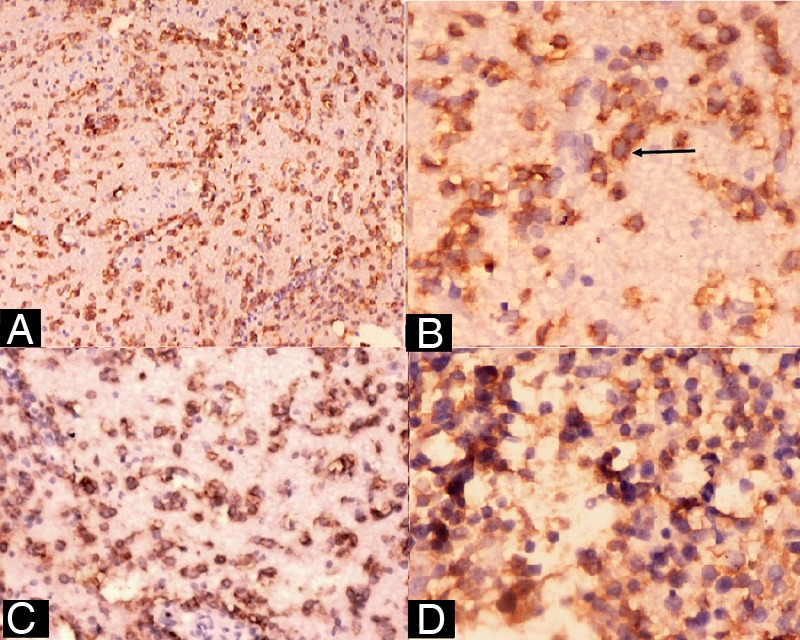
(A) CD-45 positivity of tumour cells (×50), (B) CD-20 positivity of tumour cells (arrow) (×1000), (C and D) CD-5 and CD-23 positivity of tumour cells, respectively ((C) ×125, (D) ×500).
Figure 6.
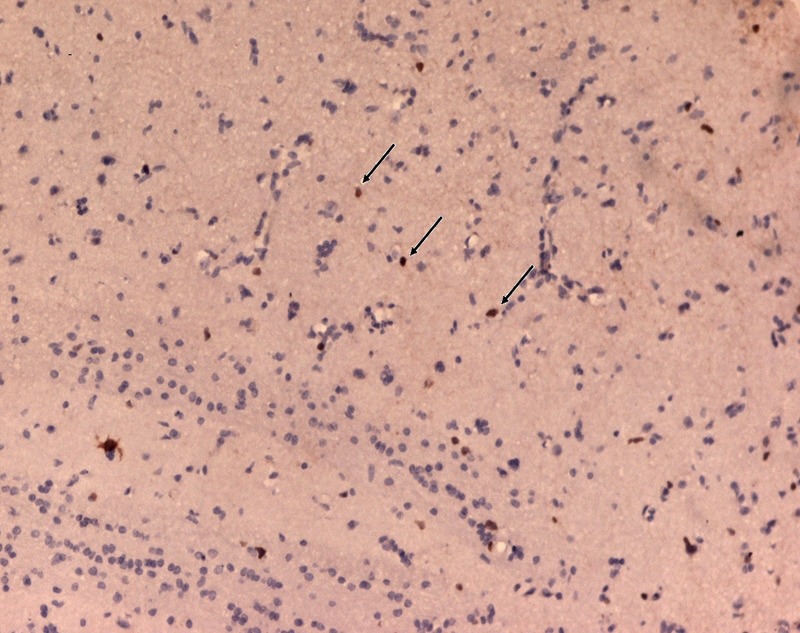
Ki-67-positive tumour cells (arrow), suggestive of low proliferation index (less than 10%) (Ki-67 antibody ×50).
Investigations
Routine haematological, urine examination and biochemical tests were found within normal limits. The patient's liver and renal function tests were also found to be normal. Serum lactate dehydrogenase level was within normal limit for age (130 U/L; reference range: 115–221 U/L).
Differential diagnosis
The differential diagnoses in the present case include benign conditions such as toxoplasmosis, demyelinating diseases-like multiple sclerosis (MS) and malignant tumours including glioblastoma multiforme (small-cell variant), small round cell tumours such as medulloblastoma, primitive neuroectodermal tumour (PNET), pineoblastoma, metastatic small cell carcinomas and other CNS lymphomas including extranodal marginal zone B-cell lymphomas, lymphoplasmacytic lymphoma, intravascular B-cell lymphomas and follicular lymphoma. The findings in the present case were consistent with a primary low-grade SLL of CNS.
Treatment
Keeping in view, the histological diagnosis of primary low-grade SLL of CNS in the present case and the neurotoxic effects of methotrexate chemotherapy, the patient was treated with localised surgical excision of the tumour with targeted precision radiotherapy without chemotherapy.
Outcome and follow-up
Postoperatively the patient was monitored for 10 days and the recovery was gradual and uneventful. The patient is being followed up and clinical signs and symptoms showed regression during the past 2 months of follow-up.
Discussion
A low-grade PCNSL is extremely rare and it is important to distinguish it from a high-grade PCNSL as its clinical course is more indolent and therefore, a less aggressive and localised targeted treatment therapy could be sufficient rather than the high-dose, neurotoxic methotrexate-based treatment, recommended for a high-grade PCNSL.5
A delay in establishment of diagnosis of a low-grade PCNSL may be possible due to its wide and variable clinical and radiological features which may overlap with high-grade CNS lymphomas and other primary malignant CNS tumours. A patient with PCNSL usually presents with cognitive dysfunction, psychomotor slowing, personality changes and disorientation. However, these symptoms were absent in the present case. Presentation with seizures, as was seen in this case, is uncommon and have been reported in only 14–20% patients in different studies.3 6
Radiologically, a low-grade PCNSL often shows hyperintensity on T2-weighted MRI, as was observed in the present case. However, hyperintensity may also be possible with a high-grade PCNSL, causing diagnostic difficulty. Previous studies documented that there appears to be no correlation of lymphoma histology to imaging and histological characterisation based on neuroradiological appearance was not possible.7 A low-grade PCNSL usually shows heterogenous postcontrast enhancement, compatible with the present case, rather than homogeneous enhancement seen in high-grade lymphomas.5 8 However, low-grade lymphomas have also been reported to show faint or absent postcontrast enhancement, in contrast to the present case in which intense enhancement was noticed.5 8
It has been reported that occult systemic lymphoma may be detected in up to 7% of patients initially presenting with brain lymphoma and in 27% patients at relapse.9 Therefore, it is recommended to perform a thorough clinical examination along with bone marrow examination, CT of the chest and abdomen, testicular ultrasound in male patients, ophthalmology examination by slit-lamp and CSF analysis for pleocytosis and cytomorphological evidence of lymphoma cells for leptomeningeal infiltration in order to detect occult systemic lymphoma.10 11 In the present case, any evidence of occult systemic lymphoma was not observed, confirming its ‘primary’ origin in the CNS.
Diagnosis confirmation and further histological typing and specification of PCNSL is difficult as usually only a small amount of biopsy material is submitted which is often a problem in brain tumours diagnosed by stereotactic biopsy. Histologically, majority of PCNSL were reported to be diffuse large B-cell lymphomas (DLBCL) with predominance of immature, blastic cells with a high proliferative index >50%.2 6 Presently, the tumour cells were diffusely proliferating monomorphic small lymphocytes with proliferation index of less than 10%, compatible with a low-grade lymphoproliferative tumour. Although B-cell markers such as CD-20 and CD-19 are positive in DLBCL, its strong MUM-1 and bcl-6 positivity along with non-reactivity to CD-23 and CD-5 differentiates it from SLL.
A low-grade PCNSL should be differentiated from benign conditions such as toxoplasmosis and demyelinating disorders-like MS. Clinical improvement and radiographic resolution of abnormal imaging in PCNSL to corticosteroid can be misinterpreted as steroid-induced remitting brain lesions of MS.12 Also, no specific imaging method can differentiate aggressive MS from PCNSL and biopsy is usually required.12 Histologically, MS is differentiable from PCNSL by predominant population of histiocytes and absence of malignant lymphoid cells.
Toxoplasmosis pose a diagnostic challenge and may manifest concomitantly with PCNSL in patients with AIDS. However, toxoplasmosis is distinguishable from PCNSL as it has more radiologically identifiable lesions such as ring enhancement, target-shaped on T2-weighted MRI.13
CNS neoplasms that cause diagnostic confusion with low-grade SLL are medulloblastoma, PNET, pineoblastomas. Histologically, these are round cell tumours, comprising of sheets of undifferentiated tumour cells, show fibrillarity and synaptophysin positivity of tumour cells in contrast to the present case of lymphoma which was non-fibrillar and synaptophysin negative.
Metastatic small cell carcinomas can have parenchymal invasion but histologically tumour cells show nuclear moulding, high N : C ratio, more cohesion as compared with lymphomas and demonstrable epithelial differentiation with cytokeratin and EMA immunomarker.
PCNSL can sometimes deceptively give a fibrillar appearance as cells can infiltrate parenchyma singly and mix with reactive gliosis leading to resemblance with gliomas. Radiologically, high-grade gliomas-like glioblastoma multiforme (GBM) are hyperintense on T2-weighted MRI similar to PCNSL but show marked peritumoral oedema, in contrast to the present case in which minimal oedema was observed. However, histologically, the small cell variant of GBM may cause diagnostic difficulty as it is characterised by proliferating monomorphic uniform small cells and may also show absence of characteristic identifiable features of a typical GBM like endothelial proliferation and necrosis with pseudopalisading by tumour cells. Immunohistochemically, it is distinguishable from low-grade SLL by GFAP-positive cytoplasmic processes of its tumour cells, high-proliferative index and negativity to immunomarkers CD-5, CD-23 and B-cell markers CD-20 and CD-19.
Extranodal marginal zone lymphoma and lymphoplasmacytic lymphoma of CNS are low-grade indolent lymphomas, characterised by proliferating lymphocytes with variable degrees of plasmacellular differentiation and show positivity to B-cell markers CD-20 and CD-19. However, non-reactivity to CD-5 and CD-23 and positivity of plasma cells to CD-138, differentiates these lymphomas from SLL.14 15
Intravascular lymphoma is a variant of extranodal DLBCL and can occur in CNS. It is angiotropic and is distinguishable from SLL by presence of atypical cells in the lumen of vessels and immunoreactivity to MUM-1 and bcl-6.16
Follicular lymphomas may involve CNS and express B-cell markers CD-20 and CD-19 but is differentiable from SLL by its immunohistochemical positivity to CD10 and bcl-6 and non-reactivity to CD-5.17 Similarly, though mantle cell lymphoma shows CD-5 positivity, it is characteristically non-reactive to CD-23.
Learning points.
Primary low-grade diffuse small lymphocytic lymphoma of central nervous system (CNS) is extremely rare.
The diagnosis of a primary low-grade CNS lymphoma (PCNSL) is difficult as clinical and neuroradiological features are wide and variable and may overlap with high-grade PCNSL and other malignant CNS tumours.
Low-grade PCNSL must be distinguished from a high-grade PCNSL as its clinical course is more indolent and thus, may require a less aggressive and localised targeted treatment, with the advantage of reduced risk of neurotoxicity.
The diagnosis of primary low-grade SLL should be concluded by extensive histological and immunohistochemical examinations to distinguish it from high-grade PCNSL and other malignant CNS tumours, for early, appropriate treatment and management.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Plasswilm L, Herrlinger U, Korfel A, et al. Primary central nervous system (CNS) lymphoma in immunocompetent patients. Ann Hematol 2002;81:415–23 [DOI] [PubMed] [Google Scholar]
- 2.Fine HA, Mayer RJ. Primary central nervous system lymphoma. Ann Intern Med 1993;119:1093–104 [DOI] [PubMed] [Google Scholar]
- 3.Bhagavathi S. Primary central nervous system lymphoma. Arch Pathol Lab Med 2008;132:1830–4 [DOI] [PubMed] [Google Scholar]
- 4.Jahnke K, Korfel A, O'Neill BP, et al. International study on low-grade primary central nervous system lymphoma. Ann Neurol 2006;59:755–62 [DOI] [PubMed] [Google Scholar]
- 5.Jahnke K, Schilling A, Heidenreich J, et al. Radiologic morphology of low-grade primary central nervous system lymphoma in immunocompetent patients. Am J Neuroradiol 2005;26:2446–54 [PMC free article] [PubMed] [Google Scholar]
- 6.Paul TR, Challa S, Tandon A, et al. Primary central nervous system lymphomas: Indian experience, and review of literature. Indian J Cancer 2008;45:112–18 [DOI] [PubMed] [Google Scholar]
- 7.Lanfermann H, Heindel W, Schaper J, et al. CT and MR imaging in primary cerebral non-Hodgkin's lymphoma. Acta Radiol 1997;38:259–67 [DOI] [PubMed] [Google Scholar]
- 8.Braks E, Urbach H, Pels H, et al. Primary central nervous system immunocytoma: MRI and spectroscopy. Neuroradiology 2000;42:738–41 [DOI] [PubMed] [Google Scholar]
- 9.Mohile NA, DeAngelis LM, Abrey LE. The utility of body FDG PET in staging primary central nervous system lymphoma. Neuro Oncol 2008;10:223–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abrey LE, Batchelor TT, Ferreri AJM, et al. Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol 2005;23:5034–43 [DOI] [PubMed] [Google Scholar]
- 11.Kiewe P, Fischer L, Martus P, et al. Meningeal dissemination in primary CNS lymphoma: diagnosis, treatment, and survival in a large monocenter cohort. Neuro Oncol 2010;12:409–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeAngelis LM. Primary central nervous system lymphoma imitates multiple sclerosis. J Neurooncol 1990;9:177–81 [DOI] [PubMed] [Google Scholar]
- 13.Miguel J, Champalimaud JL, Borges A, et al. Cerebral toxoplasmosis in AIDS patients, CT and MRI images and differential diagnostic problems. Acta Med Port 1996;9:29–36 [PubMed] [Google Scholar]
- 14.Sengos AP, Rodriguez JW, Wang HY, et al. Rare case of a primary non-dural central nervous system low grade B-cell lymphoma and literature review. Int J Clin Exp Pathol 2012;5:89–95 [PMC free article] [PubMed] [Google Scholar]
- 15.Abbi KK, Muzaffar M, Gaudin D, et al. Primary CNS lymphoplasmacytic lymphoma: a case report and review of literature. Hematol Oncol Stem Cell Ther 2013;6:76–8 [DOI] [PubMed] [Google Scholar]
- 16.Zuckerman D, Seliem R, Hochberg E. Intravascular lymphoma: the oncologist's “Great Imitator”. Oncologist 2006;11:496–502 [DOI] [PubMed] [Google Scholar]
- 17.Spectre G, Gural A, Amir G, et al. Central nervous system involvement in indolent lymphomas. Ann Oncol 2005;16:450–4 [DOI] [PubMed] [Google Scholar]


